Revista
Brasileira
de
Hematologia
e
Hemoterapia
Brazilian
Journal
of
Hematology
and
Hemotherapy
w w w . r b h h . o r g
Original
article
Acute
myeloid
leukemia:
survival
analysis
of
patients
at
a
university
hospital
of
Paraná
Sergio
Lunardon
Padilha,
Emannuely
Juliani
dos
Santos
Souza,
Marcela
Coriolano
Cruz
Matos
∗,
Natália
Ramos
Domino
UniversidadeFederaldoParaná(UFPR),Curitiba,PR,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received20December2013 Accepted16June2014
Availableonline21November2014
Keywords:
Acutemyeloidleukemia Survivalanalysis Prognosis Adult Cytogenetics
a
b
s
t
r
a
c
t
Objective:Theaimofthisstudywastoanalyzetheprognosticfactorscorrelatedwithsurvival ofpatientswithacutemyeloidleukemiaattheHospitaldeClínicas,UniversidadeFederal doParanábetween2003and2009,aswellastoinvestigatetheclinicalandepidemiological profile.
Methods:Theoverallsurvivalanddisease-freesurvivalwerestatisticallyevaluatedusing theKaplan–Meiermethod,thelog-ranktestandmultivariateevaluationbyCoxregression analysis.
Results:Thestudypopulationwaspredominantlyyoungerthan60yearsold(81,6%),had intermediatecytogeneticrisk(40.8%),infirstcompleteremissionafterinduction chemother-apy(46.9%),withawhitebloodcountatdiagnosisoflessthan30×109/L(57.1%)anddenovo
acutemyeloidleukemia(62.2%).Survivalcurvesshowedthatbetterprognosiswasrelated toagebelow60years(median:12,4months;p-value=0,2227;OddsRatio=0,6676),good pro-gnosticcytogeneticmarkers(median:97.7months;p-value=0.0037;OddsRatio=0.4239)and whitebloodcellcountatdiagnosisoflessthan30×109/L(mediansurvival:23.6months;p
-value=0.0001;OddsRatio=0.3651).RegardingtheFrench-American-Britishsubgroups,the medianoverallsurvivalwas23.5monthsforM0,M1andM2,97.7monthsforM3and7.4 monthsforM4,M5,M6,andM7(p-value=0.0288).
Conclusion: Prognosticfactorsstronglyinfluencedpatientsurvival,aswellasguided treat-ment.Moreover,thesefactorswereconsistentwiththeavailableliteratureadjustedforthe populationinquestion.
©2014Associac¸ãoBrasileiradeHematologia,HemoterapiaeTerapiaCelular.Published byElsevierEditoraLtda.Allrightsreserved.
∗ Correspondingauthorat:HospitaldeClínicasdaUniversidadeFederaldoParaná(UFPR),RuaGeneralCarneiro,181,AltodaGlória, 80060-900Curitiba,PR,Brazil.
E-mailaddress:marcelacoriolano@hotmail.com(M.C.C.Matos). http://dx.doi.org/10.1016/j.bjhh.2014.11.008
Introduction
Neoplasticdiseaseswerehistoricallyassociatedwith econom-icallydevelopedcountries.For approximatelyfour decades, however,thissituationhaschangedandalotoftheonusis observedindevelopingcountries.Infectiousdiseasesarenot themaincauseofdeathanymoreandcancerhasacquireda greaterdimension,becomingaglobalpublichealthproblem.1
Hematologicalmalignancies represent7%ofnewcancer caseseach year.2 According toTheBrazilianNational
Can-cerInstitute,ithasbeenestimatedthattherewere4570new cases inmen and 3940in women in 2012. Acute myeloid leukemia(AML)isthemostcommonacuteleukemiainadults andaccountsforapproximately80%ofthecasesinthisgroup, withanannualincidenceof2.7casesper100,000population.3
AMLisarelativelyrarediseasewithhighheterogeneityin theaffectedpopulationintermsofmorphology, immunophe-notype, cytogenetics and molecular abnormalities. It is a clonalproliferationofmyeloidprecursors,theresultofgenetic and epigenetic alterations that disrupt self-renewal, prolif-eration and differentiation of cells, with accumulation of leukemicblastsorimmaturecellsinthebonemarrow.4The
clinicaloutcomeisextremelyvariable,withsurvivalfrom a fewdaystoadefinitivecureofsomeclinicalandbiological aspects,whichisusefulinpredictingoutcomes.4–6
Several clinicalfeatures canpredict complete remission andtheevent-freesurvival(EFS)ofthesepatients.Themost importantprognosticfactorsregardingadverseclinical pre-sentations,includeage,cytogeneticabnormalities,secondary leukemia,whitebloodcell(WBC)countandcomplete remis-sionafterthefirstinduction.5,7
Thecasescanbemorphologicallysubclassifiedaccording tothe French-American-British(FAB) system. Thisform of organizationdoesnotprovideadditionalprognostic informa-tion,butit isimportanttosystematizeacutepromyelocytic leukemia, a biological and clinical variant of AML, classi-fied asAML M3 in the FAB system, currently calledacute promyelocyticleukemiawitht(15,17)(q24.1,q21.1),and PML-RARA,intheWorldHealthOrganization(WHO)classification system.8–10
Brazilhaspeculiaritiesregardingitsterritorialdimensions, withimportantregionaldifferencesintheoccurrenceofthe diseaseanddistributionofassociatedriskfactorsandsolocal informationisextremelyimportantforanalyticalexploration ofthismalignancy.
Prospectsforpatientshaveimprovedoverthelast30years, but despite significant progress, the treatment outcome is variableandfrequentlysuboptimal.Morethanhalfofyoung andadultpatients,andabout90%ofthedeathsofover 60-year-oldpatientsinthispopulationarediseaserelated.5,6,11,12
Thisstudy shows the indispensability ofregistries with standardized,up-to-dateandrepresentativeinformation,due theconsiderablevariationsbetweenpopulationsinrelationto survivalandepidemiologicalcharacteristicswhichcanpredict treatmentoutcome.
The aim of this study was to analyze the influence of prognosticfactorsdescribedintheliteraturecorrelatedwith survival of patients with acute myeloid leukemia treated between 2003 and 2009 at the Hematology and Oncology
ServiceoftheHospitaldeClínicas,UniversidadeFederaldo Paraná(HC-UFPR),Brazil,aswellastotracetheclinicaland epidemiologicalprofileofthepatients.
Methods
ThisretrospectiveanalyticalstudywasconductedatHC-UFPR afterbeingapprovedbytheEthicsCommitteeofthehospital. Thestudypopulationwasselectedusingrecordsfromthe ComputerInformationServiceandtheHospitalCancer Reg-istry ofHC-UFPRusing thefollowinginclusion criteria: the International Classification ofDiseases (ICD)ofAML, older than 15 yearsold anddiagnosis betweenJanuary2003and December2009.Theinitialpatientsetconsistedofpatients, predominantlytreatedwithcombinedinduction chemother-apyusingcytarabineanddaunorubicin(theso-called“7+3” regimen) fornon-M3 leukemias,and all-transretinoic acid (ATRA)aloneorcombinedwithananthracyclinefortheM3 subtype.
Patientswhowerenottreatedexclusivelyinthe Hemato-logyandOncologyService,HC-UFPRwereexcludedaswere thosewhohadbiphenotypicleukemia,Fanconianemiaeither associated with myelodysplastic syndrome or in isolation, thosewhowerediagnosedbefore2003andthosewhose med-icalrecordswerenotavailable.Theflowchartforselectionof thestudypopulationisdetailedinFigure1.
Datacollectionwasbasedonthereviewofmedicalrecords availablefromtheMedicalArchiveService(SAME),basedon theresultsofcytogeneticandimmunophenotyping examina-tionsprovidedbytherespectivelaboratories,aswellasrecords fromtheHospitalEpidemiologyServiceofHC-UFPR. Informa-tionofinterestwasinputonanExcelspreadsheettofacilitate furtheranalysisofthevariablesandtocompiletheresults.
Data forclinical andepidemiologicalcharacterizationof thestudypopulation,suchasgender,ageatdiagnosis,race, family history ofcancer, cytogenetics, the presenceof the t(15;17),completeremissionrateafterthefirstinduction,WBC count at diagnosis and type ofevolution (primary or sec-ondary) were arranged in a table of absolute and relative frequencies,withcalculationsperformedusingtheMicrosoft Excelprogram.
Regarding the analysis of overall survival (OS) and EFS of the patients, survival curves were constructed by the Kaplan–Meier method,using the statistical programPRISM (version 5.0). The definitions used for the calculation of survivalfollowedtherevisedrecommendationsofthe Interna-tionalWorkingGroupfortherapeuticstudiesinacutemyeloid leukemia.13TheOSwasdefinedasthetimeintervalbetween
thedateofdiagnosisanddateofdeathordateoflast follow-upvisit.TheEFSwastakenastheperiodbetweenthedateof diagnosisandthedateofrelapse,inductionfailureordateof deathfromanycause.
ThecurvesofOSand EFSwerealsocorrelatedwith cer-tainprognosticfactorsasreportedintheliterature,suchas ageatdiagnosis,FABclassification,cytogenetics,WBCcount at diagnosis and evolution (primary and secondary).11,12,14
120 over 15-year-old patients with ICD of acute myeloid leukemia according to patients’ charts and
Cancer Hospital records between 2003 and 2009
31 patients were excluded for the following reasons
5 patients due to biphenotypic leukemia, associated Fanconi
anemia or isolated myelodysplastic syndrome
9 patients were previously treated at other centers
14 patients were diagnosed prior
to 2003
The medical records of 3 patients were inaccessible
98 patients were analyzed
Figure1–Flowchartusedtoselectthestudypopulation.
curvesalsoincludedtheOddsRatio(OR)withrespective95% confidenceintervals(95%CI).
Multivariateanalysesofprognosticfactorsinrelationtothe OS,includingcytogenetics,gender,age,WBCcountandtypeof evolutionwereperformedusingCoxregressionanalysiswith theSPSS20.0software.
Thepoorcytogeneticprognosiscategoryincludedcomplex karyotypes(twoormoreunrelatedabnormalities),monosomy of chromosome 5, monosomy of chromosome 7, translo-cations involving the 11q23 locus, and all deletions. The intermediateprognosiscategorywasdefinedasnormal kar-yotype and trisomies of chromosomes 4, 8 and 21. The translocationst(15;17),t(8;21)andinversions(16)/t(16;16)were considered the only entities capable of predictinga favor-ableprognosis. Other factors considered as poorprognosis inthecurrentstudywereagegreaterthan60,aWBCcount atdiagnosisover30×109/L,secondaryleukemiaandthelack
ofcompleteremissionatfirstinduction.Completeremission wasconsideredtobetheabsenceofsignsandsymptomsof diseaseassociatedwithnormalcompletebloodcountandless than5%blastsinbonemarrowaspirate.
The categorization used was based on the
French-American-British(FAB)groupclassification,whichcomprises eightsubtypesofAML(M0–M7)andisbasedon morpholog-icalandcytochemicalaspects.12Tocomparesurvivalcurves,
patientsweredividedintothreegroups:GroupI(FABM0–M2), GroupII(FABM3)andGroupIII(FABM4–M7).
Results
Thefinalstudypopulationwas98individuals.Patientswere predominantlyyoungerthan60yearsold(81.6%),inthe inter-mediatecytogenetic risk group(40.8%), withfirst complete remissionafterinductionchemotherapy(46.9%),whiteblood countlessthan30×109/L(57.1%)anddenovoleukemia(62.2%).
Themeanageatdiagnosiswas44.27years.Allclinicaland epidemiologicalfeaturesarelistedinTable1.
Therewasnostatisticallysignificantdifferencewhen com-paring the survival curves (Figure 2A) related to age for patientsaboveandbelowthecut-offdeterminedforthestudy population (median survival of 12.4 months for the group agedlessthanorequalto60yearsversus8.2monthsforthe groupolderthan60years;p-value=0.2227;OR=0.6676;95% CI=0.3488–1.278).ThemedianEFS(Figure2B)was10.7months fortheyoungergroupversus7.3monthsfortheover60-year olds(p-value=0.2448;OR=0.6812;95%CI=0.3567–1.301).The survivalcurvewasnotstatisticallysignificantforthe gender-relatedSGwithamediansurvivalof15.3monthsforfemales versus26.8monthsforthemen(p-value=0.2756;OR=0.7625; 95%CI=0.4684–1.241).
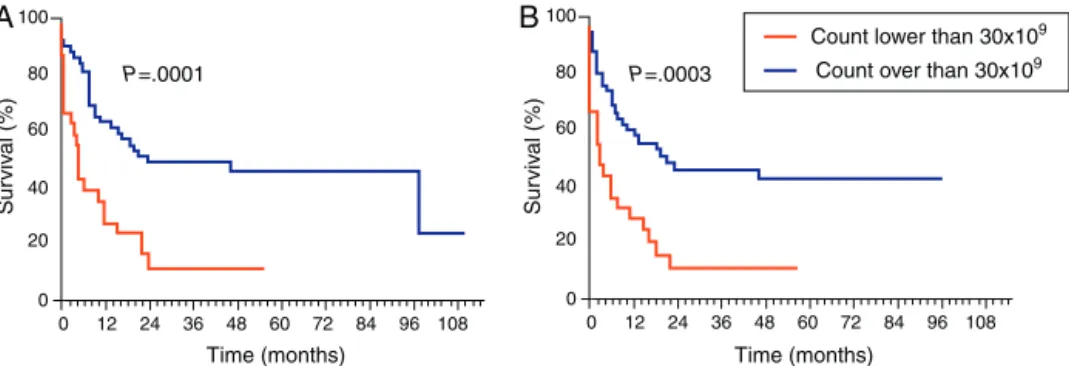
OScurvesforthetypeofevolutiondidnotshowany sta-tisticallysignificantdifferencebetweenthetwogroupswith a mediansurvivaltime forthe primary leukemia groupof 21.9monthsversus11.8monthsforthegroupwithsecondary leukemia(p-value=0.1706;OR=0.6824;95%CI=0.3951–1.179). ThisstudyfoundasignificantlyhigherOSinthegroupwith WBCcountlowerthan30×109/Latdiagnosis(Figure3A)with
amediansurvivaltimeof23.6monthsversus4.7monthsfor thegroupwithWBCcountofmorethan30×109/Lat
diag-nosis(p-value=0.0001;OR=0.3651;95%CI=0.1403–0.5160).As for EFS(Figure 3B), the groupwith leukocyte countslower than 30×109/L had asurvival time of19.3 months versus
3.0 months forthe group with aleukocyte count ofmore than30×109/Latdiagnosis(p-value=0.0003;OR=0.3042;95%
CI=0.1586–0.5834).
100 80 60 40 20 0
0 12 24 36 48 60 72 84 96 108
Time (months)
0 12 24 36 48 60 72 84 96 108
Time (months)
Survival (%)
P=.222 P=.244
Younger than 60 years
Older than 60 years
A
10080 60 40 20 0
Survival (%)
B
Figure2–Age-relatedsurvival.(A)Overallsurvivaland(B)event-freesurvival.
Sur
viv
al (%)
Sur
viv
al (%)
A
100B
80
60
40
20
0
0 12 24 36 48 60 72 84 96 108
Time (months) P=.0001
100
80
60
40
20
0
0 12 24 36 48 60 72 84 96 108
Time (months) P=.0003
Count lower than 30x109 Count over than 30x109
Figure3–Survivalinrespecttowhitebloodcellcount.(A)Overallsurvivaland(B)event-freesurvival.
100
80
60
40
20
0
0 12 24 36 48 60 72 84 96 108
Time (months)
0 12 24 36 48 60 72 84 96 108
Time (months)
Survival (%)
100
80
60
40
20
0
Survival (%)
P=.0037 P=.0005
Good prognosis
Poor and intermediate prognosis
A
B
Figure4–Survivalinrespecttocytogenetics.(A)Overallsurvivaland(B)event-freesurvival.
(97.7 versus 6.3 months; p-value=0.0005; OR=0.3591; 95% CI=0.2013–0.6407–Figure4B).
Someservicessentincompleteresultsfor immunopheno-typingandmyelogramanalysisandsoonly86%oftheresults oftheFABclassificationwereobtained.Accordingtothis clas-sification,5%ofthepatientswereintheM0subgroup,1%in M1,13%inM2,31%inM3,17%inM4,11%inM5,3%inM6and 1%ofthepatientsinM7.TheM3subgrouphadstatistically greatermedianOSandEFSasisshowninFigure5AandB (medianOSof23.5monthsforM0,M1,M2;97.7forM3and7.4 monthsforM4,M5,M6andM7;p-value=0.0288andmedian EFSof15.2monthsforM0,M1,M2;97.7forM3and6.1months forM4,M5,M6andM7;p-value=0.0047).
Multivariateanalysis wassignificant forcytogenetics(p -value=0.015).
Inthisanalysis,65.3%ofpatientsdied;25%ofthedeaths were dueto disease progression. Bleeding was the second mostcommoncauseofdeath,followedbyinfectionandthe consequencesoftreatment.
Discussion
Survival (%)
A
Survival (%)
B
100
80
60
40
20
0
100
80
60
40
20
0 0 12 24 36 48 60 72 84 96 108
Time (months)
0 12 24 36 48 60 72 84 96 108
Time (months)
P=.0288 P=.0047 M0-M2M3
M4-M7
Figure5–SurvivalinrespecttoFrench-American-Britishclassification.(A)Overallsurvivaland(B)event-freesurvival.
analyticalstudies,despitethelimitationsinherentto retro-spectivestudies.
The data shows a slight predominance of males than femalesandanabsolutemajorityofself-identifiedCaucasian patients.DataavailableforBrazilisscarce,butdataprovided
Table1–Globalclinicalandepidemiological characterization.
n %
Gender
Female 47 48
Male 51 52
Age
60yearsorless 80 81.6
Over60years 18 18.4
Race
Caucasian 91 92.9
Non-Caucasian 7 7.1
Familycancerhistory
Present 40 40.8
Absent 39 39.8
Insufficientinformation 19 19.4
Cytogenetics
Goodprognosis 21 21.4 Intermediaryprognosis 40 40.8 Poorprognosis 23 23.5 Insufficientinformation 14 14.3
Translocation(15;17)
Present 17 17.3
Absent 81 82.7
Completeremissionafterfirstinduction
Present 46 46.9
Absent 43 43.9
Insufficientinformation 9 9.2
Whitebloodcellcount
Lowerthan30×109/L 56 57.1
Overthan30×109/L 26 26.6
Insufficientinformation 16 16.3
Typeofleukemia
Primary 61 62.2
Secondary 36 36.7
Insufficientinformation 1 1.1
bytheAmericanCancerSocietyshowthatAMLis1.7times
moreprevalentinmenandslightlyhigherinthenon-Hispanic whitepopulationthanotherraces.3Thehighnumberof
self-identifiedCaucasian patientsmayberelatedtotheequally highprevalenceofthisraceinthesouthernregionofthe coun-try, wherethe researchwas conducted, orthis is the kind ofinformationprovidedbypatientswho,forhistorical and culturalreasons,tendtoclassifythemselvesasWhite.
Themeanageatdiagnosiswaslowerthanthatfoundin theliterature.3,15Asthereisnocleardefinitionofthecut-off
agetodefinetheprognosisofAML,becauseofthestatistical significanceoftheresults,thisstudyestablishedthelimitof60 yearstostratifythepopulationintotwogroups.The disagree-mentwiththeliteratureregardingthestatisticalsignificance forthisfactormayberelatedtotheincorporationofAMLM3, prevalentinyoungerpatients,withothersubtypesofAML.3
TheM3subtypeisaverypeculiarformofleukemiaandmay beconsideredaparticulardisease,bothbytheclinical char-acteristicsandbythegoodprognosis.9However,duetothe
limitednumber ofsubjects, thissubtypewasgroupedwith otherentities.
Olderindividualshadlowerratesofcomplete remission, OSandEFSwhencomparedwithyoungerpatients.AMLin elderlyisabiologicallyandclinicallydistinctentity.Basedon theanalysisofmolecularandcytogeneticdata,itisknown thattheleukemiccellsinolderpatientsareintrinsically resis-tanttoconventionalchemotherapy.Duetocomorbiditiesand the poor reserve of stem cells in the bone marrow, older adultsdonottoleratemyelosuppressivechemotherapywell andthereisahightreatment-relatedmortalityrateand evo-lutionofthedisease.3,15,16
Therewasnostatisticalsignificancerelatedtofamily his-toryofcancerandthetypeofevolution(primaryorsecondary) of the disease in the prognosis of these patients. Familial occurrenceisrareanditsroleinthedevelopmentofthe dis-ease isuncertain,sincetherole ofthetypeofevolutionis wellestablishedintheliterature.Historyofprior myelodys-plasiaormyeloproliferative diseasesiscommon(24–40%of cases) inelderlypatientswithacutemyeloidleukemia.17–19
Theabsenceofanyprognosticcorrelationmayberelatedto thehigherproportionofyoungpatientsinthestudy popula-tion.
Thelimitof30×109/Lwasadoptedduetothehigher
statis-tical significancefoundforthis valueinthe current study, althoughtraditionallythedecisiveprognosticscoreiscloser to20×109/L.20
ThisstudyfoundacorrelationbetweenhigherWBCcounts andreduced OSand EFS.Onepossibleexplanationforthis findingisthatveryhighWBCcountsareassociatedwithan increasedriskoftumorlysissyndromeandleukostasis.Both theseare consideredoncologicemergenciesandareableto affecttheprognosisofpatients.21Inamultivariateanalysis,
thisfactor didnotshowthe samesignificance,despitethe borderlinevalue(p-value=0.063).
Cytogeneticsisakeypointinthediagnosis,treatmentand prognosisofAML.22,23Inthepresentstudy,thesurvivalcurves
ofpatientswithcytogeneticsrelatedtopoorprognosisand intermediateprognosisweregroupedtogetherwiththe prog-nosisbeingpoorduetooverlapping.
Cytogeneticswasstatisticallysignificantbothinunivariate analysisandinmultivariateanalysis,showingthat, irrespec-tiveofotherfactors,thisisanimportantprognosticfactor. Consistentwiththe literature,cytogeneticsrelated togood prognosisconferredabettersurvivalforpatients.22–24There
wasasignificantdropinthesurvivalcurveofpatientswith cytogeneticsrelatedtopoorprognosis;survivalforthisgroup wascloseto20%withinthe firsttwoyears.TheOSrelated togoodprognosisbecamestableatbetween70%and80%in eightyearsoffollowup.Thisdatanotoriouslyrevealstherole ofcytogeneticsintheprognosisofpatients.
Currently,theclassificationrecommendedforAMLisone establishedbytheWHOin2008,whichassessesthe morpho-logical,immunophenotypicandgeneticcombinationsaswell asclinicalmanifestationsofthedisease.7,25Sincethisisa
ret-rospectivestudyandprevioustoWHOdefinitions,thisstudy was based exclusively on the FAB classification associated withcytogeneticstoestablishsubtypes.8
Acutepromyelocyticleukemia (knownasM3intheFAB classification) is characterized bythe translocationt(15;17) andisregardedasaparticulartypeofAML.9Inthepresent
study,similartootherpublications,thiskindofleukemia con-ferredbettersurvivalratesthanthoseobservedfortheother subtypes.26–28
Ahigherdeathratewasobservedrelatedtothe FABM3 classificationinthefirst daysfollowingdiagnosis,probably duetothehighfrequencyofcomplications,suchas dissemi-natedintravascularcoagulation(characteristicofthisclassof AML).Subsequentdeathsweremostlyrelatedtoaggressive treatment.Moreoverthesurvivalcurve showsthatpatients whosurvivedthefirststagesoftheillnessandtreatmenthad highersurvivalratesthanthoseofothersubgroups.Wemust stressthatinthisstudythepatientsweregroupedinrespect totheFABclassificationduetotheoverlapofthecurvesand thesizeofthepopulationstudied.
Conclusion
Prognostic factors significantly influenced the survival of patients,aswellasguidedtreatment.Moreover,theoutcomes wereconsistentwiththeliterature,adjustedforthe popula-tion inquestion. Clinicaland epidemiological dataprovide
important toolsfora possibledevelopment ofsurveillance systems forAML,so thatthe necessitiesofthe population consultedatHC-UFPRareprioritizedandeffectivelytreated bypublichealthpolicies.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
r
e
f
e
r
e
n
c
e
s
1.Estimativa2012:incidênciadecâncernoBrasil[Internet]; 2011.Availablefrom:www2.inca.gov.br/wps/wcm/connect/ inca/portal/home[cited30.6.12].
2.GuerraMR,MouraGalloCV,Mendonc¸aGA.Riscodecâncerno Brasil:tendênciaseestudosepidemiológicosmaisrecentes. RevBrasCancerol.2005;51(3):227–34.
3.SiegelR,NaishadhamD,JemalA.Cancerstatistics,2012.CA CancerJClin.2012;62(1):10–29.
4.EsteyEH.Acutemyeloidleukemia:2013updateon risk-stratificationandmanagement.AmJHematol. 2013;88(4):318–27.
5.GrimwadeD.Thechangingparadigmofprognosticfactorsin acutemyeloidleukaemia.BestPractResClinHaematol. 2012;25(4):419–25.
6.MuratiA,BrecquevilleM,DevillierR,MozziconacciMJ, Gelsi-BoyerV,BirnbaumD.Myeloidmalignancies:mutations, modelsandmanagement.BMCCancer.2012;12:304.
7.SekeresMA,PetersonB,DodgeRK,MayerRJ,MooreJO,LeeEJ, etal.Differencesinprognosticfactorsandoutcomesin AfricanAmericansandwhiteswithacutemyeloidleukemia. Blood.2004;103(11):4036–42.
8.BennettJM,CatovskyD,DanielMT,FlandrinG,GaltonDA, GralnickHR,etal.Proposedrevisedcriteriaforthe classificationofacutemyeloidleukemia.Areportofthe French-American-BritishCooperativeGroup.AnnInternMed. 1985;103(4):620–5.
9.WarrellRP,deThéH,WangZY,DegosL.Acutepromyelocytic leukemia.NEnglJMed.1993;329(3):177–89.
10.SwerdlowSH,CampoE,HarrisNL,JaffeES,PileriSA,SteinH, etal.TheWorldHealthOrganizationclassificationoftumors ofhematopoieticandlymphoidtissues.IARCPress;2008. 11.FerraraF,SchiffCA.Acutemyeloidleukaemiainadults.
Lancet.2013;381(9865):484–95.
12.LiesveldJ.ManagementofAML:whodowereallycure?Leuk Res.2012;36(12):1475–80.
13.ChesonBD,BennettJM,KopeckyKJ,BüchnerT,WillmanCL, EsteyEH,etal.RevisedrecommendationsoftheInternational WorkingGroupforDiagnosis,StandardizationofResponse Criteria,TreatmentOutcomes,andReportingStandardsfor TherapeuticTrialsinAcuteMyeloidLeukemia.JClinOncol. 2003;21(24):4642–9.
14.SoutoFilhoJT,PortugalRD,LoureiroM,PulcheriW,NucciM. Characterizationandanalysisoftheoutcomeofadultswith acutemyeloidleukemiatreatedinaBrazilianUniversity hospitaloverthreedecades.BrazJMedBiolRes. 2011;44(7):660–5.
15.EsteyE.Acutemyeloidleukemiaandmyelodysplastic syndromesinolderpatients.JClinOncol.2007;25(14):1908–15. 16.StoneRM.Thedifficultproblemofacutemyeloidleukemiain
theolderadult.CACancerJClin.2002;52(6):363–71.
17.OwenC,BarnettM,FitzgibbonJ.Familialmyelodysplasiaand acutemyeloidleukaemia–areview.BrJHaematol.
18.YagasakiH,MugishimaH.[Hereditarydiseaseswith propensitytomyeloidmalignancy].NihonRinsho. 2009;67(10):1884–8.
19.WahlinA,MarkevärnB,GolovlevaI,NilssonM.Prognostic significanceofriskgroupstratificationinelderlypatients withacutemyeloidleukaemia.BrJHaematol.
2001;115(1):25–33.
20.SuL,LiW,CuiJW,TanYH,YangY,LiuXL,etal.[Correlationof NPM1,FLT3-ITDmutationswithleukocytecountand myeloblastspercentageinAMLpatientswithnormal karyotype].ZhongguoShiYanXueYeXueZaZhi. 2013;21(3):571–5.
21.vanBuchemMA,teVeldeJ,WillemzeR,SpaanderPJ. Leucostasis,anunderestimatedcauseofdeathinleukaemia. Blut.1988;56(1):39–44.
22.MrózekK,MarcucciG,NicoletD,MaharryKS,BeckerH, WhitmanSP,etal.PrognosticsignificanceoftheEuropean LeukemiaNetstandardizedsystemforreportingcytogenetic andmolecularalterationsinadultswithacutemyeloid leukemia.JClinOncol.2012;30(36):4515–23.
23.GrimwadeD,HillsRK,MoormanAV,WalkerH,ChattersS, GoldstoneAH,etal.Refinementofcytogeneticclassification inacutemyeloidleukemia:determinationofprognostic significanceofrarerecurringchromosomalabnormalities among5876youngeradultpatientstreatedintheUnited KingdomMedicalResearchCounciltrials.Blood. 2010;116(3):354–65.
24.DöhnerH,EsteyEH,AmadoriS,AppelbaumFR,BüchnerT, BurnettAK,etal.Diagnosisandmanagementofacute myeloidleukemiainadults:recommendationsfroman internationalexpertpanel,onbehalfoftheEuropean LeukemiaNet.Blood.2010;115(3):453–74.
25.VardimanJW,ThieleJ,ArberDA,BrunningRD,BorowitzMJ, PorwitA,etal.The2008revisionoftheWorldHealth Organization(WHO)classificationofmyeloidneoplasmsand acuteleukemia:rationaleandimportantchanges.Blood. 2009;114(5):937–51.
26.AsouN,AdachiK,TamuraJ,KanamaruA,KageyamaS, HiraokaA,etal.Analysisofprognosticfactorsinnewly diagnosedacutepromyelocyticleukemiatreatedwith all-transretinoicacidandchemotherapy.JapanAdult LeukemiaStudyGroup.JClinOncol.1998;16(1): 78–85.
27.HiornsLR,SwansburyGJ,MehtaJ,MinT,DaintonMG, TreleavenJ,etal.Additionalchromosomeabnormalities conferworseprognosisinacutepromyelocyticleukaemia.Br JHaematol.1997;96(2):314–21.