New heterogeneous catalyst for the esterification of fatty acid produced
by surface aromatization/sulfonation of oilseed cake
Eleonice Moreira Santos
a,⇑, Ana Paula de Carvalho Teixeira
a, Flávia Gontijo da Silva
a,
Thérèse Ebambi Cibaka
a, Maria Helena Araújo
a, Willian Xerxes Coelho Oliveira
a, Felipe Medeiros
a,
Alex Nogueira Brasil
b, Leandro Soares de Oliveira
a, Rochel Montero Lago
a,⇑a
Departamento de Química, Instituto de Ciências Exatas, Universidade Federal de Minas Gerais, Belo Horizonte-MG 31270-901, Brazil
bBiominas Engineering Industry, Itaúna, MG, Brazil
h i g h l i g h t s
Simple sulfonation of biodiesel waste cake in mild conditions produces a new and active heterogeneous acid catalyst. The catalytic activity is comparable to H2SO4and the catalyst can be reused several times.
The catalyst combines a carbon-sulfonic acid surface with a very hydrophilic cellulose surface responsible for water removal.
a r t i c l e
i n f o
Article history:
Received 30 September 2014
Received in revised form 6 February 2015 Accepted 8 February 2015
Available online 16 February 2015
Keywords:
Acid catalyst Oleic acid Esterification Biodiesel cake Aromatization Sulfonation
a b s t r a c t
In this work, an efficient heterogeneous acid catalyst for the esterification of oleic acid was prepared directly from oilseed cake by a simple sulfonation with concentrated H2SO4. Characterization by SEM/EDS, IR, Raman, TG, TG/MS, potentiometric titration showed that treatment with H2SO4for 1, 2 and 4 h at 120°C partially dehydrates the cake to form a carbon/cellulose composite which is sulfonated to produce strongASO3H acidic sites. These surface sites were active for the esterification of oleic acid with yields ca. 84%, 88% and 94% in the presence of 5, 10 and 20 wt% catalyst, respectively. These results are comparable to 98% yield obtained with 1 wt% H2SO4 and higher than 75% observed for a high surface area (880 m2g 1) sulfonated activated carbon with similar number of
ASO3H active groups. These results
are discussed in terms of two effects: (i) the number of sulfonic surface acidic groups and (ii) the presence of a hydrophilic cellulosic fraction in the catalyst that adsorbs/traps water formed in the reaction shifting the esterification equilibrium and improving the yield.
Ó2015 Elsevier Ltd. All rights reserved.
1. Introduction
The transesterification reaction to produce biodiesel in the pres-ence of basic homogeneous catalysts, e.g. KOH, NaOH or methox-ides, has been extensively investigated in the last decade[1–4]. It is well established that this alkaline catalyzed transesterification is strongly affected by the presence of free fatty acids[4,5]. The presence of these acids in concentrations higher than ca. 2% can hinder the reaction and form surface active molecules with sig-nificant complications in the purification step due to the formation of stable emulsions[6].
An alternative route to deal with acidic oils is typically a previ-ous esterification in the presence of H2SO4as catalyst[7]. However, sulfuric acid is corrosive and cannot be recovered [8,9]. In this respect, the development of an active acid heterogeneous catalyst to produce biodiesel using acidic oils is of considerable interest. Heterogeneous catalysts can be easily removed and reused avoid-ing the washavoid-ing step which simplifies the process[10–13].
Different types of acidic materials, such as zeolites [14–16]; mesoporous silica[17–19], resins[20,21], oxides, e.g. zinc, titani-um, strontium oxides[22–24], zirconia[25–28], supported carbon nanotubes[29] and minerals such as a mordenite, kaolins, hal-loysite[30–32]have been investigated as catalyst for the esterifi-cation reaction. Also promising carbon based acid catalysts have been prepared by pyrolysis followed by sulfonation with sulfuric acid, using different precursors such as carbohydrates, lignin
[33,34], sugar cane bagasse [35], fibers [36], biochar [37], resin
http://dx.doi.org/10.1016/j.fuel.2015.02.027 0016-2361/Ó2015 Elsevier Ltd. All rights reserved.
⇑Corresponding authors at: Departamento de Química, Universidade Federal de Minas Gerais – UFMG, Av. Antônio Carlos, 6627, Pampulha, Belo Horizonte-MG 31270-901, Brazil. Tel.: +55 31 3409 5719.
E-mail address:rochel@ufmg.br(R.M. Lago).
Contents lists available atScienceDirect
Fuel
[38], bean vermicelli [39], de-oiled canola [40], polymers [41], D-glucose and sulfonated ordered mesoporous carbons [42–46]. These catalysts have shown great potential to replace the tradition-al homogeneous H2SO4catalysis.
In this work, we present a new and simple reaction procedure to prepare an active heterogeneous catalyst from oilseed cake, a biodiesel byproduct. Biodiesel cakes are solid materials obtained after oil extraction by mechanical pressing, consisting mainly of lignocellulosic fibers. The high concentrations of lignocellulosic material, deficiency of proteins and the presence of some toxic compounds strongly limit the use of some biodiesel cakes in feed blends for ruminant animals[47,48]. The active acid catalyst can be produced by the direct reaction of the cake with sulfuric acid according to the simplified scheme shown inFig. 1.
The reaction with concentrated sulfuric acid with the lignocel-lulosic waste seems to promote surface reactions likely based on dehydration with aromatization followed by sulfonation producing the acid catalytic sites. The synthesis, characterization and use of this heterogeneous acid catalyst for the esterification of oleic acid in bench and pilot/ultrasound scale are described below.
2. Material and methods
Different biodiesel cakes obtained after extraction of sunflower, castor, jatropha, curcas and macaw palm oil can be used as solid lignocellulosic precursor. In a typical procedure the biodiesel cake (1 g, ground to particles smaller than 2 mm and dried overnight at 80°C) was mixed with concentrated H2SO4(min 98%, 8 mL, Syn-thÒ
), under stirring, at room temperature for 1 h (sample CK1rt) and at 120 ± 5°C for 1, 2 and 4 h (samples CK1, CK2 and CK4, respectively). Due to the exothermicity of the reaction, the process should be well controlled in order to avoid overheating and dissi-pate possible hot spots generated on the precursor surface. Caution should be taken to control after the reaction the black solid was extensively washed with water (until reaching pH7) and dried
at 80°C for 12 h. Raman spectroscopy measurements were made using a Senterra Raman spectrometer from Bruker using a CCD detector, equipped with an optical microscope (OLYMPUS BX51) and a laser at 633 nm. Thermal analyses (TG/DTG) were performed in a Shimadzu 60H under nitrogen flow (100 mL min 1) and heat-ing rate of 10°C min-1 up to 900°C. Scanning Electron Microscopy (SEM/EDS) results were obtained in a Quanta 200 – FEG – FEI 2006. FTIR spectra were obtained with KBr pellets in a Perkin Elmer FTIR GX instrument. Potentiometric titration was carried out in an auto-matic titrator Metrhomn 670 with a mixture of 25 mg of the sam-ple dispersed in 0.01 mol/L of HCl and 0.1 mol/L of NaCl and titrated with 0.010 mol/L of NaOH solution. The thermogravimet-ric-mass spectrometry (TG–MS) analysis were performed in a NETZSCH TGA model STA 449 F3, coupled to a mass spectrometer
NETZSCH Aëolos model QMS 403C. About 20 mg of the sample was used an argon flow in the purge and protective lines, both at 20 mL/min, and with heating rate of 10°C min 1up to 900°C. Gas-eous species released from the sample during the heating were drawn into an alumina tube fixed inside the furnace of the ther-mobalance close to the sample, connected to a capillary silica col-umn heated at 300°C. The gases were then directly sucked into the ionization chamber of the mass spectrometer.
The catalytic activity of the materials was tested in the esterifi-cation of pure oleic acid in a round bottom flask fitted with a reflux condenser at 60°C for 2 h, with a ratio of 12:1 methyl alcohol:oleic acid and different catalyst concentrations (5, 10 or 20 wt%, with respect of oleic acid concentration). After the reaction the catalyst (ca. 1 g) was washed with ethanol (30 mL at room temperature) and dried at 80°C for 4 h and tested in the reuse experiments. The conversion of methyl esters was analyzed by the Ca 5a-40/ AOCS method and confirmed by 1H NMR. (RMN Bruker Advance DPX 200). The signals used as references were of the methoxy groups in the methyl ester (3.7 ppm) and of the
a
-carbonylmethy-lene groups present in the oil and biodiesel (2.1 ppm) [49]. A calibration curve from the 1H NMR spectra of oil/biodiesel mix-tures using the 3.7 ppm and 2.3 ppm area ratio was obtained.
The catalytic activity of the materials was also investigated at a pilot plant scale using an ultrasound promoted reactor (see Supple-mentary Material –Fig. 1S)[50].
Commercially available activated carbon (from coconut shell, 880 m2g 1, Sulfal) was used as a catalyst in a control reaction study. The activated carbon was sulfonated using the same condi-tions used for preparation of CK2 (sulfonation with concentrated H2SO4 for 2 h at 120°C under stirring and washed with water (pH7) and dried at 80°C for 12 h). The water absorption capacity
of the CK2 material was determined by the ‘‘tea bag’’ method by weighing the water retained by the material[51].
3. Results and discussion
Biodiesel cakes from different sources, e.g. sunflower, castor, jatropha curcas and macaw palm are composed mainly of lignocel-lulosic fibers. In contact with sulfuric acid the lignocellignocel-lulosic mate-rial becomes completely black suggesting a strong dehydration process to form polyaromatic/carbon on the surface. The reaction with H2SO4was carried out at room temperature for 1 h (sample CK1rt) and at 120°C for 1, 2 and 4 h (samples CK1, CK2 and CK4 respectively).
defects or amorphous carbon [52] confirming the carbonization process of the cake material. These bands are not observed in the material obtained at room temperature, CK1rt, indicating no sig-nificant reaction with H2SO4. Moreover, due to exothermicity of the reaction at 120°C local temperatures might significantly raise to generate hot spots which can contribute to the pyrolysis process.
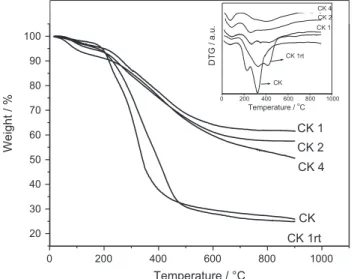
TG and DTG analyses (in N2) of the dry cake before reaction (Fig. 3) showed a weight loss between 200 and 500°C ofca.80% associated to the decomposition of lignocellulose to produce mainly CO2, H2O and other small volatile molecules[53]. Similar TG profile was obtained for the sample CK1rt. On the other hand, the materials after reaction at 120°C showed weight losses of only ca. 35–50%. These smaller weight losses are likely related to the decomposition of the remaining lignocellulose present in the materials.
FTIR spectra (Fig. 4) of the cake showed typical absorptions observed in lignocellulosic materials. The intense band at 3340 cm 1is assigned to the O
AH stretching vibration, bands at 2893 and 1431 cm 1characteristic of C
AH stretching and bands at 1190 and 1000 cm 1 are related to the saccharide structure [54]. After acid dehydration a significant change in the IR spectrum was observed with strong changes in the absorptions at 1190 and 1000 cm-1and a narrowing of the band at 3340 cm 1. On the other hand, new bands appeared, i.e. 1029 and 1160 cm 1, related to the
symmetric stretching vibrations of O@S@O in ASO3H groups [38,55–57]and another at 620 cm 1related to the bending vibra-tion of –OH groups hydrogen bonded to -SO3H[57], mainly in CK1 and CK2 samples. In addition, intense absorptions are observed
between 1500 and 1800 cm 1 likely related to aromatic C
@C (1600 cm 1) and C@O from various functional groups as carboxyl (1729 cm 1) and quinone (1550–1680 cm 1)[58].
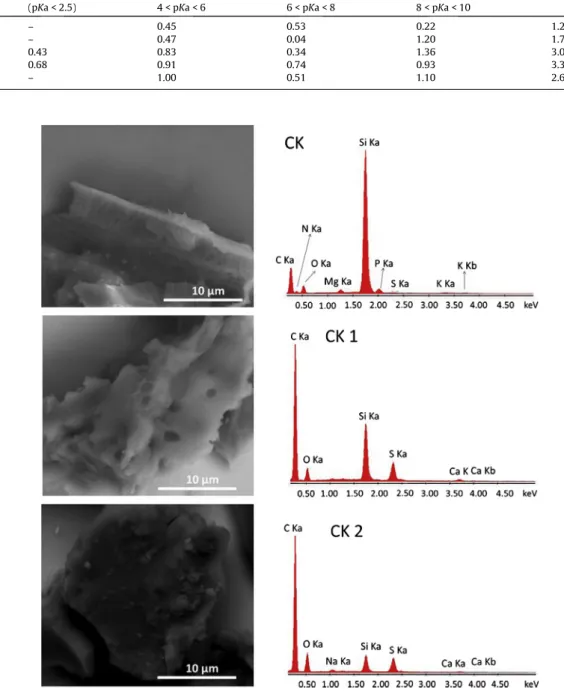
Fig. 5shows the potentiometric titration curves of the CK cata-lysts with NaOH. The number of acidic sites and pKa obtained are displayed inTable 1.
It can be observed that the CK and CK1rt showed total groups concentration of 1.2–1.7 mmol g 1, mainly as weak acidic groups. As the cake was treated with H2SO4, more acidic groups were formed, 3.0–3.3 mmol g 1. It can be observed that very acidic groups are formed with pKa lower than 2. These groups are likely related toASO3H.
It is interesting to observe that longer reaction times, i.e. 4 h, led to a decrease of the number of acidic groups. This is probably relat-ed to the hydrolysis/decomposition of the surface groups[51].
SEM analyses (Fig. 6) suggests that the surface texture of the cake does not change significantly after H2SO4 reaction. In fact, the BET surface area (ca. 0 m2g 1) and porosity of the precursor CK also did not change after H2SO4 treatment. EDS data clearly showed the presence of sulfur after reactions with H2SO4due to the sulfonation reaction.
500 1000 1500 2000 2500 3000 3500 4000
Intensity / a.u.
Raman Shift / cm-1
CK
CK1rt CK1 CK2 CK4
Fig. 2.Raman spectra of CK samples after reaction with concentrated H2SO4.
0 200 400 600 800 1000
20 30 40 50 60 70 80 90 100
0 200 400 600 800 1000 Temperature / o
C CK
CK 1rt CK 1 CK 2 CK 4
DTG / a.u.
CK 1
CK 2
CK 1rt CK
Weight / %
Temperature / °C
CK 4
Fig. 3.TG and DTG analyses of the materials CK, CK1rt, CK1, CK2, CK4, under nitrogen atmosphere.
3500 3000 2500 2000 1500 1000 500
Transmitance / a.u.
Wavenumber / cm-1
CK CK1rt
CK1 CK2
Sulfoxide
CK4
Fig. 4.FTIR spectra of the materials CK, CK1rt, CK1, CK2, CK4.
0 2 4 6 8 10 12 14 16
2 4 6 8 10 12
pH
NaOH (0.010 M) / mL
HCl CK1rt CK1 CK2 CK4
The CK and CK2 samples were also characterized by TG/MS.
Fig. 7shows the TG/MS profiles for some specific molecules. It is possible to observe that the CK sample loses m/z 18 near 100°C related to adsorbed water. It can also be observed a water loss 150–350°C related to cellulose decomposition. After sulfonation for 2 h (CK2) the TG/MS profiles suggests the presence of some adsorbed water but the cellulose decomposition signal is much less intense, suggesting that H2SO4has previously dehydrated the cake. The profile for m/z 44 signal also decreases in intensity after sul-fonation which is also likely due to deoxygenation reactions caused by sulfuric acid. It is interesting to observe an intense m/z signal 64 related to SO2 produced in the sulfonated CK2 material which suggests the presence ofASO3H groups.
The esterification of oleic acid was investigated using the sam-ple CK2 since it showed the highest concentration of surface acid
sites. Different catalyst concentrations were used, i.e. 5, 10 and 20 wt% relative to oleic acid. The esterification yields after 2 h reac-tion are shown inFig. 8(seeSM Fig. S3results for ester yields vs reaction time).
It can be observed yields near 84, 88 and 94% in the presence of 5, 10 and 20 wt% of catalyst, respectively. It is interesting to observe that the use of the classical homogeneous reaction with 1 wt% H2SO4showed yield near 98%. Sulfuric acid (H2SO4) is very acidic with pKa of 3 (first ionization) and 2 (second ionization)
[59]and potentially affords 2 H+for the reaction. Catalyst concen-tration of 1wt% H2SO4corresponds to 20 mmol H+/100 g of oleic acid. For the CK2 heterogeneous catalyst it can be considered as catalytically active sites sulfonic acid surface groups which should have pKa near between 2 and 3 (see for example benzene sul-fonic acid with pKa of 2.8)[59]. According to titration results Table 1
Results of potentiometric titrations for biodiesel cake, before and after reaction with H2SO4.
Sample Acidic functional groups (mmol g 1) Total of acids (mmol g1)
(pKa < 2.5) 4 < pKa < 6 6 < pKa < 8 8 < pKa < 10
CK – 0.45 0.53 0.22 1.2
CK1rt – 0.47 0.04 1.20 1.7
CK1 0.43 0.83 0.34 1.36 3.0
CK2 0.68 0.91 0.74 0.93 3.3
CK4 – 1.00 0.51 1.10 2.6
the surface groups with pKa < 2.5 corresponds to ca. 0.68 mmol g 1 for the catalyst CK2 (seeTable 1). Therefore, 5, 10 and 20 wt% of catalyst in the reaction corresponds to 3.4, 6.8 and 13.6 mmol H+/ 100 g oleic acid. It is interesting to observe that the CK2 shows similar catalytic activity with even lower concentration of catalytic species. It is also interesting to compare the CK2 catalyst (near no surface area ca. 0 m2g 1) with a high surface area activated carbon (890 m2g 1) sulfonated under the same conditions (2 h at 120°C with H2SO4concentrated). This catalyst showed total acid concen-tration of 3.15 mmol g 1however with lower esterification yield, 75%, compared with the CK2. Turnover number calculation after 2 h reaction showed for the catalyst CK2 (20%) 4.6 molestermolsite whereas the activated carbon AC-SO3showed TON of 3.9 molester molsite. This result suggests that some catalytic acid sites of the activated carbon are not active for the reaction likely due to the location inside the not accessible small micro pores.
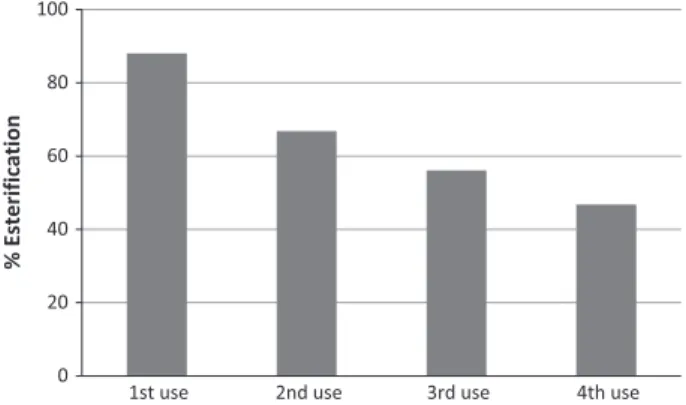
Catalyst reuse was carried out with the sample CK2 at 10 wt% under mechanical stirring without any treatment between the reactions (Fig. 9).
After the first use the yield decrease from 88% toca. 66%. In the subsequent cycles there was a decrease of the 10% in ester yield every cycle. The catalyst used in the 4th reaction was regenerated by ethanol washing characterized by thermal analysis in nitrogen atmosphere (seeSM Fig. S4). It is possible to observe a weight loss of 4% at temperatures below 150°C, relating to the loss of water/al-cohol adsorbed. In addition, the material still lost 35% weight at temperatures between 150 and 300°C. This loss can be attributed to the contamination of the catalyst by oleic acid or biodiesel.
Preliminary scale up experiments in ultrasonic reactor pro-duced by the company Biominas (see reactor inSM, Fig. S1) were carried out for the esterification of oleic acid (Fig. 10).
It can be observed for pilot scale ultrasound stirring similar ester yields obtained by mechanical stirring bench experiments. Reactions in the presence of 5, 10 and 20% showed similar behavior as in mechanical stirring bench experiments (seeSM – Fig. S5). The 20% CK2 heterogeneous catalyst showed esterification yield higher than 90% which was comparable to 1% H2SO4used as control.
The reuse of the catalyst CK2 (10%) under ultrasound stirring showed a decrease from 86% to 81%, 56% and 48% for the 1st, 2nd, 3rd and 4th reuse.
Previous works used sulfonated carbons as heterogeneous cata-lysts for the esterification of fatty acids. The carbons used were produced by pyrolysis of different vegetable precursors such as lignin[33], cane bagasse[35], mung bean [39], canola[40] and sugars[60]. In all these studies, the precursors had to be previously carbonized at high temperature (400–800°C) under controlled atmosphere and only after pyrolysis underwent sulfonation. The obtained results showed only moderate yields of ca. 80% which could be increased to higher than 90% at high temperatures and prolonged reactions (5–24 h).
The catalysts prepared in this work did not need a preliminary carbonization step and the sulfonation was carried out directly on the lignocellulosic precursor waste under mild reaction conditions. Another feature of the CK catalysts is that the carbonization and sulfonation is only partial and part of the lignocellulosic structure remains in the material as suggested by IR and TG analyses. This lignocellulosic structure has a high concentration of surface OH groups and shows a strong hydrophilic character. This hydrophilic surface can have the effect of water adsorption which can be
0 200 400 600 800 1000
Water of cellulose decomposition
Cellulose decomposition
Intensity / a.u.
m=64 SO2 (CK) m=18 H2O (CK)
m=18 H2O (CK2)
m=44 CO2 (CK)
m=44 CO2 (CK2)
m=64 SO2 (CK2)
Temperature / oC SO3H decomposition Water adsorbed
Fig. 7.TG/MS profiles of the samples CK and CK2.
0 20 40 60 80 100
CK2 5% CK2 10% CK2 20% 1% H2SO4 20% AC-SO3
% Es
te
rific
a
ti
on
% Catalyst
2h
Fig. 8.Esterification yield of oleic acid using the catalyst CK2, H2SO4, and sulfonated commercial activated carbon (AC-SO3) by mechanical stirring after 2 h reaction.
0 20 40 60 80 100
1st use 2nd use 3rd use 4th use
% Esterification
Fig. 9.Esterification yields for different reuses with 10 wt% of CK2 after 2 h reaction with mechanical stirring.
0 20 40 60 80 100
1% H2SO4 (reference)
CK2 (10%) 1st use
CK2 (10%) 2nd use
CK2 (10%) 3rd use
CK2 (10%) 4th use
% Esterification
beneficial for the esterification reaction since the water molecules are directly involved in the reaction equilibrium.
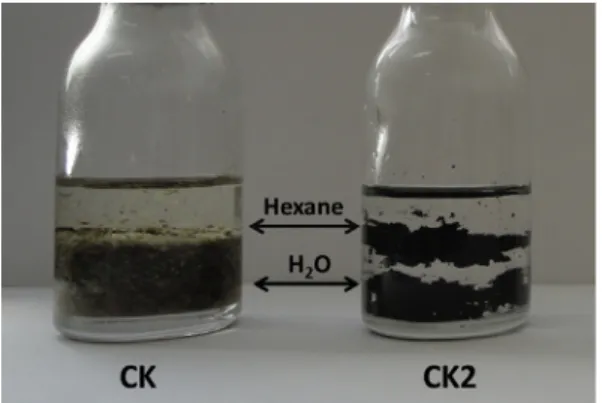
In fact, water absorption experiments showed that the CK2 mate-rial can absorb a high amount of water, i.e. 30 gH2O/gcatalyst. (seeSM – Fig. S6). This water absorption can be a potential advantage over the homogeneous H2SO4catalyst that does not have any effect on the H2O formed in the reaction. On the other hand, the carbonized sur-face has a more hydrophobic character which is important for the interaction/interface with the oil or oleic acid.Fig. 11shows the behavior of materials CK (cake without sulfonation) and CK2 in the presence of water/hexane two phases mixture. It is possible to observe that the cake CK, before sulfonation, presents a hydrophilic character and immediately goes to the aqueous phase. For the CK2 (after the sulfonation) the material initially stays at the interface water/hexane suggesting an amphiphilic character due to the com-bination of hydrophilic cellulose fraction with the carbonized sur-face. However, after few minutes the black solid migrates to the aqueous phase due to the wetting of the hydrophilic surface. The reaction medium is an emulsion of the hydrophobic oleic acid and the hydrophilic methanol. An amphiphilic catalyst is very important to contact these reactants and promote the reaction. The hydrophilic surface is important to interact with the polar methanol.
4. Conclusion
Cakes obtained after oil extraction can be converted by a simple process of aromatization/sulfonation with H2SO4into a new type of heterogeneous catalyst for the esterification of free fatty acids. The produced catalyst showed activities comparable to the homoge-neous H2SO4catalyst and could be recovered and reused several times. The catalytic activity is discussed in terms of sulfonic groups (ASO3H) combined with an amphiphilic surface composed of carbon (more hydrophobic) which interacts well with the fatty acid and non-decomposed cellulose (more hydrophilic) which is responsible for water adsorption during the esterification reaction. Preliminary tests, showed that this heterogeneous catalyst can also be used in the production of biodiesel from low quality/high free acidity oils with the important advantage of eliminating the washing step.
Acknowledgements
The authors acknowledge financial support from CNPq, CAPES, FAPEMIG and Petrobras.
Appendix A. Supplementary material
Supplementary data associated with this article can be found, in the online version, athttp://dx.doi.org/10.1016/j.fuel.2015.02.027.
References
[1]Maddikeri GL, Pandit AB, Gogate PR. Intensification approaches for biodiesel
synthesis from waste cooking oil: a review. Ind Eng Chem Res
2012;51:14610–28.
[2]Guan G, Kusakabe K. Development of advanced biodiesel fuel production
process. J Jpn Petrol Inst 2012;55:171–81.
[3]Gole VL, Gogate PR. A review on intensification of synthesis of biodiesel from
sustainable feed stock using sonochemical reactors. Chem Eng Process
2012;53:1–9.
[4]Sharma YC, Singh B, Korstad J. Advancements in solid acid catalysts for
ecofriendly and economically viable synthesis of biodiesel. Biofuel Bioprod
Bior 2011;5:69–92.
[5]Erickson DR. Neutralization. In: Erickson DR, editor. Practical handbook of
soybean production and utilization. Champaign, Illinois: AOCS Press; 1995. p.
184–202.
[6]Sharma Y, Singh B, Upadhyay S. Advancements in development and
characterization of biodiesel: a review. Fuel 2008;87:2355–73.
[7]Alptekin E, Canakci M, Sanli H. Evaluation of leather industry wastes as a
feedstock for biodiesel production. Fuel 2012;95:214–20.
[8]Gürü M, Koca A, Can Ö, Çınar C, Sßahin F. Biodiesel production from waste
chicken fat based sources and evaluation with Mg based additive in a diesel
engine. Renew Energy 2010;35:637–43.
[9]Alptekin E, Canakci M. Optimization of pretreatment reaction for methyl ester
production from chicken fat. Fuel 2010;89:4035–9.
[10] Borges ME, Diaz L. Recent developments on heterogeneous catalysts for
biodiesel production by oil esterification and transesterification reactions: a
review. Renew Sust Energy Rev 2012;16:2839–49.
[11]Atadashi IM, Aroua MK, Aziz ARA, Sulaiman NMN. The effects of catalysts in
biodiesel production: a review. J Ind Eng Chem 2013;19:14–26.
[12]Islam A, Taufiq-Yap YH, Chu C-M, Chan E-S, Ravindra P. Studies on design of
heterogeneous catalysts for biodiesel production. Process Saf Environ
2013;91:131–44.
[13]Tariq M, Ali S, Khalid N. Activity of homogeneous and heterogeneous catalysts,
spectroscopic and chromatographic characterization of biodiesel: a review.
Renew Sust Energy Rev 2012;16:6303–16.
[14]Nandiwale KY, Sonar SK, Niphadkar PS, Joshi PN, Deshpande SS, Patil VS, et al.
Catalytic upgrading of renewable levulinic acid to ethyl levulinate biodiesel using dodecatungstophosphoric acid supported on desilicated H-ZSM-5 as
catalyst. Appl Catal a-Gen 2013;460:90–8.
[15]Patel A, Narkhede N. 12-Tungstophosphoric acid anchored to zeolite H beta:
synthesis, characterization, and biodiesel production by esterification of oleic
acid with methanol. Energy Fuel 2012;26:6025–32.
[16]Babajide O, Musyoka N, Petrik L, Ameer F. Novel zeolite Na-X synthesized from
fly ash as a heterogeneous catalyst in biodiesel production. Catal Today
2012;190:54–60.
[17]Mbaraka IK, McGuire KJ, Shanks BH. Acidic mesoporous silica for the
catalytic conversion of fatty acids in beef tallow. Ind Eng Chem Res 2006;45:
3022–8.
[18]Mbaraka IK, Shanks BH. Design of multifunctionalized mesoporous silicas for
esterification of fatty acid. J Catal 2005;229:365–73.
[19]Mbaraka IK, Shanks BH. Conversion of oils and fats using advanced
mesoporous heterogeneous catalysts. J Am Oil Chem Soc 2006;83:79–91.
[20] Lotero E, Liu YJ, Lopez DE, Suwannakarn K, Bruce DA, Goodwin JG. Synthesis of
biodiesel via acid catalysis. Ind Eng Chem Res 2005;44:5353–63.
[21]Feng Y, He B, Cao Y, Li J, Liu M, Yan F, et al. Biodiesel production using
cation-exchange resin as heterogeneous catalyst. Bioresource Technol
2010;101:1518–21.
[22]Kim M, Lee H-s, Yoo SJ, Youn Y-S, Shin YH, Lee Y-W. Simultaneous synthesis of
biodiesel and zinc oxide nanoparticles using supercritical methanol. Fuel
2013;109:279–84.
[23]Shao GN, Sheikh R, Hilonga A, Lee JE, Park Y-H, Kim HT. Biodiesel production by
sulfated mesoporous titania-silica catalysts synthesized by the sol-gel process
from less expensive precursors. Chem Eng J 2013;215:600–7.
[24]Chen C-L, Huang C-C, Tran D-T, Chang J-S. Biodiesel synthesis via
heterogeneous catalysis using modified strontium oxides as the catalysts.
Bioresource Technol 2012;113:8–13.
[25]Park Y-M, Chung S-H, Eom HJ, Lee J-S, Lee K-Y. Tungsten oxide zirconia as solid
superacid catalyst for esterification of waste acid oil (dark oil). Bioresource
Technol 2010;101:6589–93.
[26]Lee J-S, Saka S. Biodiesel production by heterogeneous catalysts and
supercritical technologies. Bioresource Technol 2010;101:7191–200.
[27]Kim M, DiMaggio C, Yan S, Wang H, Salley SO, Ng KYS. Performance of
heterogeneous ZrO2 supported metaloxide catalysts for brown grease
esterification and sulfur removal. Bioresource Technol 2011;102:2380–6.
[28]Rattanaphra D, Harvey AP, Thanapimmetha A, Srinophakun P. Kinetic of
myristic acid esterification with methanol in the presence of triglycerides over
sulfated zirconia. Renew Energy 2011;36:2679–86.
[29]Shuit SH, Yee KF, Lee KT, Subhash B, Tan SH. Evolution towards the utilisation
of functionalised carbon nanotubes as a new generation catalyst support in
biodiesel production: an overview. RSC Advances 2013;3:9070–94.
[30] SathyaSelvabala V, Varathachary TK, Selvaraj DK, Ponnusamy V, Subramanian
S. Removal of free fatty acid in Azadirachta indica (Neem) seed oil using phosphoric acid modified mordenite for biodiesel production. Bioresource
Technol 2010;101:5897–902.
[31]do Nascimento LAS, Angelica RS, da Costa CEF, Zamian JR, da Rocha Filho GN. Comparative study between catalysts for esterification prepared from kaolins.
Appl Clay Sci 2011;51:267–73.
[32]Zatta L, Ferreira da Costa Gardolinski JE, Wypych F. Raw halloysite as reusable
heterogeneous catalyst for esterification of lauric acid. Appl Clay Sci
2011;51:165–9.
[33]Guo F, Xiu Z-L, Liang Z-X. Synthesis of biodiesel from acidified soybean
soapstock using a lignin-derived carbonaceous catalyst. Appl Energy
2012;98:47–52.
[34]Pua Fl, Fang Z, Zakaria S, Guo F, Chia C-h. Direct production of biodiesel from
high-acid value Jatropha oil with solid acid catalyst derived from lignin.
Biotechnol Biofuels 2011;4:1–8.
[35]Chin LH, Abdullah AZ, Hameed BH. Sugar cane bagasse as solid catalyst for
synthesis of methyl esters from palm fatty acid distillate. Chem Eng J
2012;183:104–7.
[36]Fu Z, Wan H, Hu X, Cui Q, Guan G. Preparation and catalytic performance of a
carbon-based solid acid catalyst with high specific surface area. React Kinet
Mech Cat 2012;107:203–13.
[37]Dehkhoda AM, West AH, Ellis N. Biochar based solid acid catalyst for biodiesel
production. Appl Catal A-Gen 2010;382:197–204.
[38]Chang B, Fu J, Tian Y, Dong X. Soft-template synthesis of sulfonated
mesoporous carbon with high catalytic activity for biodiesel production. RSC
Advances 2013;3:1987–94.
[39]Mar WW, Somsook E. Sulfonic-functionalized carbon catalyst for esterification
of high free fatty acid. Procedia Eng 2012;32:212–8.
[40]Rao BVSK, Chandra Mouli K, Rambabu N, Dalai AK, Prasad RBN. Carbon-based
solid acid catalyst from de-oiled canola meal for biodiesel production. Catal
Commun 2011;14:20–6.
[41]Xia P, Liu FJ, Wang C, Zuo SF, Qi CZ. Efficient mesoporous polymer based solid
acid with superior catalytic activities towards transesterification to biodiesel.
Catal Commun 2012;26:140–3.
[42]Stellwagen DR, van der Klis F, van Es DS, de Jong KP, Bitter JH. Functionalized
carbon nanofibers as solid-acid catalysts for transesterification. Chemsuschem
2013;6:1668–72.
[43]Zhou L, Dong B, Tang S, Ma H, Chen C, Yang X, et al. Sulfonated carbon
catalyzed oxidation of aldehydes to carboxylic acids by hydrogen peroxide. J
Energy Chem 2013;22:659–64.
[44]Chang B, Fu J, Tian Y, Dong X. Multifunctionalized ordered mesoporous carbon
as an efficient and stable solid acid catalyst for biodiesel preparation. J Physl
Chem C 2013;117:6252–8.
[45] Zhang X, Wang Y, Hao C, Wu P, Jiang Z, Study on the preparation and catalytic property of sulfonated ordered mesoporous carbon. In: Kida K, editor, Advanced Materials and Engineering Materials Ii, 2013. p. 484–87.
[46]Hou K, Zhang A, Gu L, Liu M, Guo X. Efficient synthesis and sulfonation of
ordered mesoporous carbon materials. J Colloid Interf Sci 2012;377:18–26.
[47]Ayrilmis N, Kaymakci A, Ozdemir F. Sunflower seed cake as reinforcing filler in
thermoplastic composites. J Appl Polym Sci 2012:1170–8.
[48]Fernández-Cegrí V, Raposo F, de la Rubia MA, Borja R. Effects of chemical and
thermochemical pretreatments on sunflower oil cake in biochemical methane
potential assays. J Chem Technol Biotechnol 2013;88:924–9.
[49]Gelbard G, Bres O, Vargas RM, Vielfaure F, Schuchardt UF. H-1
nuclear-magnetic-resonance determination of the yield of the transesterification of
rapeseed oil with methanol. J Am Oil Chem Soc 1995;72:1239–41.
[50] Brasil AN, Usina portátil para simulação industrial de processos de produção de biodiesel por irradiação por ultrassom, in: Energias BEd, editor, Brasil, 2011.
[51]Vieira Grossi C, de Oliveira Jardim E, de Araújo MH, Lago RM, da Silva MJ.
Sulfonated polystyrene: a catalyst with acid and superabsorbent properties for
the esterification of fatty acids. Fuel 2010;89:257–9.
[52]Dresselhaus MS, Jorio A, Souza AG, Saito R. Defect characterization in graphene
and carbon nanotubes using Raman spectroscopy. Philos Trans R Soc A-Math
Phys Eng Sci 2010;368:5355–77.
[53]Evon P, Vandenbossche V, Rigal L. Manufacturing of renewable and
biodegradable fiberboards from cake generated during biorefinery of sunflower whole plant in twin-screw extruder: Influence of thermo-pressing
conditions. Polym Degrad Stabil 2012;97:1940–7.
[54]Kacurakova M, Wilson RH. Developments in mid-infrared FT-IR spectroscopy
of selected carbohydrates. Carbohydr Polym 2001;44:291–303.
[55]Chen G, Fang B. Preparation of solid acid catalyst from glucose–starch mixture
for biodiesel production. Bioresource Technol 2011;102:2635–40.
[56]Rao BVSK, Mouli KC, Rambabu N, Dalai AK, Prasad RBN. Carbon-based solid
acid catalyst from de-oiled canola meal for biodiesel production. Catal
Commun 2011;14:20–6.
[57]Chang B, Tian Y, Shi W, Liu J, Xi F, Dong X. Magnetically separable porous
carbon nanospheres as solid acid catalysts. RSC Adv 2013;3:20999–1006.
[58] Özçimen D. An Approach to the Characterization of Biochar and Bio-Oil. Yildiz Technical University, 2013.
[59]Guthrie JP. Hydrolysis of esters of oxy acids: pKa values for strong acids;
Brønsted relationship for attack of water at methyl; free energies of hydrolysis of esters of oxy acids; and a linear relationship between free energy of
hydrolysis and pKa holding over a range of 20pK units. Can J Chem
1978;56:2342–54.
[60]Toda M, Takagaki A, Okamura M, Kondo JN, Hayashi S, Domen K, et al. Biodiesel