www.bjorl.org
Brazilian
Journal
of
OTORHINOLARYNGOLOGY
ORIGINAL
ARTICLE
Cross-cultural
adaptation
and
validation
of
the
Sinus
and
Nasal
Quality
of
Life
Survey
(SN-5)
into
Brazilian
Portuguese
夽
Priscila
Regina
Candido
Espinola
Uchoa
a,∗,
Thiago
Freire
Pinto
Bezerra
b,c,
Élcio
Duarte
Lima
a,
Marco
Aurélio
Fornazieri
c,
Fabio
de
Rezende
Pinna
c,
Fabiana
de
Araújo
Sperandio
b,
Richard
Louis
Voegels
caInstitutodeMedicinaIntegralProfessorFernandoFigueira(IMIP),Recife,PE,Brazil
bInstitutodeMedicinaIntegralProfessorFernandoFigueira(IMIP),DepartamentodeOtorrinolaringologia,Recife,PE,Brazil cUniversidadedeSãoPaulo(USP),FaculdadedeMedicina,SãoPaulo,SP,Brazil
Received19October2015;accepted10November2015 Availableonline13February2016
KEYWORDS Qualityoflife; Validationstudies; Rhinitis;
Sinusitis; Childhealth
Abstract
Introduction:Theconceptofqualityoflifeissubjectiveandvariabledefinition,whichdepends ontheindividual’sperceptionoftheirstateofhealth.Qualityoflifequestionnairesare instru-mentsdesignedtomeasurequality oflife,butmostaredevelopedinalanguageotherthan Portuguese.Questionnairescanidentifythemostimportantsymptoms,focusonconsultation, and assist in defining the goals of treatment. Some of these have been validated for the Portugueselanguage,butnoneinchildren.
Objective:Tovalidatethetranslationwithcross-culturaladaptationandvalidationoftheSinus andNasalQualityofLifeSurvey(SN-5)intoPortuguese.
Methods:Prospective study of children aged 2---12 years with sinonasal symptoms of over 30days.Thestudycomprisedtwostages:(I)translationandcross-culturaladaptationofthe SN-5intoPortuguese(SN-5p);and(II)validationoftheSN5-p.Statisticalanalysiswasperformed toassessinternalconsistency,test-retestreliability,andsensitivity,aswellasconstructand discriminantvalidityandstandardization.
Results:TheSN-5wastranslatedandadaptedintoPortuguese(SN-5p)andtheauthorofthe original versionapproved the process. Validation was carried outby administration ofthe SN-5pto51pediatric patientswith sinonasalcomplaints(meanage,5.8±2.5years;range, 2---12years).The questionnaireexhibitedadequate constructvalidity (0.62,p<0.01), inter-nalconsistency(Cronbach’salpha=0.73),anddiscriminantvalidity(p<0.01),aswellasgood
夽 Pleasecitethisarticleas:UchoaPR,BezerraTF,LimaÉD,FornazieriMA,PinnaFR,SperandioFA,etal.Cross-culturaladaptationand validationoftheSinusandNasalQualityofLifeSurvey(SN-5)intoBrazilianPortuguese.BrazJOtorhinolaryngol.2016;82:636---42.
∗Correspondingauthor.
E-mail:priscilaespinola@hotmail.com(P.R.Uchoa).
http://dx.doi.org/10.1016/j.bjorl.2015.11.013
test---retestreproducibility(Goodman---Kruskalgamma=0.957,p<0.001),goodcorrelationwith avisualanalogscale(r=0.62,p<0.01),andsensitivitytochange.
Conclusion: Thisstudyreportsthesuccessfultranslationandcross-culturaladaptationofthe SN-5instrumentintoBrazilianPortuguese.Thetranslatedversionexhibitedadequate psycho-metric propertiesforassessmentofdisease-specificqualityoflifeinpediatricpatientswith sinonasalcomplaints.
© 2016Associac¸˜aoBrasileira de Otorrinolaringologiae CirurgiaC´ervico-Facial.Publishedby Elsevier EditoraLtda.Thisisanopenaccess articleundertheCCBY-NC-NDlicense(http:// creativecommons.org/licenses/by-nc-nd/4.0/).
PALAVRAS-CHAVE Qualidadedevida; Estudosdevalidac¸ão; Rinite;
Sinusite; Saúdedacrianc¸a
Adaptac¸ãoevalidac¸ãotransculturaldaSinusandNasalQualityofLifeSurvey(SN-5) paraoportuguêsbrasileiro
Resumo
Introduc¸ão: Oconceitodequalidadedevidaésubjetivoededefinic¸ãovariável;dependeda percepc¸ãodoindivíduoquantoaoseuestadodesaúde.Osquestionáriosparaqualidadedevida sãoinstrumentosplanejadosparamediraqualidadedevida,masamaioriafoidesenvolvida emlínguasdiferentesdoportuguês.Osquestionáriospodemidentificarossintomasmais impor-tantes,seremfocadasnaconsultaeajudarnadefinic¸ãodasmetasterapêuticas.Algunsdesses instrumentosforamvalidadosparaoidiomaportuguês,masnenhumemcrianc¸as.
Objetivo: Validaratraduc¸ãocomadaptac¸ãotransculturalevalidac¸ãodoquestionárioSN-5para oidiomaportuguês.
Método: Estudoprospectivodecrianc¸ascomidadesentre2e12anoscomsintomassinonasais commaisde30diasdedurac¸ão.Oestudoconsistiuemdoisestágios:(I)traduc¸ãoeadaptac¸ão transcultural do SN-5para o idioma português(SN-5p); e(II) validac¸ãodo SN5-p. Foi real-izada análiseestatística paraavaliac¸ão daconsistência interna,confiabilidade deretestee sensibilidade,bemcomoconstrutoevalidadediscriminanteedepadronizac¸ão.
Resultados: OquestionárioSN-5foitraduzidoeadaptadoparaoidiomaportuguês(SN-5p)e oautor daversãooriginalaprovou oprocesso.A validac¸ãofoirealizada pelaadministrac¸ão doSN-5pa51pacientespediátricoscomqueixassinonasais(mediadeidade,5,8±2,5anos; variac¸ãode2---12anos).Oquestionárioexibiuvalidadedeconstrutoadequada(0,62,p<0,01), consistência interna (alfa de Cronbach=0,73) e validade discriminante (p<0,01), além de boa reprodutibilidade de teste-reteste (gama de Goodman---Kruskal=0,957, p<0,001), boa correlac¸ãocomumaescalaanalógicavisual(r=0,62,p<0,01)esensibilidadeàmudanc¸a. Conclusão:Opresenteestudorelataumabem-sucedidatraduc¸ãoeadaptac¸ãotransculturaldo questionárioSN-5paraoidiomaportuguêsbrasileiro.Aversãotraduzidaexibiupropriedades psi-cométricasadequadasparaavaliac¸ãodaqualidadedevidaespecíficaparadoenc¸asempacientes pediátricoscomqueixassinonasais.
©2016Associac¸˜ao BrasileiradeOtorrinolaringologiaeCirurgiaC´ervico-Facial.Publicado por Elsevier EditoraLtda. Este ´eum artigo Open Accesssob umalicenc¸a CC BY-NC-ND(http:// creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Theconceptofqualityoflifeissubjectiveand,therefore, variously defined. Itis related tothe individual’s percep-tion of his or her health status in the major domains or dimensionsoflife.1,2
The main instruments used inassessment of qualityof lifearequalityoflifequestionnaires.Theseinstrumentsare intendedtoassessthevariousaspectsanddimensionsofa patient’slife,includingphysical,psychological,social,role performance,pain,andsleepquality,inadditiontospecific symptoms.1---6
Sinonasalsymptomsandtheircorrelativediseases,such asrhinitisandrhinosinusitis,accountforasignificantportion of visits tohealth care facilities. Rhinitis is estimated to affect approximately 500 million people worldwide.7 The
International Study of Asthma and Allergies in Childhood (ISAAC)revealedanupwardtrendforrhinitisprevalencein Brazilianchildren,withratesincreasingfrom10.3%to17.4% among6---7year-olds andfrom8.9%to28.5% among13 to 14year-oldsbetween1996and2002.8Rhinosinusitisaffects
approximately31million people every year inthe United Statesalone,generatinganannualcostofUS$6million,and isoneoftheleadingcausesofantibioticprescriptionandof absenteeism.9---11Itsprevalenceisestimatedat14%inadults
and7.6%inthepediatricpopulation.12Nationwidedataare
onlyavailablefortheadultBrazilianpopulation.Prevalence is estimated at 5.5% according tohousehold surveys con-ductedinSãoPaulo.13
andthe Sino-NasalOutcome Test-20and-22(SNOT-20and -22).15TheSinusandNasalQualityofLifeSurvey(SN-5)was
thefirstvalidatedquestionnaireforassessmentof disease-specific quality of life related to sinonasal symptoms in pediatricpopulations.16TheSN-5isashort,straightforward,
andeasilyadministeredinstrument.Sinceitsvalidation,it hasbeenemployedinepidemiologicalstudies17and,
partic-ularly,intrialsofclinical18andsurgical19,20interventions.
Methods
This prospective, observational study wasconducted at a hospital,between October2013andJune 2014. Participa-tionwasvoluntary,andallpatientsandtheirlegalguardians providedwritteninformedassentandconsentrespectively, asapprovedbythelocalresearchethicscommittee(CAAE: 05955813.6.0000.5201).
Questionnaire
TheSN-5consistsofaseriesoffivequestionstobeanswered bythe patient’s parents.Each item isscored on a seven-pointscaledesignedtoassesssymptomfrequencyduringthe precedingfourweeks. Theitems assesssymptoms related to: (a) nasal obstruction; (b) sinus infection; (c) allergy symptoms;(d) emotional distress; and (e) activity limita-tions.Attheendofthequestionnaire,overallqualityoflife wasassessedbymeansofavisualanalogscale(VAS)from0 to10.16
Recruitment
The parents of children seen at the outpatient otorhin-olaryngology clinic who met the inclusion criteria were invitedtotakepartvoluntarily.Theinclusioncriteriawere: (a) child aged 2---12 years; (b) presence of one or more of the following symptoms for at least one month at the time of assessment: rhinorrhea or postnasal drip, nasal congestion, nasalobstruction, daytime cough, and halito-sis; and (c) caregiver ability to read and understand the Portugueselanguage.The exclusioncriteriawere:(a) pri-marydiagnosisofobstructivesleepapneasyndrome(OSAS) caused by tonsillar hyperplasia; (b) developmental delay orcognitiveimpairmentand/orcraniofacialabnormalities; (c) secondary chronic rhinosinusitis: fungus ball, invasive fungal disease, granulomatous diseases, vasculitides, iso-lated mucocele, malignant or benign sinonasal tumors, congenitalabnormalities (e.g., primary ciliary dyskinesia, cystic fibrosis), and oroantral fistula; and (d) primary or secondaryimmunedeficiency.
Cross-culturaladaptation
Cross-culturaladaptation of theoriginal, English-language SN-5(Fig.1)intoPortuguese (Fig.2)followed a standard-izedprocess.21Theintermediateandfinalversionsresulting
fromthis processwere sent tothe authorof the original instrumenttoensurethattheoriginalmeaningoftheitems waspreserved.
ValidationoftheSN-5p
ThePortugueseversionoftheinstrumentwasadministered atthreetimepoints,asintheoriginalstudy:inperson,at theinitialpatientencounter;bytelephone,oneweeklater; andagainin personafterfourweeks.Test---retest reliabil-itywasassessedbymeansoftheGoodman---Kruskalgamma coefficient()betweentheresultsoftheinitialencounter
andtheone-weektimepoints.
Statisticalanalysis
Theminimumsamplesizewasestimatedat45patients,with a correlation coefficient of 0.20 asthe outcome of inter-est.Analphavalueof5%(p<0.05)wasdeemedsignificant forallstatisticaltests.16 AnalyseswereperformedinPASW
Statisticsv.18(Chicago,IL,UnitedStates).
Internalconsistency reliabilitywasestimated by calcu-lation of Cronbach’s alpha and inter-item and item-total correlations,andwasconsideredacceptableif>0.70.
Test---retest reliability of the SN-5 questionnaire was assessed by means of Spearman’s correlation coefficient, comparing responses to the initial questionnaire to the responses of patients who did not exhibit any change in overallqualityoflifescoreasassessedontheVAS.
Discriminantvaliditywasassessed bymeansofthe dif-ferenceinSN-5scoresbetweentwogroups:patientsinthe studygroupand25patientsseenatthestudyclinicforother reasons andwithnosinonasalcomplaints(control group). TheMann---WhitneyUtestwasusedforthiscomparison.
Thesensitivitytochangeoftheinstrumentwasassessed bycalculationofthemeaneffectsize.6
Results
ThePortugueseversionoftheSN-5(SN-5p)wasadministered toa group ofpatients withsinonasal complaintsbetween October 2013andJune2014.Overall, 51participantsmet theinclusioncriteria,ofwhom28(54.9%)weremaleand23 (45.1%)werefemale.Themeanagewas5.82±2.51years (range,2---12years).
TheSN-5pwasadministeredtotheselectedpatientsand, afterassessmentofapplicability,wasnotfoundtorequire modificationofanyitems.
The internal consistencyof the SN-5p, asmeasured by Cronbach’s alpha, was 0.73 (total scale). Item-item and item-total correlation analysisshowedadequateconstruct validity.
Discriminantvaliditywasstatisticallysignificant(median [interquartilerange])=0.20[0.20]vs.3.40[1.80],U=752.5,
p<0.01;Fig.3).Test---retestreproducibilityoneweekafter initial interview was adequate (=0.957, p<0.001).
Sig-nificant correlation was observed between the VAS and theSN-5p, asassessedby Spearman’scoefficient (r=0.62,
p<0.01)(Fig.4).Theeffectsizewas2.03.
Figure1 QuestionnaireEnglish-languageSN-5.
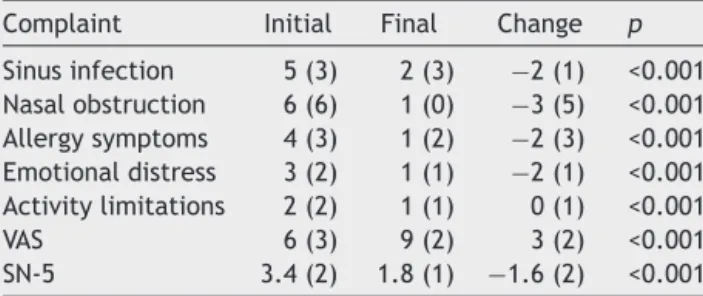
Table 1 Median and interquartile range sinonasal com-plaintscoresoninitialandfinalassessment.
Complaint Initial Final Change p
Sinusinfection 5(3) 2(3) −2(1) <0.001
Nasalobstruction 6(6) 1(0) −3(5) <0.001
Allergysymptoms 4(3) 1(2) −2(3) <0.001
Emotionaldistress 3(2) 1(1) −2(1) <0.001
Activitylimitations 2(2) 1(1) 0(1) <0.001
VAS 6(3) 9(2) 3(2) <0.001
SN-5 3.4(2) 1.8(1) −1.6(2) <0.001
VAS,visualanalogscale;SN-5,SinusandNasalQuality ofLife Survey.
Discussion
Assessment of quality of life in pediatric patients usually posesachallenge.Those whoshouldbethemostreliable informants---patientsthemselves---maybeunabletoexpress theirperceptionsofqualityoflifeasclearlyasadultscan. Parentshavetheirownperceptionsoftheirchildren’s qual-ityoflife,butfromastandpointthatmaybebiasedbytheir own experiences, by the concern they wish toconvey to
theclinician,andbytheiraffectionalbondswiththechild. These facts clearly demonstrate theunique challenges of qualityoflifeassessmentinpediatricpatientsandtheneed toraiseawareness ofthe translation,cross-cultural adap-tation,andvalidationoftheSN-5instrumentinPortuguese (Fig.2).
Severalquestionnairesexistfortheassessmentofoverall qualityoflifein pediatricpatients,suchasthe Autoques-tionnaire de Qualité de Vie Enfant Imagé (AUQEI),22 but
these instruments do not provide precise information as comparedwithquestionnairescontainingitemsdesignedto assessaspecificdisease.Oneofthefirstpediatric health-relatedqualityoflife(HRQoL)questionnaireswastheTNO AZL Child Quality Of Life (TACQOL) questionnaire, devel-opedin 1992. Thus far, few attempts had been made to developinstrumentsspecificallyfortheassessmentof pedi-atricpatients.23
Oneofthefirstsuchpublicationsinthefieldof otorhin-olaryngology was a 1998 French questionnaire devised to assessthe cumulativeeffect of recurrent child ear,nose, andthroatinfectionsonparents’qualityoflifeduringthe winterseason.24
Figure2 QuestionnairePortuguese-languageSN-5.
dailylivingandissuesatworkandatschool,particularlyin patientswithsymptomsclassifiedasmoderatetosevere.7
The patient’s involvementin the proposedtreatment and the need for a broader assessment of how and to which extentagivendiseaseormedicalinterventionaffects qual-ityoflifeareessential factorsinany healthcaresetting.1
Measurementofqualityoflifecanhelpscreenandmonitor patientswithaltered clinicalstatus, demonstrate popula-tionperceptionsofdifferenthealthproblems,andmeasure theoutcomesofmedicalinterventions.1
Sinonasal complaints and their correlative diseases, suchasrhinitisandrhinosinusitis,accountforasignificant portionofvisitstohealthcarefacilities.Patientswiththese
complaints can present with symptoms such as sneezing, nasaldischarge,itching,nasalobstruction,facialpain,and coughing,aswellasfatigue,mooddisorders,andcognitive disturbances.
Theneedfor adisease-specificqualityoflife question-nairetoassesstheimpactofsinonasalsymptomsinchildren hasbeenmetbythedevelopmentandvalidationoftheSN-5 instrument.TheSN-5wasselectedfortranslationbecause it is easily and quickly administered.16 The cross-cultural
6.00
4.00
2.00
.00
Controls Patients
51 60
Group
SN-5
Figure3 Discriminantvalidity.SN-5,SinusandNasalQuality ofLifeSurvey.
1.00
.00
–1.00
–2.00
–3.00
–4.00
–2.00 .00 2.00 4.00
VAS outcome
SN-5 outcome
6.00 8.00 10.00
Figure4 Scatterplot. VAS,visual analogscale; SN-5,Sinus andNasalQualityofLifeSurvey.
instrumentthatis notequivalenttothe original,and this lackofequivalencelimitscomparisonofresponsesobtained in different populations. The process of cross-cultural adaptation of the SN-5 employed a detailed, systematic procedureforformalassessmentofsemanticequivalence, aspublishedelsewhere.21
Afteritsdevelopment,theSN-5punderwentaseriesof semantic validation procedures, which are important and necessary as ameans of ensuringconceptual equivalence withtheoriginalinstrument.Theoverallimpressionofthe SN-5pwas positive, confirming the relevance of its items to assessment of quality of life in pediatric patients, its properunderstanding,andtheadequacyoftheitemscoring scale.
Theinternalconsistencyreliabilityofthetotalscale,as measured by Cronbach’s alpha coefficient, was adequate (0.76).Cronbach’salphaisan importantmeasurebecause itassessesthe extenttowhichquestionnaire items corre-lateamongthemselves andwiththe overallresultsof the
study; it usuallyrepresents a measure of itemreliability. The original, English-language version of theinstrument16
hadaCronbach’salphaof0.62.Therefore,thecoefficient obtainedforthePortugueseversionsuggestsgoodreliability andshowsthattheseveralquestionnaireitemsdesignedto measurethe sameconstructyieldedsimilarresults,which is a finding relevant to the applicability of the instru-ment.
The SN-5p exhibited test-retest reliability, with a Goodman---Kruskalgamma of 0.957(p<0.001). This statis-ticsuggestsgoodreproducibilityofthequestionnairewhen itwasre-administered topatientsoneweekafterthefirst encounter.
When theSN-5pisadministeredtothesameindividual repeatedlyovertime,changesinscoreusuallyprovide indi-rectestimatesofanoverall changeinqualityoflife,with directestimatesofclinicalchangereportedbythecaregiver. Translationandvalidationof theSN-5pprovidesclinicians and investigators with a useful, user-friendly instrument thatmeetsapressingneedinviewofthehighprevalence of sinonasal complaints in the pediatric population. Indi-rectchangesinhealth statuscanbemeasured aschanges in score, as obtained through completion of the instru-mentafteranintervention,andthiscanbeusedclinically toevaluate the quality of life of pediatric patients with sinonasaldisease.The sizeof thechangein scorereflects thedegree ofchangeinqualityoflifeexperiencedbythe individual.
TotalSN-5pscoresalsocorrelatedwellwithVASscores, withacoefficientof0.62(p<0.001).Thisdemonstratesthe extenttowhichVASscorescorrespondtotheoverallclinical pictureofthepatientandthat questionnaireitemsarein factconsistentwiththe phenomenaof interest,providing evidenceofthereliabilityofthequestionnaire.
Analysisofdiscriminantvaliditybetweenthecontroland patientgroups(median[interquartilerange]=0.20[0.20]vs.
3.40,U=752.5,p<0.01) demonstratedgoodability ofthe questionnairetodiscriminatebetweenindividualswithand withoutsinonasalsymptoms.
Effect sizesrevealed that theinstrumentwassensitive tochange,asdemonstratedbytheratioofmeanscoresand theirstandarddeviations. Theeffectsizeof2.02suggests adequatesensitivitytolongitudinalchanges.
The overall impression of theSN-5pwaspositive, con-firmingtherelevanceofitsitemstoassessmentof quality oflifeinpediatricpatients,itsproperunderstanding,and theadequacyoftheitemscoringscale.
Conclusion
TheSN-5pwassuccessfully translatedand cross-culturally adaptedintoBrazilian Portuguese,andthetranslated ver-sionexhibitedadequateproperties.Thequestionnairewas effectiveinassessingthequalityoflifeofpediatricpatients withsinonasalcomplaints,andcanbeusedforthispurpose bothintheclinicalsettingandinfutureresearch.
Conflicts
of
interest
References
1.FitzpatrickR,FletcherA,GoreS,JonesD,SpiegelhalterD,Cox D. Quality oflife measures inhealth care. Applications and issuesinassessment.BMJ.1992;305:1074---7.
2.FerrazMB.Qualidadedevida.Conceitoeumbrevehistórico. RevJovemMed.1998;3:219---22.
3.CarrAJ,ThompsonPW,KirwanJR.Qualityoflifemeasures.Br JRheumatol.1996;35:275---81.
4.WymanJF.Qualityoflifeofolderadultswithurinary inconti-nence.JAmGeriatrSoc.1998;46:778---9.
5.KelleherCJ,CardozoLD,KhullarV,SalvatoreS.Anew question-nairetoassessthequalityoflifeofurinaryincontinentwomen. BrJObstetGynaecol.1997;104:1374---9.
6.DuBeauCE,KielyDK,ResnickNM.Qualityoflifeimpactofurge incontinenceinolderpersons:anewmeasureandconceptual structure.JAmGeriatrSoc.1999;47:989---94.
7.BousquetJ,KhaltaevN,CruzAA,DenburgJ,FokkensWJ,Togias A,etal.AllergicRhinitisanditsimpactonasthma(ARIA)2008 update(incollaborationwiththeWorldHealthOrganization). Allergy.2008;63:8---160.
8.Solé D, Camelo-Nunes IC, Wandalsen GF, Rosário Filho NA, Naspitz CK, Brazilian ISAAC’s Group. Prevalence of rhinitis amongBrazilianschoolchildren:ISAACphase3results. Rhinol-ogy.2007;45:122---8.
9.BenningerMS,FergusonBJ,HadleyJA,HamilosDL,JacobsM, Kennedy DW, et al. Adult chronic rhinosinusitis: definitions, diagnosis,epidemiology,andpathophysiology.OtolaryngolHead NeckSurg.2003;129:S1---32.
10.OsguthorpeJD.Adultrhinosinusitis:diagnosisandmanagement. AmFamPhysician.2001;63:69---76.
11.European Academy of Allergology and Clinical Immunology. Europeanpositionpaperonrhinosinusitisandnasalpolyps. Rhi-nolSuppl.2005:1---87.
12.Aitken M,Taylor JA.Prevalence of clinicalsinusitisin young childrenfollowedupbyprimarycarepediatricians.ArchPediatr AdolescMed.1998;152:244---8.
13.BezerraTF,PiccirilloJF,FornazieriMA,dePilanMRR,AbdoTR, deRezendePinnaF,etal.Cross-culturaladaptationand vali-dationofSNOT-20inPortuguese.IntJOtolaryngol.2011. 14.Juniper EF, Guyatt GH, Andersson B, Ferrie PJ. Comparison
of powder and aerosolized budesonide in perennialrhinitis:
validationofrhinitisqualityoflifequestionnaire.AnnAllergy. 1993;70:225---30.
15.Piccirillo JF, Merritt MG Jr, Richards ML. Psychometric and clinimetric validity of the 20-Item Sino-Nasal Out-comeTest(SNOT-20).OtolaryngolHeadNeckSurg.2002;126: 41---7.
16.KayDJ, RosenfeldRM. Quality oflife for childrenwith per-sistent sinonasal symptoms. Otolaryngol Head Neck Surg. 2003;128:17---26.
17.ErwinEA,FaustRA,Platts-MillsTA,BorishL.Epidemiological analysisofchronic rhinitisinpediatric patients.AmJRhinol Allergy.2011;25:327---32.
18.WeiJL,SykesKJ,JohnsonP,HeJ,MayoMS.Safetyand effi-cacyofonce-dailynasalirrigationforthetreatmentofpediatric chronic rhinosinusitis. Laryngoscope. 2011;121:1989---2000, http://dx.doi.org/10.1002/lary.21923.
19.RamadanHH,TerrellAM.Ballooncathetersinuplastyand ade-noidectomy in childrenwith chronic rhinosinusitis. Ann Otol RhinolLaryngol.2010;119:578---82.
20.RudnickEF,MitchellRB.Long-term improvementsin quality-of-lifeafter surgicaltherapy for pediatric sinonasaldisease. OtolaryngolHeadNeckSurg.2007;137:873---7.
21.BeatonDE,BombardierC,GuilleminF,FerrazMB.Guidelines fortheprocessofcross-culturaladaptationofself-report meas-ures.Spine.2000;25:3186---91.
22.Assumpc¸ão FB, Kuczynski E, Sprovieri MH,Aranha EM. Qual-ityof lifeevaluation scale(AUQEI–Autoquestionnaire Qualité deVie EnfantImagé).Validity and reliabilityof aquality of lifescale for children4 to12years-old.Arq Neuropsiquiatr. 2000;58:119---27.
23.VogelsT,VerripsGH,Verloove-VanhorickSP,FekkesM,Kamphuis RP,KoopmanHM,etal.Measuringhealth-relatedqualityoflife inchildren:thedevelopmentoftheTACQOLparentform.Qual LifeRes.1998;7:457---65.
24.BerdeauxG,HerviéC,SmajdaC,MarquisP.Parentalquality oflifeandrecurrentENTinfectionsintheirchildren: develop-mentofaquestionnaire.RhinitisSurveyGroup.QualLifeRes. 1998;7:501---12.