www.jped.com.br
ORIGINAL
ARTICLE
A
randomized
controlled
trial
of
the
laryngeal
mask
airway
for
surfactant
administration
in
neonates
夽
,
夽夽
Rosilu
F.
Barbosa
a,b,∗,
Ana
C.
Simões
e
Silva
a,
Yerkes
P.
Silva
a,caUniversidadeFederaldeMinasGerais(UFMG),FaculdadedeMedicina,DepartamentodePediatria,LaboratórioInterdisciplinar
deInvestigac¸ãoMédica,BeloHorizonte,MG,Brazil
bMaternidadeUNIMED-BH,UnidadedeTerapiaIntensivaNeonatal,BeloHorizonte,MG,Brazil cHospitalLifecenter,DepartamentodeAnestesia,BeloHorizonte,MG,Brazil
Received23May2016;accepted17August2016 Availableonline25January2017
KEYWORDS Laryngealmask airway; Preterminfant; Pulmonarysurfactant
Abstract
Objective: Tocomparetheshort-termefficacyofsurfactantadministrationbylaryngealmask airwayversusendotrachealtube.
Methods: Preterminfants(28---35weeksofgestationalage),weighing1kgormore,with respi-ratorydistresssyndrome,requiringnasalcontinuouspositiveairwaypressure,withincreased respiratory effort and/orfraction ofinspiredoxygen (FiO2)≥0.40tomaintain oxygen
satu-ration 91---95%,were randomized to receive surfactant by LMA following nCPAP or by ETT followingmechanicalventilation(MV). Theprimaryoutcomewasaclinicalresponsedefined asFiO2≤0.30threehoursaftersurfactant.SecondaryoutcomesforLMAgroupwere:needof
surfactant retreatmentduringthefirst24h,MVrequirement, andpresence ofsurfactant in gastriccontent.
Results: Forty-eightpatientswererandomized;26intheLMAgroupand22intheETTgroup. Six of26 patients (23%)inthe LMAgroup andfiveof22 patients (22.7%)intheETTgroup didnotmeettheprimaryoutcome(p=0.977).Fourteen(53.8%)oftheLMApatientswerenot intubatednorventilated;12(46.1%)wereventilated:forsurfactantfailure(23%),for nCPAP failure (11.5%),and for late complications(11.5%). Groupswere similar regardingprenatal status,birthconditions,andadverseevents.Nosignificantgastriccontentwasfoundin61.5% oftheLMApatients.Oxygenandseconddosesurfactantrequirements,arterial/alveolarratio, andmorbiditiesweresimilaramonggroups.
夽
Pleasecitethisarticleas:BarbosaRF,SimõeseSilvaAC,SilvaYP.Arandomizedcontrolledtrialofthelaryngealmaskairwayforsurfactant
administrationinneonates.JPediatr(RioJ).2017;93:343---50.
夽夽
StudyconductedatUniversidadeFederaldeMinasGerais(UFMG),FaculdadedeMedicina,BeloHorizonte,MG,Brazil.
∗Correspondingauthor.
E-mail:rosilu@gmail.com(R.F.Barbosa). http://dx.doi.org/10.1016/j.jped.2016.08.007
Conclusions: SurfactantadministrationbyLMAshowedshort-termefficacy,withsimilar sup-plementaryoxygenneedcomparedtosurfactantbyETT,andlowerMVrequirement.Further studieswithlargersamplesizearenecessarytoconfirmtheseresults.
©2017SociedadeBrasileiradePediatria.PublishedbyElsevierEditoraLtda.Thisisanopen accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/ 4.0/).
PALAVRAS-CHAVE Máscaralaríngea; Neonatoprematuro; Surfactantepulmonar
Ensaiocontroladorandomizadodemáscaralaríngeaparaadministrac¸ão desurfactantesemneonatos
Resumo
Objetivo: Comparar aeficácia decurtoprazodaadministrac¸ãodesurfactante pormáscara laríngeaemcomparac¸ãoaotuboendotraqueal.
Métodos: Neonatosprematuros(28---35semanasdeidadegestacional),pesando1kgoumais, comSíndromedoDesconfortoRespiratório,necessitandopressãopositivanasalcontínuanasvias aéreas,comaumentodoesforc¸orespiratórioe/oufrac¸ãodeoxigênioinspirado(FiO2)≥0,40
paramanterasaturac¸ãodeoxigênio 91---95%,foramrandomizadospara recebersurfactante porMLseguidopornCPAPouporTEseguidoporventilac¸ãomecânica(VM).Oresultadoclinico primáriofoidefinidocomoFiO2≤0,30trêshorasapósosurfactante.Osresultadossecundários
dogrupodeMLforam: necessidadedesegundadosedesurfactantenasprimeiras 24horas, necessidadedeVMepresenc¸adesurfactantenoconteúdogástrico.
Resultados: Quarentaeoitopacientesforamrandomizados;26nogrupodeMLe22nogrupo deTE.Seisdentreos26pacientes(23%)dogrupodeMLecincodentre22pacientes(22,7%)do grupodeTEnãoapresentaramoresultadoprimário(p=0,977).Quatorze(53,8%)dospacientes dogrupodeMLnãoforamintubadosnemventilados;12(46,1%)foramsubmetidosaVM:por falhadosurfactante (23%),por falhadanCPAP(11,5%)epor complicac¸ões tardias (11,5%). Osgruposforamsemelhantesem relac¸ãoàscondic¸õespré-nataisedenascimentoea ocor-rênciadeeventosadversos.Nãofoiencontradoconteúdogástricosignificativoem61,5%dos pacientesdogrupodeML.Asnecessidadesdeoxigênioedasegundadosedesurfactante,o índicearterial/alveolareasmorbidadesforamsemelhantesentreosgrupos.
Conclusões: Aadministrac¸ãodesurfactanteporMLmostroueficáciadecurtoprazocom neces-sidadecomplementardeoxigêniosemelhanteaosurfactanteporTEemenornecessidadede VM.Serãonecessáriosestudosadicionaiscomtamanhodaamostramaiorparaconfirmaresses resultados.
©2017SociedadeBrasileiradePediatria.PublicadoporElsevierEditoraLtda.Este ´eumartigo OpenAccesssobumalicenc¸aCCBY-NC-ND(http://creativecommons.org/licenses/by-nc-nd/4. 0/).
Introduction
Surfactanttreatment inneonatologypracticehas substan-tially reduced mortality and improved the prognosis of respiratorydistresssyndrome(RDS).1,2Traditionally,
surfac-tanthasbeen givenbyendotrachealtube (ETT),however, superimposed lung injury from facemask-bag or ETT-bag positive pressure ventilation (PPV) followed by mechani-cal ventilation (MV) may impair surfactant function and trigger an inflammatory response in the lung, leading to bronchopulmonarydysplasia(BPD).1,3Intheeraof
noninva-siverespiratorysupport,neonatologistsbegantosearchfor newtechniquesthatmight allownasalcontinuouspositive airwaypressure(nCPAP)andsurfactantatthesame time, withoutETTandMV.4---6
The laryngeal mask airway (LMA) is a supraglottic air-waydevicedesignedtomaintainasealaroundthelaryngeal inletto deliver PPV in situations of difficult airway man-agementandanesthesiapractice.7---9Fornewborns,LMAhas
shownitspotentialinavarietyofcircumstances,mainlyin
neonatal resuscitation and drug administration.6---10 A few
observational studies and a small randomized controlled trial (RCT)havereportedthe administrationofsurfactant throughLMA.3,6,10 Until now, therehas been nopublished
prospective RCT comparing surfactant administration by LMAvs.ETTandMV.
The aim of the present study was to assess if surfac-tantadministrationthroughLMAfollowedbynCPAPhasthe sameshort-termclinical efficacyforRDStreatmentasthe conventionaltreatmentbyETTfollowedbyMV.
Methods
Studydesignandpatients
committeeoftheinstitutionapprovedthestudyandwritten informed consent was obtained from the patient’s par-ents/guardians.Preterminfantswereassessedforeligibility and enrolled for randomization if they had the following characteristics:gestationalage(GA)atbirthbetween28---35 weeks and birth weight ≥1kg; <8h of age; requirement ofnCPAP, Silverman-Anderson(SA)scoregreater thanfour and/or respiratory frequency >60bpm and/or fraction of inspiredoxygen(FiO2)≥0.40tomaintainoxygensaturation (SpO2)91---95%;clinicaldiagnosisofRDS; typicalRDSchest
X-ray.11 The exclusioncriteriawere:GA>35weeks,major
congenitalanomalies;previousETT;Apgarscore<3at5min; chorioamnionitis,feverand/orruptureofmembranes>18h.
Randomizationandmasking
By using a table of random numbers, 60 patients were randomizedtooneoftwotreatmentarmsbeforedata col-lection.Attendingneonatologistsidentifiedeligiblepatients accordingtotheinclusionandexclusioncriteriaafter clin-icalevaluation,umbilicalveincatheterization,chestX-ray, arterial blood gas analysis, and assignment of surfactant treatment.Aftereligibilityandenrollment,only thesame investigator checked the sequential randomization, per-formedtheLMAor ETTinsertion,administeredsurfactant, and followed all patients for the next six hours. Those responsible for the dataanalysis were notmasked to the treatmentgroup.
Procedures
Prenatal, perinatal, and postnatal patient conditions and laboratory findings (vital signs, SA score, arterial blood gas analysis, Neonatal Infant Pain Scale (NIPS),12 and
arterial/alveolar (a/A) ratio) were registered before the procedures. All patients received the same surfactant preparation (poractant-␣, 120mg/1.5mL) and the same
dose (200mg/kg), bybolus instillationwithin thefirst 8h ofage.ThechestX-rayfindingswereevaluatedbeforeand sixhoursaftersurfactant.
Patients randomized to the oral ETT group received premedication (remifentanil and midazolam bolus) and theViby---Mogensen scorewasusedtoevaluateintubation quality.13TubeplacementwasverifiedbychestX-raybefore
surfactantinstillation,whichwasfollowedbyconventional MV. Patients were extubated as soon aspossible (inspira-torypressurelessthan20cmH2O,respiratoryfrequency30
orless,andFiO2<0.40).
Patients randomized tothe LMA group were on nCPAP 5---6cmH2O and received surfactant through a ProSealTM
(Teleflex®,NC,USA)sizeoneLMA(PLMA;IntaventOrthofix --- Maidenhead,United Kingdom),inserted by the classical techniquedescribedbyBrain.7,8Gastriccontentswere
aspi-rated and the orogastric tube was removed before LMA insertion. Lidocainegel was utilizedaround theLMA cuff tolubricateandtopreventdiscomfort.Afterinsertion,the proximalendoftheairwaydevicewasconnectedtoa self-inflatedbagforPPV,duringoneortwominutes,timeenough toachieveSpO2>88%andheartrate(HR)>100beats/min.
Afterward,athinsiliconesize6-Fcatheter(shortened previ-ouslytobeapproximately1cmbiggerthantheairwaydevice
length)wasintroduced throughthe LMA airwaytube asa conduitforsurfactantadministrationintwotofouraliquots, according topatient tolerance and surfactantreflux, fol-lowedbyLMA-bagPPVduringoneortwominutestoobtain SpO2andHRimprovement.14 When fulldose ofsurfactant
wasadministered,LMA wasremovedandnCPAPrestarted. AthinsiliconeorogastrictubewasreplacedafterLMA with-drawal to measure gastric contents. Any gastric volume aspirated wasassumed to be surfactantbecause patients hadnotbeenfedpriortoenrollment.
Cardio-respiratory adverse events during both proce-dureswereregistered.Justaftertheprocedures,NIPSwas recorded again. Likewise, cardio-respiratory signs, nCPAP pressure,andventilatorsettingsweremonitored and reg-istered soon after procedures and at five, 20, 35, and 60min after surfactant and then every 30min until six hoursafterwards.Arterialblood gasanalysisanda/Aratio wereobtainedthreehoursaftersurfactant.Respiratory sup-portandFiO2wereprogressivelyreducedintheETTgroup
during the first six hours, based on HR, blood pressure, respiratoryeffort,SpO2,andblood gasanalysis.The CPAP
pressureofLMApatientswasmaintainedat5---6cmH2Oand
FiO2wasreducedaccordingtothesamecardio-respiratory
parameters. After the first six hours of study, attending neonatologistsmadedecisionsforbothgroupsregardingthe need for retreatment and/or MV. A second dose of sur-factant was administered six to 33h after the first dose if any infant presented the following: increasing respi-ratory effort, hemodynamic instability, frequent apneas (≥two/hour),pH<7.20,partialarterialpressureofcarbon
dioxide(PaCO2)>65mmHg,partialarterialpressureoxygen
(PaO2)<50mmHg,SpO2<91%,orFiO2≥0.50.11LMApatients
receivedaseconddosebyETTfollowedbyMV.
Outcomes
FiO2≤0.30threehoursaftersurfactantwastheprimary out-comesinceoxygenneed reductionresults fromsurfactant responseas gas exchangeimproves. It wasalso based on currenttherapyprotocolsandmanufacturer’slow-threshold criteriaforretreatmentthatindicateanadditional surfac-tantadministrationwhentheFiO2neededis>0.30afterthe
firstdose.15,16Thesamplesizewascalculatedas30patients
pergrouptoobtainapowerof85%indetectingsignificant differences(alpha=0.05)inFiO2betweengroups.17
Further-more,aninterimanalysiswasperformedwhen48patients wereenrolled andrandomized. This analysis showedthat both groups were equivalent. Therefore, due to ethical reasons,itwasdecidedtointerruptthestudysincethe pri-maryoutcomewasachievedwith26patientsinLMAgroup. Indeed,aposthocanalysisshowedthat20patientsineach groupweresufficienttodetectaminimumdifferenceofFiO2
betweengroupsassmallas8%(aboveorevenbelow; two-tailed)for abetaerror of20%andanalphaerror of5%.17
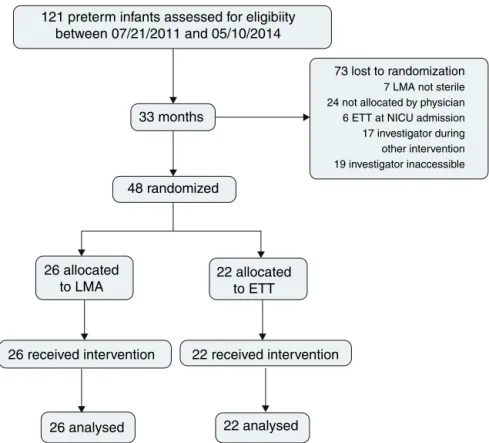
121 preterm infants assessed for eligibiity between 07/21/2011 and 05/10/2014
33 months
48 randomized
26 allocated to LMA
26 analysed 22 analysed
26 received intervention 22 received intervention 22 allocated
to ETT
73 lost to randomization
7 LMA not sterile 24 not allocated by physician 6 ETT at NICU admission 17 investigator during other intervention 19 investigator inaccessible
Figure1 Flowdiagramofparallel-randomizedtrial oftheLMAandETTtreatmentgroups.LMA,laryngealmaskairway; ETT, endotrachealtube;NICU,neonatalintensivecareunit.
events during procedures, mortality, duration of oxygen, nCPAP, MV and hospitalization,the incidence of intraven-tricularhemorrhage(IVH),pneumothorax,BPD,retinopathy ofprematurity(ROP),andearlyand/orlateonsetsepsis.
SurfactanttreatmentfailurebytheLMA wasdiagnosed when a second dose was indicated and administered by ETTatanytime.Insucha case,asinsufficientsurfactant shouldhavebeendeliveredtothelungsbyLMA,thestudy designestablishedforpatientsecurityandhigherchanceof response,thatthe seconddose shouldbeadministeredby ETT.CPAPfailurewasdiagnosedinLMApatientstreatedfor RDSwhentheydidnotneedsurfactantretreatment accord-ingtoradiologicaland bloodgas analysisfindings,but MV wasindicatedduetorespiratoryworsening.
Statisticalanalysis
Data were stored in Microsoft Excel (Microsoft®, WA, USA) spreadsheet and analyzed using Minitab version 16 (Minitab®, PA, USA). For categorical variables, the chi-squaredtestorFisher’sexacttestwasused.Forquantitative variables,theRyan---Joinertestwasusedtocheckfordata normality,followedbyparametric Student’st-test or non-parametricMann---Whitney.17Inallstatisticaltests,thelevel
ofsignificancewassetatp<0.05.
Results
During the study period, 121 preterm infants were born and assessed for eligibility. Surfactant treatment for RDS wasindicatedfor all patientsonnCPAP;48 patientswere
randomized,26forLMAinsertionandnCPAPand22forETT andMV;73werelostpriortorandomization(Fig.1).
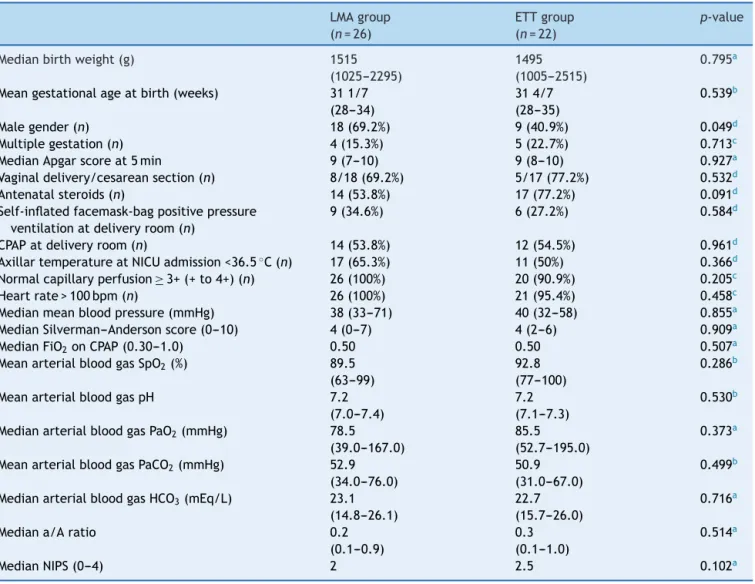
Table1presentsdemographicandclinicalcharacteristics atbirth,inadditiontopain,cardiovascular,andrespiratory supportdatabeforeLMAinsertionor ETT,whichwerenot significantlydifferent.
Soon after LMA and laryngoscope removal, NIPS eval-uation showed a significantly lower score for intubated patientswhohadjustreceivedpremedication(p=0.0001). There were no serious adverse events associated with ETTorLMAinsertionandsurfactantadministration.During theprocedures,patientsofbothgroups equallypresented transitorycardiovascularevents(HR<100beats/minand/or SpO2<80%) managed by PPV with immediate recovery
(p=0.594).
The respiratoryeffort measured by the SA scorethree and six hours after surfactant were significantly higher in LMA patients, but the a/A ratio, FiO2 on nCPAP or
MV, and PaCO2 values were equivalent in both groups
(Table2).
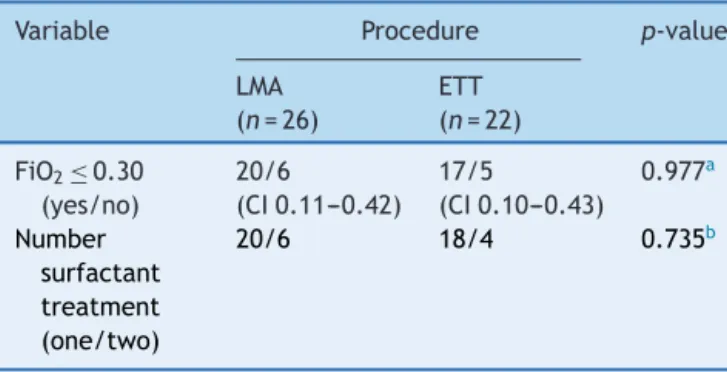
Elevenout48patients(22.9%)didnotmeettheprimary outcome: FiO2 was>0.30threehoursafter surfactant;six
(23.0%)intheLMAgroupvs.five(22.7%)intheETTgroup. Tenout48patients(20.8%)receivedasecond doseof sur-factant:six(23%)previouslytreatedbyLMAandfour(18.1%) byETT(Table3).FourLMApatientsreceivedaseconddose of surfactant in thefirst day of life, sixto 12h after the firstone;tworeceivedretreatmentduringtheseconddayof lifebecausetheywereintubatedlateron,duetoincreasing respiratoryeffort.
Table1 DemographicandclinicalcharacteristicsatbirthofpatientsfromtheLMAandETTgroups.Pain,cardiovascular,and respiratorysupportdatabeforeLMAinsertionorendotrachealintubation.
LMAgroup (n=26)
ETTgroup (n=22)
p-value
Medianbirthweight(g) 1515
(1025---2295)
1495 (1005---2515)
0.795a
Meangestationalageatbirth(weeks) 311/7 (28---34)
314/7 (28---35)
0.539b
Malegender(n) 18(69.2%) 9(40.9%) 0.049d
Multiplegestation(n) 4(15.3%) 5(22.7%) 0.713c
MedianApgarscoreat5min 9(7---10) 9(8---10) 0.927a
Vaginaldelivery/cesareansection(n) 8/18(69.2%) 5/17(77.2%) 0.532d
Antenatalsteroids(n) 14(53.8%) 17(77.2%) 0.091d
Self-inflatedfacemask-bagpositivepressure ventilationatdeliveryroom(n)
9(34.6%) 6(27.2%) 0.584d
CPAPatdeliveryroom(n) 14(53.8%) 12(54.5%) 0.961d
AxillartemperatureatNICUadmission<36.5◦C(n) 17(65.3%) 11(50%) 0.366d
Normalcapillaryperfusion≥3+(+to4+)(n) 26(100%) 20(90.9%) 0.205c
Heartrate>100bpm(n) 26(100%) 21(95.4%) 0.458c
Medianmeanbloodpressure(mmHg) 38(33---71) 40(32---58) 0.855a
MedianSilverman---Andersonscore(0---10) 4(0---7) 4(2---6) 0.909a
MedianFiO2onCPAP(0.30---1.0) 0.50 0.50 0.507a
MeanarterialbloodgasSpO2(%) 89.5
(63---99)
92.8 (77---100)
0.286b
MeanarterialbloodgaspH 7.2 (7.0---7.4)
7.2 (7.1---7.3)
0.530b
MedianarterialbloodgasPaO2(mmHg) 78.5
(39.0---167.0)
85.5 (52.7---195.0)
0.373a
MeanarterialbloodgasPaCO2(mmHg) 52.9
(34.0---76.0)
50.9 (31.0---67.0)
0.499b
MedianarterialbloodgasHCO3(mEq/L) 23.1
(14.8---26.1)
22.7 (15.7---26.0)
0.716a
Mediana/Aratio 0.2 (0.1---0.9)
0.3 (0.1---1.0)
0.514a
MedianNIPS(0---4) 2 2.5 0.102a
LMA,laryngealmaskairway;ETT,endotrachealtube;NICU,neonatalintensivecareunit;CPAP,continuouspositiveairway pressure;
bpm,beatsperminute;FiO2,fractionofinspiredoxygen;SpO2,oxygensaturation;PaO2,partialarterialpressureofoxygen;PaCO2,
partialarterialpressureofcarbondioxide;HCO3,bicarbonate;a/A,arterial/alveolarratio;NIPS,neonatalinfantpainscale.
a Mann---Whitneytest.
b t-Test.
c Fisher’sexacttest.
d X2test.
ofage,while,duetoprogressiverespiratoryeffortwithout retreatment,intubationwasperformedintwoofthem.One patientwasneverintubatednorventilatedandremainedon nCPAP.
Among 20 patients of LMA group whometthe primary outcome,seven(35%)wereintubatedafterward:threefor retreatment (one before and two after 24h of life); the remainingfourwereintubatedduetorespiratoryeffortor apneaandsepsisafter24h.
Overall, twelve (46.1%) LMA patients were intubated and mechanically ventilated, but only six patients (23%) experienced treatment failure requiring another dose of surfactant. CPAP failure was characterized in three patients (11.5%) who required MV without retreatment; anotherthreepatients(11.5%)wereintubatedduetolate
complications (pleural effusion and sepsis). Moreover, 14 LMApatients (53.8%) were neverintubatedor ventilated, independentofbirthweight.
Table2 Silverman---AndersonscoreandFiO23and6hafter surfactantinpatientsfromtheLMAandETTgroups;a/Aratio andPaCO23haftersurfactantinbothgroups;NIPSafterLMA insertionandendotrachealintubation.
SAscoreandFiO2---3 and6hafter surfactant.
a/AratioandPaCO2 ---3haftersurfactant. NIPS---afterLMAor ETT
Procedure p-value
LMA (n=26)
ETT (n=22)
SAscore3h Median
2.0 0.0 0.0001a
SAscore6h Median
0.5 0.0 0.017a
FiO23h
Median
0.30 0.25 0.111a
FiO26h
Median
0.30 0.25 0.468a
a/Aratio3h Mean
0.4 0.4 0.950b
PaCO23h
Median
44.5 41.0 0.425a
NIPSafter Median
2.0 0.0 0.0000a
FiO2, fraction of inspired oxygen; LMA, laryngeal mask
air-way;ETT,endotrachealtube;a/A,ratioarterial/Alveolarratio;
PaCO2,partialarterialpressurecarbondioxide;NIPS,neonatal
infantpainscale;SAscore,Silverman---Andersonscore.
aMann---Whitneytest.
b t-Test.
Table3 Primaryoutcomeandnumberofsurfactantdoses inpretermpatientsdiagnosedwithRDSfromLMAandETT groups.
Variable Procedure p-value
LMA (n=26)
ETT (n=22)
FiO2≤0.30
(yes/no) 20/6 (CI0.11---0.42) 17/5 (CI0.10---0.43) 0.977a Number surfactant treatment (one/two)
20/6 18/4 0.735b
RDS,respiratorydistresssyndrome;LMA,laryngealmaskairway;
ETT,endotrachealtube;FiO2, fractionofinspiredoxygen;CI,
confidenceinterval.
a
2test.
b Fisher’sexacttest.
Discussion
Since 1994, a gentler approach to MV in RDS treatment has been sought. Initially, the strategy of intubation sur-factantextubation(INSURE)wasintroduced,butitrequires laryngoscopy, ETT,and PPV and maycause pain and lung injury.18 Several trials demonstrated that INSURE did not
decreasedrates of mortalityor BPD and wasnotsuperior
to early nCPAP.4 Besides, the experience of being
intu-bated is unpleasant and painful; the adverse effects of laryngoscopyandETTincludelaryngospasm,bronchospasm, hypoxia, bradycardia, pulmonary and systemic hyperten-sion,raisedintracranialpressure,andincreasedriskofIVH. Theuseofpremedicationmayattenuatesucheffects,butit maycauserespiratorydepressionanddelayextubation.18---21
The assumption that infants did not feel pain has been proventobeuntrue;reducingpainisahumanandethical obligation.20,21In2010,theAmericanAcademyofPediatrics
recommendedpremedicationtobeusedforallintubations inneonates,exceptduringresuscitation.21
In2004,Kattwinkeletal.describedamethodof nasopha-ryngealsurfactantinstillationsoonafterbirth.However,no RCTs have proven its efficacy.22 Göpel etal. and Kanmaz
etal. demonstratedtheeffectiveness ofsurfactant deliv-eryfor spontaneouslybreathingpreterminfantsthrougha thin catheter.2,23,24 It could be an alternative to INSURE,
butthemethodstillrequireslaryngoscopy,usuallywithout analgesia.1 AsmallRCTbyAttridge etal.in2013 showed
an abrupt decrease in supplemental oxygen requirements following surfactant through LMA when compared to the controlgrouponnCPAPwithoutsurfactant.3Recently,
Pin-heiroetal.,inanon-blindedtrial,compared(withasimilar sampletothecurrentstudy)somewhatearlyorlate(4---48h oflife)surfactanttreatmentbyLMAafteratropinetoINSURE after atropine and morphine. They found a substantially higherrateofearlyfailure(within1hoftreatment)toavoid mechanicalventilationintheINSUREgroup,suggestingthat respiratory depression was responsible for the superiority oftheLMAstrategy.Enrollmentwasstoppedbecausethere wereimportantdifferencesbetweengroups.25
The present study compared a less invasive RDS sur-factant treatment (first 8h) by LMA to the conventional treatment. It was demonstrated that surfactant by LMA hadthesameshort-timeclinicalefficacyofETTtreatment, because it was followed by similar supplemental oxygen need and lower MV requirements. The authors sought an efficient,safe,andlessinvasivemethodforRDStreatment that prevents premedication, laryngoscopy, ETT, and MV. Because of all the adverse effects of laryngoscopy and ETT,theauthorsdidnotcompareLMAtoINSURE.Rather,a newlessinvasivemethodofsurfactantadministrationwas compared withthe worldwide-validated,ethical, invasive methodofRDStreatment.
Sinceitwasnotpossibletoblindtheprocedures,inorder to avoid the methodological bias of randomization, only oneinvestigatorhadaccesstothesequentialrandomization after enrollment.To preventbias ofdifferent individuals’ abilities and tostandardize techniques, thesame investi-gator wasthe only one responsible for LMA insertion and for all procedures and data collectionduring the first six hoursaftersurfactant.The observed60.3%lossofeligible patientswasattributedtoinitialresistanceor misinforma-tion of the attendingstaff about the methodology, which wasdependent ononlyoneinvestigator’savailability.The samplesizewouldhavebeenlarger,especiallyifthestudy designhadpermittedtheattendingneonatologiststobealso responsiblefortheproceduresandinterventions.
surfactant administration.3,7 Sedative and analgesic drugs
were alsonot required for LMA insertionin observational studies conducted by Trevisanuto et al. and in Brima-combe’sorinAttrigde’strials.3,6,10Althoughsometransient
bradycardia and desaturation occurred during the LMA insertionand/orsurfactantinstillation,theobstruction-like symptoms were judged to be similar to those frequently encounteredintreatmentbyETT,asdidAttridgeetal.3,7,26
Pinheiroetal.alsodidnotfindadverse effectsrelatedto LMA insertion,buttheyusedavagolytic agentbeforethe procedure.25
Inthecurrentstudy,higherSAscore3and6hafter sur-factant by LMA suggested slower clinical response,which maybedue tosmaller concentrationsreachingthe lungs. However,thereducedrespiratoryeffortfoundinthecontrol groupmayalsobeduetoaresidualeffectofthe premedi-cation.
IntheCOINtrial, >50%ofthepreterminfants failedon CPAPinthefirstthreedaysoflife.IntheCURPAPand SUP-PORTtrials,48.5%and67.1%,respectively,wereintubated to receive surfactant.1,2,11,15,16,27---29 Even though withless
immaturepatients,thepresentstudyfoundnCPAPfailurein 11.5%andsurfactantfailurein23%ofLMApatients.These respiratoryoutcomesmighthavebeenassociatedwith insuf-ficientsurfactantreachingthelungs,orperhapswithmore severeRDS.
Surfactantresponseisclinicallymeasurableinregardsto ventilation (pCO2),oxygenation(SpO2 anda/A ratio),and
supplementaloxygenneed(FiO2).Duringthefirstsixhours
aftersurfactantadministration, theinvestigator’sgoalfor bothgroupswastomanagerespiratoryandoxygensupport toreducethemandtoachieveFiO2≤0.3andSpO291---95%
withprogressiverespiratoryeffort reduction,withinthree hours after surfactant. Other authors also observed oxy-genationandFiO2responses inLMAandINSUREtreatment
groups25 orLMA-treatedpatientsandnon-surfactantnCPAP
groups.3
Consideringthefrequencyandvolumeofgastriccontent measuredaftersurfactantadministrationthroughLMA, oxy-gen requirements, and clinical outcomes, it seems that sufficient amounts of the drug reached the lungs. Brima-combe estimated that at least half of the surfactant by LMAdid.10 Attridgeetal.providedobjectiveevidencethat
surfactant deliveredby LMA reaches the lungs becauseit resultedinanimmediatedecreaseintheFiO2requirement.3
Pinheiroetal.alsofoundsmallpost-surfactantgastric aspi-ratevolumes.25
Maintenance forMV wassignificantlyhigherintheLMA group, especially due to the outcomes of four patients; three presented severe RDS and one sepsis. Possibly, when surfactant administered by LMA did not sufficiently reached the lungs, ventilation/oxygenation improvement was delayed, with impact on disease severity and MV duration.
The sample size wasnot large enough for a definitive conclusionregardingmorbiditiesandsecondary outcomes. A major limitation of LMA surfactantdelivery is that the smallestdevice availableis toolarge forthe infantsmost likelytohaveRDS,lessthan1kgand28weeksatbirth.6,10
Thelimitationsofthisstudywere:asinglecenter,variable GAandbirthweightrange,oneinvestigatorresponsiblefor all procedures, noblinding for data analysis, and lack of
specificprotocol for post-LMAsurfactantandof followup afterNICUdischarge.
In this study, the treatment groups were statistically equivalentintheirsupplementaloxygenneedsthreehours after surfactant. Fourteen infants (53.8%) from the LMA groupwereneverintubatedorventilated.Themanagement ofinfantsafterLMAsurfactanttreatmentwillrequirelarger multicentersamplesandalearningcurveregardingunknown features,includingtargetpopulation, besttreatment tim-ing,instillationmethodandsurfactantdose,analgesianeed, andrespiratoryandspecialradiologicaloutcomes.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
References
1.Bohlin K. RDS---CPAP or surfactant or both. Acta Paediatr. 2012;101:24---8.
2.HertingE.Lessinvasivesurfactantadministration(LISA)---ways todeliversurfactantinspontaneouslybreathinginfants.Early HumDev.2013;89:875---80.
3.AttridgeJT,StewartC,StukenborgGJ,KattwinkelJ. Adminis-trationofrescuesurfactantbylaryngealmaskairway:lessons fromapilottrial.AmJPerinatol.2013;30:201---6.
4.Abdel-LatifME,OsbornDA.Laryngealmaskairwaysurfactant administration for prevention of morbidity and mortality in preterminfantswithoratriskofrespiratorydistresssyndrome. CochraneDatabaseSystRev.2011:CD008309.
5.BibanP,ChiniL,BonettiP,SaccoF,ZagliaF,SantuzP. Exoge-noussurfactantreplacement:howtodeliverit?ActaBiomed. 2012;83:27---32.
6.TrevisanutoD,GrazzinaN,FerrareseP,MicaglioM,VergheseC, ZanardoV.Laryngealmaskairwayusedasadeliveryconduit for the administrationof surfactantto preterminfants with respiratorydistresssyndrome.BiolNeonate.2005;87:217---20.
7.BarataI.Thelaryngealmaskairway:prehospitalandemergency departmentuse.EmergMedClinNAm.2008;26:1069---83,xi.
8.TrevisanutoD,MicaglioM,FerrareseP,ZanardoV.Thelaryngeal maskairway:potentialapplicationsinneonates.ArchDisChild FetalNeonatalEd.2004;89:F485---9.
9.Schmölzer GM, Agarwal M,Kamlin CO,Davis PG. Supraglot-ticairwaydevicesduringneonatalresuscitation:anhistorical perspective,systematicreview,andmeta-analysisofavailable clinicaltrials.Resuscitation.2013;84:722---30.
10.BrimacombeJ,GandiniD,KellerC.Thelaryngealmaskairway foradministrationofsurfactantintwoneonateswith respira-torydistresssyndrome.PaediatrAnaesth.2004;14:188---90.
11.SUPPORT Study Group of theEunice Kennedy Shriver NICHD NeonatalResearchNetwork,FinerNN,CarloWA,WalshMC,Rich W,GantzMG,etal.EarlyCPAPversussurfactantinextremely preterminfants.NEnglJMed.2010;362:1970---9.
12.LawrenceJ,AlcockD,McGrathP,KayJ,MacMurraySB,Dulberg C.Thedevelopmentofatooltoassessneonatalpain.Neonatal Netw.1993;12:59---66.
13.Viby-MogensenJ,EngbaekJ,ErikssonLI,GramstadL,JensenE, JensenFS,etal.Goodclinicalresearchpractice(GCRP)in phar-macodynamicstudiesofneuromuscularblockingagents.Acta AnaesthesiolScand.1996;40:59---74.
15.RojasMA,LozanoJM,RojasMX,LaughonM,BoseCL,Rondon MA, et al. Very early surfactant without mandatory ventila-tioninprematureinfantstreatedwithearlycontinuouspositive airway pressure: a randomized, controlled trial. Pediatrics. 2009;123:137---42.
16.Kattwinkel J, Bloom BT, Delmore P, Glick C, Brown D, Lopez S,et al.High-versuslow-thresholdsurfactant retreat-ment for neonatal respiratorydistress syndrome.Pediatrics. 2000;106:282---8.
17.ArmitageP,BerryG.Theplanningofstatisticalinvestigations. In:ArmitageP,BerryG,editors.Statisticalmethodsinmedical research.2nded.Oxford:Blackwell;1987.p.179---85.
18.AguarM,CernadaM,BrugadaM,GimenoA,GutierrezA,Vento M. Minimallyinvasivesurfactant therapy withagastric tube is as effectiveas theintubation, surfactant,and extubation techniqueinpretermbabies.ActaPaediatr.2014;103:e229---33.
19.VenkateshV,PonnusamyV,AnandarajJ,ChaudharyR,Malviya M,ClarkeP,etal.Endotrachealintubationinaneonatal pop-ulationremainsassociatedwithahighriskofadverseevents. EurJPediatr.2011;170:223---7.
20.BarringtonK.Premedicationforendotrachealintubationinthe newborninfant.PaediatrChildHealth.2011;16:159---71.
21.AllenKA.Premedicationfor neonatalintubation:which med-ications are recommended and why. Adv Neonatal Care. 2012;12:107---11.
22.Kattwinkel J, Robinson M, Bloom BT, Delmore P, Ferguson JE. Technique for intrapartum administration of surfactant
without requirement for an endotrachealtube. J Perinatol. 2004;24:360---5.
23.GöpelW,Kribs A,ZieglerA,LauxR,HoehnT,WiegC,et al. Avoidanceofmechanicalventilationbysurfactanttreatmentof spontaneouslybreathingpreterminfants(AMV):anopen-label, randomised,controlledtrial.Lancet.2011;378:1627---34.
24.Kanmaz HG, Erdeve O, Canpolat FE, Mutlu B, Dilmen U. Surfactant administration via thin catheter during spon-taneous breathing: randomized controlled trial. Pediatrics. 2013;131:e502---9.
25.PinheiroJM,Santana-RivasQ,PezzanoC.Randomizedtrialof laryngealmaskairwayversusendotrachealintubationfor sur-factantdelivery.JPerinatol.2016;36:196---201.
26.Brimacombe JR, Berry AM, White PF. The laryngeal mask airway: limitations and controversies. Int Anesthesiol Clin. 1998;36:155---82.
27.MorleyCJ,DavisPG,DoyleLW,BrionLP,HascoetJM,CarlinJB, etal.NasalCPAPorintubationatbirthforverypreterminfants. NEnglJMed.2008;358:700---8.
28.DunnMS,KaempfJ,deKlerkA,deKlerkR,ReillyM,Howard D,etal.Randomizedtrialcomparing3approachestothe ini-tialrespiratorymanagementofpretermneonates.Pediatrics. 2011;128:e1069---76.