AURENÍVIA BONIFÁCIO
RESPOSTAS OXIDATIVAS EM PLANTAS DE ARROZ
DUPLAMENTE SILENCIADAS EM APX CITOSÓLICA
AURENÍVIA BONIFÁCIO
RESPOSTAS OXIDATIVAS EM PLANTAS DE ARROZ (
Oryza
sativa
L.) DUPLAMENTE SILENCIADAS EM APX CITOSÓLICA
SUBMETIDA A ESTRESSES ABIÓTICOS
Tese de doutorado apresentada à coordenação do Curso de
Pós-Graduação em Bioquímica, da
Universidade Federal do Ceará, como requisito parcial para a obtenção do grau de Doutor em Bioquímica.
Orientador: Prof. Dr. Joaquim Albenísio Gomes da Silveira
Ficha catalográfica
Setor de Processos Técnicos da Biblioteca Central – UFC
_______Bonifácio, Aurenívia
Respostas oxidativas em plantas de arroz (Oryza sativa L.) duplamente silenciadas em APX citosólica submetida a estresses abióticos / Aurenívia Bonifácio. – 2008.
192f. :il.
Orientador: Joaquim Albenísio Gomes da Silveira
Tese (Doutorado em Bioquímica) - Universidade Federal do Ceará. Departamento de Bioquímica e Biologia Molecular. Inclui anexo e bibliografia.
CDD
1. Oryzasativa L. 2. Salinidade
3. Peroxidase do ascorbato 4. Catalase
5. Enzimas
Tese de Doutorado apresentada a Coordenação do Programa de Pós-Graduação em Bioquímica como parte dos requisitos para obtenção do grau de Doutor em Bioquímica, área de concentração Bioquímica Vegetal, outorgado pela Universidade Federal do Ceará e se encontra a disposição dos interessados na Biblioteca desta Universidade.
A transcrição de qualquer trecho desta Tese é permitida, desde que feita de acordo com as normas da ética cientifica.
Tese aprovada em _____ / _____ / _______
Aurenívia Bonifácio
Joaquim Albenísio Gomes da Silveira, Dr. UFC/DBBM - Orientador
Victor Alexandre Vitorello, Dr. USP/CENA – Conselheiro
André Dias de Azevedo Neto, Dr.
UFRB/CETEC – Conselheiro
AGRADECIMENTOS
A Deus pela minha existência.
A minha mãe Severina; meu pai Pereira (in memoriam); meus irmãos Audenir, Audenise, Pereira Junior, Israel Lima; meus sobrinhos Luiz Henrique, Maria Fernanda e Carlos Fernando; e aos meus cunhados Nivaldo Batinga e Izabela Gomes que acompanharam mesmo de longe todas as etapas dessa jornada. Agradeço por todo amor e compreensão.
Ao meu querido orientador Prof. Joaquim Albenísio Gomes da Silveira, pelo qual tenho enorme carinho, respeito e admiração. Obrigada por proporcionar todo suporte que precisei durante o desenvolvimento deste trabalho, por todos os conselhos e pelos ensinamentos humanos e científicos.
A Profa. Marcia Pinheiro Margis, chefe do Laboratório de Genética da UFRGS, pela valiosa orientação e colaboração na pesquisa e por todos os ensinamentos e receptividade que teve quando estive em seu laboratório.
Aos amigos Karina Guimarães, Luiz Evandro da Silva, Gilberto Silva Junior, Hedwiges Guadallupi Bezerra, Marcio Martins, Milton Costa Lima Neto, Ana Karla Lobo, Fabricio Eulálio Carvalho, Lurian Duarte, Cecília Renata Sales e Leonília Ferreira (in memoriam) pela valiosa amizade, boas conversas, conselhos, broncas, ensinamentos, apoio e colaboração científica.
A Rafaela Watanabe, Daniela Queiroz Zuliani, Ayrles Fernanda Brandão e Raiana Cabral pela paciência, companheirismo diário e conversas em casa.
Aos que fazem ou fizeram parte dos Laboratórios de Metabolismo de Plantas (LABPLANT) que de forma direta ou indireta contribuíram para a realização deste trabalho: Ana Karla, Fabricio Eulálio, Marcio, Milton, Cristina, Sérgio, Raquel Ribeiro, João Victor, Cinthya, Tathiana, Adilton, Jamille, Rachel, Naélia, Suyanne, Evandro, Josemir, Naiara, André, Luiz Aguiar e Rafael.
A Maria Edinilda Nascimento (Dona Nêga) e ao Marcio Souza (secretário da Pós-Graduação em Bioquímica/UFC) pela amizade e colaboração.
A Carolina Ribeiro, Andréia Caverzan, João Abreu Neto, Gisele Passaia e demais membros do Laboratório de Genética da UFRGS pela ajuda concedida.
SUMÁRIO
LISTA DE FIGURAS... ix
LISTA DE TABELAS ... xi
RESUMO GERAL ... xii
1. Introdução e revisão de literatura ... 13
1.1 O estresse oxidativo nas espécies vegetais ... 14
1.2 Processos fotossintéticos e produção de EROs em espécies vegetais ... 19
1.3 O arroz como modelo vegetal ... 22
2. Bibliografia ... 24
Capítulo I ... 30
Photosynthetic modulations prevent oxidative stress in rice plants submitted to combined salinity and heat stress ... 30
Abstract ... 32
1. Introduction ... 33
2. Material and methods ... 35
2.1 Plant material, growth conditions and treatments ... 35
2.2 Photosynthetic and chlorophyll a fluorescence parameters ... 35
2.3 Electrolyte leakage and sodium and potassium content ... 36
2.4 Lipid peroxidation and hydrogen peroxide determinations ... 37
2.5 Reduced ascorbate and glutathione determinations ... 38
2.6 Enzymatic extraction ... 38
2.7 Enzyme activity assays ... 39
2.8 Statistical analysis ... 41
3. Results ... 41
4. Discussion ... 44
5. References ... 48
Capítulo II ... 59
Role of peroxidases in the compensation of cytosolic ascorbate peroxidase knockdown in rice plants under abiotic stress ... 59
Application of the 3-aminotriazole treatment ... 84
CO2 photosynthetic assimilation parameters ... 85
Pigment determination... 85
Membrane damage and lipid peroxidation... 85
Determination of H2O2 content and ascorbate and glutathione redox states .... 86
Sample extraction and enzyme activity assays ... 87
Statistical analysis and experimental design ... 90
Results ... 90
Discussion ... 93
References ... 97
ANEXOS ... 109
LISTA DE FIGURAS Figura 1 – Ciclo Ascorbato-Glutationa na célula vegetal. Abreviações: SOD, dismutase de superóxido; APX, peroxidase de ascorbato; CAT, catalase; GPX, peroxidase de glutationa; GSH e GSSG, glutationa reduzida e oxidada; MDA, monodehidroascorbato; DHA, dehidroascorbato; GR, redutase de glutationa; PrxR, peroxirredoxina; GLR, glutarredoxina; Trx, tiorredoxina; MDAR, redutase de MDA; DHAR, redutase de DHA (Fonte: MITTLER e POULOS, 2005)... 17
Figura 2 – Sítios de produção de EROs durante a transferência de elétrons entre os PSII e PSI (Adaptado de FOYER e NOCTOR, 2011).... 20
Figure 3. Modifications in electrolyte leakage (A), sodium (B) and potassium (C) ions content in rice plants under multiples stress (heat, salt and salt+heat). Determinations were performed in leaves of rice plants grown under experimental conditions. Different lower case letters represent significant differences at p<0.05. ………... 56
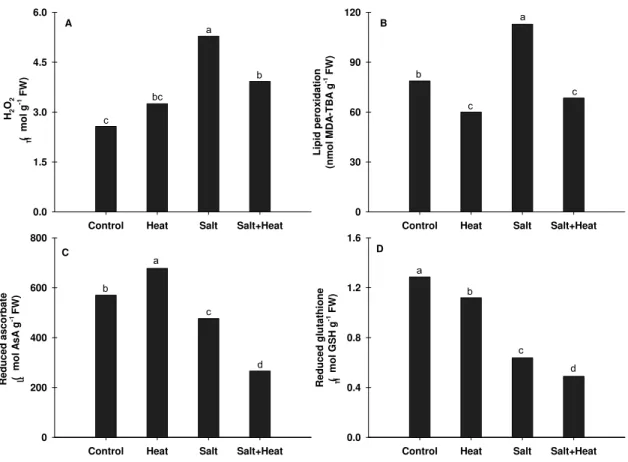
Figure 4. Changes in hydrogen peroxide (A), lipid peroxidation (B), reduced ascorbate (C) and reduced glutathione (D) in rice plants under multiples stress (heat, salt and salt+heat). Determinations were performed in leaves of rice plants grown under experimental conditions. Different lower case letters represent significant differences at p<0.05…. 57
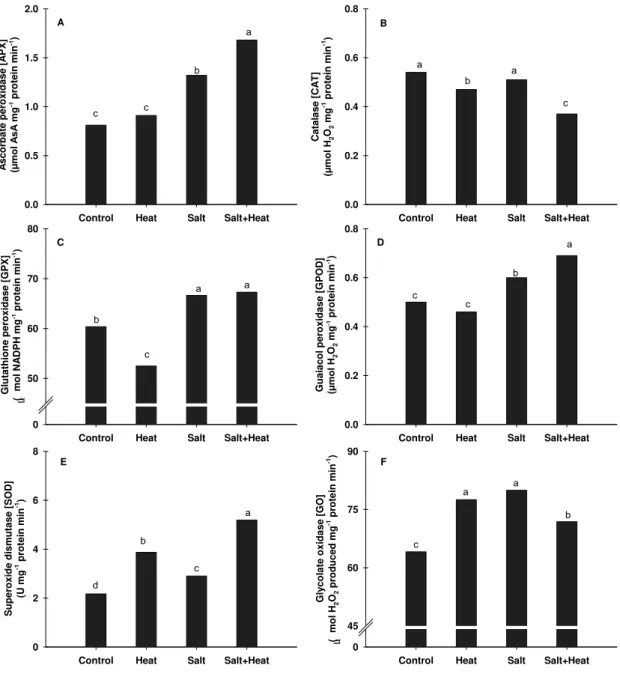
Figure 5. Changes in ascorbate peroxidase (A), catalase (B), glutathione peroxidase (C), guaiacol peroxidase (D), superoxide dismutase (E) and glycolate oxidase (F) in rice plants exposed to multiples stress (heat, salt and salt+heat). Determinations were performed in leaves of rice plants grown under experimental conditions. Different lower case letters represent significant differences at p<0.05…. 58
Capítulo III
Figure 1. (A) Membrane damage, (B) photosynthesis, (C) transpiration and (D) stomatal conductance in leaves of NT and APx1/2s plants exposed to 3-AT treatment. Different capital letters represent significant differences among the NT and APx1/2s plants whereas different lowercase letters represents significant differences among control and the 3-AT treatments, at a confidence of 0.05. Data are mean of four replicates and were compared by Tukey‟s test……….. 103
Figure 2. (A) Chlorophyll, (B) pheophytin a, (C) anthocyanin and (D) carotenoid in leaves of NT and APx1/2s plants exposed to 3-AT treatment. Different capital letters represent significant differences among the NT and APx1/2s plants whereas different lowercase letters represents significant differences among control and the 3-AT treatments, at a confidence of 0.05. Asterisk indicates significant differences at a confidence of 0.05. Data are mean of four replicates and were compared by Tukey‟s test……… 104
Figure 4. (A) Reduced ascorbate, (B) reduced glutathione, (C) ascorbate redox state and (D) glutathione redox state in leaves of NT and APx1/2s plants exposed to 3-AT treatment. Different capital letters represent significant differences among the silenced and NT plants whereas different lowercase letters represents significant differences among control and the 3-AT treatments, at a confidence of 0.05. Data are mean of four replicates and were compared by Tukey‟s test………… 106
Figure5.Enzymatic activity of (A) catalase and (B)ascorbate peroxidase in leaves of NT and APx1/2s plants exposed to 3-AT treatment. Different capital letters represent significant differences among the NT and APx1/2s plants whereas different lowercase letters represents significant differences among control and the 3-AT treatments, at a confidence of 0.05. Data are mean of four replicates and were
compared by Tukey‟s test………..… 107
Figure 6. Enzymatic activity of (A) glutathione peroxidase, (B) guaiacol peroxidase, (C) superoxide dismutase and (D) glycolate oxidase in leaves of NT and APx1/2s plants exposed to 3-AT 5 mM treatment. Different capital letters represent significant differences among the NT and APx1/2s plants whereas different lowercase letters represents significant differences among control and the 3-AT treatments, at a confidence of 0.05. Data are mean of four replicates and were compared by Tukey‟s test.………...……… 108
LISTA DE TABELAS
Tabela 1 – Características químicas das principais espécies reativas de oxigênio (EROs) produzidas durante o metabolismo oxidativo... 15
RESUMO GERAL
1. Introdução e revisão de literatura
Os estresses ambientais, tais como seca, salinidade, temperaturas altas
ou baixas, limitam o desenvolvimento das espécies vegetais devido à redução
da produção e acúmulo de biomassa (XIONG et al., 2002; MAHAJAN e
TUTEJA, 2005). Em condições de campo, as plantas estão comumente
expostas a mais de um fator ambiental adverso como, por exemplo, a
combinação de seca e calor que ocorrem simultaneamente (GUO et al., 2006;
MITTLER, 2006). Embora os efeitos dos estresses abióticos isolados no
metabolismo vegetal sejam bem conhecidos, os seus efeitos e consequências
quando eles ocorrem simultaneamente ainda não são bem entendidos
(RIZHSKY et al., 2002; MILLER et al., 2007).
Em condições de estresses isolados ou combinados, as espécies
vegetais podem ainda sofrer os efeitos deletérios do estresse oxidativo, um
estresse secundário causado pelo acúmulo excessivo de espécies reativas de
oxigênio (EROs) (GUO et al., 2006; MØLLER et al., 2007). As EROs estão
presentes naturalmente dentro de diversos compartimentos celulares
participando de processos vitais, como a fotossíntese e a respiração, e é o desbalanço entre a produção e eliminação destas EROs que leva ao estresse
oxidativo (MÜLLER et al., 2001; GUO et al., 2006). Assim, as EROs podem ser
controle a fim de evitar seus efeitos danosos (MITTLER, 2002; MØLLER et al.,
2007). Para tal, a célula possui um sistema de defesa composto por
antioxidantes enzimáticos e não enzimáticos que são responsáveis em manter
os níveis aceitáveis das EROs levando a proteção contra os danos oxidativos
(del RIO et al., 2002; VRANOVÁ et al., 2002). O sistema não enzimático é
constituído principalmente por componentes hidrofílicos, como o ascorbato e a
glutationa, enquanto que a proteção enzimática é dada por um grupo de
enzimas, que estão presentes em várias organelas e atuam de forma
coordenada para proporcionar a proteção oxidativa (ARORA et al, 2002).
Embora exista um crescente interesse em compreender os processos
envolvidos na resposta ao estresse oxidativo, ainda não é possível afirmar
quais são os “níveis críticos” dos indicadores que levam à sinalização ou dano
oxidativo (SILVEIRA et al., 2005). Neste contexto, um melhor conhecimento
acerca da participação das isoformas de APX e CAT pode fornecer subsídios
que ajudem a compreender os mecanismos utilizados na defesa antioxidativa e
ainda fornecer variáveis que ajudem a uma melhor adaptação das espécies
vegetais a ambientes adversos.
1.1 O estresse oxidativo nas espécies vegetais
As plantas estão, de modo geral, adaptadas a conviverem com certos
peróxido de hidrogênio (H2O2), radical hidroxílico (•OH) e oxigênio “singleto”
(1O2), e estão presentes na célula vegetal como subprodutos normais do
metabolismo aeróbico e de processos fotoxidativos (ARORA et al., 2002;
MITTLER, 2002; GILL e TUTEJA, 2010; ver Tabela 1), sendo produzidas em
diferentes compartimentos celulares, tais como cloroplastos, mitocôndrias,
membrana plasmática, peroxissomos, entre outros (APEL e HIRT, 2004).
Tabela 1 – Características químicas das principais espécies reativas de oxigênio (EROs) produzidas durante o metabolismo oxidativo.
Espécie química Meia vida spins
Oxigênio singleto 1O2 ~10-6 s
Radical ânion superóxido O2-● ~10-6 s
Radical hidroperoxil HO2-● ~10-6 s
Peróxido de hidrogênio H2O2 ~10-3 s
Radical hidroxila ●OH ~10-9 s
Fontes: SCANDALIOS (2005); GILL e TUTEJA (2010).
Para conter possíveis danos oxidativos causados pelas EROs em
condições normais, um complexo sistema de antioxidantes enzimáticos e
não-enzimáticos existe na célula vegetal (MITLER, 2002; FOYER e NOCTOR,
Apesar do papel deletério que tem sido atribuído a grande parte das
EROs, alguns estudos mostram um papel de “sinalizador molecular” das
condições ambientais, em particular, ao peróxido de hidrogênio (H2O2) (XIONG
et al., 2002; MITTLER et al., 2004; MØLLER et al., 2007; VEAL et al., 2007). O
H2O2 pode ser removido pelas CAT e APX por meio de diferentes mecanismos
que resultam igualmente em água (MITTLER, 2002; FOYER e NOCTOR, 2003;
GILL e TUTEJA, 2010). Quando o H2O2 é produzido nos cloroplastos, este é
eliminado pelas enzimas APX, enquanto que aquele produzido nos
peroxissomos/glioxissomos é removido pelas CAT (MITTLER et al., 2004;
KOTCHONI e GACHOMO, 2006). Esta especificidade entre as enzimas e o
H2O2 reflete as diferentes afinidades existentes dentre estes, onde a APX teria
alta afinidade (µM) e a CAT baixa afinidade (mM) pelo H2O2. Assim, as APX
seriam responsáveis pela modulação fina destas EROs, enquanto que as CAT
seriam responsáveis pela remoção do excesso de EROs durante o estresse.
Desta forma, a CAT e a APX apresentam extrema importância na célula
vegetal e foco importante em estudos que visam compreender suas interações
sob os diferentes tipos de estresses ambientais (SHIGEOKA et al., 2002;
FOYER e NOCTOR, 2003).
As CAT são enzimas tetraméricas, contendo um grupo heme-protéico
em cada subunidade, que convertem o H2O2 em água e oxigênio molecular
reação de eliminação de H2O2, protegendo as células contra os danos
oxidativos e fotooxidativos (MITTLER, 2002; SHIGEOKA et al., 2002), sendo
encontradas principalmente nos cloroplastos e no citosol, e ainda nas
mitocôndrias, peroxissomos e no apoplasto (ASADA, 2006).
Além das CAT e APX e de outras enzimas do sistema antioxidativo,
ascorbato e glutationa, os mais importantes compostos antioxidativos não
enzimáticos presentes nas células vegetais, são requeridos como doadores de
elétrons para que alguns sistemas de defesa antioxidativa enzimática possam
atuar eliminando as EROs do interior das células e compõem o ciclo
Ascorbato-Glutationa (Figura 1). Estes metabólitos se encontram em alta concentração no
interior das células, estando distribuídos no citosol, nas mitocôndrias, nos
peroxissomos, no apoplasto e, principalmente, no interior dos cloroplastos
A síntese de ascorbato ocorre em mitocôndrias, enquanto a de
glutationa pode ocorrer nos cloroplastos e/ou no citosol (FOYER e NOCTOR,
2011). O ascorbato tem sido classificado como componente chave do sistema
antioxidativo das plantas, estando relacionado aos estresses biótico e abiótico
(SMIRNOFF et al., 2001; DIPIERRO et al., 2005). A manutenção da
concentração de ascorbato e de glutationa, em plantas submetidas à
determinada condição de estresse, envolve complexa interação entre síntese,
degradação, transporte e armazenamento no interior das células (FOYER e
NOCTOR, 2003) e é de fundamental importância para as plantas.
Como as EROs são constantemente produzidas nas células, seus níveis
basais devem ser mantidos fortemente controlados. Tal controle é fornecido por
uma complexa rede gênica que atua em compartimentos sub-celulares e por
intermédio de um elaborado “feedback” entre oxidantes e antioxidantes
(GADJEV et al., 2006; CHINNUSAMY et al., 2007) que pode resultar num
aumento da atividade de enzimas envolvidas na remoção das EROs e, desta
forma, potencializar as defesas dos vegetais sob situações de estresse
(MITTLER, 2006; ASADA, 2006).
O aumento da produção de EROs pode causar danos a proteínas, DNA
e lipídios, além de resultar na sinalização molecular nas plantas e, portanto,
numa mudança no padrão de expressão de genes (SCANDALIOS, 2002; APEL
de um sinal de estresse às respostas genômicas e podem resultar em
tolerância ao estresse (KOTCHONI e GACHOMO, 2006).
A alta concentração de EROs pode causar alterações no metabolismo
vegetal devido a uma restrição dos processos fotossintéticos (CATTIVELLI et
al., 2008). Sob condições de estresse, tais como seca, salinidade e/ou calor, a
fotossíntese é um dos processos do metabolismo vegetal que pode ser
primariamente afetada. Isso se dá tanto de forma direta, através da restrição
estomática e consequente baixa disponibilidade de CO2, ou de forma indireta,
pelo desbalanço entre a produção e remoção de EROs produzidas durante o
processo fotossintético - principalmente o H2O2 - que culminam no estresse
oxidativo (MØLLER et al., 2007; CHAVES et al., 2009; GILL e TUTEJA, 2010).
1.2 Processos fotossintéticos e produção de EROs em espécies vegetais
A fotossíntese constitui a base da produção de uma cultura e é um
processo de vital importância para as espécies vegetais. Nas plantas
superiores, a captura e o armazenamento de energia luminosa, que ocorre
durante a fotossíntese, são processos realizados pela associação dos
pigmentos captores de luz e do transporte de elétrons do fotossistema II (PSII)
para o fotossistema I (PSI). Este processo de transferência de elétrons que
durante o processo de dissipação do excesso de energia de moléculas de
clorofila excitadas associadas ao PSII (MÜLLER et al., 2001; MIYAKE e
OKAMURA, 2003; MORADI e ISMAIL, 2007). Isso se dá quando a luz
absorvida num determinado comprimento de onda excita as moléculas de
clorofila para um estado singleto e o excesso de energia é dissipado pela
emissão de calor, fluorescência, processo fotoquímico, ou então, pela formação
de clorofila no estado tripleto (3Chl*) que pode transferir energia para o oxigênio
molecular (O2) gerando o oxigênio singleto (1O2) (MÜLLER et al., 2001;
CHINNUSAMY et al., 2007).
Figura 2 – Sítios de produção de EROs durante a transferência de elétrons entre os PSII e PSI (Adaptado de FOYER e NOCTOR, 2011).
Quando as plantas são expostas a condições ambientais adversas, a
et al., 2008; CHAVES et al., 2009). Assim, o aparato fotossintético pode ser
danificado e a fotorrespiração favorecida, levando a geração de EROs, em
especial o H2O2 (ARORA et al., 2002; VEAL et al., 2007). Sob condições de
estresse salino, o aparato fotossintético pode também ser danificado, tanto por
um componente osmótico intrínseco da salinidade como também pelo excesso
de íons (MIYAKE e OKAMURA, 2003; CHAVES et al., 2009). Em condições de
alta temperatura, a Rubisco pode ter suas propriedades cinéticas alteradas
levando a uma diminuição de sua afinidade pelo CO2 e consequentemente num
aumento da fotorrespiração (MØLLER et al., 2007).
Além dos estresses isolados, os estresses múltiplos também levam a
alterações na fotossíntese (RIZHSKY et al., 2002; 2004). As alterações
metabólicas resultantes da exposição simultânea aos estresses de seca e
calor, por exemplo, são únicas, e não podem ser obtidas a partir dos efeitos
isolados dos respectivos estresses (MITTLER, 2006). Segundo Rizhsky et al.
(2002), quando plantas de tabaco são expostas ao calor ocorre um estimulo da
respiração e aumento da condutância estomática e temperatura foliar,
enquanto que sob estresse hídrico registra-se a redução da respiração,
fotossíntese e condutância estomática. No entanto, quando se combina a seca
e a alta temperatura, a respiração e a temperatura foliar nestas plantas são
(PAN et al., 2006). Estes danos podem ser peroxidação de lipídios, degradação
de proteínas, quebra da dupla fita do DNA e ainda pode resultar na morte
celular (APEL e HIRT, 2004; MØLLER et al., 2007; NGUYEN et al., 2009).
Uma forma simples, rápida e não destrutiva de analisar a absorção e uso
da energia luminosa pelos vegetais é a análise da fluorescência da clorofila
(MIYAKE e OKAMURA, 2003; van der TOL et al., 2009). A eficiência da
utilização da luz absorvida por cada fotossistema regula as reações de fixação
de CO2 e a geração de ATP pelas reações luminosas (MIYAKE e YOKOTA,
2001). Neste contexto, esse parâmetro pode ser utilizado para examinar o
desempenho fotossintético de plantas sob diferentes condições de estresse
isolado e também sob estresses múltiplos (BAKER e ROSENQVIST, 2004;
MORADI e ISMAIL, 2007; ELSHEERY e CAO, 2008; RYANG et al., 2009).
1.3 O arroz como modelo vegetal
O arroz pertence à família das gramíneas e é um importante cereal
cultivado mundialmente. Cerca de 70% da população do mundial,
principalmente na Ásia, África e América Latina, têm o arroz como principal
fonte de alimento (DEMIRAL e TÜRKAN, 2005) e diz-se que ele compõe
aproximadamente 20% de energia alimentar da população mundial (KOCHIAN
et al., 2004; YADAV e JINDAL, 2008).
organismo diploide com 24 cromossomos e que possui um genoma muito
pequeno de cerca de 420 Mb (GOFF et al., 2002) e com isso, este é
considerado um modelo para as monocotiledôneas sendo bastante utilizado em
estudos de expressão gênica (JAIN, 2009).
Foram identificados no genoma do arroz oito genes que codificam para a
enzima APX (APxs de 1 a 8) e variam de acordo com a localização sub-celular:
APx1 e 2 são codificadoras das APXs citosólicas; APx3 e 4 das peroxissomais;
APx5 e 6 das mitocondriais; e APx7 e 8 das cloroplastídicas (TEIXEIRA et al.,
2004; 2006; ver Tabela 2). Enquanto que para a CAT, três genes são relatados:
CatA, CatB e CatC (IWAMOTO et al., 2000; MENEZES-BENAVENTE et al.,
2004). As isoformas de CAT são localizadas nos peroxissomos e/ou
glioxissomos (SCANDALIOS, 2005). As enzimas APX e CAT são removedoras
de H2O2 e compreender o balanço entre elas ao nível gênico e bioquímico, e
como se dá a sincronia das atividades dessas enzimas na proteção
antioxidativa celular é de grande importância na fisiologia do estresse.
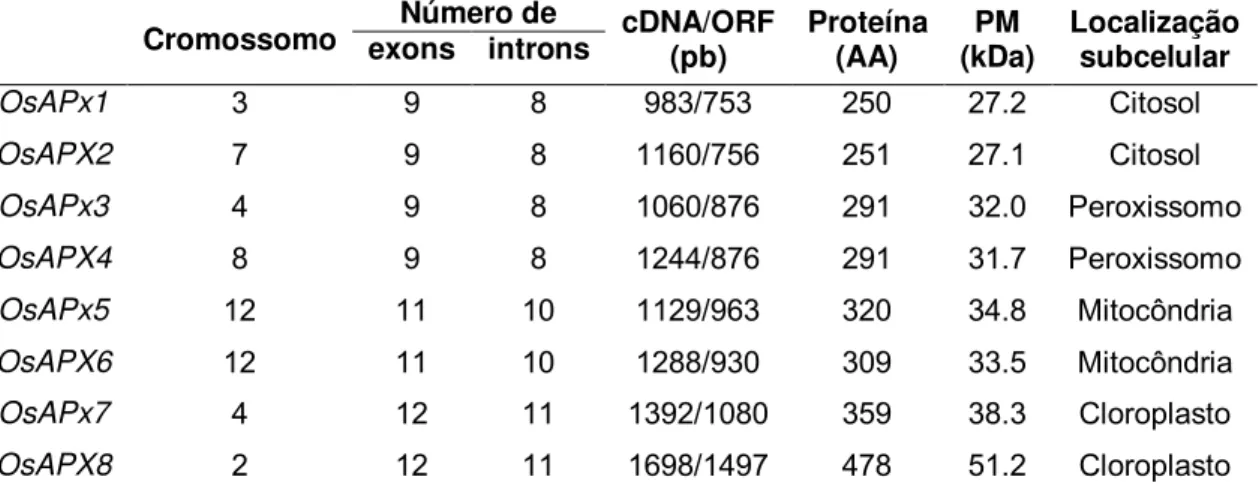
Tabela 2 – Estrutura e principais características dos genes das isoformas de APX presentes em plantas de arroz (Adaptado de TEIXEIRA et al., 2004).
Cromossomo exons introns Número de cDNA/ORF (pb) Proteína (AA) (kDa) PM Localização subcelular
OsAPx1 3 9 8 983/753 250 27.2 Citosol
OsAPX2 7 9 8 1160/756 251 27.1 Citosol
O estudo de como a ausência de um dos genes codificadores de APX
e/ou CAT pode alterar o metabolismo oxidativo de plantas transformadas de
arroz parece ser um enfoque importante. Pesquisas com plantas de
Arabidopsis sem APx1 (citosólica) mostraram um aumento nos níveis de H2O2
juntamente com a ocorrência de oxidação de proteínas do cloroplasto e
redução da atividade fotossintética, além de induzir um aumento da expressão
de genes ligados a CAT (ASAI et al., 2004; DAVLETOVA et al., 2005). De
forma inesperada, Rizhsky et al. (2002), mostraram que plantas de tabaco que
apresentam APX e CAT suprimidas exibiram melhor performance sob
condições de estresse em relação às plantas que apresentavam ausência de
apenas uma destas enzimas.
Tendo em vista o papel duplo exercido pelo H2O2– sinalizador e espécie
reativa de oxigênio capaz de causar danos celulares (MØLLER et al., 2007;
VEAL et al., 2007), saber como isoformas de APX e CAT contribuem para a
sinalização gênica e proteção oxidativa tem se tornado uma pergunta biológica
importante tanto para o arroz como também para outras espécies agricultáveis
e que pode resultar em dados úteis para pesquisas de melhoramento genético
(BENNETZEN, 2002).
2. Bibliografia
ASADA, K. 2006. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiology, v. 141, p. 391-396.
ASAI, N.; MATSUYAMA, T.; TAMAOKI, M.; NAKAJIMA, N.; KUBO, A.; AONO, M.; KATO, T.; TABATA, S.; SHIRANO, Y.; SHIBATA, D. 2004. Compensation for lack of a cytosolic ascorbate peroxidase in an Arabidopsis mutant by activation of multiple antioxidative systems. Plant Science, v. 166, n. 6, p. 1547-1554.
BAKER, N.; ROSENQVIST, E. 2004. Applications of chlorophyll fluorescence can improve crop production strategies: an examination of future possibilities.
Journal of Experimental Botany, v. 55, p. 1607-1621.
BENNETZEN, J. 2002. Opening the door to comparative plant biology.
Science, v. 296, p. 60-63.
CATTIVELLI, L.; RIZZA, F.; BADECK, F.W.; MAZZUCOTELLI, E.; MASTRANGELO, A.M.; FRANCIA, E.; MARÉ, C.; TONDELLI, A.; STANCA, A.M. 2008. Drought tolerance improvement in crop plants: An integrated view from breeding to genomics. Field Crops Research, v. 105, p. 1-14.
CHAVES, M.M.; FLEXAS, J.; PINHEIRO, C. 2009. Photosynthesis and salt stress: regulation mechanisms from whole plant to cell. Annals of Botany, v. 103, p. 551-560.
CHINNUSAMY, V.; ZHU, J; ZHU, J.K. 2007. Cold stress regulation of gene expression in plants. Trends in Plant Science, v. 12, p. 444-451.
DAVLETOVA, S.; RIZHSKY, L.; LIANG, H.J.; ZHONG, S.Q.; OLIVER, D.J.; COUTU, J.; SHULAEV, V.; SCHLAUCH, K.; MITTLER, R. 2005. Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis. Plant Cell, v. 17, n. 1, p. 268-281.
FOYER, C.H.; NOCTOR, G. 2003. Redox sensing and signaling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria.
Physiologia Plantarum, v. 119, n. 3, p. 355-364.
FOYER, C.H.; NOCTOR, G. 2011. Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiology, v. 155, p. 93-100.
FOYER, C.H.; BLOOM, A.J.; QUEVAL, G.; NOCTOR, G. 2009. Photorespiratory metabolism: genes, mutants, energetics, and redox signaling.
Annual Review of Plant Physiology, v. 60, p. 455-484.
GADJEV, I.; VANDERAUWERA, S.; GECHEV, T.S.; LALOI, C.; MINKOV, I.N.; SHULAEV, V.; APEL, K.; INZE, D; MITTLER, R.; BREUSEGEM, F.V. 2006. Transcriptomic footprints disclose specificity of reactive oxygen species signaling in Arabidopsis. Plant Physiology, v. 141, p. 436-445.
GILL, S.S.; TUTEJA, N. 2010. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology and
Biochemistry, v. 48, p. 909-930.
GOFF, S.A.; RICKE, D.; LAN, T.H.; PRESTING, G.; WANG, R.; DUNN, M. et al. 2002. A draft sequence of the rice genome (Oryza sativa L. ssp. japonica).
Science, v. 296, p. 92-100.
GUO, Y.P.; ZHOU, H.F.; ZHANG, L.C. 2006. Photosynthetic characteristics and protective mechanisms against photoxidation during high temperature stress in two citrus species. Scientia Horticulturae, v. 108, p. 260-267.
IWAMOTO, M.; HIGO, H.; HIGO, K. 2000. Differential diurnal expression of rice catalase genes: the 5%-flanking region of CatA is not sufficient for circadian control. Plant Science, v. 151, p. 39-46.
JAIN, M. 2009. Genome-wide identification of novel internal control genes for normalization of gene expression during various stages of development in rice.
Plant Science, v. 176, p. 702-706.
KIM, Y.H.; KIM, C.Y.; LEE, H.S.; KWAK, S.S. 2009. Changes in activities of antioxidant enzymes and their gene expression during leaf development of sweet potato. Plant Growth Regulation, v. 58, p. 235-241.
KOCHIAN, L.V.; HOEKENGA, O.A.; PIÑEROS, M.A. 2004. How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous
MAHAJAN, S.; TUTEJA, N. 2005. Cold, salinity and drought stresses: An overview. Archives of Biochemistry and Biophysics, v. 444, n. 2, p. 139-158. MENEZES-BENAVENTE, L.; TEIXEIRA, F.K.; KAMEI, C.L.A.; MARGIS-PINHEIRO, M. 2004. Salt stress induces altered expression of genes encoding antioxidant enzymes in seedlings of a Brazilian indica rice (Oryza sativa L.).
Plant Science, v. 166, p. 323-331.
MITTLER, R. 2002. Oxidative stress, antioxidants and stress tolerance. Trends
in Plant Science, v. 7, n. 9, p. 405-410.
MITTLER, R. 2006. Abiotic stress, the field environment and stress combination. Trends in Plant Science, v. 11, n. 1, p. 15-19.
MITTLER, R.; VANDERAUWERA, S.; GOLLERY, M.; van BREUSEGEM, F. 2004. Reactive oxygen gene network of plants. Trends in Plant Science, v. 9, n. 10, p. 490-498.
MILLER, G.; SUZUKI N.; RIZHSKY L.; HEGIE A.; KOUSSEVITZKY S.; MITTLER R. 2007. Double mutants deficient in cytosolic and thylakoid ascorbate peroxidase reveal a complex mode of interaction between reactive oxygen species, plant development, and response to abiotic stresses. Plant
Physiology, v. 144, p. 1777-1785.
MIYAKE, C.; OKAMURA, M. 2003. Cyclic electron flow within PSII protects PSII from its photoinhibition in thylakoid membranes from spinach chloroplasts.
Plant Cell Physiology, v. 44, n. 4, p. 457-462.
MIYAKE, C.; YOKOTA, A. 2001. Cyclic flow of electrons within PSII in thylakoid membranes. Plant Cell Physiology, v. 42, n. 5, p. 508-515.
MØLLER, I.M.; JENSEN, P.E.; HANSSON, A. 2007. Oxidative modifications to cellular components in plants. Annual Review of Plant Biology, v. 58, p. 459-481.
MORADI, F.; ISMAIL, A.M. 2007. Responses of photosynthesis, chlorophyll fluorescence and ROS-scavenging systems to salt stress during seedling and reproductive stages in rice. Annals of Botany, v. 99, p. 1161-1173.
cell death and pollen abortion. Journal of Agronomy & Crop Science, v. 195, p. 157-164.
PAN, Y.; WU, L.J.; YU, Z.L. 2006. Effect of salt and drought stress on antioxidant enzymes activities and SOD isoenzymes of liquorice (Glycyrrhiza uralensis Fisch). Plant Growth Regulation, v. 49, p. 157-165.
RIZHSKY, L.; LIANG, H.; MITTLER, R. 2002. The combined effect of drought stress and heat shock on gene expression in tobacco. Plant Physiology, v. 130, n. 3, p. 1143-1151.
RIZHSKY, L.; LIANG, H.; SHUMAN, J.; SHULAEV, V.; DAVLETOVA, S.; MITTLER. R. 2004. When defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress. Plant Physiology, v. 134, p. 1683-1696.
RYANG, S.Z.; WOO, S.Y.; KWON, S.Y.; KIM, S.H.; LEE, S.H.; KIM, K.N.; LEE, D.K. 2009. Changes of net photosynthesis, antioxidant enzyme activities, and antioxidant contents of Liriodendron tulipifera under elevated ozone.
Photosynthetica, v. 47, n. 1, p. 19-25.
SILVEIRA, J.A.G.; LIMA, J.P.M.S.; CAVALCANTI, F.R.; MAIA, J.M.; VIÉGAS, R. A. 2005. Salt induced oxidative response in plants: Damage or Protection? In: NOGUEIRA, R.J.M.C.; ARAÚJO, E.L.; WILLADINO, L.G.; CAVALCANTE, U.M.T. (Ed.). Estresses Ambientais: Danos e Benefícios em Plantas. Recife, PE: MXM Gráfica e Editora. p. 106-117.
SCANDALIOS, J.G. 2002. The rise of ROS. Trends in Biochemical Sciences,
v. 27, p. 483-486.
SCANDALIOS, J.G. 2005. Oxidative stress: molecular perception and transduction of signals triggering antioxidant gene defenses. Brazilian Journal
of Medical and Biological Research, v. 38, p. 995-1014.
SHIGEOKA, S.; ISHIKAWA, T.; TAMOI, M.; MIYAGAWA, Y.; TAKEDA, T.; YABUTA, Y.; YOSHIMURA, Y. 2002. Regulation and function of ascorbate peroxidase isoenzymes. Journal of Experimental Botany, v. 53, n. 372, p. 1305-1319.
ascorbate peroxidase gene family: Inferences from the rice genome. Journal of
MolecularEvolution, v. 59, p. 761-770.
TEIXEIRA, F.K.; MENEZES-BENAVENTE, L.; GALVÃO, C.V.; MARGIS, R.; MARGIS-PINHEIRO, M. 2006. Rice ascorbate peroxidase gene family encodes functionally diverse isoforms localized in different subcellular compartments.
Planta, v. 224, p. 300-312.
VAIDYANATHAN, H.; SIVAKUMAR, P.; CHAKRABARTY, R.; THOMAS, G. 2003. Scavenging of reactive oxygen species in NaCl-stressed rice (Oryza sativa L.) - differential response in salt-tolerant and sensitive varieties. Plant
Science, v. 165, p. 1411-1418.
van der TOL; C. VERHOEF, W.; ROSEMA, A. 2009. A model for chlorophyll fluorescence and photosynthesis at leaf scale. Agricultural and Forest
Meteorology, v. 149, p. 96-105.
VEAL, E.A.; DAY, A.M.; MORGAN, B.A. 2007. Hydrogen peroxide sensing and signaling. Molecular Cell, v. 26, p. 1-14.
VRANOVÁ, E.; INZE, D.; van BREUSEGEM, F. 2002. Signal transduction during oxidative stress. Journal of Experimental Botany, v. 53 n. 372, p. 1227-1236.
XIONG, L.; SCHUMAKER, K.S.; ZHU, J.K. 2002. Cell Signaling during cold, drought, and salt stress. The Plant Cell, suplemento, p. S165-S183.
Capítulo I
Photosynthetic modulations prevent oxidative stress in rice plants submitted to
Title
Photosynthetic modulations prevent oxidative stress in rice plants submitted to combined salinity and heat stress
Authors
Aurenivia Bonifacio1, Marcio O. Martins1, Milton C. Lima Neto1, Fabricio E. L. Carvalho1, Ana Karla M. Lobo1, Joaquim A. G. da Silveira1,2
Institute of origin
1
Departamento de Bioquímica e Biologia Molecular, Universidade Federal do
Ceará, Brasil
2
Instituto Nacional de Ciência e Tecnologia em Salinidade (INCTsal/CNPq)
Corresponding author
Joaquim A. G. Silveira. Departamento de Bioquímica e Biologia
Molecular/Instituto Nacional de Ciência e Tecnologia em Salinidade
(INCTsal/CNPq), Universidade Federal do Ceará, Laboratório de Metabolismo
e Estresse de Plantas (LABPLANT), Av. Humberto Monte, s/n, CP 6004, CEP
60451-970, Fortaleza, Ceará, Brasil. Tel: +55 85 3366 9821. E-mail:
Abstract
Plants in the field are exposed to multiple stresses, such as salinity, drought, high light, heat/cold and others, resulting in different physiological responses. To evaluate the consequences of some of these stresses to the photosynthetic apparatus and antioxidative metabolism, 30 day old rice plants, were submitted to the following treatments: control (without NaCl and at 27 °C), heat stress (without NaCl and at 42 °C), salt stress (with 100 mM NaCl and at 27 °C) and combined stress (salt+heat). The control and salt stress treatments lasted 8 days and the heat and combined stress treatments lasted 6 hours. At the end of the experimental period, gas exchange, chlorophyll fluorescence and electrolyte leakage were measured and the leaves were collected for biochemical determinations. Isolated salt and heat stresses were not sufficient to cause damage in the photochemical apparatus and heat stress only modified stomatal aperture. In combined heat and salt stress, the results indicate that photosynthetic processes were affected at the level of CO2 assimilation and quantum efficiency. Electrolyte leakage, TBARS and H2O2 content were elevated in salt+heat treatment, but in isolated heat stress the TBARS was decreased. Reduced ascorbate and glutathione were similarly decreased in plants exposed to the combination of salt and heat. All enzymes examined here were differently modulated in experimental treatments. Taken together, the data indicates more intense impairment of photosynthesis in rice plants in the combination of the salt and heat, however the effective modulation of the antioxidative system was effective in establishing a new redox homeostasis and providing tolerance to abiotic stress.
1. Introduction
Plants in the field are exposed to multiple stresses, such as salinity,
drought, high light, heat/cold and others, and their responses to these various
stressful conditions determines their capacity to survive (Dombrowski, 2003;
Davletova et al., 2005; Miller et al., 2010). In nature, salt stress is often
accompanied by high temperatures and these stressful conditions, depending
on the duration and intensity of exposure, can cause various physiological and
biochemical responses in plants such as reduction in leaf expansion, alteration
in photosynthetic apparatus, enhanced oxidative stress and modify the
antioxidant system (Foyer et al., 2009; Hussain et al., 2010; Miller et al., 2010).
Alterations in photosynthetic apparatus in plants under combined salt and
high temperature stresses are mostly due to the increase of the reactive oxygen
species (ROS) content in chloroplasts (Miller et al., 2009, 2010), mainly
superoxide radical (O2•-) and hydrogen peroxide (H2O2) produced during
electron transfer between PSII and PSI which can lead to damage in PSII
(Foyer et al. 2009). Furthermore, photorespiration may increase when
carboxylation reactions in the chloroplast are impaired by the effect of combined
stresses, increasing ROS production, especially H2O2. The H2O2 can migrate in
the cell via transport across the membranes (Veal et al., 2007).
causing oxidative damage and impairs normal performance of cells. To protect
cells from oxidative damage caused by the excess of ROS, plants have
developed a series of enzymatic and non-enzymatic detoxification systems
(Apel and Hirt, 2004; Munns and Tester, 2008). Ascorbate, α-tocopherol,
carotenoids and glutathione are responsible for the non-enzymatic control of
ROS, while superoxide dismutase (SOD), catalase (CAT) and ascorbate
peroxidase (APX) are the main enzymes involved in the control of ROS and
regulate some metabolic pathways (Foyer and Noctor, 2003; Miller et al., 2010).
The combination of stresses can alter plant metabolism in a different way
compared to a single stress (Rizhsky et al., 2004; Mittler, 2006; Volkov et al.,
2006; Xu and Zhou, 2006; Munns and Tester, 2008). Under high temperature
stress the PSII component is considered the most sensitive and its activity is
significantly inhibited. However, when prior abiotic stress exposure occurs this
sensitivity is reduced (Chaves et al., 2009). Information about the impact of
salinity on the physiology and biochemistry of plant species are well
documented (Munns and Tester, 2008; Chaves et al., 2009), however plant
responses under combined salt and heat stress are still little known. Knowledge
of the mechanisms by which plants perceive the environment and activate
adaptive responses at the cellular level is of fundamental importance for biology
(Penfield, 2008; Kolodyazhnaya et al., 2009). In this context, in this study we
2. Material and methods
2.1 Plant material, growth conditions and treatments
Rice seeds (Oryza sativa spp. Japonica; cv. Nipponbare) were
germinated in Germitest® paper under 240 µmol m-2 s-1 photosynthetically active
radiation (PAR), 27 ± 2 °C, 80% relative humidity and 12-h photoperiod. Eight
days after sown, rice seedlings were transferred to 2 L plastic pots filled with ¼
strength Hoagland-Arnon‟s nutritive solution (Hoagland and Arnon, 1950). The
seedlings were grown initially in a greenhouse (average maximum PAR of
800 µmol m-2 s-1; 29 ± 2 C; 12-h photoperiod; and 68% relative humidity).
When plants were 31 days old, they were transferred to a growth chamber at
27 °C with a PAR of 600 µmol m-2 s-1. At this time, two treatments were
imposed: nutrient solution + 27 °C (control) and 100 mM NaCl dissolved in
nutrient solution + 27 °C (salt stress). The NaCl was supplied in two steps of 50
mM each per day. After 8 days of treatment, the chamber temperature was
gradually elevated to 42 °C (4 °C hour-1). At this time, two more conditions were
imposed: nutrient solution + 42 °C (heat stress) and 100 mM NaCl + 42 °C
(salt+heat stress). The plants were subjected to these conditions for 6 h. At the
end of the experimental period, leaf discs were harvested to determine
electrolyte leakage. Then, the leaves were frozen in liquid N2 and stored at
flow of 200 mL min-1. Photosynthesis (PN), transpiration (E) and stomatal
conductance (gs) were measured as described previously (Silva et al., 2010).
With a modulated fluorometer (FMS1; Hansatech; England), fluorescence
measurements were taken by means of the saturation pulse method (Schreiber
et al., 1994) for light and 30 min-dark-adapted completely expanded leaves.
Leaf gas exchange and chlorophyll fluorescence were measured
simultaneously, in fully expanded and mature leaves of plants exposed to PPFD
of 260 µmol m-2 s-1. The intensity and duration of the saturation light pulse were
18,000 µmol m-2 s-1 and 0.7 s, respectively. The following fluorescence
parameters were obtained: the maximum quantum yield of photosystem II (PSII)
[Fv/Fm = (Fm - Fo)/Fm], the electron transport rate (ETR) [ETR = ΔF/Fm' x PPFD x
0.5 x 0.84)], the excitation capture efficiency of PSII open centers [Fv'/Fm' = (Fm'
- Fo')/Fm'], the effective quantum yield of PSII [ΔF/Fm' = (Fm' - Fs)/Fm'] and the
photochemical (qP) and non-photochemical quenching coefficient [qNP=(Fm
-Fm')/Fm'], where Fm and Fo are, respectively, maximum and minimum
fluorescence of dark-adapted leaves; Fm' and Fs are, respectively, maximum
and steady state fluorescence in the light-adapted state and Fo' is minimum
fluorescence after far-red illumination of the previously light-exposed leaves.
The ratio ETR/PN was calculated to estimate the use of electrons in other
processes not related to photosynthetic CO2 assimilation rate (Ribeiro et al.,
tubes containing 20 mL deionized water. The tubes were incubated in a shaking
water bath at 25 °C for 6 h and the electric conductivity of the medium (L1) was
measured. After that, the discs were boiled at 95 °C for 60 min, cooled to 25 °C
and the electric conductivity (L2) was measured. Relative electrolyte leakage
(EL) was estimated using the formula: EL[%]=L1/L2×100.
Sodium and potassium contents were determined according Cavalcanti
et al. (2004). Dry leaves were finely grinded and 50 mg samples were extracted
with 20 mL of deionized water at 95 °C for 60 min in hermetically closed tubes.
After cooling, the extract was filtered through cotton cloth and the
determinations were performed by a flame photometer (Micronal, Brazil).
2.4 Lipid peroxidation and hydrogen peroxide determinations
Lipid peroxidation was assayed by measuring thiobarbituric acid-reactive
substances (TBARS) in accordance with Cakmak and Horst (1991), with minor
modifications as described previously (Rosa et al., 2010). The concentration of
TBARS was calculated using the absorption coefficient of 155 mM-1 cm-1 and
the results were expressed as ηmol MDA-TBA g FW-1.
Hydrogen peroxide content was detected by the titanium tetrachloride
method in accordance to Brennan and Frenkel (1977). Fresh leaf samples were
2.5 Reduced ascorbate and glutathione determinations
Reduced ascorbate content was assayed according to Kampfenkel et al.
(1995). Fresh leaf samples were homogenized in 5% (w/v) TCA, centrifuged at
12,000 g (4 °C) for 20 min and the supernatant was then used. The assay is
based on the reduction of Fe3+ to Fe2+ by ascorbate (AsA) and the detection by
spectrophotometry of the Fe2+ complex with 2,2`-bipirydyl and read at 525 nm.
The reduced ascorbate content was expressed as µmol AsA g FW-1.
The reduced glutathione (GSH) content was assayed as described by
Griffith (1980). Fresh leaf samples were homogenized in 5% (w/v) TCA,
centrifuged at 12,000 g (4 °C) for 20 min and the supernatant was then used.
The assay mixture was prepared by adding extract, DTNB
(5,5-dithio-bis-(2-nitrobenzoic acid)) and 150 mM phosphate potassium buffer. The mixture was
stabilized at 30 °C for 10 min. Then the absorbance was read at 412 nm in the
spectrophotometer and the GSH content was expressed as µmol GSH g FW-1.
2.6 Enzymatic extraction
Leaf samples (0.5 g FW) were ground to fine powder in presence of
liquid N2 in a mortar and pestle and extracted in 3 mL of ice-cold 100 mM
K-phosphate buffer pH 6.8 for 5 min, containing 0.1 mM EDTA and 1 mM
ascorbate. After filtration through cheesecloth, the homogenate was centrifuged
at 4 °C at 15,000 g for 15 min and the obtained extract was used for
2.7 Enzyme activity assays
Ascorbate peroxidase (APX; EC 1.11.1.1) activity was measured
following ascorbate (AsA) oxidation by the decrease in absorbance at 290 nm
(Nakano and Asada, 1981). APX activity was assayed in a reaction mixture
containing 0.5 mM ascorbate (AsA) and 0.1 mM EDTA dissolved in 100 mM
K-phosphate buffer (pH 7.0) and enzyme extract. The reaction was started by
addition of 30 mM H2O2. The enzyme activity was measured by the decrease in
absorbance at 290 nm at 25 °C over 300 s. APX activity was estimated utilizing
the molar extinction coefficient of AsA (2.8 mM-1 cm-1) and expressed as µmol
H2O2 mg protein-1 min-1.
Glutathione peroxidase (GPX; EC 1.11.1.9) activity was measured by the
method of Awasthi et al. (1975) with cumene hydroperoxide as a substrate.
Aliquots (0.1 mL) of the enzyme extract were mixed with a reaction mixture
consisting of 4 mM GSH, 0.2 mM NADPH, 0.05 U of GR (type II from wheat;
Sigma) and 0.5 mM substrate in phosphate buffer (0.1 M; pH 7.0). The GPX
activity was determined by the decrease of NADPH absorption at 340 nm. The
nonspecific NADPH decrease was corrected by using additional measurements
without substrate. The GPX activity was estimated utilizing the molar extinction
coefficient of NADPH (6.22 mM-1 cm-1) and expressed as µmol NADPH mg
hydrogen peroxide and enzyme extract. In order to avoid APX interference, two
determinations were carried out in parallel as described above for APX activity
assay. GPOD activity was estimated utilizing the molar extinction coefficient of
pyrogallol (2.47 mM-1 cm-1) and expressed as µmol H2O2 mg protein-1 min-1.
Catalase (CAT; EC 1.11.1.6) activity was measured following the
oxidation of H2O2 at 240 nm. CAT was determined after the reaction of the
enzymatic extract in the presence of 50 mM potassium phosphate buffer (pH
7.0) containing 20 mM H2O2. The reaction took place at 30 °C, with monitoring
of the absorbance at 240 nm over 300 s (Havir and Mchale, 1987). The CAT
activity was calculated using the molar extinction coefficient of H2O2 (36 mM-1
cm-1) and expressed as µmol H2O2 mg protein-1 min-1.
Superoxide dismutase (SOD; EC 1.15.1.1) activity was determined by
inhibition of blue formazane production by means of the NBT photoreduction.
SOD was measured by adding leaf extract to a mixture containing 50 mM
potassium phosphate buffer (pH 7.8), 0.1 mM EDTA, 13 mM L-methionine, 2
µM riboflavin and 75 µM p-nitro blue tetrazolium chloride (NBT) in the dark. The
reaction was carried out under illumination (30 watt fluorescent lamp) at 25 C
for 6 min. The absorbance was measured at 540 nm (Giannopolotis and Ries,
1977). One SOD activity unit (U) was defined as the amount of enzyme required
to inhibit 50% of the NBT photoreduction and the activity was expressed as
8.3), 40 mM glycolic acid, 100 mM L-cysteine and 100 mM phenylhydrazine.
The reaction was started with the addition of the 1 mM FMN and the
absorbance was monitored over 300 s. The GO activity was calculated using
the molar extinction coefficient of the glyoxylate-phenylhydrazone complex (17
mM-1 cm-1) and expressed as ηmol H2O2 produced mg protein-1 min-1.
2.8 Statistical analysis
The experiment was arranged in a completely randomized design, with
four independent replicates, each consisting of one pot containing three plants.
Data were analyzed by ANOVA and means were compared by the Tukey´s test
at the 0.05 level of confidence.
3. Results
In the present study, rice plants were exposed to 100 mM NaCl in the
root medium, followed or not by heat stress after 8 days of salt treatment. Under
the experimental conditions, alterations in photosynthetic parameters and
chlorophyll fluorescence were observed. Rice plants submitted to isolated heat
stress did not show alterations in photosynthesis and transpirations parameters;
photosystem II, photochemical (qP) and apparent electron transport rate (ETR),
which are variables related to photochemical activity, were all decreased in rice
plants submitted to salt+heat treatment (Fig. 2A, 2B, 2C and 2E). The
non-photochemical quenching (NPQ) and ETR/PN ratio were increased by 65% and
4-fold, respectively, due to combination of salt and high temperature when
compared to controls (Fig. 2D and 2F).
Electrolyte leakage increased significantly in rice plants submitted to salt
and salt+heat stress, but not in plants exposed to heat stress in comparison to
control plants (Fig. 3). In rice plants subjected to the combination of salt and
heat stress, electrolyte leakage increased by about 71% in relation to control,
while in plants exposed to isolated salt stress this parameter was increased by
53% (Fig. 3A). Sodium content showed a pattern similar to electrolyte leakage
in rice plants subjected to experimental conditions (Fig. 3B). Plants submitted to
salt+heat stress showed 16-fold increases in sodium content in comparison to
control, while in plants exposed only to salt stress this increase was about 8-fold
(Fig. 3B). The potassium ion content was elevated in rice leaves submitted to
salt stress alone (35%), but not in plants exposed to salt+heat stress in relation
to control (Fig. 3C). The Na/K ratio was similar in plants submitted to salt and
salt+heat stress (varying by about 0.07).
The leaf H2O2 concentration increased significantly in rice plants exposed
significantly reduced by about 25% when compared to control (Fig. 4B). Plants
exposed to isolated salt stress exhibited elevation in lipid peroxidation by
around 45% in comparison to control.
When compared to control plants, reduced forms of ascorbate and
glutathione (non-enzymatic antioxidants) showed significant alterations.
Reduced ascorbate (AsA) showed decrease in rice plants under salt stress by
about 20%, while under salt+heat stress this reduction reached 50% (Fig. 4C).
Glutathione (GSH) content varied in saline treatments similarly to AsA, where
salt stress alone was responsible for a 50% reduction when compared to
untreated plants. In plants submitted to salt+heat treatment, GSH decreased
62% in relation to control plants. Under heat stress, GSH content was reduced
by 13% and AsA content exhibited a 19% increase in comparison to control.
In the present study, the activity of APX, GPX, GPOD, GO and SOD was
increased in the leaves of rice plants exposed to salt+heat treatment (Fig. 5).
APX and GPOD, enzymes that degrade H2O2, were increased by 29% and 38%
in comparison to control, respectively (Fig. 5A and 5D). GPX, which is also
involved in H2O2 degradation was slightly stimulated in relation to non-treated
plants (Fig. 5C). APX, GPX and GPOD enzymes were stimulated only in plants
exposed to salt stress (isolated or combined with heat; see Fig. 5). SOD activity
treated plants (Fig. 5B). For GO activity, a slight increase (12%) was observed
in salt+heat stress in relation to control (Fig. 5F).
4. Discussion
Salinity is known to cause an imbalance in physiological and biochemical
processes in plant species. According to Munns and Tester (2008), salts at the
outside of roots have an immediate effect on cell growth and associated
metabolism and an accumulation of the salts inside the plants may affect their
metabolic functions. Under salt stress, the photosynthetic responses may be
affected by the ionic component of salinity or by disturbances in the water
relations (Munns, 2002; Kolodyazhnaya et al., 2009). In this work,
photosynthetic restriction accompanied by stomatal closure, alterations in
transpiration and chlorophyll fluorescence parameters were observed in plants
exposed to salt+heat stress. This response, which was exclusive to combined
salt and heat stress, seems to be associated with increased sodium
concentrations in leaves mediated by the elevated temperature. In accordance
to Wang et al. (2003), salt ions may be transported from root to shoot via the
transpiration stream and accumulated in leaf apoplast following water
evaporation from the leaf. Furthermore, salts may build up in the chloroplast
exerting a direct toxic effect on photosynthetic processes (Wang et al., 2003;
and the Mehler reaction (Noctor et al., 2002; Makino et al., 2002; Ribeiro et al.,
2009). Photochemical reactions are considered the most heat sensitive and
photosystem II is the critical site of damage caused by a variety of stress factors
such as salinity, drought, low and high temperatures, high light and UV radiation
(Allakhverdiev et al., 2008; Chaves et al., 2009). The photochemistry
parameters, indicated by chlorophyll a fluorescence parameters such as
effective (ΔF/Fm‟) and potential (Fv/Fm) quantum efficiency of photosystem II,
non-photochemical quenching (NPQ) and photochemical quenching (qP), may
be assessed non-destructively in vivo to indicate the impact of abiotic stresses
on the photosynthetic apparatus (Makino et al., 2002; Baker, 2008; Dias and
Brüggemann, 2010).
Some studies with salt stress have shown that actual photochemical
efficiency of PSII, a parameter of the chlorophyll a fluorescence usually
assessed by ΔF/Fm‟, may be inhibited by salinity (Hasegawa et al., 2000;
Munns, 2002; Ashraf and Shahbaz, 2003), while other authors have sustained
that salinity has no effect on this parameter (Abadía et al., 1999; Lu and Zhang,
2000). According to Dias and Brüggemann (2010), ΔF/Fm‟ and Fv/Fm are usually
not changed by mild drought stress, however, under severe drought stress
these parameters are strongly altered in C3 plants. The ΔF/Fm‟ indicates the
with an increase in NPQ in response to a combination of salinity and heat. The
NPQ is an important photoprotective mechanism to avoid light-induced damage
in plant tissues, where its increase is related to non-radioactive dissipation of
light energy and development of trans-thylakoidal ΔpH, since cyclic electron
flow around PSI does not produce any harmful ROS species (Makino et al.,
2002; Allakhverdiev et al., 2008).
Imbalance between photosynthetic CO2 assimilation and photochemical
activity under abiotic stresses, such as salinity and high temperature, can
provoke increases in the production of reactive oxygen species (ROS) and alter
the properties of plant cell membranes (Penfield, 2008; Forman et al., 2010).
Electrolyte leakage reflects damage to cellular membranes and the elevation of
this parameter indicates higher membrane permeability and reduced cell
tolerance to temperature change (Campos et al., 2003; Kolodyazhnaya et al.,
2009). In this work, electrolyte leakage, measured by electric conductivity, was
increased in rice plants, mainly in plants submitted to salt combined with heat.
However, this response may probably be due to higher sodium content in
vacuoles that overstepped with the disruption of cell (Antunes and Sfakiotakis,
2008), since the accumulation of ions apoplast in the salt stressed leaves,
mainly Na+, will contribute to electrical conductivity increase although they are
not involved in cellular efflux (Ghoulam et al., 2002) and resulting in a false idea
Lipid peroxidation is a process that occurs by chain reactions initiated by
ROS, such as singlet oxygen, superoxide radicals and hydrogen peroxide.
Once started it spreads rapidly and affects a great number of lipid molecules
(Bor et al., 2003; Campos et al., 2003; Demiral and Turkan, 2005; Khan and
Panda, 2008). To contain this process during normal metabolism and
particularly under stress, the plant cell has antioxidant compounds, such as
ascorbate (AsA) and glutathione (GSH) and ROS-scavenging enzymes (Apel
and Hirt, 2004; Mittler et al., 2004; Miller et al., 2010). AsA reacts directly with
ROS in photosynthetic tissues, recycles α-tocopherol, protects enzymes with
prosthetic metal ions and is utilized as a substrate for ascorbate peroxidase
(APX) which catalyzes H2O2 detoxification (Mittler and Poulos, 2005; Khan and
Panda, 2008; Foyer and Noctor, 2011). Besides this, the GSH also plays a
protective role due to its important role as a redox buffer and in the expression
of defense genes (Gomez et al., 2004; Foyer and Noctor, 2011). In our study,
the AsA and GSH contents were reduced in rice plants exposed to salt stress,
combined or not with high temperature, indicating that these antioxidant
compounds could have been used by the antioxidant enzymes (Mittler and
Poulos, 2005; Asada, 2006; Chang et al., 2009) or used directly to contain the
levels of ROS and lower lipid peroxidation (Demiral and Türkan, 2005; Hussain
(APX), catalase (CAT), glutathione peroxidase (GPX) and phenol peroxidases
(GPOD) – a type III peroxidase (Cavalcanti et al. 2004; Mittler and Poulos,
2005; Møller et al., 2007; Forman et al., 2010). APX, CAT and/or GPOD remove
H2O2, which easily permeates cell membranes, produced during salt stress
(Asada, 2006; Penfield, 2008). In this work, APX, GPX and GPOD were
increased in rice plants submitted to salt stress combined with heat, in relation
to control, and this response was associated with a significant reduction in H2O2
content and lower TBARS levels. Despite the increase in SOD and GO, H2O2
-producing enzymes, the data indicates that antioxidative system was effective in
establishing a new redox homeostasis and providing tolerance to abiotic stress.
5. References
Abadía A., Belkohodja R., Morales F. & Abadía J. (1999) Effects of salinity on the photosynthetic pigment composition of barley (Hordeum vulgare L.) growth under a triple-line-source sprinkler system in the field. Journal of
Plant Physiology 154, 392-400.
Allakhverdiev S.I., Kreslavski, V.D., Klimov, V.V., Los, D.A., Carpentier R. & Mohanty P. (2008) Heat stress: an overview of molecular responses in photosynthesis. Photosynthesis Research 98(1-3): 541-550.
Amako K., Chen G.X. & Asada K. (1994) Separate assays specific for ascorbate peroxidase and guaiacol peroxidase and for the chloroplastic and cytosolic isozymes of ascorbate peroxidase in plants. Plant Cell Physiology 35: 497-504.
Ashraf M. & Shahbaz M. (2003) Assessment of genotypic variation in salt tolerance of early cimmyt hexaploid wheat germplasm using photosynthetic capacity and water relations as selection criteria. Photosynthetica 41(2): 273-280.
Awasthi Y.C., Beutler E. & Srivastava S.K. (1975) Purification and properties of human erythrocyte Glutathione Peroxidase. The Journal of Biological
Chemistry 250: 5144-5149.
Baker A.L. & Tolbert N.E. (1966) Glycolate oxidase (ferredoxin-containing form).
Methods in Enzymology 9: 339-340.
Baker N.R. (2008) Chlorophyll fluorescence: a probe of photosynthesis in vivo.
Annual Review of Plant Biology 59, 89-113.
Beauchamp C. & Fridovich I. (1971) Superoxide dismutase: Improved assay applicable to acrylamide gels. Analytical Biochemistry 44: 2762-2787. Blum A. & Ebercon A. (1981) Cell membrane stability as a measure of drought
and heat tolerance in wheat. Crop Science 21: 43-47.
Bor M., Ozdemir F. & Turkan I. (2003) The effect of salt stress on lipid peroxidation and antioxidants in leaves of sugar beet Beta vulgaris L. and wild beet Beta maritime L. Plant Science 164: 77-84.
Bradford M.M. (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding.
Analytical Biochemistry 722: 248-254.
Brennan T. & Frenkel C. (1977) Involvement of hydrogen peroxide in the regulation of senescence in pear. Plant Physiology 59: 411-416.
Cakmak I. & Horst W.J. (1991) Effect of aluminum on lipid-peroxidation, superoxide-dismutase, catalase and peroxidase-activities in root-tips of soybean (Glycine max). Physiologia Plantarum 83: 463-468.
Campos P.S., Quartin V., Ramalho J.C. & Nunes M.A. (2003) Electrolyte leakage and lipid degradation account for cold sensitivity in leaves of Coffea sp. plants. Journal of Plant Physiology 160: 283-292.
Chaves M.M., Flexas J. & Pinheiro C. (2009) Photosynthesis and salt stress: regulation mechanisms from whole plant to cell. Annals of Botany 103: 551-560.
Davletova S., Rizhsky L. & Liang H.Z. (2005) Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis.
The Plant Cell 17, 268-281.
Demiral, T. & Türkan, I. 2005 Comparative lipid peroxidation, antioxidant defense systems and proline content in roots of two rice cultivars differing in salt tolerance. Environmental and Experimental Botany, v. 53, p. 247-257. Dias M.C. & Brüggemann W. (2010) Limitations of photosynthesis in Phaseolus
vulgaris under drought stress: gas exchange, chlorophyll fluorescence and Calvin cycle enzymes. Photosynthetica 48(1): 96-102.
Dombrowski J.E. (2003) Salt Stress Activation of Wound-Related Genes in Tomato Plants. Plant Physiology 132: 2098-2107.
Forman H.J., Maiorino M. & Ursini F. (2010) Signaling functions of reactive oxygen species. Biochemistry 49: 835-42.
Foyer C.H. & Noctor G. (2003) Redox sensing and signaling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiologia
Plantarum 119, 355-364.
Foyer C.H. & Noctor G. (2011) Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiology 155, 93-100.
Foyer C.H., Bloom A.J., Queval G. & Noctor G. (2009) Photorespiratory metabolism: genes, mutants, energetics, and redox signaling. Annual
Review of Plant Physiology 60: 455-484.
Ghoulam C., Foursy A. & Fares K. (2002) Effects of salt stress on growth, inorganic ions and proline accumulation in relation to osmotic adjustment in five sugar beet cultivars. Environmental and Experimental Botany 47: 39-50.
Giannopolotis C.N. & Ries S.K. (1977) Superoxide dismutases: I. Occurrence in Higher Plants. Plant Physiology 59, 309-314.
Gomez L.D., Noctor G., Knight M. & Foyer C. (2004) The intercellular distribution of glutathione synthesis and its response to chilling in maize.