(Epi)genetic characterization of the AZFc region of the Y chromosome : new links to male fertility
Texto
(2) Cover image: Paulo Navarro Costa / Tribute to Ian / 2009 Electropherogram of the b3_SFV genetic marker for the Y chromosome. DNA sample: author’s own.. Disclaimer As opiniões expressas nesta publicação são da exclusiva responsabilidade do seu autor..
(3) UNIVERSIDADE DE LISBOA FACULDADE DE MEDICINA. (EPI)GENETIC CHARACTERIZATION OF THE AZFc REGION OF THE Y CHROMOSOME: NEW LINKS TO MALE FERTILITY. Paulo Alexandre Navarro Costa. DOUTORAMENTO EM CIÊNCIAS BIOMÉDICAS (Ciências Morfológicas). 2009.
(4) A impressão desta dissertação foi aprovada pela Comissão Coordenadora do Conselho Científico da Faculdade de Medicina de Lisboa em reunião de 22 de Setembro de 2009..
(5) UNIVERSIDADE DE LISBOA FACULDADE DE MEDICINA. (EPI)GENETIC CHARACTERIZATION OF THE AZFc REGION OF THE Y CHROMOSOME: NEW LINKS TO MALE FERTILITY. Paulo Alexandre Navarro Costa. DOUTORAMENTO EM CIÊNCIAS BIOMÉDICAS (Ciências Morfológicas) Tese co-orientada pelo Prof. Doutor Carlos E. Plancha e Doutor João Gonçalves. 2009.
(6)
(7) IN MEMORIAM • J.S.M.B & N.D.F.
(8) Resumo da tese de Doutoramento. A região AZFc (do inglês, AZoospermia Factor c) do cromossoma Y humano contém um conjunto de genes necessários a uma correcta espermatogénese. A região localiza-se no braço longo do Y, perfazendo um total aproximado de 3.5 megabases. Um total de três famílias génicas (BPY2, DAZ e CDY1) foram mapeadas neste intervalo, desempenhando diversas funções durante o processo de formação do gâmeta masculino. AZFc é um dos domínios estruturalmente mais complexos do genoma humano. Tal complexidade advém do facto da região ser essencialmente constituída por um conjunto de unidades genéticas altamente homólogas entre si. Estes blocos de DNA designam-se por amplicões e estão organizados em cinco famílias que variam na sequência e no número de cópias das unidade base que as compõem. Apesar dos amplicões serem estruturas genómicas relativamente extensas (a dimensão varia entre os 100 e os 700 kilobases), os diferentes membros de cada família apresentam entre si elevadíssimos níveis de identidade genética, superando mesmo em média os 99%. A existência destas unidades de homologia tem duas grandes consequências funcionais. Em primeiro lugar, implica que os genes da região estejam organizados em cópia múltipla. Em segundo lugar, potencia a ocorrência de recombinação intracromossómica. Tal é particularmente relevante na medida em que a localização de AZFc fora das regiões pseudo-autossómicas do Y traduz-se na impossibilidade de recombinação canónica com um cromossoma homólogo. Neste âmbito, a estrutura genómica de AZFc pode ser vista como um factor chave na geração de diversidade genética dentro do intervalo. No entanto, algumas destas alterações podem representar um risco para o sucesso do programa espermatogénico. De facto, deleções completas da região AZFc, resultado de recombinação entre os amplicões terminais do domínio, são comprovadamente uma das causas genéticas mais frequentes de infertilidade masculina. No entanto, o evento recombinacional pode envolver amplicões mais internos da sequência, dando origem a padrões de deleção.
(9) parcial. Estes são ainda mais frequentes que os da deleção completa, não obstante as suas consequências efectivas para a fertilidade masculina serem menos claras. Efectivamente, existe uma considerável variabilidade fenotípica imputável a estas alterações. Deleções parciais em AZFc já foram detectadas num leque diverso de perfis espermatogénicos que variam da normospermia até à completa ausência de gâmetas no ejaculado. Adicionalmente, esta variabilidade reflecte-se também a nível do resultado final dos diversos screenings populacionais realizados, já que estes são inconclusivos quanto à existência ou não de uma associação entre tais rearranjos e um risco acrescido de anomalias espermatogénicas. Esta dificuldade no estabelecimento de padrões consistentes de correlação genótipo-fenótipo poderá advir não só de uma efectiva variabilidade biológica associada às alterações, como também a um efeito metodológico imputável às ferramentas moleculares disponíveis para as caracterizar. Nesse âmbito, tanto a análise aprofundada dos factores genéticos e epigenéticos que modulam as manifestações fenotípicas de AZFc, como a utilização de novas plataformas analíticas para descrever com maior detalhe a diversidade genética da região são dois pressupostos essenciais para uma maior compreensão do papel desempenhado por AZFc na fertilidade masculina. O objectivo central da presente tese de Doutoramento é a caracterização (epi)genética da região AZFc do cromossoma Y, correlacionando os diferentes padrões moleculares analisados com o potencial fértil do indivíduo. Pretende-se desta forma detectar perfis genéticos de AZFc que representem per se um risco de infertilidade, assim como identificar factores extrínsecos ao intervalo que possam modular o risco associado a uma variante específica. Para tal, estabeleceram-se três linhas de investigação: ireavaliação crítica e pormenorizada de estudos anteriormente publicados sobre a associação entre deleções parciais em AZFc e fertilidade masculina; ii- análise da heterogeneidade genética dos produtos de deleção parcial em AZFc, tanto dentro do intervalo como noutros domínios do cromossoma Y; e iii- caracterização epigenética dos genes DAZ da região AZFc, em linhagens espermatogénicas normais e defectivas. Os resultados obtidos reflectem um extenso conjunto de dados que suportam a existência de uma complexa regulação multifactorial de AZFc. Primeiramente, a.
(10) complementação dos resultados do screening de deleções parciais na população Portuguesa com uma análise ponderada da literatura permitiu estabelecer que o risco de infertilidade masculina associado a esta alteração genómica depende do tipo de linhagens evolutivas do cromossoma Y presentes na população. Mais especificamente, este risco é apenas efectivo para populações com baixa prevalência de linhagens fixadas para estes variantes. Tal é o caso da população Portuguesa, onde se detectou um odds ratio de 5.6 (IC 95%: 1.6–30.1) de possuir uma deleção parcial em AZFc e ser-se infértil. A caracterização de regiões AZFc parcialmente deletadas revelou igualmente uma elevada variabilidade genética a nível de sequência. Neste contexto, podemos afirmar que os nossos resultados demonstraram pela primeira vez a extensão da diversidade genética inerente a deleções parciais em AZFc num subgrupo populacional com clara relevância clínica. Para tal, o design e aplicação de um novo painel de marcadores genéticos específicos de amplicão revelou-se determinante. De facto, a utilização desses marcadores foi preponderante na identificação de fenómenos de conversão de sequência e de variabilidade no ponto de quebra das deleções como potentes geradores de diversidade em AZFc. Contudo, mesmo com este painel de genotipagem de alta resolução, os resultados obtidos não corroboram a existência de uma correlação genótipo-fenótipo evidente. Tal resultado sugere que a expressão fenotípica de um determinado variante AZFc parcialmente deletado é variável, dependendo a mesma da acção de um conjunto de factores moduladores. Os presentes resultados apontam para a linhagem evolutiva do Y, conforme definida ao nível de haplotipo, poder exercer um tal efeito. Assim, com base nas evidências recolhidas, propõe-se que a história evolutiva do cromossoma Y à escala de haplogrupo seja determinante para a existência ou não de risco efectivo de infertilidade associado à deleção, enquanto que à escala de haplotipo possa desempenhar um papel modulador da extensão desse mesmo risco. A caracterização epigenética da região AZFc a nível do padrão de metilação de DNA do gene DAZ mostrou que este factor é eficazmente regulado tanto em casos de espermatogénese normal como deficitária. No entanto, o seu homólogo autosómico (DAZL) apresenta um aumento significativo de erros de metilação quando se compara o.
(11) perfil epigenético de espermatozóides recolhidos a partir de indivíduos com espermatogénese alterada com aquele de indivíduos normospérmicos. Este resultado é de relevância na medida em que representa a primeira vez que se associam erros na metilação de genes da linhagem germinativa com defeitos na espermatogénese. Globalmente, os resultados apresentados nesta dissertação de Doutoramento representam um pequeno mas significativo contributo para uma melhor caracterização da região AZFc, tanto a nível da sua regulação (epi)genética como também das consequências fenotípicas inerentes à sua heterogeneidade genética. Tal conhecimento é relevante no âmbito do acompanhamento clínico de pacientes com deleções parciais em AZFc e poderá ser sinónimo de futuros avanços terapêuticos que beneficiarão uma fracção considerável de casais inférteis. Palavras-chave: Reprodução humana / Infertilidade de causa masculina / Espermatogénese / Cromossoma Y / Regiões AZF / Epigenética.
(12) Ph.D. thesis abstract. The AZFc (AZoospermia Factor c) region of the human Y chromosome harbours genetic determinants required for normal spermatogenesis. AZFc is constituted by highly homologous. DNA. blocks. that. potentiate. the. occurrence. intrachromosomal. recombination. Indeed, complete AZFc deletions represent one of the most frequent genetic causes of male infertility. Partial AZFc deletions are even more prevalent, yet their clinical consequences are controversial. The extremely variable results reported in the literature most likely stem both from intrinsic biological variability and a still imprecise knowledge of the (epi)genetic regulation of the AZFc interval. The main objective of this Ph.D. thesis was to characterize AZFc (epi)genetic regulation in the context of male fertility by: i- performing an in-depth critical reassessment of previously published association studies on partial AZFc deletions; iiillustrating the fine-scale genetic heterogeneity of partial AZFc deletion products; and iiiassessing the epigenetic regulation of AZFc determinants in normal and defective spermatogenesis. Results confirm that the male infertility risk imputable to partial AZFc deletions depends on the distribution of Y chromosome evolutionary lineages in the tested population. Evidence for partial AZFc deletion products displaying striking sequence diversity owing to the contribution of different recombination forces was also demonstrated. In this regard, sequence conversion emerged as a significant source of AZFc genetic variability. Data did not corroborate an association between specific partial deletion profiles and (in)fertility phenotypes. Yet, a tentative Y chromosome haplotype effect in the modulation of the infertility risk associated to such deletions may be invoked. Additionally, epigenetic disturbances in a key autosomal germline gene (DAZL) but not in its AZFc homologue (DAZ) were shown to be associated to abnormal sperm..
(13) Globally, these results represent a small but significant contribution for a better understanding of the (epi)genetic regulation of the AZFc region. Ultimately, this knowledge may translate into clinical benefits for a considerable fraction of infertile couples. Keywords: Human reproduction / Male infertility / Spermatogenesis / Y chromosome / AZF regions / Epigenetics.
(14) "(…) THE ONLY THINGS INDISPENSABLE TO HUMAN LIFE ARE. FOOD, DRINK AND EXCRETION; AND THE SEARCH FOR TRUTH.. THE REST IS OPTIONAL." Jonathan Littell - Les Bienveillantes.
(15) Preface. “There are many sources of joy in this world but few pleasures - and even fewer of them are harmless”. Michel Houellebecq. This year marks important anniversaries of two defining milestones in the field of Arts & Science. Indeed, the year 2009 represents the 150th anniversary of the publication of On the Origin of Species by Charles Darwin and the 30th anniversary of the release of Joy Division’s debut album, Unknown Pleasures. Little seems to connect the work of the prominent naturalist to the efforts of the four Mancunian musicians apart from the fact that both correspond to pivotal landmarks in their respective context. Yet, closer examination reveals subtle but meaningful associations that tightly weave book and album together. The works of Darwin and Joy Division are above all essays in observation. In both cases the authors relied on the methodical analysis of their surroundings in order to operate paradigmatic shifts from readily available data. While Darwin based his work on the observation of regional peculiarities in different animal species as well as on the theoretical postulates of Charles Lyell and Thomas Malthus; Joy Division were attentive to the bleak isolation produced by industrial Britain and fully immersed themselves in the aesthetic nihilism of the then incipient punk and electronic music scene. The results were groundbreaking: Darwin formed what essentially is the unifying theory of biology, while Joy Division set the sonic blueprint for “modern music”, an ambiguous term if ever there was one..
(16) The paradox of these works is that although both bear the distinctive intellectual idiosyncrasies of their authors, On the Origin of Species and Unknown Pleasures would have most certainly come to be regardless of the actual existence of Darwin or Joy Division. Appropriately, just as Alfred Wallace was threading similar territory to that of Darwin, several other bands in the late 1970s were experimenting with notions of unused space and the cadenced minimalism that defined Joy Divison’s approach to popular music. In this regard, the two outstanding works are not only a product of brilliance, but also of opportunity. Perhaps one could go as far as to say that what set Charles Darwin and Joy Division apart from their contemporaries was communication. Both were savant enough to rapidly translate l’air du temps into accessible cultural objects that were already subconsciously in demand by segments of the population. The parallels between book and album are not restricted to the creative process. In fact, it can be argued that the similarities extend from the cognitive determinants responsible for their conception (observation and communication) to an overlapping notion of consequence. The effects of this particular type of consequence can be felt along two main dimensions. One, both works represent a schism with past formulas, introducing paradigmatic shifts in their respective domains. In other words, they replaced the previous status quo with a new set of referentials. This illustrates the societal consequence of the works and accounts for the merit and recognition credited to both. Two, the development of On the Origin of Species and Unknown Pleasures also conveyed personal consequences for their authors, particularly in physical terms. Indeed, both Darwin’s chronic ill health and the suicide of Joy Division’s frontman Ian Curtis can be partially attributed to the gruelling strain associated to the creative process. Appropriately, such bi-dimensional notion of consequence (outside/inside) is another trait uniting these apparently unrelated works. The Ph.D. dissertation you are about to read does not have any pretention to compare itself to either milestone. That would only dampen its consequence, and consequence is a function of scale. On the contrary, the present thesis, like many others before, wishes to celebrate the virtues of observation. Throughout this work a.
(17) comprehensive observation of newly generated and previously available data is undertaken. It is the author’s belief that such approach is essential to shed new light into controversial topics. Communication will be the third pillar of the thesis. This will be reflected by a text that aims to be appealing to both experts and the general public, and by the use of design aesthetics celebrating the innovative artwork of Unknown Pleasures. It is this triumvirate of references that I believe is central to Arts & Science. It is this triumvirate that will define the present Ph.D. dissertation.. Paulo Navarro Costa Lisboa, 28th July 2009.
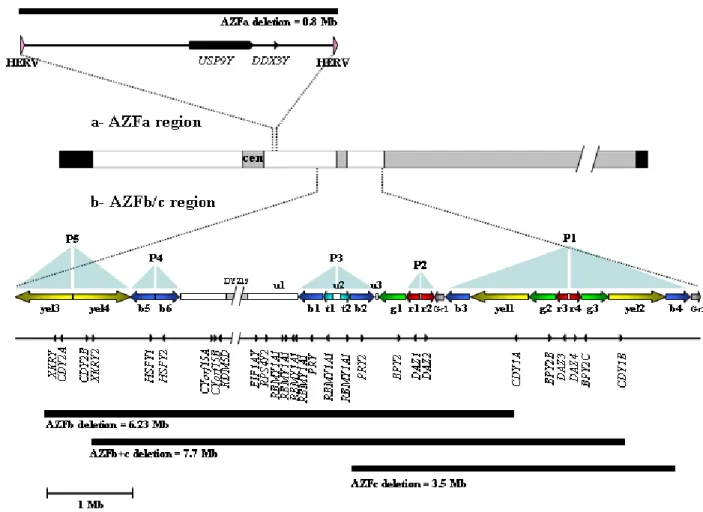
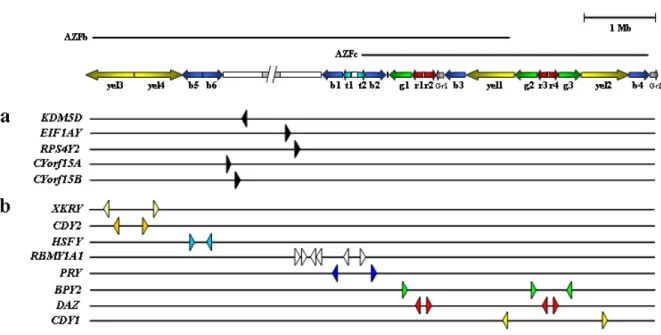
(18) Index. List of abbreviations _________________________pp.21-23 Introduction ______________________________pp.24 -174 1- Infertility: from male factor to genetics (p25) 2- (Epi)genetic control of spermatogenesis (p31) 2.1- Establishment of the germ cell lineage in developing embryos 2.2- Male germ cell differentiation: paternal imprinting and retrotransposon silencing 2.3- Spermatogenesis 2.4- The male fertility genetic network: importance of the Y chromosome 3- The human Y chromosome: history, structure & future (p46) 3.1- Historical perspective on the Y chromosome 3.2- Y chromosome structure and sequence classes 3.3- Y chromosome evolution: past and future 4- The AZF regions of the human Y chromosome (p64) 4.1- Identification and mapping of the three AZF regions 4.2- The AZFa region of the Y chromosome 4.3- The AZFb region of the Y chromosome 4.4- The AZFc region of the Y chromosome.
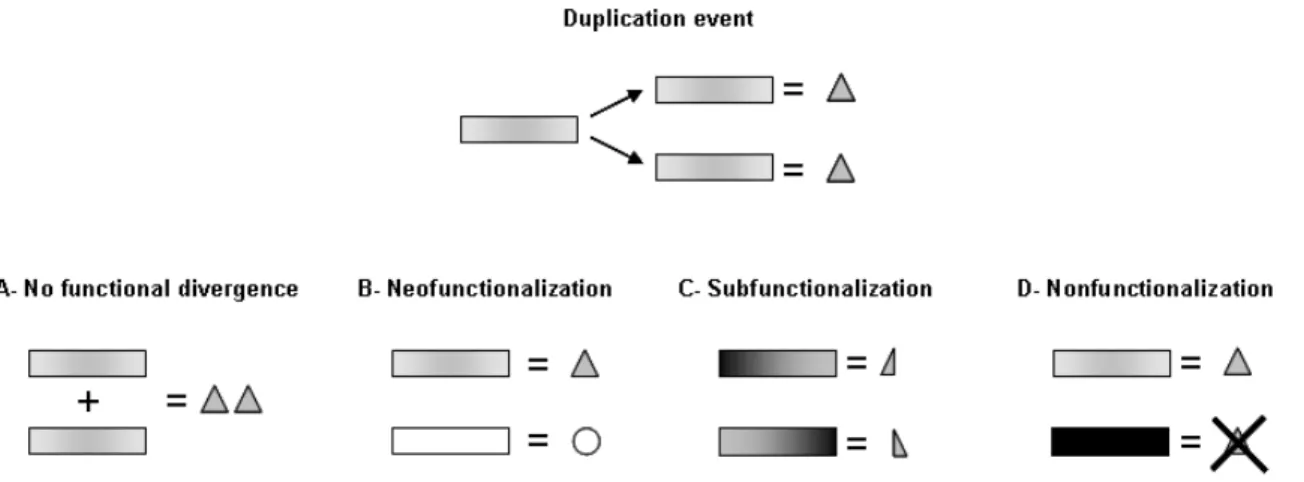
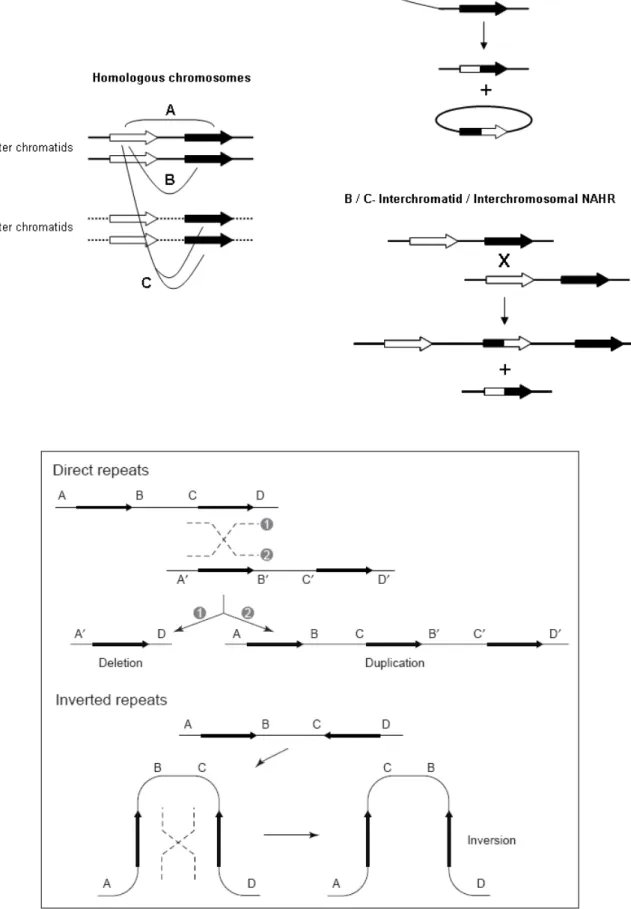
(19) 5- From duplicated sequences to Y variation (p99) 5.1- Dynamics of duplicated genes 5.2- Sequence conversion in the Y chromosome 5.3- Copy number variation and duplicated sequences 5.4- Non-allelic homologous recombination (NAHR) 5.5- Non homologous DNA end joining (NHEJ) 6- Clinical relevance of AZF genetic diversity (p118) 6.1- Genomic rearrangements in AZF: consequences for assisted reproduction techniques (ART) 6.2- Clinical features of AZF-deleted men not related to spermatogenic function 6.3- Karyotypic constitution of AZF-deleted men and its relevance to ART 6.4- AZF deletions and ART success rates 6.5- Safety of ART in AZF-deleted couples: “will our baby be healthy”? 6.6- Is ART changing the evolutionary dynamics of AZF deletions? 6.7- Molecular genetic diagnosis of AZF deletions: tools of the trade 6.8- Novel trends in AZF deletion diagnosis 7- Partial AZFc deletions: from research to clinic? (p140) 7.1- Genetic plasticity of partial AZFc deletions 7.2- Partial AZFc deletions and Y chromosome evolutionary lineages 7.3- Testing an association between partial AZFc deletions and spermatogenic potential 7.3.1- Illustrating heterogeneity: the Han Chinese results 7.4- Partial AZFc deletion association studies: an integrated approach 7.5- Partial AZFc deletion sub-typing.
(20) 7.6- Clinical relevance of partial AZFc deletion screening. Results: (epi)genetic characterization of AZFc__pp.175-228 - Characterizing partial AZFc deletions of the Y chromosome with ampliconspecific sequence markers (p178). -. Erroneous DNA methylation of the DAZL promoter CpG island is associated to abnormal human sperm (p208). On the significance of the recorded results____pp.229 - 243 A) Partial AZFc deletions were confirmed as a male infertility risk in a population largely devoid of Y chromosome lineages fixed for such rearrangements B) Sequence conversion is a significant source of AZFc diversity C) gr/gr deletion products display striking sequence diversity owing to the contribution of different recombination forces D) Specific Y chromosome haplotypes may confer a protective effect against the male infertility risk associated to partial AZFc deletions E) Epigenetic disturbances in a key autosomal germline gene but not in its AZFc homologue are associated to abnormal sperm. Reference list____________________________pp.244 - 274. Acknowledgements_______________________pp.275 - 277.
(21) List of abbreviations. ART – Assisted reproduction techniques ATP – Adenosine-5'-triphosphate AZF – Azoospermia factor AZFa; AZFb; AZFc; AZFd – Azoospermia factor region a to d, respectively BAC – Bacterial artificial chromosome cDNA – Complementary DNA CGI – CpG island CNV – Copy number variation CpG – Dinucleotide constituted by a cytosine followed by a guanine DNA – Deoxyribonucleic acid DNMT – DNA methyltransferase DNMT3A; 3B; 3L – DNA methyltransferase 3A, 3B and 3L. DSB – Double strand DNA break FISH – Fluorescence in situ hybridization FSH – Follicle-stimulating hormone γH2AX – Histone H2A variant phosphorylation at Ser139 H3K9me2 – Histone H3 lysine 9 dimethylation H3K27me3 – Histone H3 lysine 27 trimethylation HDAC – Histone deacetylase HERV – Human endogenous retrovirus ICSI – Intracytoplasmic sperm injection IR – Inverted repeat IVF – In vitro fertilization kb – Kilobase.
(22) Mb – Megabase mL – Millilitre MMEJ – Microhomology-mediated end joining MNPD – Mean number of pairwise differences mRNA – Messenger ribonucleic acid MSY – Male-specific region of the Y chromosome NAHR – Non-allelic homologous recombination NHEJ – Nonhomologous DNA end joining NORF – No long open reading frame NRY – Non-recombining region of the Y chromosome nt – Nucleotide NZ – Normozoospermia OAT – Oligoasthenoteratozoospermia PAR – Pseudoautosomal region of the Y chromosome PAR1; PAR2 – Pseudoautosomal region in Yp and Yq, respectively PCR – Polymerase chain reaction PGC – Primordial germ cell PGD – Preimplantation genetic diagnosis piRNA – PIWI-interacting RNA PIWI – Mouse P element wimpy testis-induced QF-PCR – Fluorescence PCR qPCR – Quantitative real time PCR RNA – Ribonucleic acid RNAP II – RNA polymerase II RRM – RNA recognition motif SCOS – Sertoli cell-only syndrome SFV – Sequence family variant SNP – Single nucleotide polymorphism ssPCR – Single strand PCR.
(23) STS – Sequence-tagged site STR – Short tandem repeat TESE – Testicular sperm extraction TGCT – Testicular germ cell tumour UK – United Kingdom US – United States (of America) UTR – Untranslated region WHO – World Health Organization X – X chromosome Y – Y chromosome Yp / Yq – Short and long arm of the Y chromosome, respectively.
(24) INTRODUCTION. ”I’VE BEEN WAITING FOR A GUIDE TO COME AND TAKE ME. BY THE HAND” Joy Division - Disorder.
(25) 1- Infertility: from male factor to genetics. The desire to procreate is a social and biological cornerstone of human populations. A fairly recent survey of Swedish university students indicated that approximately 95% of the 271 inquired individuals expressed the desire to have children (Lampic et al., 2006). Although attitudes towards parenthood are bound to vary between different populations, this figure is particularly remarkable since Sweden has one of the lowest population annual growth rates in the world: 0.38% for the 2000-2005 interval (United Nations Population Division, 2007). This apparent yearning for parenthood in the overwhelming majority of young Swedish adults underlies the profound importance human civilizations have historically attributed to fertility. In accordance, failure to conceive is considered a major social and psychological burden. The latest estimates indicate that infertility affects 72.4 million people throughout the globe (Boivin et al., 2007). This figure stems from a global median prevalence of approximately 9%, calculated by Boivin and colleagues based on a thorough selection and analysis of previously published reports. One of the most intriguing and debated aspects of this study is that the prevalence of infertility does not seem to significantly vary between more and less developed countries. Even if the similar prevalence scores may reflect an ascertainment bias effect, particularly in sub-Saharan Africa, this result highlights the global nature of infertility and the need for a concerted, wide-spread response. Not surprisingly, the allocation of appropriate infertility healthcare services is dependent on socio-economical levels. This is particularly evident in the latest report on the worldwide availability of assisted reproduction techniques (ART): a 1800 fold.
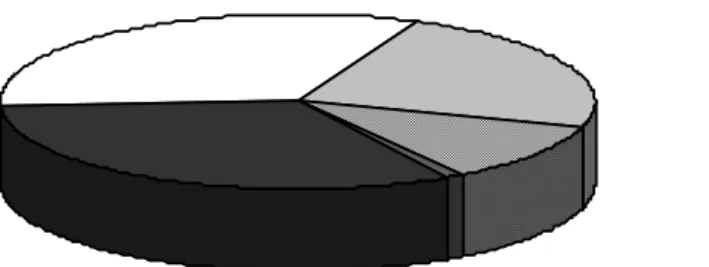
(26) difference was the gap recorded between the countries with highest and lowest number of ART cycles per million (de Mouzon et al., 2009). Such dramatic differences can partially account for the fact that of the estimated 72.4 million infertile people, approximately 56.5 million will not receive medical care (Boivin et al., 2007). Taking into consideration both the societal importance attributed to parenthood and the significant worldwide prevalence of infertility, the identification of infertility causes and the necessary therapeutic approaches to tackle them represent a key area of biomedical research. Infertility is defined in clinical terms as the absence of conception after 12 months of regular, unprotected intercourse (Marchbanks et al., 1989). Yet, the exact duration of this “normal fertility window” may be prone to some variation depending on different populations or criteria. For instance, the sharp decline in oocyte quality associated to advanced maternal age has prompted the reduction of this time-frame to 6 months if the female is over the age of 35 (Gougeon, 2005; Committee on Gynecologic Practice of American College of Obstetricians and Gynecologists and Practice Committee of American Society for Reproductive Medicine, 2008). On the other hand, since many couples conceive without treatment after more than 12 months (Collins et al., 1983), the World Health Organization (WHO) recommends an extension to 24 months as the preferred definition (Rowe et al., 1993). This apparent lack of uniformity arises from a conflict of interests between clinical practice and epidemiological research: while in the former treatment should start as soon as possible in order to increase the probability of success, in the latter the objective is to reduce the number of false positives by increasing the normal fertility window. The prevailing view in the field is that the reasons for infertility are evenly split between the male and female partner (Figure 1.1). The latest Human Fertilisation and Embryology Authority figures for UK couples undergoing ART in 2006 point to an identical rate of 32.5% for either male or female factor infertility (Human Fertilisation and Embryology Authority, 2008). In the remaining 35% the causes are largely unknown (idiopathic infertility: 23.1%), although combined male and female contribution (10.8%).
(27) and other factors (1.1%) can also be invoked. Another interesting observation can be made from the evolution of these figures: in the 2000-2006 period only the male factor and idiopathic infertility categories showed an increase in their particular contributions (+4.9% and +5.6%, respectively). Despite being restricted to a specific regional context and limited time interval, the latter figure demonstrates that regardless of the recent advances in the identification of fertility determinants, the cause of infertility in a significant fraction of couples remains unknown. This is a reflection of a still limited understanding of the intricate biological mechanisms regulating human reproduction. Furthermore, the reported increase in both male factor and idiopathic infertility gives support to the notion that basic and clinical research in these categories may be of pivotal importance.. Male factor Female factor Unknown Male + Female Other. Figure 1.1- Reasons for infertility in the UK in 2006. Data was collected from 70 ART centres and compiled by the Human Fertilisation and Embryology Authority. Isolated male and female factor represent 32.5% each, and multiple male and female contribution 10.8%. Unknown/unexplained origin accounts for 23.1% and other cases for 1.1%. Publication date: October 2008. Male factor infertility can be the result of defects in sperm production/function, sex determination/development, endocrine regulation, immunological tolerance and penile/ejaculatory function. These biological mechanisms represent the central targets for male fertility-limiting agents. Of these, sperm defects are the most frequent. In effect, the descriptive analysis of semen properties is a key test in the context of male infertility diagnosis. This test is referred to as spermiogram and is performed according to a set of international guidelines that also define the cut-off values for normal semen.
(28) properties (World Health Organization, 1999). Despite the assessment of indicators of genital tract function (such as semen volume or pH), the major parameters in a spermiogram are the concentration, morphology and motility of the sperm population. Since these parameters will be frequently alluded to in the present thesis, a brief overview of the three concepts will follow. Sperm concentrations below 20x106 cells per mL of semen are considered abnormal (although this figure is likely to be reduced in the near future). Concentrations below such threshold are referred to as oligozoospermia and the most extreme case of no sperm in the ejaculate is defined as azoospermia. Azoospermia is divided in non-obstructive azoospermia (when attributable to defects in male gamete production) or obstructive azoospermia (when sperm are indeed produced but do not reach the ejaculate due to conformational disorders in the ducts). A set of structural properties of the sperm head, middle piece and tail are evaluated when assessing sperm morphology. Under the Tygerberg criteria currently recommended by the WHO, a sperm sample is considered morphologically normal if 14% or more of the observed gametes have no morphological defects (Kruger et al., 1988). Values below such threshold are considered as indicative of teratozoospermia. Sperm motility is assessed by grading cell movement and trajectory according to predefined categories. If fewer than 50% of sperm display forward progression or if fewer than 25% have fast linear motility, these serve as indicators of asthenozoospermia. In this regard, a normal semen profile, or normozoospermia, implies that multiple variables, particularly those relating to sperm characteristics, are above the defined thresholds. Likewise, an abnormal semen profile requires the occurrence of just one or of a conjunction of the above defects, such as the case of oligoasthenoteratozoospermia (OAT) - the simultaneous occurrence of defects in sperm number, motility and morphology. Identifying the underlying cause for the spermatogenic disruption phenotype is extremely complex in a significant number of infertile men. As recently enumerated by Visser and colleagues, hyperprolactinemia, hypogonadotrophic hypogonadism, bilateral cryptorchidism, congenital absence of vas deferens, orchitis, bilateral orchidectomy, chemo/radiotherapy, numerical genetic defects and Y chromosome microdeletions.
(29) represent the list of casual factors for abnormal semen quality (Visser et al., 2009). However, these are considered to represent only a fraction of the actual range of aetiologies that underscore male infertility. Although environmental/occupational exposures are a considerable source of spermatogenic deregulation, male infertility is regarded as a clear-cut example of a complex disease with a significant genetic basis (Vogt, 2004b; Ferlin et al., 2007b). Accordingly, it is estimated that 15 to 30% of male infertility cases are due to genetic defects (Schultz et al., 2003). The relevance of genetics for male infertility is a natural consequence of the broad gene network required for normal sperm production. In fact, approximately 1 in 25 mammalian genes is expressed in the male germline (Venables and Cooke, 2000), with over 4000 estimated to play a role in human spermatogenesis (Matzuk and Lamb, 2008). Despite the identification of several mutations causing male infertility in mammalian models, such a list is extremely reduced in humans. This observation suggests the need for the screening of novel determinants in infertile males. In light of these data, the present thesis will specifically address the (epi)genetic aspects regulating male fertility, with a particular interest in the contribution of Y chromosome factors. Accordingly, a brief introduction to the (epi)genetic determinants required for normal sperm production will be the focus of the following section..
(30) ”WHERE WILL IT END?”. Joy Division – Day of the Lords.
(31) 2- (Epi)genetic control of spermatogenesis. Spermatogenesis is the process through which the male germ cell - the sperm, is formed. The process occurs in the male gonad (the testis), which couples its gametogenic role with the synthesis and secretion of male sex hormones. In this section a comprehensive outlook into the molecular mechanisms orchestrating spermatogenesis will be undertaken. 2.1- Establishment of the germ cell lineage in developing embryos In order to fully appreciate the biology of mammalian male germ cells it is necessary to take into consideration the events responsible for germ lineage determination in the early embryo. Following the development of the three germinal layers, a specific subset of pluripotent cells emerges on the epiblast - a tissue originating from the embryo inner cell mass (Ginsburg et al., 1990). These correspond to primordial germ cell (PGC) precursors that are induced into the PGC specification pathway via extra-embryonic signalling (Tam and Zhou, 1996; Ying et al., 2001; Yoshimizu et al., 2001 and references therein). This signalling results in the expression of PR domain containing 1, with ZNF domain (PRDM1), a transcription factor essential for both the suppression of the somatic program and commitment to the germline fate (Ohinata et al., 2005; Ohinata et al., 2008). The exact mechanism by which PRDM1 exerts this level of regulation is still poorly characterized, although the epigenetic repression of somatic genes via histone modifications remains a likely pathway (Sasaki and Matsui, 2008). Subsequently, a rapid.
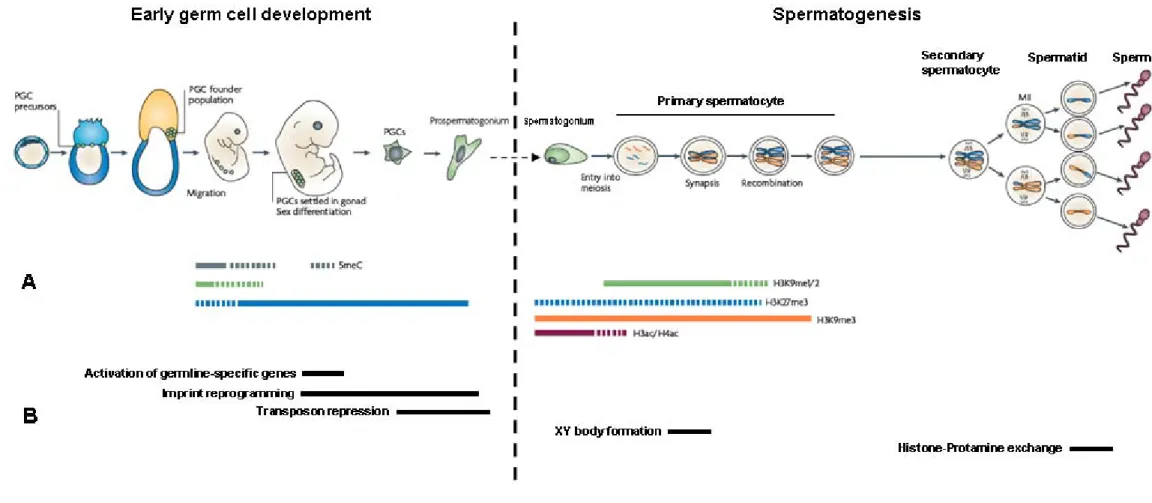
(32) proliferative event under the control of the transforming growth factor-β (TGF-β) signalling system accounts for the formation of the PGC founder population (a cluster of fully committed cells), as evidenced by defects in PGC development recorded in knockout models for both TGF-β ligands [bone morphogenetic protein-4 (Bmp4) and 8b (Bmp8b)] and SMAD transcription factors (Smad1 and Smad5) (Lawson et al., 1999; Ying et al., 2000; Chang and Matzuk, 2001; Tremblay et al., 2001). The PGC founder population starts to migrate in a journey that will take them along the hind-gut into the genital ridge, where they will eventually settle in the region that is to differentiate into the gonad. Although chemical stimuli and cell adhesion factors have been linked to this migrational event in humans, precise genetic determinants have only been detected in rodent models, where mutations in both the Kit ligand and receptor (Kitl and Kitr, respectively) have been shown to block PGC migration (Matzuk and Lamb, 2002 and references therein). During migration, PGCs are also subjected to a dramatic genome-wide reprogramming event that will lead to the establishment of a germline-specific chromatin state (Figure 2.1). These changes arise from the activation of pluripotency genes: Pou5f1, Nanog, Dppa3 and Sox2 (Surani et al., 2004). Indeed, prior to migration, the levels of three major transcriptionally-repressive marks [DNA methylation, histone H3 lysine 9 dimethylation (H3K9me2) and histone H3 lysine 27 trimethylation (H3K27me3)] are indistinguishable between PGCs and somatic cells (Seki et al., 2005). Yet, shortly after initiating migration, DNA methylation levels are reduced followed by a decrease in H3K9me2 (Seki et al., 2007; Hajkova et al., 2008). This significant decrease in repressive marks does not lead to gene expression deregulation since RNAP II-dependent transcription is transiently repressed and several key transcription factors are presumably unavailable. In addition, the level of H3K27me3 as well as of several histone acetylation marks increases prior to cells arriving at the genital ridge (Hajkova et al., 2008). Therefore, after settling in the future gonad region, PGCs display a complex pattern of regulation of chromatin-associated repressive marks that clearly distinguishes them from the somatic lineage (Seki et al., 2007; Hajkova et al., 2008)..
(33) Figure 2.1 – Epigenetic changes occurring in the male germline. Left-hand side panels depict germ cell development during the initial embryonic stages and those in the right the spermatogenic process as activated at puberty. A- Regulation of the major epigenetic marks during the male germline reprogramming events. Dashed lines indicate decreased levels when compared to the period represented by solid lines. Please consult text for abbreviations. B- Timing of occurrence of developmental mechanisms under epigenetic control. Adapted from Sasaki and Matsui, 2008..
(34) The analysis of mammalian models has also shown that one of the major developmental steps in post-migratory PGCs is the activation, via DNA demethylation, of germline-specific genes: deleted in azoospermia-like (Dazl), synaptonemal complex protein 3 (Sycp3) and DEAD box polypeptide 4 (Ddx4) (Maatouk et al., 2006). These genes and corresponding flanking regions escape the global DNA demethylation wave occurring during PGC migration, highlighting their intricate regulation in order to ensure a precise timing of expression. Additionally, another germline-specific gene, DEAH box polypeptide 38 (Dhx38), is also activated by histone modifications presumably stemming from the translocation of the repressive PRDM1-PRMT5 complex to the cytoplasm (Ancelin et al., 2006). The activation of these germline genes is associated with a second wave of asynchronous DNA demethylation that ensures the erasure of parental imprints without the loss of a certain degree of control in retrotransposon repression. This reprogramming stage occurs in an already morphologically distinguishable gonad. 2.2- Male germ cell differentiation: paternal imprinting and retrotransposon silencing Parallel to the beginning of sex determination in the gonadal region, germ cells also embark in their respective differentiation program, yielding the establishment of G1arrested prospermatogonia in developing males (McLaren and Buehr, 1990). These cells will progressively acquire new imprints throughout foetal development, as well as silence retrotransposon sequences. Both events are mediated by de novo DNA methylation, mainly conducted by DNA methyltransferase 3A (DNMT3A) and 3B (DNMT3B) (Kaneda et al., 2004; Kato et al., 2007). While imprint acquisition relies heavily on DNMT3A activity (only a single imprinted locus seems to specifically require DNMT3B), a more balanced contribution of these factors is required for silencing the different retrotransposon classes. Moreover, another member of the DNMT family, DNMT3L, is required for both processes (Bourc'his et al., 2001; Bourc'his and Bestor, 2004). DNMT3L lacks DNA methyltransferase activity but potentiates the activities of DNMT3A and DNMT3B by the formation of protein complexes..
(35) Recent studies have identified a link between small RNA pathways and retrotransposon silencing (Aravin et al., 2007). In fact, the regulation of gametogenesis via non protein coding sequences represents one of the most striking advances in the field of reproduction. The proteins responsible for the synthesis of these small RNAs predominantly localize to ribonucleoprotein particles, particularly nuage/germinal granules in male germ cells (Kotaja et al., 2006). Of these proteins, a specific subset, the mouse P element wimpy testis-induced (PIWI) homologues are involved in the processing of a class of small RNAs, the aptly named PIWI-interacting RNAs (piRNAs) (Lau et al., 2006). piRNAs are small RNAs composed of approximately 27 nucleotides that are generated from a long precursor molecule. Fittingly, several thousands of piRNA species are expected to exist (O'Donnell and Boeke, 2007; Ro et al., 2007; Brennecke et al., 2008). piRNAs have been shown to play a role in the regulation of retrotransposon activity: piwi-like homolog 2 (Drosophila) (PIWIL2; also known as MILI), is expressed prior to germ cell sexual differentiation and is required for proper spermatogenesis since mice lacking PIWIL2 show erroneous retrotransposon silencing and meiotic defects (Kuramochi-Miyagawa et al., 2004; Aravin et al., 2006; Carmell et al., 2007). Nevertheless, despite this molecular activity, prospermatogonia remain largely quiescent throughout development until they are reactivated at puberty, at the onset of spermatogenesis. 2.3- Spermatogenesis Spermatogenesis results in an average daily production of 2x108 sperm in healthy adult males, with a full spermatogenic cycle lasting approximately 64 days. Unlike female gametogenesis it is a continuous process, owing to spatiotemporal organization of the program along the seminiferous tubules, and it does not halt with advancing age. Spermatogenesis can be divided in 3 main phases: mitotic proliferation (production of a large number of precursor cells), meiosis (haploidization and generation of genetic.
(36) diversity) and spermiogenesis (morphological specialization of the male gamete for fertilization). An overview of these stages will be focus of the following paragraphs. The quiescent prospermatogonia are located in the basal compartment of the seminiferous tubules and, upon activation by suitable hormonal stimuli, acquire the status of spermatogonial stem cells. These enter a series of mitotic cycles with the resulting cells following one of two possible fates: either they continue to divide as stem cells (type A spermatogonia) or they embark in a differentiation pathway (type B spermatogonia). Rodent models have demonstrated that the initial proliferative stage is tightly regulated, with a fine balance between genes involved in growth (particularly Kit, Bmp4, Bmp8a and Bmp8b) and genes involved in apoptosis [both pro- (Bax) and anti-apoptotic (members of the BCL2 family)] being crucial for the success of program (Rodriguez et al., 1997; Rossi et al., 2000; Jahnukainen et al., 2004; Itman and Loveland, 2008). The switch from the proliferative to the differentiation pathway seems to require modulation of translational control as mediated by the PUF family RNA-binding proteins (more specifically, PUM1, PUM2 and PUMILIO) (Parisi and Lin, 1999; Crittenden et al., 2002; Moore et al., 2003; Urano et al., 2005). PUF family members interact with another RNA-binding protein, NANOS and this interaction, also involving DDX4, is required for the maintenance of the germ stem cell population (Jaruzelska et al., 2003; Ehrmann and Elliott, 2005; GinterMatuszewska et al., 2009). Equally important for such maintenance is the action of the RNA-binding factor NANOS2, mutations in which seem to be linked to male infertility (Kusz et al., 2009). After commitment to the differentiation pathway, type B spermatogonia adopt the role of a progenitor cell as they are subjected to clonal expansion via successive mitotic cycles. Studies show that RBM plays a role in the mitotic regulation of these pre-meiotic germ cells (Szot et al., 2003; Tsuei et al., 2004). The mitotic divisions in developing spermatogonia are characterized by incomplete cytokinesis. The functional consequence of such peculiar division dynamics is that all resulting cells are clonally linked via cytoplasmic bridges, adopting the configuration of a syncytium. These cytoplasmic bridges are to play a pivotal role during subsequent meiotic and post-meiotic developmental stages, since they will allow the diffusion of X and Y-.
(37) encoded gene products to cells that would otherwise be bereft of them (due to chromosomal segregation), as well as spatially synchronize the development of the cell cohort. In fact, the syncytial organization is a hallmark of all subsequent developmental stages up to the individualization of the fully mature gametes. A recent study in the rodent model has shown that the product of testis expressed gene 14 (Tex14) is essential for the formation of these cellular bridges (Greenbaum et al., 2007). After successive clonal divisions, type B spermatogonia differentiate into primary spermatocytes - diploid cells with 4n of DNA (“n” indicates the DNA content of a single set of chromosomes). Upon synthesis of the 4n DNA complement, these cells migrate in the direction of the adluminal compartment of the seminiferous tubules. In this migration, cells cross the blood-testis barrier by transiently disrupting the zonular junctions between adjacent Sertoli cells. Primary spermatocytes immediately enter the prophase of the first meiotic division, the longest stage of the meiotic program (taking approximately 22 days to be completed). From such division arise secondary spermatocytes, resulting from the segregation of homologous chromosomes to opposite poles and subsequent partial cytokinesis. The latter cell stage is rapidly replaced by that of round spermatids after completion of the second meiotic division without DNA synthesis. Seeing that the segregation products correspond to sister chromatids, spermatids are haploid (1n) cells. In addition to its role as a haploidization mechanism, meiosis is also crucial for the generation of genetic diversity. Such diversity can stem not only from the random segregation dynamics, but also from genetic crossing over events occurring in the extended prophase stage of the first meiotic division. The latter is classically divided in 5 sub-stages: leptotene, zygotene, pachytene, diplotene and diakinesis, with the attachment of chromosomes to the inner membrane of the nuclear envelope signalling its start. Subsequently, the synapsis (physical pairing) of homologous chromosomes begins by the development of a synaptonemal complex that mediates pairing from the nuclear attachment points. The end result of full synapsis is that homologous stretches of paternal and maternal DNA are in close contact with each other. This allows genetic crossing-over.
(38) (the exchange of segments between non-sister chromatids) to take place during the pachytene sub-stage. Since genetic crossing-over is associated to chromatid breakage and rejoining, these are reflected by the occurrence of chiasmata (singular: chiasma) - points of chromatin connection between chromosomes that signal the localization of the crossing-over. Accordingly, despite the disassembly of the synaptonemal complex being necessary for chromosome disjunction and condensation in the following prophase substages, such disassembly is delayed in the chiasma regions. The success of this process is tightly. correlated. with. effective. retrotransposon. silencing,. since. unmasked. retrotransposons lead to non-homologous synapsis (Sasaki and Matsui, 2008). In this regard, mutations in Dnmt3a, Dnmt3l and Piwil2 lead to meiotic block (Bourc'his and Bestor, 2004; Kuramochi-Miyagawa et al., 2004; Webster et al., 2005a). Due to a more restricted level of homology, the pairing and crossing-over mechanisms are slightly different in the sex chromosomes. In fact, by the early pachytene sub-stage the X and Y chromosomes form a specific structure - the XY body. This structure is marked by a particular level of chromatin condensation and transcriptional inactivation (Jablonka and Lamb, 1988; Turner, 2007). In spatial terms, the X and the Y form two discrete domains, with the chromosomal ends being joined by synaptonemal pairing between the homologous pseudoautosomal regions (PARs) (Metzler-Guillemain et al., 2000). Consequently, the four telomeres acquire a characteristic clustered spatial organization from which the two chromosomal domains emerge. The synaptonemal pairing may extend from the PARs into non-homologous domains, yet sequence exchanges are confined to the PARs, particularly to a 2.5 megabase (Mb) stretch adjacent to the short arm telomeres (Handel, 2004; Yogev et al., 2004). Indeed, both significant chromatin condensation and pairing impairment are the main strategies to prevent recombination between non-homologous sequences of the X and Y. Additionally, chromosome inactivation may also prevent the eliciting of meiotic checkpoint systems by masking the extensive X-Y unsynapsed domains (Fernandez-Capetillo et al., 2003). The occurrence of a single genetic crossing-over event in the short arm PAR (PAR1), is required for successful meiosis since it maintains the sex chromosomes paired in the.
(39) metaphase plate of the first division. Although recombination rates are also elevated for the long arm PAR (PAR2), there is no crossing-over requirement (Burgoyne, 1986; Flaquer et al., 2008). Regarding the (epi)genetic determinants regulating XY body formation, histone H2A variant phosphorylation at Ser139 (γH2AX) seems to play a central role. γH2AX co-localizes with ataxia telangiectasia and Rad3 related (ATR) and accumulates in the XY body prior to the pachytene sub-stage (Fernandez-Capetillo et al., 2003; Turner et al., 2004). This pronounced chromatin remodelling is also linked to histone deacetylation (in histones H3 and H4) and methylation (in histone H3) (Khalil et al., 2004). Accordingly, mutations in PR domain containing 9 (Prdm9), a histone H3K4 methyltransferase, leads to defects in XY body formation in mammalian models (Hayashi et al., 2005). Fittingly, the use of such models has been pivotal for the identification of genes involved in meiotic control (reviewed in: Hunt and Hassold, 2002; Matzuk and Lamb, 2008). Mutations in sporulation protein meiosis-specific SPO11 homolog (S. cerevisiae) (Spo11), mutL homolog 1 (E. coli) (Mlh1), mutS homolog 4 (E. coli) (Msh4) and 5 (Msh5), DMC1 dosage suppressor of mck1 homolog, meiosis-specific homologous recombination (yeast) (Dmc1), and postmeiotic segregation increased 2 (S. cerevisiae) (Pms2) have been linked to meiotic block. Furthermore, mutations in synaptonemal complex protein 3 (Sycp3), ataxia telangiectasia mutated homolog (human) (Atm), cyclin A1 (Ccna1), meiosis defective 1 (Mei1), deleted in azoospermia-like (Dazl) and DEAD (Asp-Glu-Ala-Asp) box polypeptide 4 (Ddx4) are also involved, among others, in early meiotic disruption. Less severe meiotic phenotypes have been recorded for mutations in cytoplasmic polyadenylation element binding protein 1 (Cpeb1) and Fanconi anemia complementation group A (FANCA), the latter being also detected in infertile men (Wong and Buchwald, 2002). More recently, a role of Aurora Kinase C (AURKC) in the regulation of male meiotic cytokinesis has been proposed based on the identification of a gene mutation in men with large-headed polyploid multi-flagellar sperm (Dieterich et al., 2007)..
(40) An interesting property of the germline genetic program can be observed in genes that are also expressed in somatic tissues. In these cases, transcript structure and regulation tend to vary between the germ and somatic lineages (Hecht, 1998). Such an effect may be a reflection of the particular developmental requirements for gametogenesis. Although differences in transcription start site, polyadenylation cues and alternative splicing may account for some of the differences, the use of different promoters is the main mechanism responsible for this variability (Eddy and O'Brien, 1998). In fact, germline-specific promoters tend to alter the structure of the 5’-UTR in order to modulate translational efficiency and timing. Accordingly, the consequent formation of secondary structures or the incorporation of upstream reading frames, cis regulatory elements and localization signals, are all strategies vying for gene expression control in male gametogenesis (for a review: Kleene, 2001). Parallel to the orchestration of genetic determinants for meiosis, male germ cells also dramatically remodel their epigenetic constitution in preparation for the process. A significant increase in the number of histone variants (namely TH2A, TH2B, TH3, H3.3A, H3.3B and HT1) is detected in the nucleosomes of pre-meiotic germ cells (Meistrich et al., 1985; Kimmins and Sassone-Corsi, 2005). This represents a possible mechanism for the priming of chromatin into a meiosis-compatible conformation. Furthermore, it fits with the extensive histone methylation dynamics required for the completion of early prophase. In accordance, mutations in genes coding for histone H3K9 methyltransferases are associated to meiotic arrest. This has already been demonstrated in rodent models for the suppressor of variegation 3-9 homolog 1 (Drosophila) (Suv39h1) and 2 (Suv39h2) and euchromatic histone lysine N-methyltransferase 2 (Ehmt2) (Peters et al., 2001; Tachibana et al., 2007). Two other genes are also required for XY body formation and autosomal synapsis and recombination: mutations in PRDM9 and sex comb on midleg homolog 1 (SCMH1) result in pachytene block (Hayashi et al., 2005; Takada et al., 2007). These chromatin changes also seem to favour the elevated transcriptional activity recorded in pachytene spermatocytes. In fact, spermatocytes are characterized by overexpression of most mRNA species, with transcript levels far.
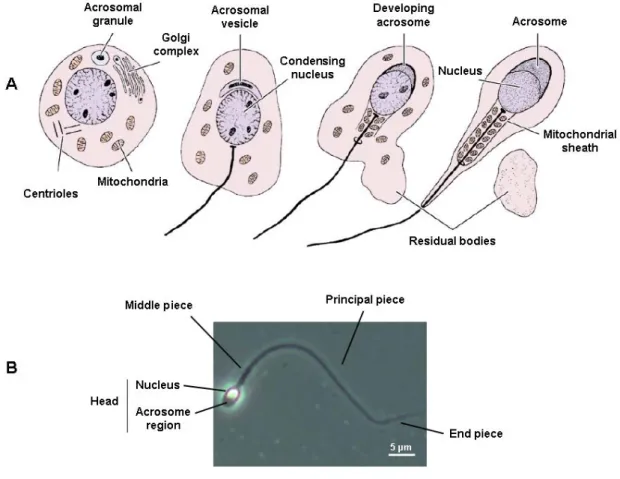
(41) exceeding those of the corresponding proteins (Kleene, 2001). It can be speculated whether the chromatin changes can partially account for the elevated expression levels by increasing DNA accessibility. The high sensitivity to DNase I digestion recorded in pachytene spermatocytes seems to favour this view, but the causality of the association is difficult to ascertain (Raman et al., 1988). Chromatin accessibility is equally a prerequisite for meiosis-associated events such as induction of double strand breaks, synapsis and genetic crossing over. Indeed, an apparent correlation between testicular expression of specific sequence tags and frequency of recombination in males prompted a model in which the increased transcription levels are a requirement for successful pairing and synapsis of homologous chromosomes (Jones et al., 1997). The last phase of the spermatogenic process is referred to as spermiogenesis (Figure 2.2). It corresponds to a dramatic cytodifferentiation program that during approximately 35 days will gradually shape the round spermatid into a highly polarized and motile cell the sperm. Spermiogenesis occurs in the adluminal compartment of the seminiferous tubules, with the developing spermatids being embedded in Sertoli cell cytoplasmic crypts. The first significant event in spermiogenesis is the progressive development of a vesicle that will envelop the anterior part of the sperm nucleus - the acrosome. This specialized organelle contains hydrolytic enzymes required for the fertilization process. During acrosome formation, centrioles migrate to the pole opposite to the acrosomal vesicle, whereby the distal centriole differentiates into a long concentric array of microtubule doublets, the axoneme. The axoneme serves as scaffold for the sperm tail, the latter also requiring the zonal deposition of keratin-containing outer dense fibers, a fibrous and a mitochondrial sheath. In fact, the disruption of genes associated to any of these structures has already been identified in infertile men (Chemes et al., 1987; Turner et al., 2001; Schwabe et al., 2008; Zuccarello et al., 2008). Nuclear condensation is another hallmark of spermiogenesis. This chromatin remodelling event is essential for the remarkable compaction of the sperm nucleus. The latter ensures genome protection in the harsh reproductive tract milieu and an increased cell density suitable for fast progressive motility. Nuclear remodelling in developing.
(42) spermatids relies heavily on the modification of chromatin-bound proteins. Methylation, phosphorylation and ubiquitinization of canonical histones seem to recruit histone variants (some of them testis-specific, such as H1T2 and HILS1) to replace them in the nucleosome (Govin et al., 2004; Martianov et al., 2005; Sasaki and Matsui, 2008). These in turn elicit additional bouts of histone modification, with acetylation included in the repertoire. In fact, the latter mechanism serves as driving force for histone replacement with transition proteins 1 and 2 (TNP1 and TNP2). Again, such organization represents a transient chromatin state, with the final replacement of transition proteins with protamines 1 and 2 (PRM1 and PRM2) being mediated by phosphorylation/ dephosphorylation events (Rousseaux et al., 2005). The protamine units derive from previously synthesized transcripts that were produced en masse and stored as ribonucleoprotein particles in earlier developmental stages (Kleene, 1996). This strategy for massive transcript synthesis and storage under the guise of stable protein complexes is also extended to other genes required for spermiogenesis. The process can be considered a natural consequence of the progressive shut-down of transcriptional activity in the male germ cell nucleus, stemming from chromatin remodelling. In fact, translational delay in spermatids represents an additional level of genetic control in the gametogenic program, with the premature expression of developmentally regulated transcripts being linked to spermatogenic arrest (Lee et al., 1995). Interestingly, the existence of histone-rich domains in the condensed sperm nucleus suggests that some genomic regions may be available for transcription in the last stages of spermatogenesis (Wykes and Krawetz, 2003). The completion of spermiogenesis is signalled not only by nuclear condensation, but also by the final alignment of mitochondria along the middle piece of the sperm tail and the Sertoli cell-mediated phagocytosis of a large part of the cell’s cytoplasm (the residual body). Only after these steps are complete, mature sperm are released in the lumen of the tubule in a process designated as spermiation. The still immotile mature gametes will then be embedded in testicular fluid and directed to the epididymis for storage prior to ejaculation..
(43) 5 µm. Figure 2.2 – Spermiogenesis: from round spermatids to mature sperm. A- Major cytological modifications occurring during spermiogenesis. Four key structural changes are operated: idevelopment of the acrosome; ii- nuclear condensation, iii- development of the tail, and ivshedding of residual cytoplasm. B- Sperm structure. The cell can be broadly divided in the head, middle piece and tail segments, the latter further subdivided into a principal and end piece. Adapted from Junqueira & Carneiro, Basic Histology: Text & Atlas, 11th ed. (2005).. 2.4- The male fertility genetic network: importance of the Y chromosome As expected from the pivotal importance of the sex determining region Y (SRY) for sexual development, XX mice carrying a Sry transgene have a male phenotype (Bradbury, 1983). Yet, such mice also display a spermatogenic block. This readily validates previous assertions on the importance of the Y for male fertility, stressing the fact that it contains genes involved in the sperm production pathway (Tiepolo and Zuffardi, 1976). Deletions in the Y chromosome, particularly microdeletions in its long arm, represent one of the most frequent genetic causes of spermatogenic failure (Ferlin et al.,.
(44) 2007a). Since the core focus of this thesis will be the analysis of genetic determinants of the Y, the following sections will deal with some of the most significant aspects of Y chromosome dynamics. These will include an historical perspective on how our understanding of its role in human reproduction has evolved, as well as a structural and genetic overview of its properties..
(45) ”I TRAVELED FAR AND WIDE THROUGH MANY DIFFERENT TIMES,. WHAT DID YOU SEE THERE?”. Joy Division - Wilderness.
(46) 3- The human Y chromosome: history, structure & future. If gene quantity was the sole criterion for geneticists to choose their research interests, the human Y chromosome would amount to little more than a footnote in the literature. In fact, the Y harbours just ~0.3% of all known human protein-coding genes (Ensembl, 2009). Nevertheless, this intriguing genetic entity is not only one of the most intensely debated chromosomes in the genome but also preferential study matter for reproductive biologists. At the centre of this interest is the Y chromosome’s role in sex determination and spermatogenesis. Yet, views on its functional importance have dramatically changed over the past hundred years. In this regard, a brief historical perspective on the study of the Y is a fitting start to this section. 3.1- Historical perspective on the Y chromosome Pioneering studies at the beginning of the 20th century had identified an association between chromosomal constitution and phenotypical sex (Wilson, 1905). Yet, this association was considered minor by a significant part of the scientific community, which proposed that quantitative differences in metabolic rates were the de facto sexdetermining system. Such views were consistent with observations of the Y as “condensed chromosome nucleoli”, therefore prone to a less active role during male development (Wilson, 1905). Yet, in a paper published in 1910, Edmund Wilson elegantly changes his.
(47) position on the quantitative vs. qualitative sex determination debate; cautiously adopting the view that the sex chromosomes do contain different male and female determinants, even going as far as considering them genes (Wilson, 1910). Impervious to such dispute, considerable advances in the field of cytogenetics were recorded in the 1920s. In this regard, the contributions of Theophilus Painter would prove to be decisive. Painter provided evidence for a total of 46 chromosomes being the diploid number in our species (Painter, 1921). Additionally, he identified that half of the sperm carried a Y chromosome and the other half an X, as a consequence of segregation dynamics in the first spermatocyte division. These observations were further extended to other primates with particular care being taken in the analysis of the XY constitution of male somatic and germ lineages (Painter, 1923). These advances were coincident with a significant breakthrough by Johannes Schmidt that recorded, for the first time, an instance of Y-linked inheritance (Schmidt, 1920). This initial study on the genetics of a pigment spot in fishes was followed by a considerable list of reports describing the transmission of several traits by the Y chromosome in the most diverse species (for a review: Stern, 1957). It was only natural that the concept of Y-linked inheritance would be extended to humans, and indeed in 1922 two independent studies suggested such a mechanism for the transmission of the webbed toes phenotype (Castle, 1922; Enriques, 1922). This triggered such a series of reports on the identification of novel Y-linked traits that by 1946 almost twenty different phenotypes were deemed to be attributable to Y genes (Gates, 1946). This somewhat extensive list of traits stood in stark contrast from the prevailing view at the start of the century of the Y as a metabolically inefficient X. Despite the flimsy evidence supporting most claims, some interesting observations were made in this period, particularly Fisher’s suggestion that the Y would selectively contain alleles that would favour male over female functions (Fisher, 1931). Nevertheless, the reported instances of Y-linked inheritance were based on the observation of a very restricted number of pedigrees and consequently displayed little robustness. This very situation had prompted, a few years earlier, Enriques’ quip to one particular study author (and affected family member), asking him.
(48) to “produce many children, many male children and many female children, for the love of science” (Enriques, 1922). In fact, in his review of Y-linkage in man, Curt Stern performed a critical analysis of all available data supporting previous associations, and concluded that evidence was insufficient to corroborate such type of heredity (Stern, 1957). Fittingly, current evidence points to a paucity of Y-linked diseases: other than defects in sex determination and spermatogenic function, a genetic basis for deafness and retinitis pigmentosa has been proposed, the latter based on limited evidence (Zhao et al., 1995; Wang et al., 2004). Stature defects have also been recorded, but in this case the genetic determinant is also present in the X chromosome (for a review: Marchini et al., 2007). While one can only imagine the busy reproductive activity among the webbed toe community (for the noble values of science, no less), 1959 yielded a major breakthrough on the genetics of the Y. In fact, two independent studies in patients with abnormal sex chromosome constitution (XXY and XO) provided solid evidence that the Y chromosome was a male determinant (Ford et al., 1959; Jacobs and Strong, 1959). These observations validated the postulates of Alfred Jost, that already by the late 1940s had argued that sex chromosome constitution determined the phenotypical sex of the individual by controlling gonadal differentiation (Jost, 1947). After the publication of those two seminal papers, thirty years had to pass before the identity of this determinant was ascribed to the SRY gene (Sinclair et al., 1990). Yet, the existence of a functional locus regulating sex determination in the Y was viewed more as an exception than a rule. The mechanistic bases of this concept were put forward in 1967 in one of the most thought-provoking essays of contemporary biology. According to Susumu Ohno, the mammalian sex chromosomes originated from a standard autosomal pair after the acquisition of a sex determining locus in one of the homologues (Ohno, 1967). This led to a gradual emptying of genes in the sex determining chromosome due to progressive loss of recombination with the homologous partner. A more thorough analysis of this postulate and particularly of its consequences for the evolutionary fate of the Y chromosome will be dealt with in greater detail in a subsequent subsection..
(49) Refinements in karyotype analysis in the 1970s translated into crucial insight on the role of the Y chromosome in sex-related functions. The shift from pedigree-based association studies to cytogenetic analysis proved to be essential for a better understanding of Y function. Accordingly, by the beginning of the decade, an association between cytologically visible Y chromosome lesions and defects in spermatogenic function was recorded (Neu et al., 1973). These observations were later confirmed by the identification of a genetic determinant in the long arm of the Y (Yq) controlling spermatogenesis (Tiepolo and Zuffardi, 1976). Such determinant was considered to be an azoospermia factor, since its deletion resulted in the lack of sperm in the ejaculate. Appropriately, by providing evidence that the Y also ensured male gamete production, Tiepolo and Zuffardi’s work demonstrated that this chromosome was involved in other biological processes besides sex determination. The link between the Y and spermatogenesis was later identified in several animal models, highlighting the evolutionary conservation of this strategy (Hardy et al., 1981; Levy and Burgoyne, 1986). With the advent of more powerful molecular biology techniques, several efforts were undertaken during the 1980s to establish Y chromosome deletion maps (illustrating examples: Affara et al., 1986; Vergnaud et al., 1986). The goal of these maps was to ascribe the different genes to specific chromosome intervals. Nonetheless, this strategy was met with limited success mainly due to the restriction to naturally-occurring deletions (which dramatically decreased the mapping templates), and the repetitive nature of the chromosome. Only the use of larger patient sets and single-copy sequence markers in the 1990s led to a progressive understanding of the complex genetic organization of the Y (Ma et al., 1992; Kobayashi et al., 1994; Vogt et al., 1996). Major advances were recorded in the early 2000s, first with the establishment of a high-resolution genetic map, then with the sequencing of the chromosome (Tilford et al., 2001; Skaletsky et al., 2003; Ross et al., 2005). The latter represented a milestone that led to a more precise understanding of the Y. This new level of information was paramount not only to elucidate the full gene content of the chromosome, but also to.
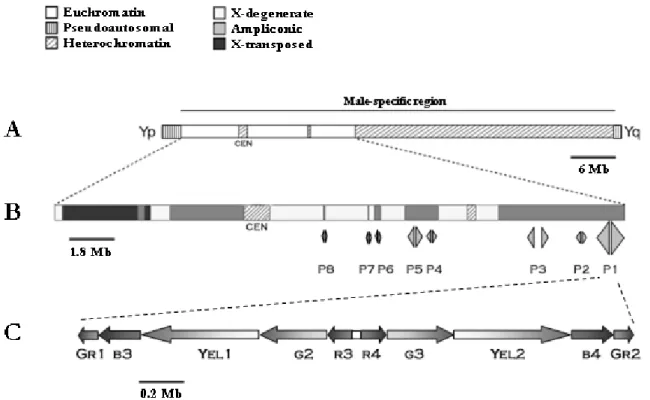
(50) identify some striking structural properties that will be discussed in more detail in the ensuing subsection. 3.2- Y chromosome structure and sequence classes The human Y is a small submetacentric chromosome harbouring according to the latest Ensembl estimates 86 protein-coding genes and 282 pseudogenes (Ensembl, 2009). Although prone to significant size variation as consequence of a polymorphism in a large heterochromatin block, the Y spans approximately 60 Mb. Structurally, the Y chromosome can be divided in four hallmarks: the PARs, the male-specific region, the centromeric domain and the large Yq heterochromatin block, with the latter two localizing to the male-specific region (Figure 3.1). The heterochromatin block extends over an average of 30 Mb and is constituted by arrays of the DYZ1 [3.4 kilobase (kb)], DYZ2 (2.5 kb) and DYZ18 (2.8 kb) repeat families (Cooke et al., 1983). This region exhibits significant size polymorphism among human populations with the DYZ sequences constituting up to 70% of the DNA content of some Y chromosomes (Repping et al., 2006). Interestingly, the use of large heterochromatic domains to significantly expand the sequence of the Y chromosome seems to be an evolutionarily conserved strategy (Carvalho et al., 2003a). It can be speculated that such motifs may serve a structural role during X-Y pairing. The PARs correspond to two homology domains between the sex chromosomes ensuring successful meiotic pairing. These blocks of sequence identity are located at both ends of the X and Y chromosomes, with PAR1 mapping to the short arm of the Y (Yp) and PAR2 to Yq. Altogether, PAR1 and PAR2 account for about 5% of the Y, with an estimated size of 2.7 and 0.33 Mb for PAR1 and PAR2, respectively (Ross et al., 2005). Both sequences diverge significantly in terms of structural properties, a likely consequence of the requirement for genetic crossing over in PAR1 but not in PAR2. PAR1 is characterized by a higher GC content than PAR2, as well as a specific repeat element signature: low levels of L1 family repeats compensating a much higher incidence.
(51) of Alu sequences (Ciccodicola et al., 2000). This sequence organization likely potentiates the intense recombination activity recorded in PAR1. Of the 86 protein-coding genes in the Y chromosome, 28 or 29 (depending on the selected criteria) map to the PARs (24 in PAR1 and 4 or 5 in PAR2) (Blaschke and Rappold, 2006; Helena Mangs and Morris, 2007). Accordingly, the PARs exhibit a much higher gene density than the rest of the Y. These encode for products related to diverse biological themes, with three clinical conditions already linked to disturbances in this region: isolated short stature, Leri-Weill dyschondrosteosis and Langer mesomelic dysplasia (corresponding to the homozygous state of Leri-Weill dyschondrosteosis) (Ballabio et al., 1989; Rao et al., 1997; Shears et al., 1998). All these conditions are linked to mutations in a single gene: short stature homeobox (SHOX) (Rappold et al., 2002). On the other hand, the loss of PAR1 is associated to male infertility due to meiotic block, as stated in the previous section. The male-specific region of the Y (MSY) represents one of the most intriguing sequences of the human genome. It spans approximately 23 Mb (8 Mb in Yp and the remaining in Yq) and contains 78 protein-coding genetic units (Skaletsky et al., 2003). It should be noted that if we consider the latest Ensembl figures this number drops to 58. Of these, only 18 are present as single copy sequences, with the vast majority being arranged as members of 9 multi-copy gene families. Therefore, if one is to consider the number of different protein-coding sequences in the Y, this figure amounts to 55 or 56 (27 in the MSY + 28 or 29 in the PAR regions). One of the most interesting aspects of the MSY is that it is constituted by a lattice of 3 distinct sequence classes differing not only in genomic origin but also in structural properties (Figure 3.1). These correspond to the X-transposed, X-degenerate and ampliconic classes, each with a characteristic gene density reflecting the evolutionary history of the Y. Such observations were the result of the MSY sequencing project, a tremendous effort undertaken by David Page’s lab (Skaletsky et al., 2003), which is central to our current knowledge of the Y chromosome. Accordingly, most of the following data on the MSY sequence classes stems from this study..
Imagem
Documentos relacionados
The probability of attending school four our group of interest in this region increased by 6.5 percentage points after the expansion of the Bolsa Família program in 2007 and
Quanto ao conhecimento dos estudantes sobre o período que a gestante deve iniciar o uso do ácido fólico como método de prevenção de defeitos no fechamento do tubo neural os
Por isto, é de es- pecial importância o conhecimento tanto das con- d ições ecológicas d a célula administrativa, como dos fatôres geográficos que determinam sua
keywords Digital images analysis, feature extraction, image segmentation, classifica- tion, content-based image retrieval, similar images, image histogram, edge detection
Ousasse apontar algumas hipóteses para a solução desse problema público a partir do exposto dos autores usados como base para fundamentação teórica, da análise dos dados
This log must identify the roles of any sub-investigator and the person(s) who will be delegated other study- related tasks; such as CRF/EDC entry. Any changes to
Além disso, o Facebook também disponibiliza várias ferramentas exclusivas como a criação de eventos, de publici- dade, fornece aos seus utilizadores milhares de jogos que podem
didático e resolva as listas de exercícios (disponíveis no Classroom) referentes às obras de Carlos Drummond de Andrade, João Guimarães Rosa, Machado de Assis,