w w w . r b h h . o r g
Revista
Brasileira
de
Hematologia
e
Hemoterapia
Brazilian
Journal
of
Hematology
and
Hemotherapy
Original
article
Mature
B
cell
neoplasms:
retrospective
analysis
of
93
cases
diagnosed
between
2011
and
2014
in
a
University
Hospital
in
southern
Brazil
Chandra
Chiappin
Cardoso,
Ana
Carolina
Rabello
de
Moraes,
Joanita
Angela
Gonzaga
Del
Moral,
Maria
Claudia
Santos-Silva
∗UniversidadeFederaldeSantaCatarina(UFSC),Florianópolis,SC,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received12November2015 Accepted7February2016 Availableonline9March2016
Keywords:
MatureB-cellneoplasms Diagnosis
Prognosis
Treatmentresponse
a
b
s
t
r
a
c
t
Background:Accordingtothe2008WorldHealthOrganizationclassification,matureB-cell neoplasmsareaheterogeneousgroupofdiseasesthatincludeB-celllymphomasandplasma cell disorders.Theseneoplasmscanhaveverydifferentclinicalbehaviors,fromhighly aggressivetoindolent,andthereforerequirediversetreatmentstrategies.
Objective:Theaim ofthisstudywastoassesstheprofileof93patientsdiagnosedwith matureB-cellneoplasmsmonitoredbetween2011and2014.
Methods:Areviewofpatients’chartswasperformedandlaboratoryresultswereobtained usingtheonlinesystemoftheUniversidadeFederaldeSantaCatarina.
Results:Thestudyincluded93adultpatientswithmatureB-cellneoplasms.Themost fre-quentsubtypesweremultiplemyeloma,chroniclymphocyticleukemia,diffuselargeB-cell lymphoma,follicularlymphoma,andBurkitt’slymphoma.Themedianageatdiagnosiswas 58yearswithamale-to-femaleratioof1.3:1.Therewerestatisticaldifferencesintermsof ageatdiagnosis,lactatedehydrogenaseactivityandKi-67expressionamongthesubtypes ofB-celllymphoma.Accordingtotheprognosticindexes,themajorityofmultiplemyeloma patientswerecategorizedashighrisk,whilethemajorityofchroniclymphocyticleukemia patientswereclassifiedaslowrisk.
Conclusions: ThisstudydemonstratestheprofileofpatientsdiagnosedwithmatureB-cell neoplasmsinasouthBrazilianuniversityhospital.OftheB-celllymphoma,Burkitt’s lym-phomapresentedparticularfeaturesregardinglactatedehydrogenaseactivitylevels,Ki-67 expression,ageatdiagnosis,andhumanimmunodeficiencyvirusinfection.
©2016Associac¸ãoBrasileiradeHematologia,HemoterapiaeTerapiaCelular.Published byElsevierEditoraLtda.Allrightsreserved.
∗ Correspondingauthorat:Divisãode AnálisesClínicas,HospitalUniversitário,UniversidadeFederaldeSanta Catarina(UFSC),Rua
ProfessoraMariaFloraPausewang,s/n◦,Trindade,88036-800Florianópolis,SC,Brazil.
E-mailaddress:maria.claudia.silva@ufsc.br(M.C.Santos-Silva).
http://dx.doi.org/10.1016/j.bjhh.2016.02.003
Introduction
MatureB-cellneoplasms(MBCN)areaheterogeneousgroupof diseasesthatcomprisesB-celllymphomas(BCL)andplasma cellneoplasms(PCN).1AboutfifteensubtypesofBCLandfive
subtypes ofPCN are categorized in the 2008World Health Organization(WHO)TumorsofHematopoieticandLymphoid Tissues.1MBCNpresentvariableclinicalpresentationsfrom
highlyaggressivetoindolent,andthereforerequiredifferent treatmentstrategies.2
EpidemiologicaldataplaceMBCNasasubstantialglobal healthproblem.IntheWesternworldwithabout20newcases oflymphomabeingdiagnosedper100,000peopleannually,2
whichaccountsfor3–4%ofallmalignancies.3Moreover,
mul-tiplemyeloma(MM)accountsfor1%ofallmalignancies,with aglobalincidenceofnearly120,000casesperyear.4
Similartoothertypesofcancer,MBCNarisebymultistep accumulationofgeneticaberrationsthatinduceaselective growthadvantageofthemalignantclone.Commonly,the ini-tialstepsinthemalignanttransformationarechromosomal translocations,whichmightoccurduringdifferentstagesof B-celldifferentiation.5
Inadditiontothe recentadvancesintheunderstanding ofgenetic and genomic characteristics ofB-cell malignan-cies,remarkableefforthasbeenmadetounderstandpotential risk factors that accountfor the increase in the incidence of MBCN, particularly in relation to the environment and lifestyle.Severe immunosuppressionresulting from immu-nodeficiencysyndromesandafterorgantransplantation,and someinfectiousagentsareknownriskfactorsforthe devel-opment of some lymphoma subtypes.6 Much attention is
beingpaidtothehigh riskoflymphomainhuman immu-nodeficiency virus (HIV)-positive patients (60 to 200-fold).7
In addition, studies have suggested there is an increased riskoflymphomasand MMinrelationto theuse ofsome pesticides.8 Additionally,the contributionofenvironmental
factors,suchassmoking,andlifestyleincludingdietremain unclear.6
ConsideringthelackofBrazilianstudiesonthisissueand thelackofepidemiologicaldata,thepresentstudywas con-ductedtoprovide informationaboutthe profileofpatients whohavedevelopedthistypeofneoplasm.Thus,theaimof thisstudywastoassesstheprofileof93patientsdiagnosed withMBCNmonitoredbetween2011and2014inauniversity hospitalinsouthernBrazil.
Methods
Studyparticipants
ThesubjectscomprisedadultpatientsdiagnosedwithMBCN duringthe periodcoveredbythe study (June2011–October 2014) and monitoredatthe university hospitalofthe Uni-versidadeFederaldeSantaCatarina(UFSC),Florianópolis,SC, Brazil.Patientswereexcludediftheirrecordswere unavail-ableoriftheydidnotsigntheconsentform.Thisstudywas approvedbytheEthicsCommitteeoftheinstitution.The uni-versityhospitalofUFSCtreatsanaverageof250,000patients
peryear.Fromthistotal,anaverageof2400patientsperyear areattendedattheHematologyClinicandaround240patients peryeararehospitalizedbytheHematologyService.
Design
Clinical and personaldatawere obtainedthroughareview ofpatientchartsfrom thehospitalwiththe confidentiality ofinformationbeingpreserved.Laboratorytestresultswere obtainedthroughtheonlinehospitalsystem.Clinicaland lab-oratorydatawererecordedonadatacollectionformandlater compiledforstatisticalanalysis.
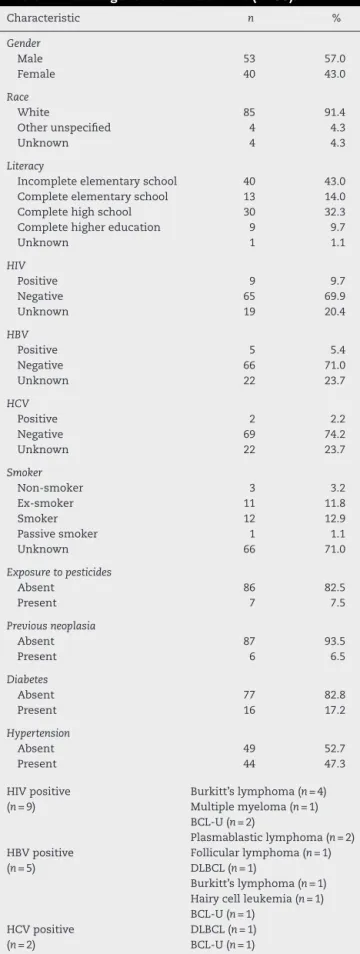
Collected dataincludedvariablessuchasageat diagno-sis,yearofdiagnosis,gender,race/ethnicity,literacy,serology, clinical manifestationsatdiagnosis,co-morbidities, labora-toryexamsatdiagnosis,neoplasmstageorstratification,and outcome.Outcome wasclassified asoneoftwocategories: complete/partialremission(CR/PR)andrelapsed/progressive disease (PD) basedon previous establishedcriteria.9,10 The
endofthedatacollectionperiodwasestablishedasFebruary 2015.
Statisticalanalysis
Databasepreparationandstatisticalanalysiswereperformed usingtheStatisticalPackageforSocialSciences(SPSS®version
17.0)andMedCalc® (version12.3.0.0).Dataarepresentedas
descriptivestatisticsincludingabsoluteandrelative frequen-ciesforcategoricalvariablesandmeasuresofcentraltendency anddispersionofcontinuousvariables.Continuousvariables were tested for normality using the Kolmogorov–Smirnov andShapiro–Wilktests.Numericalvariableswerecompared betweengroupsusingtheMann–WhitneyUtestorStudent’s
t-test.Acomparisonofthreeormorevariableswasperformed employing the Kruskal–Wallis test or one-way analysis of variance (ANOVA). Thefrequencies obtained incategorical variableswerecomparedbetweenpatientsbythechi-square orFisher’sexacttest.Asignificancelevelof5%(p-value<0.05) wasconsideredsignificant.
Results
MatureBcellneoplasms
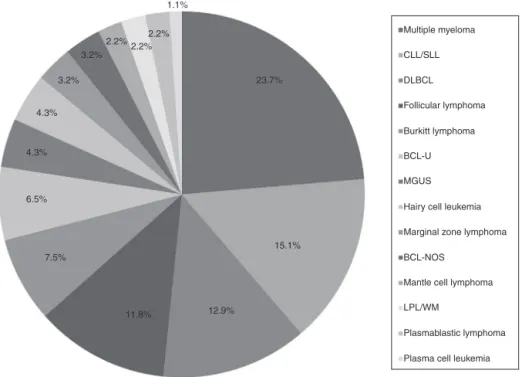
Atotalof93patientswerediagnosedwithasubtypeofMBCN, includingBCLandPCN,from2011to2014.Themedianage atdiagnosiswas58years(range:20–93years)witha male-to-femaleratioof1.3:1.Patientgeneralcharacteristicsarelisted inTable1andprevalenceofthedifferentsubtypesofMBCN casesareshowninFigure1.Thecorrespondingage distribu-tionsareshowninFigure2.
Multiple myeloma
CLL/SLL
DLBCL
Follicular lymphoma
Burkitt lymphoma
BCL-U
MGUS
Hairy cell leukemia
Marginal zone lymphoma
BCL-NOS
Mantle cell lymphoma 15.1%
12.9% 11.8%
7.5% 6.5% 4.3% 4.3%
3.2% 3.2%
2.2%2.2%2.2%
1.1%
23.7%
LPL/WM
Plasmablastic lymphoma
Plasma cell leukemia
Figure1–PrevalenceofthematureB-cellneoplasmssubgroups.
CLL/SLL:chroniclymphocyticleukemia/smalllymphocyticlymphoma;DLBCL:diffuselargeB-celllymphoma;BCL-U:B-cell lymphoma,unclassifiable,withfeaturesintermediatebetweenDLBCLandBurkitt’slymphoma;MGUS:monoclonal gammopathyofundeterminedsignificance;BCL-NOS:B-celllymphoma,nototherwisespecified;LPL/WM:
lymphoplasmacyticlymphoma/Waldenströmmacroglobulinemia.
Plasmablastic lymphoma Plasma cell leukemia LPL/WM BCL-U BCL-NOS Hairy cell leukemia
Multiple myeloma Marginal zone lymphoma Mantle cell lymphoma Burkitt lymphoma DLBCL Follicular lymphoma CLL/SLL
20 18 63
19
44 59
Min Median Max
1st quartile 3rd quartile 100
Age at diagnosis
MBCN subtype
MGUS
Figure2–AgeatdiagnosisofmatureB-cellneoplasms.
CLL/SLL:chroniclymphocyticleukemia/smalllymphocyticlymphoma;DLBCL:diffuselargeB-celllymphoma;BCL-U:B-cell lymphoma,unclassifiable,withfeaturesintermediatebetweenDLBCLandBurkitt’slymphoma;MGUS:monoclonal gammopathyofundeterminedsignificance;BCL-NOS:B-celllymphoma,nototherwisespecified;LPL/WM:
lymphoplasmacyticlymphoma/Waldenströmmacroglobulinemia.
B-celllymphoma
Overall,66patients werediagnosedwithasubtypeofBCL. Therewerestatisticaldifferencesintermsofageatdiagnosis betweenBLandchroniclymphocyticleukemia/small lympho-cyticlymphoma(CLL/SLL –p-value=0.001),BL andBCL not otherwisespecified(BCL-NOS–p-value=0.041),and BLand mantlecell lymphoma(MCL – p-value=0.016). In addition,
intermsofserum lactatedehydrogenase(LDH)astatistical difference(p-value=0.003)wasfoundbetweenthedifferent groups(Table2).
Table1–Patientgeneralcharacteristics(n=93).
Characteristic n %
Gender
Male 53 57.0
Female 40 43.0
Race
White 85 91.4
Otherunspecified 4 4.3
Unknown 4 4.3
Literacy
Incompleteelementaryschool 40 43.0
Completeelementaryschool 13 14.0
Completehighschool 30 32.3
Completehighereducation 9 9.7
Unknown 1 1.1
HIV
Positive 9 9.7
Negative 65 69.9
Unknown 19 20.4
HBV
Positive 5 5.4
Negative 66 71.0
Unknown 22 23.7
HCV
Positive 2 2.2
Negative 69 74.2
Unknown 22 23.7
Smoker
Non-smoker 3 3.2
Ex-smoker 11 11.8
Smoker 12 12.9
Passivesmoker 1 1.1
Unknown 66 71.0
Exposuretopesticides
Absent 86 82.5
Present 7 7.5
Previousneoplasia
Absent 87 93.5
Present 6 6.5
Diabetes
Absent 77 82.8
Present 16 17.2
Hypertension
Absent 49 52.7
Present 44 47.3
HIVpositive (n=9)
Burkitt’slymphoma(n=4) Multiplemyeloma(n=1) BCL-U(n=2)
Plasmablasticlymphoma(n=2) HBVpositive
(n=5)
Follicularlymphoma(n=1) DLBCL(n=1)
Burkitt’slymphoma(n=1) Hairycellleukemia(n=1) BCL-U(n=1)
HCVpositive (n=2)
DLBCL(n=1) BCL-U(n=1)
MBCN:mature B-cellneoplasm; HIV:human immunodeficiency virus;HBV:hepatitisBvirus;HCV:hepatitisCvirus;DLBCL:diffuse largeB-celllymphoma;BCL-U:B-celllymphoma,unclassifiablewith featuresintermediatebetweenDLBCLandBurkitt’slymphoma.
Table2–Serumlactatedehydrogenaseactivityin
patientsdiagnosedwithB-celllymphoma(n=66).
BCLsubtype LDH(IU/L)
Median(range)
CLL/SLL 188(116–332)a
DiffuselargeB-celllymphoma 406(184–2739)b
Follicularlymphoma 229(150–315)a
Burkitt’slymphoma 1534(243–7239)b
BCL-U 464(170–6121)b
Hairycellleukemia 152(141–402)a
Marginalzonelymphoma 137(113–397)a
BCL-NOS 215(182–232)a
Mantlecelllymphoma 222
LPL/WM 214(128–300)a
Plasmablasticlymphoma 174(148–199)a
LDH: lactate dehydrogenase; CLL/SLL: chronic lymphocytic leukemia/small lymphocytic lymphoma;BCL-U: BCL, unclassifi-able,withfeaturesintermediatebetweenDLBCLandBL;BCL-NOS: BCL not otherwisespecified; LPL/WM: lymphoplasmacytic lym-phoma/Waldenströmmacroglobulinemia.
There was a significant difference between ‘a’ and ‘b’ by the Kruskal–WallisandMann–WhitneyUtests.
infiltration.Inaddition,12patientswereevaluatedregarding central nervous system (CNS) involvement. Three patients withBLandtwowithBCL-UhadCNSinfiltration.
Ki-67 expression was evaluated by immunohistochem-istry(IHC)in30patients.SimilartoLDHlevels,astatistical difference was foundbetween groups (p-value=0.013).The subtypes with the highest Ki-67 expressions were DLBCL, BL,andBCL-UwithmedianKi-67expressionof80%(range: 50–95%), 92.5% (range: 50–95%), and 80% (range: 30–95%), respectively.ThemedianexpressionofKi-67fortheCLL/SLL group was 30% (range: 10–50%) and for the follicular lym-phomagroupitwas35%(range:10–50%).
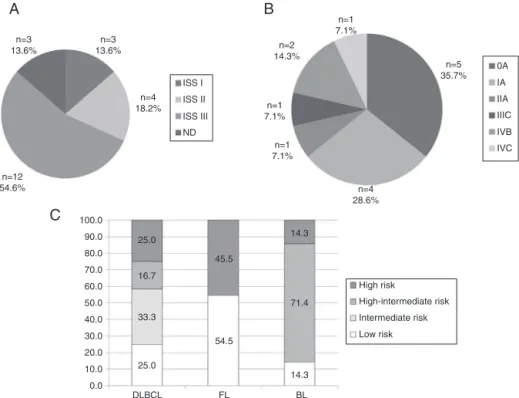
MostcommonmatureBcellneoplasms
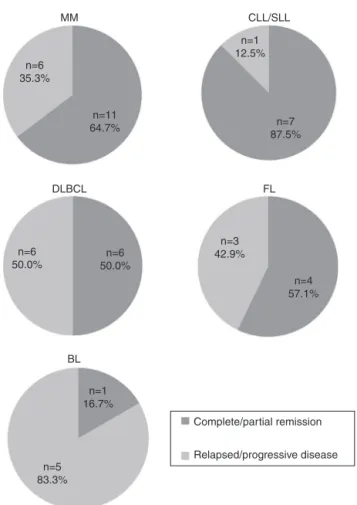
Themostcommonsubtypes ofMBCNfoundinthepresent studyinvolved66patients:MM(n=22),CLL/SLL(n=14),DLBCL (n=12),FL(n=11)andBL(n=7).Figure3showsstratificationof patientsbyMBCNsubtype.Responsetotreatmentwas evalu-atedasCR/PRandPD(Figure4).CLL/SLLpresentedthehighest frequencyofCR/PR(87.5%),whileBLpresentedthehighest frequencyofPD(83.3%).
Discussion
Thepresent studyretrospectivelyanalyzedtheclinical and laboratorialdataof93MBCNpatientsinaBrazilian univer-sityhospital.ThefrequenciesoftheMBCNsubtypesfoundin thisstudyaresimilartothosedescribedininternationaland Brazilianstudies.11,12
A
B
C
n=3
13.6% n=2
14.3%
n=1 7.1%
n=1 7.1%
n=5 35.7%
0A
IA
IIA
IIIC
IVB
IVC
n=4 28.6% n=1
7.1% n=3
13.6%
n=4 18.2%
n=12 54.6%
100.0
90.0
80.0
70.0
60.0
50.0
40.0
30.0
20.0
10.0
0.0
DLBCL 25.0 33.3 16.7 25.0
45.5
54.5
14.3 71.4 14.3
FL BL
High risk
High-intermediate risk
Intermediate risk
Low risk ISS I
ISS II
ISS III
ND
Figure3–Stratificationofpatientswith(A)multiplemyeloma,(B)chroniclymphocyticleukemia/smalllymphocytic lymphoma,and(C)diffuselargeB-celllymphoma,follicularlymphomaandBurkitt’slymphoma.
DLBCL:diffuselargeB-celllymphoma;FL:follicularlymphoma;BL:Burkitt’slymphoma;ND:notdefinedbecauseofthe absenceoftheresultof2-microglobulin.
cases),CLL/SLL(835cases),FL(446cases),marginalzone lym-phoma(390cases),MCL(100cases),BL(54cases),andhairy cellleukemia(48cases).11Thedatathatdifferscomparedto
thepresentstudyisthefrequencyofBL,whichinthisreport wasthefifthmostcommonMBCNsubtype.
InBrazil, astudy published in 2011inthe State ofSão Pauloontheprevalenceofnon-Hodgkinlymphoma(NHL) sub-typesin546casesreportedthefollowingfrequencies:DLBCL in49.45%ofthecases,FLin7.69%,BLin6.41%,diffuseand smallcelllymphoma(unclassifiable)in6.41%,andCLL/SLLin 6.23%.12Thus,theprevalenceofBLintheStateofSãoPaulo
(SoutheasternregionofBrazil)issimilartothatfoundinthe presentstudy;however,theprevalenceofCLL/SLLis discord-ant.
Moreover,Queirogaetal.demonstratedthedistributionof BLinthefiveBrazilianregionsandanalyzedthecasesinterms ofage(pediatricoradult),amongotherparameters. Interest-ingly,BLinthesouthernregionmainlyaffectsadultpatients (67%),whileintheother fourregionsthemajorityofcases arepediatricpatients.13Thisdatacorroboratesthehigher
fre-quencyofBLinthepresentstudy,whichiscomposedentirely byadultpatientsfromsouthernBrazil.
Accordingtodata fromthe MinistryofHealth ofBrazil, thecity ofFlorianopolisisthirdintheranking ofBrazilian citiesintermsofHIVdetectionandmortalityrates;infact,the southleadstherankingofthehighestdetectionrates(∼2.3×
thecountryrate).14ThehighlocalprevalenceofHIVinfection
mayhaveledtothehighrateofBLinthepresentstudy,since thereisanincreasedriskofdevelopingBLinpatientswithHIV infection.15
ThefrequencyofpatientswithMBCNwhowere seropos-itive for HIV was 9.7%, which is higher than the results foundbyShielsetal.Theseauthorsassessed115,643North American NHL cases and observed a rate of 5.9% of HIV infection.16 MBCNsubtypesthat havebeen associatedwith
thisinfection includeDLBCL,BL,Hodgkin’slymphoma(HL), primaryeffusionlymphomaandplasmablasticlymphomaof theoralcavity.7Ofthesubjectsinthecurrentstudywhowere
seropositive forHIV, four had BL, twohad BCL-U and two hadplasmablasticlymphomaoftheoralcavity.Ontheother hand,PCNarenotcommonlyassociatedwithHIVinfection andtheseneoplasmsusuallyaffectolderindividuals.17
How-ever,therearereportsofMMdevelopinginyoungerpatients withHIV,afindingthatiscompatiblewithacaseofMMina 43-year-oldHIV-positivesubjectinthisseries.
Studieshavedemonstratedanassociationbetween smok-ingandthedevelopmentoflymphomas,specificallyFL.18,19
Ofthe12currentsmokersincludedinthisstudy,fourwere diagnosed with FL.In addition, four cases were diagnosed withCLL/SLL;however,noassociationbetweenCLL/SLLand smokingwasfoundintheliterature.
According to the 2008 data from the Ministry of Agri-culture,BrazilsurpassedtheUnitedStatesinpesticideuse, anditisnowtheworld’slargestmarket.Thisfacthighlights the importance ofstudies thatinvestigate the relationship between thesepotentialcarcinogens and thehealth ofthe populationexposedtothem.20 Inthisstudy,sevenpatients
MM
DLBCL
BL n=6 35.3%
n=11 64.7%
n=1 12.5%
n=7 87.5%
n=3 42.9% n=6
50.0%
n=1 16.7%
Complete/partial remission
Relapsed/progressive disease n=5
83.3% n=6 50.0%
n=4 57.1% CLL/SLL
FL
Figure4–Treatmentresponseassessedascomplete/partial remissionandrelapsed/progressivedisease.
MM:multiplemyeloma;CLL/SLL:chroniclymphocytic leukemia/smalllymphocyticlymphoma;DLBCL:diffuse largeB-celllymphoma;FL:follicularlymphoma;BL: Burkitt’slymphoma.
thosefromapreviousstudy,whichfoundapositive associ-ationbetweenindividualsexposedtospecificpesticidesand thedevelopmentofMMandCLL/SLL.8
Inthis study,themedianage atdiagnosiswas 58years similar to another Brazilian study that reported a median age of 50 years for NHL patients.12 Conversely, a
world-wideepidemiologicalstudy showedthatthemedianageof patientsdiagnosedwithlymphomasisaround70years,with onlyafewsubtypes,suchasFL,BL andprimary mediasti-nallymphoma, affecting younger patients.3 Indeed, inthe
presentstudyBLpatientspresentedamedianageof33years which was statistically different to that ofother subtypes ofBCL.
MBCNmayhaveaclinicalcourserangingfromindolentto extremelyaggressive.2,21Thus,apartfromcorrectdiagnosis,
prognosticstratificationisfundamentaltodefinethemedical strategy,whichcanrangefromjustawatch-and-wait strat-egytocombinedchemotherapyregimens.22ConsideringBCL,
themostimportantlaboratoryteststoevaluatethedegreeof neoplasmaggressivenessareserumLDHenzymeactivityand thepercentageofKi-67proteinevaluatedusingIHC.Sincethe 1970s,highserumLDHactivityisconsideredanindependent poorprognosticfactor.23 Inthe present study,asignificant
differencebetweenBCLsubtypeswasobservedinrespectto thisparameter.ThehighestvalueswereobservedinBLwith amedianof1534IU/L(range:243–7239IU/L).Moreover,serum LDHactivitywasindividuallyanalyzedamongBLpatientsand showedthatinonlyonecasetheactivityofthisenzymeat diagnosiswaslessthan500IU/L(243IU/L).Interestingly,this wasalsotheonlypatientwhoachievedCR/PRwithtreatment, andisinagoodgeneralcondition.
Morerecently,theevaluationofKi-67expressionhasbeen consideredaprognosticfactorforlymphomas,sinceit indi-cates the cell proliferation rate.24 Thus, as found for LDH
activity,thehighestvaluesofKi-67expressionwereobserved incasesofBL,DLBCL,andBLC-U.Theseresultsaresimilarto thosereportedbyBroydeetal.,whoshowedtheexpression ofKi-67inDLBCL,FL,CLL/SLL,atypicalBL,andMCLas67.5%, 32.1%,10.8%,96.5%,and40.2%,respectively.24
Furthermore,themostcommonsubtypesofMCBNwere stratifiedaccordingtoprognosticindexesinordertoestablish thestageofthetumoratdiagnosis.OftheMMcases(n=22), 54.6%(n=12)werestratifiedasinternationalstagingsystem (ISS)III,i.e.,thehighestriskcategoryforthistypeofneoplasm. Ofthese12patients,fourdiedwithin,onaverage,11monthsof diagnosis.Adifferentprofilewasfoundwhenprognostic strat-ificationof756patientswasassessedinapreviousstudythat includeddatafrom 16BrazilianInstitutions withMMcases diagnosedbetween1998and2004.Theresultsshowedthat 20.1%ofpatientswerestratifiedasISSI,48.7%asISSII,and 31.2%asISSIII.25Ontheotherhand,aChinesestudy
evalu-ated264casesofMMclassifiedas8.7%ISSI,36.4%ISSII,and 54.9%ISSIII,26whichissimilartothefrequenciesfoundinthe
presentstudy.DiagnosisofMMinadvancedstagescouldbe relatedtolackofinformationandunspecificsymptomssuch asbone lesions,which leadpatientstoseek othermedical specialtiesandoftenresorttoself-medication.
Withadifferentprofile,64.3%(n=9)ofpatientsdiagnosed withCLL/SLL(n=14)werestratifiedinlowerriskstages includ-ing0andIoftheRaisystemandAoftheBinetsystem.This dataissimilartotheresultsofastudypublishedin2014that evaluated924casesofCLL/SLLandfoundthefollowing fre-quencies fortheRaisystem:58%wereclassifiedasstage0, 34% asI/II and 8%asIII/IV; and forthe Binetsystem: 93% wereclassifiedasA/B,and7%asC.Accordingtothe litera-ture,stages0andAarerelatedtoahigheroverallsurvival (OS),withanexpectedmedianofabouttenyears.RaistagesI andIIandBinetstageBhaveintermediatemedianOSoffive tosevenyears,whilethemostaggressivegroups(RaiIIIand IVandBinetC)haveamuchshortermedianOSoflessthan threeyears.27
Limitationsofthisstudyincludethesmallpatientsample sizeandtheshortfollow-upperiod.Thelackofinformationon HIV,HBVandHCVstatusisanotherpotentialsourceofbias.
Conclusion
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgement
TheauthorsthanktheCentroNacionaldeDesenvolvimento CientíficoeTecnológico(CNPq)forfinancialsupport.
r
e
f
e
r
e
n
c
e
s
1. SwerdlowSH,CampoE,HarrisNL,JaffeES,PileriSA,SteinH, etal.WHOclassificationoftumoursofhaematopoieticand lymphoidtissues.4thed.Lyon,France:InternationalAgency forResearchonCancer;2008,479pp.
2. KuppersR.MechanismsofB-celllymphomapathogenesis. NatRevCancer.2005;5(4):251–62.
3. RomanE,SmithAG.Epidemiologyoflymphomas. Histopathology.2011;58(1):4–14.
4. LudwigH,MiguelJS,DimopoulosMA,PalumboA,GarciaSanz R,PowlesR,etal.InternationalMyelomaWorkingGroup recommendationsforglobalmyelomacare.Leukemia. 2014;28(5):981–92.
5. NogaiH,DorkenB,LenzG.Pathogenesisofnon-Hodgkin’s lymphoma.JClinOncol.2011;29(14):1803–11.
6. BassigBA,LanQ,RothmanN,ZhangY,ZhengT.Current understandingoflifestyleandenvironmentalfactorsandrisk ofnon-Hodgkinlymphoma:anepidemiologicalupdate.J CancerEpidemiol.2012;2012:978930.
7. GroggKL,MillerRF,DoganA.HIVinfectionandlymphoma.J ClinPathol.2007;60(12):1365–72.
8. AlavanjaMC,HofmannJN,LynchCF,HinesCJ,BarryKH, BarkerJ,etal.Non-Hodgkinlymphomariskandinsecticide, fungicideandfumigantuseintheagriculturalhealthstudy. PLOSONE.2014;9(10):e109332.
9. ChesonBD,PfistnerB,JuweidME,GascoyneRD,SpechtL, HorningSJ,etal.Revisedresponsecriteriaformalignant lymphoma.JClinOncol.2007;25(5):579–86.
10.DurieBG,HarousseauJL,MiguelJS,BladéJ,BarlogieB, AndersonK,etal.Internationaluniformresponsecriteriafor multiplemyeloma.Leukemia.2006;20(9):1467–73.
11.SmithA,RomanE,HowellD,JonesR,PatmoreR,JackA,etal. TheHaematologicalMalignancyResearchNetwork(HMRN):a newinformationstrategyforpopulationbasedepidemiology andhealthserviceresearch.BrJHaematol.
2010;148(5):739–53.
12.GouveiaGR,SiqueiraSAC,ChamoneDAF,PereiraJ.Prevalence ofnon-HodgkinlymphomasinSãoPaulo,Brazil.RevBras HematolHemoter.2011;33(4):317.
13.QueirogaEM,GualcoG,WeissLM,DittmerDP,AraujoI, KlumbCE,etal.BurkittlymphomainBrazilischaracterized bygeographicallydistinctclinicopathologicfeatures.AmJ ClinPathol.2008;130(6):946–56.
14.BRASIL.BoletimEpidemiológicoHIVAIDS;2014.Brasília. Availablefrom:http://www.aids.gov.br/sites/default/files/ anexos/publicacao/2014/56677/boletim20141pdf60254.pdf
15.PerkinsAS,FriedbergJW.Burkittlymphomainadults. HematolAmSocHematolEducProg.2008:341–8.
16.ShielsMS,EngelsEA,LinetMS,ClarkeCA,LiJ,HallHI,etal. Theepidemicofnon-HodgkinlymphomaintheUnited States:disentanglingtheeffectofHIV,1992–2009.Cancer EpidemiolBiomarkersPrev.2013;22(6):1069–78.
17.DezubeBJ,AboulafiaDM,PantanowitzL.Plasmacell disordersinHIV-infectedpatients:frombenigngammopathy tomultiplemyeloma.AIDSRead.2004;14(7),372–4,
377–9.
18.MortonLM,HartgeP,HolfordTR,HollyEA,ChiuBC,VineisP, etal.Cigarettesmokingandriskofnon-Hodgkinlymphoma: apooledanalysisfromtheInternationalLymphoma EpidemiologyConsortium(interlymph).CancerEpidemiol BiomarkersPrev.2005;14(4):925–33.
19.GibsonTM,SmedbyKE,SkibolaCF,HeinDW,SlagerSL,de SanjoséS,etal.Smoking,variationinN-acetyltransferase1 (NAT1)and2(NAT2),andriskofnon-Hodgkinlymphoma:a pooledanalysiswithintheInterLymphconsortium.Cancer CausesControl.2013;24(1):125–34.
20.BoccoliniPdeM,BoccoliniCS,ChrismanJdeR,MarkowitzSB, KoifmanS,KoifmanRJ,etal.Pesticideuseandnon-Hodgkin’s lymphomamortalityinBrazil.IntJHygEnvironHealth. 2013;216(4):461–6.
21.ShanklandKR,ArmitageJO,HancockBW.Non-Hodgkin lymphoma.Lancet.2012;380(9844):848–57.
22.GorczycaW.Prognosticandpredictivemarkersin hematologicneoplasms.Areview.PolJPathol. 2011;62(4):189–205.
23.FerrarisAM,GiuntiniP,GaetaniGF.Serumlactic dehydrogenaseasaprognostictoolfornon-Hodgkin lymphomas.Blood.1979;54(4):928–32.
24.BroydeA,BoycovO,StrenovY,OkonE,ShpilbergO,BaireyO. RoleandprognosticsignificanceoftheKi-67indexin non-Hodgkin’slymphoma.AmJHematol.2009;84(6): 338–43.
25.HungriaVT,MaiolinoA,MartinezG,ColleoniGW,CoelhoEO, RochaL,etal.ConfirmationoftheutilityoftheInternational StagingSystemandidentificationofauniquepatternof diseaseinBrazilianpatientswithmultiplemyeloma. Haematologica.2008;93(5):791–2.
26.GengC,LiuN,YangG,LiuA,LengY,WangH,etal. Retrospectiveanalysisof264multiplemyelomapatients. OncolLett.2013;5(2):707–13.