Contents lists available atScienceDirect
Journal of A
ff
ective Disorders
journal homepage:www.elsevier.com/locate/jad
Research paper
Brain antioxidant e
ff
ect of mirtazapine and reversal of sedation by its
combination with alpha-lipoic acid in a model of depression induced by
corticosterone
Tatiana de Queiroz Oliveira
a, Caren Nádia Soares de Sousa
a, Germana Silva Vasconcelos
a,
Luciene Costa de Sousa
a, Anneheydi Araújo de Oliveira
a, Cláudio Felipe Vasconcelos Patrocínio
b,
Ingridy da Silva Medeiros
a, José Eduardo Ribeiro Honório Júnior
b, Michael Maes
c,d,
Danielle Macedo
a,e, Silvânia Maria Mendes Vasconcelos
a,⁎aNeuropharmacology Laboratory, Drug Research and Development Center, Department of Physiology and Pharmacology, Universidade Federal do Ceará, Fortaleza,
Ceará, Brazil
bLaboratory of Pharmacology, University Center Christus
–Unichristus, Fortaleza, CE, Brazil cIMPACT Strategic Research Center, Deakin University, Geelong, Australia
dDepartment of Psychiatry, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand eNational Institute for Translational Medicine (INCT-TM, CNPq), Brazil
A R T I C L E I N F O
Keywords: Mirtazapine Depression Anxiety Alpha-lipoic acid
Corticosterone model of depression Oxidative stress
A B S T R A C T
Background: Depression is accompanied by activated neuro-oxidative and neuro-nitrosative pathways, while targeting these pathways has clinical efficacy in depression. This study aimed to investigate the effects of mirtazapine (MIRT) alone and combined with alpha-lipoic acid (ALA) against corticosterone (CORT) induced behavioral and oxidative alterations.
Methods:Male mice received vehicle or CORT 20 mg/kg during 14 days. From the 15th to 21st days they were divided in groups administered: vehicle, MIRT 3 mg/kg or the combinations MIRT+ALA100 or MIRT+ALA200. On the 21st day of treatment, the animals were subjected to behavioral tests. Twenty-four hours after the last drug administration hippocampus (HC) and striatum (ST) were dissected for the determination reduced glutathione (GSH), lipid peroxidation (LP) and nitrite levels.
Results:CORT induced anxiety- and depressive-like behaviors as observed by increased immobility time in the tail suspension test and decreased sucrose consumption. MIRT or MIRT+ALA are effective in reversing anxiety-and depressive-like behaviors induced by CORT. CORT anxiety-and MIRT alone prolonged sleeping time anxiety-and this effect was reversed by MIRT+ALA. CORT significantly increased LP, which was reversed by MIRT or MIRT+ALA. Nitrite levels were increased in CORT-treated animals and reversed by MIRT+ALA200 (HC), MIRT or MIRT+ALA (ST).
Limitation:A relative small sample size and lack of a washout period between drug administration and behavioral testing.
Conclusions:MIRT or MIRT+ALA reverse CORT-induced anxiety- and depressive-like behaviors probably via their central antioxidant effects. Augmentation of MIRT with ALA may reverse sedation, an important side effect of MIRT. Randomized controlled studies are needed to examine the clinical efficacy of this combination in human depression.
1. Introduction
Depression is a disabling mental disorder, with high incidence and chronic course characterized by depressed mood (APA, 2014;Kessler et al., 2003). This disorder is a multifactorial condition due to genetic,
biochemical, psychological, social and family influences being, for this reason, studied under different approaches (APA, 2014;Who, 2001).
There is now evidence that depression is characterized by activated neuro-oxidative and neuro-nitrosative pathways, which may drive neuroprogressive processes, namely neurotoxicity and excitotoxicity,
http://dx.doi.org/10.1016/j.jad.2017.05.022 Received 24 October 2016; Accepted 6 May 2017
⁎Correspondence to: Department of Physiology and Pharmacology, Federal University of Ceará, Rua Cel. Nunes de Melo 1127, 60431-270 Fortaleza, CE, Brazil. E-mail addresses:silvania_vasconcelos@yahoo.com.br,silvania@pq.cnpq.br(S.M.M. Vasconcelos).
Available online 11 May 2017
0165-0327/ © 2017 Elsevier B.V. All rights reserved.
stimulates hypothalamic–pituitary–adrenal (HPA)-axis activity, which is in part related to attenuation of glucocorticoid negative feedback mechanisms (Mizoguchi et al., 2003; Tafet and Nemeroff, 2015). Moreover, chronic stress activates oxidative pathways thereby increas-ing lipid peroxidation and the catabolism of monoamines, while reducing antioxidant enzyme activities (Moylan et al., 2014).
Based on HPA-axis hyperactivity following stress and depression, a preclinical model of depressive-like alterations was developed, whereby depressive behaviors were induced by repeated administration of corticosterone (CORT) (Zhao et al., 2008a). The rodents submitted to this depression model show increases in immobility time in the forced swimming test, a gold standard test for the screening of antidepressant drugs (Iijima et al., 2010), suggesting that repeated administration of glucocorticoids may mimic the symptoms of depression (Johnson et al., 2006; Silva et al., 2013; Sousa et al., 2015). Chronic treatment of rodents with CORT induces multiple anxiety- and depressive-like changes in behavior, neurochemistry and brain morphology similar to those observed in treatment resistant depression (TRD) (Murray et al., 2008) suggesting that this model may be used as a TRD model (Ago et al., 2013).
Mirtazapine (MIRT) is an atypical antidepressant with noradrener-gic and serotonernoradrener-gic effects, being one of the few antidepressants that do not have major effects on the reuptake of monoamines (Thase et al., 2010). This drug was developed in 1996 and its relevance reside on the manifestation of fewer side effects when compared to tricyclic anti-depressant drugs and a faster onset of action (Peña et al., 2005). Despite this, sedation and weight gain are important side effects that limits MIRT use (Thase et al., 2010).
Nevertheless, antidepressant therapies present major limitations, most importantly the late onset of action and limited clinical efficacy (Rush, 2007). Recent reviews showed that augmentation of antidepres-sant treatments with different antioxidant compounds may increase the efficacy of antidepressants (Maes et al., 2012). In this regard, our research group have studied the antidepressant effects of alpha-lipoic acid (ALA), an endogenous natural antioxidant (Silva et al., 2016, 2014, 2013;Sousa et al., 2015). Our previous results showed that ALA alone and combined with desvenlafaxine presented antidepressant-like effects in CORT-induced model of depression (Silva et al., 2016, 2013).
Thus, in the present study we hypothesized, based on the promising results obtained in previous preclinical study evaluating ALA antide-pressant-like effects (Silva et al., 2016, 2014, 2013;Sousa et al., 2015), that the combination of ALA with MIRT would potentiate the anti-depressant effects of MIRT and/or improve CORT-induced oxidative alterations in the brain. We also aimed at addressing one important limitation related to MIRT use, i.e. sedation in these animals.
Corticosterone (CORT - Sigma-Aldrich, St Louis, MO, USA) was dissolved in a saline solution containing 0.1% dimethyl sulfoxide and 0.3% Tween-80. Corticosterone 20 mg/kg was administered as a single daily subcutaneous injection, from 09:00 to 11:30 a.m. for twenty-one consecutive days. The dosage and route of administration for CORT was selected based previous studies (Silva et al., 2013;Sousa et al., 2015; Zhao et al., 2009). Alpha-lipoic acid (ALA, Sigma-Aldrich, St. Louis, MO, USA) was dissolved in an aqueous solution of 0.2% carboxymethy cellulose and administered by gavage for 7 consecutive days at doses of 100 or 200 mg/kg according to previous studies (Silva et al., 2013). Mirtazapine (MIRT, Remeron, Schering-Plough®) was dissolved in distilled water and administered by gavage for 7 consecutive days at the dose of 3 mg/kg according to a previous study (Engel et al., 2013). In the sleeping time test, a sub-hypnotic dose of sodium pentobarbital (35 mg/kg) diluted in distilled water was intraperitoneally (i.p.) administered 30 min before the test. All solutions were administered in a volume of 0.1 mL/10 g of body weight.
2.3. Experimental design
The animals were randomly divided into eight experimental groups as shown inFig. 1. In the present study, different animals were used for biochemical tests assay and for behavioral determinations.
i) Control group: mice received daily injections of saline solution containing 0.1% dimethylsulfoxide and 0.3% Tween-80 (s.c.) for 21 consecutive days;
ii) CORT-induced model of depression: mice in this group received daily subcutaneous injections of CORT (20 mg/kg, s.c.) once a day, between 09:00 and 11:30 a.m., for 21days (Silva et al., 2016, 2013; Sousa et al., 2015;Zhao et al., 2008a, 2008b).
iii) Groups treated with MIRT alone: mice received daily injections of saline solution containing 0.1% dimethylsulfoxide and 0.3% Tween-80 (s.c.) for 14 consecutive days. From 15th to 21st days of administration, the animals received MIRT (3 mg/kg, p.o.). iv) Groups treated with MIRT and ALA associated (MIRT+ALA): mice
received daily injections of saline solution containing 0.1% di-methylsulfoxide and 0.3% Tween-80 (s.c.) for 14 consecutive days. From 15th to 21st days of administration, the animals received MIRT (3 mg/kg, p.o.) and one hour after ALA (100 or 200 mg/kg, p.o.).
v) CORT-induced depression model treated with MIRT alone (CORT+MIRT): mice received repeated injections of CORT during 14 days to induce depressive-like behavior. From the 15th to 21st days of administration, the animals received CORT and MIRT (3 mg/kg).
CORT administration, the mice were randomly divided into groups that were further treated with MIRT (3 mg/kg). One hour after MIRT administration the animals were given ALA (100 or 200 mg/ kg).
On the twenty-first day of treatment, one hour after the last drug administration, the animals were randomly divided in subgroups for the behavioral determinations. i) One subgroup underwent the openfield followed by rota rod test and twenty-four hour after the last test, animals were sacrificed by rapid decapitation and the brains were quickly removed and placed on aluminum foil in a Petri dish on ice. Hippocampus (HC) and striatum (ST) were dissected for the measure-ment of oxidative parameters; ii) Other subgroup underwent the elevated plus maze and tail suspension in this order; iii) The other subgroup underwent sucrose preference; iv) The fourth subgroup underwent sleeping time test.
2.4. Behavioral assessments
2.4.1. Tail suspension test (n=6–10 animals)
For the Tail suspension test (TST), each mouse was suspended by the tail on the edge of a shelf placed 58 cm above a table top. The mouse was secured in the place via adhesive tape positioned approximately 1 cm from the tip of the tail. The time during which the mouse remained immobile over a 6-min period was recorded. As previously described, each animal was submitted to this test only once (Steru et al., 1985).
2.4.2. Sucrose preference test (n=8–12 animals)
The test was performed as previously described (Mao et al., 2014). Briefly, 72 h before the test, mice were trained to adapt 1% sucrose solution (w/v): two bottles of 1% sucrose solution were placed in each cage, and 24 h later 1% sucrose in one bottle was replaced with tap water for 24 h. After the adaptation, mice were deprived of water and food for 24 h. Sucrose preference test was conducted at 9:00 a.m. in which mice were housed in individual cages and were free to access two bottles containing 100 mL of sucrose solution (1%w/v) and 100 mL of water. After 1 h, the volumes of consumed sucrose solution and water were recorded and the sucrose preference was calculated by the following formula:
Sucrose preference(%) Sucrose consumption
Water consumption + Sucrose consumption × 100
2.4.3. Elevated plus maze test (n=6–10 animals)
The Elevated plus maze (EPM) for mice consisted of two perpendi-cular open arms (30×5 cm) and two perpendiperpendi-cular closed arms (30×5×25 cm) connected by a central platform (5×5 cm), 45 cm above thefloor. After the respective treatments, each animal was placed at the center of the EPM with its nose in the direction of one of the closed arms. The time of observation was 5 min. The parameters evaluated were: number of entries in the open arms (NEOA) and time of permanence in the open arms (TPOA). The percentage of entries into the closed arms (%ECA) and the percentage of time into the closed arms (%TCA) were calculated as an indicative of anxiogenic effect. Anxiolytic compounds reduce the animal's natural aversion to the open arms and promote the exploration thereof. On the other hand, the forced or voluntary passages of the animal into the open arms of the EPM are associated with hormonal and behavioral changes indicative of increased anxiety (Lister, 1987).
2.4.4. Sleeping time test (n=8–12 animals)
A sub-hypnotic dose of sodium pentobarbital (35 mg/kg, i.p.) was injected twenty minutes after the administration of vehicle or drugs. The sleeping time was determined as the interval between loss and recovery of righting reflex (Ferrini et al., 1974). The loss of righting reflex is the inability of the animal to return to the normal position when placed in the supine position. The criteria for the return of the righting reflex is that the animal must roll completely over three consecutive times (Mattei et al., 1998).
2.4.5. Openfield test (n=6–10 animals)
Locomotor activity was evaluated by openfield test (Archer, 1973). This test was conducted in afield (30×30×15 cm), divided into nine equal parts. The apparatus was placed in a room with red light. The parameters observed were the number of squares crossed (with all four feet) and number of rearing for a period of 5 min.
2.4.6. Rota rod test (n=6–10 animals)
This method allows the evaluation of the motor coordination of the animals. For this test mice were placed with all four paws on a 2.5 cm diameter rod, 25 cm high from thefloor rotating 12 rpm for 1 min. It was recorded the length of stay in the swivel bar in seconds (s) (Rosland et al., 1990).
2.5. Determination of oxidative stress parameters (n=5–10 animals)
2.5.1. Determination of reduced glutathione (GSH) levels
Reduced glutathione levels were determined to estimate
boiling water bath for 30 min. After cooling, the lipid peroxidation was determined using a microplate reader (Asys model UVM 340) set at 535 nm and expressed asμmol MDA/g wet tissue.
2.5.3. Nitrite determination
Nitrite levels, an indirect measure of NO, were determined based on Griess reaction (Green and Goldman, 1981; Radenovic and Selakovic, 2005) and expressed as nM/g wet tissue.
2.6. Statistical analysis
Two-way ANOVA followed by Tukey post hoctest was used with
“CORT model” (Vehicle and CORT) and“Drugs treatment” (Vehicle, MIRT and MIRT+ALA) as factors. The significance level was set at P≤0.05. Statistical analysis was performed with GraphPad Prism 6.0
for Windows, GraphPad Software (San Diego, CA, USA).
3. Results
3.1. Effects of the administration of MIRT or MIRT+ALA in mice submitted to CORT-induced model of depression
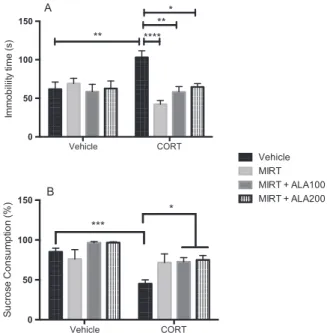
In the evaluation of depressive-like behavior by the TST, we observed a significant interaction between “CORT model” x “Drugs treatment”[F=6.90, df=3/54, p < 0.001] with significant main effect
of “Drugs treatment” [F=4.89, df=3/54, p=0.004] (Fig. 2). As expected, the administration of CORT increased the immobility time in TST when compared to control group (p=0.009) (Fig. 2A). This effect of CORT was reversed by treatment with MIRT (p < 0.001) and by the combination of MIRT+ALA 100 (p=0.005) or 200 (p < 0.05). No significant effect was observed in the groups treated with MIRT or MIRT+ALA alone when compared to control. In the sucrose preference test (Fig. 2B), repeated administration of CORT (p=0.0008) caused a significant decrease in sucrose consumption when compared to control group, characterizing an anhedonic-like behavior. This effect of CORT was reversed only by the combinations of MIRT+ALA100 (p=0.035) or 200 (p=0.025) (ANOVA: “CORT model” x “Drugs treatment”
significant main effect of “CORT model” [F =26.56, df=1/43, p < 0.001] and“Drugs treatment [F=5.15, df=3/43, p=0.004].
Again, the administration of MIRT or MIRT+ALA alone caused no significant alteration.
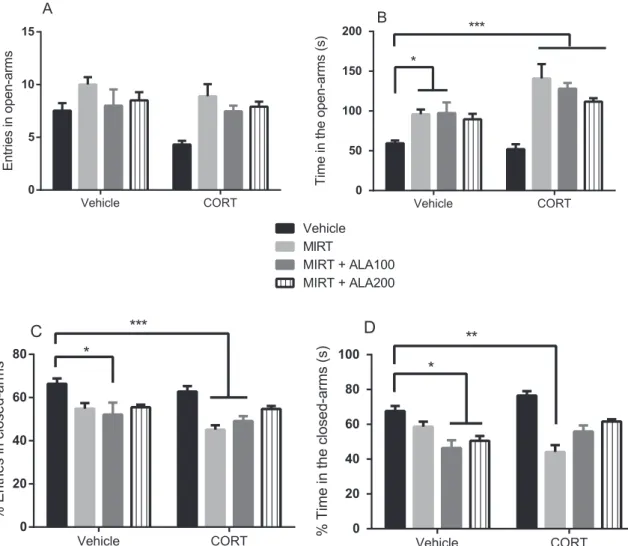
In the analysis of NEOA in the EPM test we observed significant main effects of “CORT model” [F=6.39, df=1/85, p= 0.013] and
“Drugs treatment”[F=8.91, df=3/85, p < 0.001] without significant
interaction between factors.Regarding TPOA, a significant interaction between“CORT model”דDrugs treatment”was observed [F=4.483, df=3/75, p=0.0060]. Tukey test revealed that the administration of MIRT alone (p=0.0128) or MIRT+ALA 100 (p=0.0412) increased TPOA when compared to control group (Fig. 3B). However, the
repeated administration of CORT alone caused no change in TPOA when compared to control group. All combinations studied, CORT+-MIRT, CORT+MIRT+ALA100, or 200 increased the TPOA when compared to control (p < 0.001). In the evaluation of %ECA (Fig. 3C) mice administered MIRT+ALA100 (p=0.0436), CORT+MIRT (p=0.0002) or CORT +MIRT+ALA100 (p=0.0009) presented de-creased levels of this parameter when compared to control. No significant alteration in the %ECA was observed by the comparison between control and CORT-treated animals (ANOVA %ECA: “CORT model”דDrugs treatment” [F=7.131, df=3/75), p=0.0003]). A significant interaction between “CORT model” x “Drugs treatment”
was observed in the %TCA [F=7.131, df=3/75, p=0.0003], with significant main effect of “Drugs treatment” [F=15.99, df=3/75, p < 0.0001]. According to Tukeypost hoctest, a reduction of %TCA was observed only in the group MIRT+ALA100 (p=0.0038), ALA200 (p=0.0199) or CORT+MIRT (p=0.0009) when compared to control (Fig. 3D). CORT caused no significant alteration in %TCA when compared to control animals (Fig. 3D).
In the evaluation of sleeping time (Fig. 4), a significant interaction between“CORT model”דDrugs treatment”was observed [F=4.642, df=3/60, p=0.0055]. CORT (p < 0.001) and MIRT (p < 0.001), alone or in combination (CORT+MIRT, p < 0.0001), increased this para-meter when compared to control. On the other hand, the groups CORT+MIRT+ALA100 (p < 0.05) or 200 (p < 0.01) presented a reversal of CORT-induced increase of the sleeping time, returning this parameter to control values. The same reduction of the sleeping time was observed in mice administered MIRT+ALA 100 or 200 alone when compared to MIRT animals (p < 0.05).
No significant alterations were observed in the number of crossings (Fig. 5A), rearings (Fig. 5B) or motor coordination (Fig. 5C) after the repeated administration of CORT, MIRT, MIRT+ALA 100 or 200 when compared to control group.
3.2. Effects of the administration of MIRT or MIRT+ALA on oxidative alterations induced by repeated administration of CORT
A significant interaction between “CORT model”דDrugs treat-ment”with significant main effect of“Drugs treatment”was observed in the HC and ST of mice (ANOVA HC: [F=11.94, df=3/47, p < 0.0001], main effect of “Drugs treatment” [F=48.18, df 3/47, p < 0.0001]; ST: [F=3.944, df 3/49, p=0.0134], main effect of“Drugs
treatment” [F=9.964, df 3/49, p < 0.0001]). In the HC (Fig. 6A)
according to Tukey test, the levels of GSH were increased in the groups administered MIRT+ALA100 or 200 (p < 0.0001) and CORT+MIR-T+ALA200 (p < 0.0001) when compared to control group. In the ST (Fig. 6B) this significant increase was detected in the group treated with CORT+MIRT+ALA200 when compared to control (p < 0.05).
Regarding MDA levels, a significant interaction between “CORT model”דDrugs treatment”was detected in the HC (Fig. 6C) and ST (Fig. 6D) with significant main effect of both factors (ANOVA interac-tion HC: [F=15.28, df 3/51, p < 0.0001]; ANOVA interacinterac-tion ST: [F=3.401, df 3/55, p=0.0239]. The repeated administration of CORT, CORT+MIRT or CORT+MIRT+ALA100 increased MDA levels in the HC (p < 0.0001) and ST (p < 0.0001) when compared to control group. Decreased levels of MDA were also detected in the groups MIRT+ALA100 or MIRT+ALA200 when compared to control group (p < 0.0001) in the studied areas. In the HC, CORT+MIRT or CORT+MIRT+ALA100 presented a significant decrease in MDA levels when compared to CORT (p < 0.0001). Despite this decrease, a significant reversal of CORT-induced increase in MDA levels was observed only in the group CORT+MIRT+ALA200 (p < 0.0001), reaching control values. In the ST, the group CORT+MIRT+ALA100 presented a significant decrease in MDA levels when compared to CORT (p < 0.05), although as observed in the HC, only the group CORT+-MIRT+ALA200 presented a significant reversal of CORT-induced increase in MDA levels (p < 0.0001).
Nitrite levels also presented a significant interaction between
Fig. 3.Entries in open arms (A), time in the open arms (B), % entries in closed-arms (C) and % time in the closed-arms (D) of the plus maze task of animals administered MIRT or in combination (MIRT+ALA) and submitted to the model of depression by repeated administration of CORT. Each bar represents the mean ± SEM of 6–10 animals/group. In B, *p < 0.05 vs vehicle, ***p < 0.001 vs vehicle. In C, *p < 0.05 vs vehicle, ***p < 0.001 vs vehicle. In D, *p < 0.05 vs vehicle, **p < 0.01 vs vehicle, according to two-way ANOVA followed by Tukey's multiple comparisons test. Abbreviations: ALA–alpha-lipoic acid; CORT–corticosterone; MIRT–mirtazapine.
Vehicle CORT 0
20 40 60 80
S
leeping t
im
e
(
m
in
)
Vehicle MIRT MIRT + ALA100 MIRT + ALA200 ***
*** ****
* **
** *
“CORT model”דDrugs treatment”in the HC (Fig. 6E) and ST (Fig. 6F) with significant main effect of both factors (ANOVA interaction HC: [F=47.98, df 3/42, p < 0.0001]; ANOVA interaction ST: [F=87.45, df 3/44, p < 0.0001]. Nitrite levels were significantly increased in mice submitted to repeated administration of CORT in the HC (p < 0.0001) and ST (p < 0.0001) when compared to control group. This effect of CORT was reversed in the HC by the treatment with MIRT+ALA200 (p < 0.05). However, in the ST this reversal of CORT effect was observed in the groups treated with MIRT (p < 0.0001), MIRT+A-LA100 (p < 0.0001) or 200 (p < 0.0001). Indeed, the levels of nitrite in the ST of mice administered CORT+MIRT+ALA100 or CORT+MIR-T+ALA200 were decreased, when compared to the groups MIRT+A-LA100 (p < 0.0001) or MIRT+ALA200 (p < 0.0001). Similar effect was found in the HC (p < 0.0001), but only with the highest ALA dose (CORT+MIRT+ALA200). An increase in nitrite levels was observed in the HC of mice administered MIRT+ALA100 (p < 0.0001) or MIR-T+ALA200 (p < 0.0001) and also in the ST of MIRT (p < 0.01), MIRT+ALA100 (p < 0.0001) or MIRT+ALA200 (p < 0.0001) when compared to control group.
4. Discussion
The mainfindings of the present study are i) the reversal of MIRT-induced sedation by its combination with ALA; and ii) the brain antioxidant effect of MIRT and combined with ALA. We also observed a reversal of CORT-induced depressive-like alterations by MIRT alone or combined with ALA. Regarding the behavioral evaluations, both MIRT (CORT+MIRT) and the combination (CORT+MIRT+ALA)
pre-(Chourbaji et al., 2012, 2008; Silva et al., 2016; Sousa et al., 2015). In the present study, we replicated the depressive-like alterations induced by CORT, as observed in previous studies, by showing that the animals displayed increased immobility time in the tail suspension test and decreased sucrose preference (Silva et al., 2013; Zhao et al., 2008a). Furthermore, we observed anxiogenic-like alterations induced by CORT, i.e. main effect of“CORT model”in the number of entries in the open arms of the plus maze apparatus and increase in sleeping time. These depressive- and anxiogenic-like alterations were accompanied by increased levels of lipid peroxidation and nitrite content in the HC and ST, two brain areas related to depression pathophysiology.
Recently, CORT-induced depression has been suggested as a model of treatment-resistant depression (Ago et al., 2013) and/or psychotic depression. Indeed, patients suffering from psychotic major depression present higher plasma levels of cortisol when compared to patients suffering from nonpsychotic major depression (Belanoffet al., 2001). This is confirmed by studies showing that the antidepressants desipra-mine (30 mg/kg, i.p.) andfluoxetine (30 mg/kg, i.p.), both used alone to treat treatment-resistant depression, did not show antidepressant effects in this model, whereas drugs such as mGlu2/3 receptor antagonists (Ago et al., 2013) and desvenlafaxine (Silva et al., 2016) showed efficacy. However, there are some controversies in these findings since, in a subsequent study,fluoxetine presented antidepres-sant effect in the same model (Gupta et al., 2015). Here we also replicate the findings that CORT model can induce pro-oxidative alterations in the brain (Silva et al., 2016; Zafir and Banu, 2009a).
Our research group has studied the effects of the endogenous antioxidant compound ALA against depressive-like alterations induced by CORT (Silva et al., 2016, 2013;Sousa et al., 2015). In this regard, we observed that this antioxidant augmented the antidepressant effect of desvenlafaxine (Silva et al., 2013).
In the present study, we did not observe an augmentation of MIRT antidepressant effect by its combination with ALA as observed in our previous study with the antidepressant desvenlafaxine (Silva et al., 2013). One possible explanation may be the dose of MIRT chosen for this study. In fact, MIRT alone and in combination with ALA completely reversed the depressive-like alterations induced by CORT. Thus, possibly an augmentation effect of MIRT+ALA might be seen with protocols using lower doses of MIRT. This hypothesis should be subjected to further testing.
As far as we know, this is thefirst study showing that the animal model of CORT-induced depression increases sleeping time in male mice. Alterations in sleep architecture were observed in other animal models of depression, such as chronic mild stress (Cheeta et al., 1997). Indeed, these authors observed that rats submitted to chronic mild stress presented changes in REM sleep such as increased duration and transitions into REM sleep over the sleep part of the sleep-wake cycle, and of most translational value, a reduced latency to the onset of the first REM period (Cheeta et al., 1997; Moreau et al., 1995). In fact,
reduced latency to the onset of thefirst REM period and hypersomnia (present in about 40%) are important alterations observed in young depressed adults and 10% of older patients, with a preponderance in females (Nutt et al., 2008). Further studies are needed to determine the alterations in sleep architecture induced by CORT model of depression. Based on the promising antidepressant effect obtained with ALA (Silva et al., 2013;Sousa et al., 2015) and knowing that this antioxidant enhances energy expenditure (Kim et al., 2004), we decided to determine whether ALA could reverse one of the most important side effects related to MIRT administration, i.e., sedation.
In fact, we observed in animals treated with MIRT alone and submitted to CORT-induced model an increase in pentobarbital-induced sleeping time. This increase in sleeping time was not observed in the groups administered with ALA (MIRT+ALA and CORT+MIRT+ALA). This result is interesting because it shows that ALA can reverse the sedative action of MIRT both in animals submitted to CORT-induced depression and also in not depressed animals. It is worth mentioning that none of the animals allocated in the experimental groups presented
deficits in the locomotor activity neither in the motor coordination. This absence of motor impairments induced by the drugs gives further relevance to ourfindings. It is important to highlight that circadian manipulations, such as total sleep deprivation and bright light therapy, can reverse depressive symptoms within hours, whereas conventional antidepressants usually have a delayed onset of action in the order of weeks (Bao et al., 2008;Bunney and Potkin, 2008).
Depression is characterized by intertwined oxidative, neuro-nitrosative and neuro-immune pathways, which may cause neuropro-gressive processes (Bakunina et al., 2015). Importantly, high concen-trations of glucocorticoids are associated with oxidative stress and neuroprogressive processes (McEwen, 2008; Zafir and Banu, 2009b).
In this study, repeated CORT administration induced an increase in lipid peroxidation, in both studied areas, that was reversed by MIRT (CORT+MIRT in HC and combined with ALA (CORT+MIRT+ALA) in HC and ST. Of note, the studies on the antioxidant effect of MIRT are limited. As far as we know only one study showed that MIRT presents antioxidant effects by showing that this drug prevents oxidative
the absence of the CORT, increased GSH levels, suggesting the importance of ALA in the regulation of GSH. Thisfinding may show the importance of MIRT+ALA to the brain oxidative homeostasis in depression.
Besides being a biological antioxidant, ALA is a detoxification agent and neuroprotectant. It has been used to improve age-associated cardiovascular, cognitive, and neuromuscular deficits, and has been implicated as a modulator of various inflammatory signaling pathways including the inhibition of the pro-inflammatory and pro-oxidant transcription factor NF-kappa-B (Shay et al., 2009).
In relation to nitrite, we observed that MIRT alone (CORT+MIRT) and combined with ALA (CORT+MIRT+ALA) reversed the increase in this parameter induced CORT in the ST. Interestingly, in the HC only the combination with the highest dose of ALA (CORT+MIRT+ALA 200) reversed this alteration. Nitrite is an indirect measure of nitric oxide production, which induces hypernitrosylation, thereby causing neuroprogressive processes (Moylan et al., 2014). Increased production of NO, inducible NO synthase (iNOS) and hypernitrosylation have frequently been reported in depression (Kim et al., 2006; Maes et al., 2011; Moylan et al., 2014). Mice submitted to chronic unpredictable mild stress model of depression presented high levels of brain nitrite (Gupta et al., 2015). An unexpected increase in nitrite levels was observed in the MIRT+ALA group in relation to the control, in both studied areas. However, this increase was not observed in the animals submitted to CORT-model and administered MIRT combined with the highest dose of ALA (CORT+MIRT+ALA200), suggesting that the protective action of MIRT+ALA seems to depend on the presence of a depressor stimulus.
Our study presents some limitations such as the relatively small sample size and the default of a washout period between drug administration and behavioral testing.
5. Conclusion
We found that MIRT administration alone or combined with ALA is effective in reversing depressive- and anxiety-like behaviors induced by the repeated administration of CORT. We also observed a reversal of sedation, one important side effect related to MIRT therapy by its combination with ALA. Furthermore, MIRT displays antioxidant effects in the brain. This antioxidant effect is potentiated when MIRT is combined with ALA 200 mg/kg suggesting that this combinatorial treatment may represent a new possibility to treat depression, whilst adding ALA may additionally reduce MIRT-induced sedation. Clinical randomized controlled studies are needed to examine the clinical efficacy of this combination in human depression.
Acknowledgments
This study received financial support and scholarships of the
Cortisol activity and cognitive changes in psychotic major depression. Am. J. Psychiatry 158, 1612–1616.http://dx.doi.org/10.1176/appi.ajp.158.10.1612. Bunney, J.N., Potkin, S.G., 2008. Circadian abnormalities, molecular clock genes and
chronobiological treatments in depression. Br. Med. Bull.http://dx.doi.org/10.1093/ bmb/ldn019.
Cheeta, S., Ruigt, G., Van Proosdij, J., Willner, P., 1997. Changes in sleep architecture following chronic mild stress. Biol. Psychiatry 41, 419–427.http://dx.doi.org/10. 1016/S0006-3223(96)00058-3.
Chourbaji, S., Brandwein, C., Vogt, M.A., Dormann, C., Hellweg, R., Gass, P., 2008. Nature vs. nurture: can enrichment rescue the behavioural phenotype of BDNF heterozygous mice? Behav. Brain Res. 192, 254–258.http://dx.doi.org/10.1016/j.
bbr.2008.04.015.
Chourbaji, S., Rtnagl, H.H., Molteni, R., Riva, M.A., Gass, P., Hellweg, A.R., 2012. The impact of environmental enrichment on sex-specific neurochemical circuitries – Effects on brain-derived neurotrophic factor and the serotonergic system. Neuroscience 220, 267–276.http://dx.doi.org/10.1016/j.neuroscience.2012.06.016. Dhir, A., Kulkarni, S.K., 2007. Involvement of nitric oxide (NO) signaling pathway in the
antidepressant action of bupropion, a dopamine reuptake inhibitor. Eur. J. Pharmacol. 568, 177–185.http://dx.doi.org/10.1016/j.ejphar.2007.04.028. Engel, D., Zomkowski, A.D.E., Lieberknecht, V., Rodrigues, A.L., Gabilan, N.H., 2013.
Chronic administration of duloxetine and mirtazapine downregulates proapoptotic proteins and upregulates neurotrophin gene expression in the hippocampus and cerebral cortex of mice. J. Psychiatr. Res. 47, 802–808.http://dx.doi.org/10.1016/j. jpsychires.2013.02.013.
Eren,İ., Nazıroğlu, M., Demirdaş, A., Çelik, Ö., Uğuz, A.C., Altunbaşak, A., Özmen,İ., Uz, E., 2007. Venlafaxine modulates depression-induced oxidative stress in brain and medulla of rat. Neurochem. Res. 32, 497–505. http://dx.doi.org/10.1007/s11064-006-9258-9.
Ergün, Y., Ergün, U.G.O., Orhan, F.O., Küçük, E., 2006. Co-administration of a nitric oxide synthase inhibitor and melatonin exerts an additive antidepressant-like effect in the mouse forced swim test. Med. Sci. Monit. 12, BR307–BR312.
Ferrini, R., Miragoli, G., Taccardi, B., 1974. Neuro-pharmacological studies on SB 5833, a new psychotherapeutic agent of the benzodiazepine class. Arzneimittelforschung 24, 2029–2032.
Fujisaki, C., Utsuyama, M., Kuroda, Y., Watanabe, A., Seidler, H., Watanabe, S., Kitagawa, M., Hirokawa, K., 2003. An immnosuppressive drug, cyclosporine-a acts like anti-depressant for rats under unpredictable chronic stress. J. Med. Dent. Sci. 50, 93–100.
Green, L., Goldman, P., 1981. Nitrate synthesis in the germfree and conventional rat. Science 80–212, 56–58.http://dx.doi.org/10.1126/science.6451927.
Gupta, D., Radhakrishnan, M., Kurhe, Y., 2015. Effect of a novel 5-HT3 receptor antagonist 4i, in corticosterone-induced depression-like behavior and oxidative stress in mice. Steroids 96, 95–102.http://dx.doi.org/10.1016/j.steroids.2015.01.021. Iijima, M., Ito, A., Kurosu, S., Chaki, S., 2010. Pharmacological characterization of
repeated corticosterone injection-induced depression model in rats. Brain Res. 1359, 75–80.http://dx.doi.org/10.1016/j.brainres.2010.08.078.
Johnson, S.A., Fournier, N.M., Kalynchuk, L.E., 2006. Effect of different doses of corticosterone on depression-like behavior and HPA axis responses to a novel stressor. Behav. Brain Res. 168, 280–288.http://dx.doi.org/10.1016/j.bbr.2005.11. 019.
Kessler, R., Berglund, P., Demler, O., Jin, R., Koretz, D., Merikangas, K., Rush, A., Walters, E., Wang, 2003. The epidemiology of major depressive disorder. Jama 2003, 186–187.
Kim, M.-S., Park, J.-Y., Namkoong, C., Jang, P.-G., Ryu, J.-W., Song, H.-S., Yun, J.-Y., Namgoong, I.-S., Ha, J., Park, I.-S., Lee, I.-K., Viollet, B., Youn, J.H., Lee, H.-K., Lee, K.-U., 2004. Anti-obesity effects of alpha-lipoic acid mediated by suppression of hypothalamic AMP-activated protein kinase. Nat. Med. 10, 727–733.http://dx.doi.
org/10.1038/nm1061.
Kim, Y.-K., Paik, J.-W., Lee, S.-W., Yoon, D., Han, C., Lee, B.-H., 2006. Increased plasma nitric oxide level associated with suicide attempt in depressive patients. Prog. Neuropsychopharmacol. Biol. Psychiatry 30, 1091–1096.http://dx.doi.org/10.1016/ j.pnpbp.2006.04.008.
neurotrophic properties of the standardized extract of Cocos nucifera huskfiber in mice. J. Nat. Med.http://dx.doi.org/10.1007/s11418-016-0970-8.
Lister, R.G., 1987. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology 92, 180–185.
Liu, T., Zhong, S., Liao, X., Chen, J., He, T., Lai, S., Jia, Y., 2015. A meta-analysis of oxidative stress markers in depression. PLoS One 10.http://dx.doi.org/10.1371/ journal.pone.0138904.
Liu, W., Zhou, C., 2012. Corticosterone reduces brain mitochondrial function and expression of mitofusin, BDNF in depression-like rodents regardless of exercise preconditioning. Psychoneuroendocrinology 37, 1057–1070.http://dx.doi.org/10. 1016/j.psyneuen.2011.12.003.
Maes, M., 2008. The cytokine hypothesis of depression: inflammation,
oxidative & nitrosative stress (IO & NS) and leaky gut as new targets for adjunctive treatments in depression. Neuroendocrinol. Lett (doi:NEL290308R02)(pii). Maes, M., 2011. Depression is an inflammatory disease, but cell-mediated immune
activation is the key component of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 35, 664–675.http://dx.doi.org/10.1016/j.pnpbp.2010.06.014. Maes, M., Galecki, P., Chang, Y.S., Berk, M., 2011. A review on the oxidative and
nitrosative stress (O & NS) pathways in major depression and their possible contribution to the (neuro)degenerative processes in that illness. Prog.
Neuropsychopharmacol. Biol. Psychiatry 35, 676–692.http://dx.doi.org/10.1016/j. pnpbp.2010.05.004.
Maes, M., Fi??ar, Z., Medina, M., Scapagnini, G., Nowak, G., Berk, M., 2012. New drug targets in depression: inflammatory, cell-mediate immune, oxidative and nitrosative stress, mitochondrial, antioxidant, and neuroprogressive pathways. and new drug candidates-Nrf2 activators and GSK-3 inhibitors. Inflammopharmacology.http://dx. doi.org/10.1007/s10787-011-0111-7.
Mao, Q.-Q., Huang, Z., Zhong, X.-M., Xian, Y.-F., Ip, S.-P., 2014. Brain-derived neurotrophic factor signalling mediates the antidepressant-like effect of piperine in chronically stressed mice. Behav. Brain Res. 261, 140–145.http://dx.doi.org/10. 1016/j.bbr.2013.12.020.
Mattei, R., Dias, R., Espı́nola, E., Carlini, E., Barros, S.B., 1998. Guarana (Paullinia cupana): toxic behavioral effects in laboratory animals and antioxidant activity in vitro. J. Ethnopharmacol. 60, 111–116.http://dx.doi.org/10.1016/S0378-8741(97) 00141-4.
McEwen, B.S., 2008. Central effects of stress hormones in health and disease: understanding the protective and damaging effects of stress and stress mediators. Eur. J. Pharmacol. 583, 174–185.http://dx.doi.org/10.1016/j.ejphar.2007.11.071. McIntosh, L.J., Hong, K.E., Sapolsky, R.M., 1998. Glucocorticoids may alter antioxidant
enzyme capacity in the brain: baseline studies. Brain Res. 791, 209–214.http://dx.
doi.org/10.1016/S0006-8993(98)00115-2.
Mello, B.S.F., Monte, A.S., McIntyre, R.S., Soczynska, J.K., Custódio, C.S., Cordeiro, R.C., Chaves, J.H., Vasconcelos, S.M.M., Nobre, H.V., Florenço de Sousa, F.C., Hyphantis, T.N., Carvalho, A.F., Macêdo, D.S., 2013. Effects of doxycycline on depressive-like behavior in mice after lipopolysaccharide (LPS) administration. J. Psychiatr. Res. 47, 1521–1529.http://dx.doi.org/10.1016/j.jpsychires.2013.06.008.
Mizoguchi, K., Ishige, A., Aburada, M., Tabira, T., 2003. Chronic stress attenuates glucocorticoid negative feedback: involvement of the prefrontal cortex and hippocampus. Neuroscience 119, 887–897. http://dx.doi.org/10.1016/S0306-4522(03)00105-2.
Moreau, J.-L., Scherschlicht, R., Jenck, F., Martin, J.R., 1995. Chronic mild stress-induced anhedonia model of depression; sleep abnormalities and curative effects of electroshock treatment. Behav. Pharmacol. 6, 682–687.http://dx.doi.org/10.1097/ 00008877-199511000-00003.
Moylan, S., Berk, M., Dean, O.M., Samuni, Y., Williams, L.J., O’Neil, A., Hayley, A.C., Pasco, J.A., Anderson, G., Jacka, F.N., Maes, M., 2014. Oxidative & nitrosative stress in depression: why so much stress? Neurosci. Biobehav. Rev. 45, 46–62.http://dx. doi.org/10.1016/j.neubiorev.2014.05.007.
Murray, F., Smith, D.W., Hutson, P.H., 2008. Chronic low dose corticosterone exposure decreased hippocampal cell proliferation, volume and induced anxiety and depression like behaviours in mice. Eur. J. Pharmacol. 583, 115–127.http://dx.doi.
org/10.1016/j.ejphar.2008.01.014.
Ng, F., Berk, M., Dean, O., Bush, A.I., 2008. Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int. J. Neuropsychopharmacol. 11, 851–876.http://dx.doi.org/10.1017/S1461145707008401.
Nutt, D.J., Wilson, S., Paterson, L., 2008. Sleep disorders as core symptoms of depression. Dialog. Clin. Neurosci. 10, 329–336.
O’Donnell, P., Do, K.Q., Arango, C., 2014. Oxidative/nitrosative stress in psychiatric disorders: are we there yet? Schizophr. Bull. 40, 960–962.http://dx.doi.org/10. 1093/schbul/sbu048.
Ohkawa, H., Ohishi, N., Yagi, K., 1979. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 95, 351–358.
Peña, S., Baccichet, E., Urbina, M., Carreira, I., Lima, L., 2005. Effect of mirtazapine treatment on serotonin transporter in blood peripheral lymphocytes of major depression patients. Int. Immunopharmacol. 5, 1069–1076.http://dx.doi.org/10. 1016/j.intimp.2005.02.005.
Radenovic, L., Selakovic, V., 2005. Differential effects of NMDA and AMPA/kainate
receptor antagonists on nitric oxide production in rat brain following intrahippocampal injection. Brain Res. Bull. 67, 133–141.http://dx.doi.org/10. 1016/j.brainresbull.2005.06.019.
Raison, C.L., Miller, A.H., 2003. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am. J. Psychiatry.http://dx.doi.org/10.1176/appi.ajp.160.9.1554.
REUL, J.M.H.M., DE KLOET, E.R., SLUIJS, V.A.N., RIJNBERK, F.J., ROTHUIZEN, J, A., 1990. Binding characteristics of mineralocorticoid and glucocorticoid receptors in dog brain and pituitary. Endocrinology 127, 907–915.http://dx.doi.org/10.1210/ endo-127-2-907.
Rosland, J.H., Hunskaar, S., Hole, K., 1990. Diazepam attenuates morphine antinociception test-dependently in mice. Pharmacol. Toxicol. 66, 382–386. Rush, A.J., 2007. Limitations in efficacy of antidepressant monotherapy. J. Clin.
Psychiatry.
Sedlak, J., Lindsay, R.H., 1968. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal. Biochem. 25, 192–205.
Shay, K.P., Moreau, R.F., Smith, E.J., Smith, A.R., Hagen, T.M., 2009. Alpha-lipoic acid as a dietary supplement: molecular mechanisms and therapeutic potential. Biochim. Biophys. Acta 1790, 1149–1160.http://dx.doi.org/10.1016/j.bbagen.2009.07.026. Silva, M.C.C., Sampaio, L.R.L., de Araújo, D.P., Araújo, P.V.P., Monte, A.S., Rodrigues,
F.T.S., Woods, D.J., de Sousa, F.C.F., Fonteles, M.M.F., Vasconcelos, S.M.M., 2014. Central effects of lipoic acid associated with paroxetine in mice. Am. J. Ther. 21, 85–90.http://dx.doi.org/10.1097/MJT.0b013e318235f1a4.
Silva, M.C.C., De Sousa, C.N.S., Sampaio, L.R.L., Ximenes, N.C., Araújo, P.V.P., da Silva, J.C., De Oliveira, S.L., Sousa, F.C.F., Macêdo, D.S., Vasconcelos, S.M.M., 2013a. Augmentation therapy with alpha-lipoic acid and desvenlafaxine: a future target for treatment of depression? Naunyn. Schmiede. Arch. Pharmacol. 386, 685–695.http:// dx.doi.org/10.1007/s00210-013-0867-y.
Silva, M.C.C., de Sousa, C.N.S., Gomes, P.X.L., de Oliveira, G.V., Araújo, F.Y.R., Ximenes, N.C., da Silva, J.C., Vasconcelos, G.S., Leal, L.K.A.M., Macêdo, D., Vasconcelos, S.M.M., 2016. Evidence for protective effect of lipoic acid and desvenlafaxine on oxidative stress in a model depression in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 64, 142–148.http://dx.doi.org/10.1016/j.pnpbp.2015.08.002. Sousa, C.N.S., de, Meneses, L.N., Vasconcelos, G.S., Silva, M.C.C., Silva, J.C., da, Macêdo,
D., de Lucena, D.F., Vasconcelos, S.M.M., 2015. Reversal of corticosterone-induced BDNF alterations by the natural antioxidant alpha-lipoic acid alone and combined with desvenlafaxine: emphasis on the neurotrophic hypothesis of depression. Psychiatry Res. 230, 211–219.http://dx.doi.org/10.1016/j.psychres.2015.08.042.
Steru, L., Chermat, R., Thierry, B., Simon, P., 1985. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology 85, 367–370.
Tafet, G.E., Nemeroff, C.B., 2015. The links between stress and depression: psychoneuroendocrinological, genetic, and environmental interactions. J. Neuropsychiatry Clin. Neurosci..http://dx.doi.org/10.1176/appi.neuropsych. 15030053.(appineuropsych15030053).
Thase, M.E., Nierenberg, A.A., Vrijland, P., van Oers, H.J.J., Schutte, A.-J., Simmons, J.H., 2010. Remission with mirtazapine and selective serotonin reuptake inhibitors: a meta-analysis of individual patient data from 15 controlled trials of acute phase treatment of major depression. Int. Clin. Psychopharmacol. 25, 189–198.http://dx. doi.org/10.1097/YIC.0b013e328330adb2.
Tok, A., Sener, E., Albayrak, A., Cetin, N., Polat, B., Suleyman, B., Akcay, F., Suleyman, H., 2012. Effect of mirtazapine on oxidative stress created in rat kidneys by ischemia-reperfusion. Ren. Fail. 34, 103–110.http://dx.doi.org/10.3109/0886022X.2011. 623499.
Wang, D., An, S.C., Zhang, X., 2008. Prevention of chronic stress-induced depression-like behavior by inducible nitric oxide inhibitor. Neurosci. Lett. 433, 59–64.http://dx. doi.org/10.1016/j.neulet.2007.12.041.
Who, 2001. 54th World Health Assembly. Mental Health a Call Action by World Health Ministry.
Zafir, A., Banu, N., 2009a. Induction of oxidative stress by restraint stress and corticosterone treatments in rats. Indian J. Biochem. Biophys. 46, 53–58. Zafir, A., Banu, N., 2009b. Modulation of in vivo oxidative status by exogenous
corticosterone and restraint stress in rats. Stress 12, 167–177.http://dx.doi.org/10.
1080/10253890802234168.
Zahorodna, A., Hess, G., 2006. Imipramine and citalopram reverse corticosterone-induced alterations in the effects of the activation of 5-HT(1A) and 5-HT(2) receptors in rat frontal cortex. J. Physiol. Pharmacol. 57, 389–399.
Zhao, Y., Ma, R., Shen, J., Su, H., Xing, D., Du, L., 2008a. A mouse model of depression induced by repeated corticosterone injections. Eur. J. Pharmacol. 581, 113–120. http://dx.doi.org/10.1016/j.ejphar.2007.12.005.
Zhao, Y., Shen, J., Su, H., Li, B., Xing, D., Du, L., 2008b. Chronic corticosterone injections induce a decrease of ATP levels and sustained activation of AMP-activated protein kinase in hippocampal tissues of male mice. Brain Res. 1191, 148–156.http://dx.doi. org/10.1016/j.brainres.2007.11.027.