www.bjorl.org
Brazilian
Journal
of
OTORHINOLARYNGOLOGY
ORIGINAL
ARTICLE
Mometasone
furoate
in
the
treatment
of
mild,
moderate,
or
severe
persistent
allergic
rhinitis:
a
non-inferiority
study
(PUMA)
夽
Martti
Anton
Antila
a,
Fabio
Morato
Castro
b,
Flavio
Sano
c,
Adelmir
Machado
d,
Fatima
Fernandes
e,
Nelson
Augusto
Rosário
Filho
f,
Rafael
Stelmach
b,∗aClínicadeAlergiaMarttiAntila(CMPC),Sorocaba,SP,Brazil
bUniversidadedeSãoPaulo(FMUSP),FaculdadedeMedicina,SãoPaulo,SP,Brazil cHospitalNipo-brasileiro,SãoPaulo,SP,Brazil
dUniversidadeFederaldaBahia(UFBA),InstitutodeCiênciasdaSaúde,Salvador,BA,Brazil eFundac¸ãoJoséLuizEgydioSetúbal(HospitalInfantilSabará),SãoPaulo,SP,Brazil fUniversidadeFederaldoParaná(UFPR),DepartamentodePediatria,Curitiba,PR,Brazil
Received12May2015;accepted2November2015 Availableonline15February2016
KEYWORDS
Persistentallergic rhinitis;
Mometasonefuroate; Non-inferiority
Abstract
Introduction:AllergicrhinitisisconsideredthemostprevalentrespiratorydiseaseinBraziland worldwide,withgreatimpactonqualityoflife,affectingsociallife,sleep,andalsoperformance atschoolandatwork.
Objective:Tocompare the efficacy andsafetyoftwo formulations containing mometasone furoateinthetreatment ofmild,moderate,orseverepersistentallergic rhinitisafter four weeksoftreatment.
Methods:PhaseIII,randomized,non-inferiority,national,openstudycomparingmometasone furoateintwopresentations(controldrugandinvestigationaldrug).Theprimaryendpointwas thepercentageofpatientswithreductionofatleast0.55innasalindexscore(NIS)afterfour weeksoftreatment.Secondaryoutcomesincludedtotalnasalindexscorescoreafterfourand 12weeksoftreatment;individualscoresforsymptomsofnasalobstruction,rhinorrhea, sneez-ing,andnasalpruritus;aswellasscoreforpruritus,lacrimation,andocularrednessafterfour and12weeksoftreatment.Thestudywasregisteredatclinicaltrials.govwiththereference
numberNCT01372865.
夽 Pleasecitethisarticleas:AntilaMA,CastroFM,SanoF,MachadoA,FernandesF,RosárioFilhoNA,etal.Mometasonefuroateinthe
treatmentofmild,moderate,orseverepersistentallergicrhinitis:anon-inferioritystudy(PUMA).BrazJOtorhinolaryngol.2016;82:580---8. ∗Correspondingauthor.
E-mail:rafast@uol.com.br(R.Stelmach). http://dx.doi.org/10.1016/j.bjorl.2015.11.009
Results:Theefficacyprimaryanalysisdemonstratednon-inferiorityoftheinvestigationaldrug inrelationtothecontroldrug,sincetheupperlimitoftheconfidenceinterval(CI)of95%for thedifferencebetweenthesuccessratesafterfourweeksoftreatment(12.6%)wasbelowthe non-inferioritymarginprovidedduringthedeterminationofthesamplesize(13.7%).Adverse eventswereinfrequentandwithmildintensityinmostcases.
Conclusion: Theefficacyandsafetyofinvestigationaldruginthetreatmentofpersistent aller-gicrhinitisweresimilartothereferenceproduct,demonstratingitsnon-inferiority.
© 2016 Associac¸˜ao Brasileira de Otorrinolaringologia e Cirurgia C´ervico-Facial. Published by Elsevier Editora Ltda. This is an open access article under the CC BY license (http:// creativecommons.org/licenses/by/4.0/).
PALAVRAS-CHAVE
Rinitealérgica persistente; Furoatode mometasona; Nãoinferioridade
Furoatodemometasonanotratamentoderinitealérgicapersistenteleve,moderada
ougrave:estudodenãoinferioridade(PUMA)
Resumo
Introduc¸ão: Arinitealérgicaéconsideradaadoenc¸arespiratóriamaisprevalentenoBrasile emtodoomundo,comgrandeimpactonaqualidadedevida;alémde,afetaravidasocial,o sonoetambémodesempenhonaescolaenotrabalho.
Objetivo: Compararaeficáciaeseguranc¸adeduasformulac¸õescontendofuroatode mometa-sonanotratamentodarinitealérgicapersistenteleve,moderadaougraveporumperíodode quatrosemanas.
Método: Trata-sedeumestudonacionalabertodefaseIII,randomizado,denãoinferioridade decomparac¸ãodofuroatodemometasonaemduasapresentac¸ões(medicac¸ãodecontrolee fármacosobinvestigac¸ão).Opontofinalprimáriofoiopercentualdepacientescomreduc¸ão mínimade0,55noescoredeíndicenasal(EIN)apósquatrosemanasdetratamento.Osdesfechos secundários foram:escoreNIStotalapós4e12 semanasdetratamento; escoresindividuais parasintomasdeobstruc¸ãonasal,rinorréia,espirrosepruridonasal,bemcomoescorespara prurido,lacrimejamentoehiperemiaconjuntivalapós4e12semanasdetratamento.Oestudo foiregistradoemclinicaltrials.govcomonúmerodereferênciaNCT01372865.
Resultados: A análise de eficácia primária demonstrou não inferioridade do fármaco sob investigac¸ãoemrelac¸ãoàmedicac¸ãodecontrole,vistoqueolimitesuperiordointervalode confianc¸a(IC)de95%paraadiferenc¸aentreospercentuaisdesucessoapósquatrosemanasde tratamento(12,6%)situava-seabaixodamargemdenãoinferioridadeproporcionadadurantea determinac¸ãodotamanhodaamostra(13,7%).Eventosadversosforampoucofrequentesede leveintensidadenamaioriadoscasos.
Conclusão:A eficácia e aseguranc¸a de um fármacoexperimental no tratamento darinite alérgicapersistenteforamsimilares àsdoprodutodereferência,oquedemonstrousuanão inferioridade.
© 2016 Associac¸˜ao Brasileira de Otorrinolaringologia e Cirurgia C´ervico-Facial. Publicado por Elsevier Editora Ltda. Este ´e um artigo Open Access sob uma licenc¸a CC BY (http:// creativecommons.org/licenses/by/4.0/).
Introduction
Allergic rhinitis is an allergic disease characterized by chronic inflammation of the mucous membranes of the respiratorytract.Itsmainsymptomsarenasalcongestion, sneezing, anterior and posterior rhinorrhea, nasal pruri-tus,ocularandpalatalpruritus,conjunctivalinjection,and lacrimation.1,2
Considered the most prevalent respiratory disease in Brazil and worldwide, recent estimates indicate that approximately 500 million people suffer from allergic rhinitis.1,3IntheUnitedStates,itisbelievedthat10---30%of adultsand40%ofchildrenareaffectedbyallergicrhinitis.4 In Brazil, it is estimated that the average prevalence
in adolescents and schoolchildren is 29.6% and 25.7%, respectively.3,5
Clinicalmanifestationsofallergicrhinitisoccurafterthe interactionof a specific allergen and the immune system of previouslysensitized individual. Immediate hypersensi-tivityis afastreaction withparticipationof IgEandmast cells followed by inflammation.6 Other allergic diseases maybeassociatedwithrhinitis,suchasasthmaandatopic dermatitis.7
totheduration(intermittentorpersistent)andintensityof symptoms(mildormoderate/severe).9
Althoughbenign,allergicrhinitishasasignificantimpact on quality of life, affecting social life, sleep, as well as schooland labor performance.1,8 The main goal of treat-mentistopreventorrelievesymptomswithmaximumsafety andefficacy.Ideally,thetreatmentofallergicrhinitisshould aimprimarily theaction oncells and inflammatory medi-ators, thereby minimizing the symptoms of the disease. Several classes of drugs are used in the treatment, such asoralortopicalantihistamines,intranasaldecongestants, leukotrienereceptorantagonists,andtopicalintranasal cor-ticosteroids. The latter are recognizedas thefirst-choice drugsinanti-inflammatorytreatmentofmoderatetosevere allergicdiseases.1,8,10,11
The mechanism of action of corticosteroids includes chemicalmediatorsandcellsinvolvedintheallergic inflam-matory process that establishes the rhinitis. Intranasal formulations have the advantage of local administration, with faster onset of action compared to systemic thera-pies. Moreover, it has been reported that corticosteroids mayhelpcontrolcomorbiditiesofrhinitis,suchasallergic conjunctivitisandasthma.10,12
Mometasone furoate is a synthetic glucocorticoids for topical intranasal use,13 which inhibits the formation, release, and activity of endogenous chemical mediators, also limiting cellular phase of allergic inflammation. Its intranasalapplicationcontrols the initialandlateallergic response.14
Giventhepreviouslydemonstratedefficacyof mometa-sonefuroateinthetreatmentofallergicrhinitis,thepresent studywas designedtotest the non-inferiorityof the new formulationcomparedtothecontrolproduct.Theprimary objectivewastocomparetheefficacyofbothdrugsinthe treatmentofpersistentallergicrhinitisafterfourweeks.
Methods
ThiswasaphaseIII,randomized,non-inferiority,national, multicenter(sevencenters),openstudy,aimingtocompare thenewexperimentalformulationofmometasonefuroate (Eurofarma)tothecontroldrug(Nasonex®,Schering-Plough
Pharmaceuticals Ltd.) administered in total daily dose of 200g(twospraysof100gineachnostriloncedaily).
The study was approved by the research ethics com-mitteesof the institutions involved underthe number CE No.106/2012(issuedonMay17th,2012)andallstudy par-ticipants signed an informed consent (IC). In addition, it wassponsoredbyEurofarmaLaboratóriosS.A.Patientswith mild,moderate,or severepersistentallergicrhinitiswere included,accordingtotheARIAcriteria,1,8withtotalnasal indexscore(NIS)value≥4atthescreeningvisitandonat leastfour of the seven dayspreceding this visit. Patients alsowererequiredtobe>12yearsofage,withsymptoms of allergic rhinitis for at least twoyears (confirmed by a positiveskintesttoatleastonerelevantaero-allergen con-ductedoverthepast90days),andalsohaveprescribeduse ofnasalcorticosteroid.Furthermore,patientswererequired toundergo a washout period of 14±5 days between the screening(SV)andtherandomizationvisit(RV)withoutuse ofanynasal,oral, or parenteralcorticosteroids (including
antihistamines; oral or topical nasal vasoconstrictor; and corticosteroidsinanyrouteofadministration---except cuta-neous).Iftheresearchsubjectrequiredother medication, he/shewasexcludedfromthestudytocontinuetreatment accordingtolocalpractices.
The study was conducted in open-label setting, with no blinding of interventions. Blinding prevents possible biases in clinical trials; however, sometimes it cannot be applied.Thisstudywasopenbecausethedrugsstudiedhave very differentappearances, which would make the blind-ing infeasible. Nevertheless, the symptoms diary and the additionaltestspreventedtheoccurrenceofanybiasinthe evaluationoftheresearchers.Thus,thefactthatthisstudy wasopendoesnotimpactthequalityofdatacollected.
Patients with severe comorbidities (according to the investigator criteria); moderate to severe persistent asthma;historyofrespiratorytractinfectionwithin30days prior tothe study entry;structural changes causing nasal obstruction (excessivelydeviatedseptum,nasalpolyps,or anytypeofnasalmalformation);aswellaspatientsinneed of other medicines to treat allergic rhinitis; pregnant or lactatingpatients;activesmokersinthelastthreemonths priortoenrollmentinthestudy;andthosewhoparticipated in another clinical study in the past 12 months were not includedinthestudy.
Afterstratificationaccordingtotheresearchcenterand totheintensityofallergicrhinitis(mildversusmoderateor severe),patientswererandomizedina1:1ratiotoreceive one of the study treatments.The researchsubjects were randomizedcentrally,accordingtoalistcreatedbyan appli-cation togenerate randomsequences.The randomization and allocation of treatment wasconducted electronically through an electronic case report file (CRF). Treatment was automatically registered in the appropriate field on the medicalrecordsof thestudy.All randomizedpatients receivedatleastonedoseofstudymedicationandreceived no differentdrug than that heor she wasrandomizedto receive.Thefollow-upperiodforeachpatientwas14weeks and the scheduled duration of active treatment was 12weeks.
AfterRV,patientswereassessedinfourvisits(V1,V2,V3, andFV).Duringthestudy,patientscompletedadiary con-taininginformationaboutthesymptomsofallergicrhinitis, nasalobstruction,rhinorrhea,sneezingandnasalpruritus, aswellasthescoreforpruritus,lacrimation,andocular red-nessafterfourand12weeksoftreatment,drugadherence, anduseofrescuemedications.Ateachvisitthesymptoms wererated onascale from0to3, whichwassummedto constitutetheendpointofNISinthestudyperiod.
of3,theTNSScombinesthescoreoffoursymptoms(nasal run,blockage,itchiness,andsneeze),soitcanrangefrom 0to12.AstherewasnosuchdataontheMCIDfortheNIS, andinbothscores,symptomsareindividually scoredfrom 0to3andcontributeequallytothetotalsum(nodifferent weightisattributedtodifferentsymptoms),itwasassumed thatifa0.55reductionisclinicallysignificantinascalefrom 0to12itwouldalsobeina0to9scale.Ifaproportional extrapolationofthethresholdwasdone,areductionof0.55 inthe0to12pointscale(TNSS)wouldrepresentareduction of0.41inthe0to9NISscale.Thus,anapproachusingthe thresholdof0.55pointintheNIStodefineclinicalbenefit canbeconsideredconservative.
Secondary outcomesincludedtotalNISscoreafterfour and 12 weeksof treatment; assessment of individual and overallscoresforsymptomsofnasalobstruction,rhinorrhea, andsneezingusingNISscore;and assessmentof scoresof nasalpruritusandpruritus,lacrimation,andocularredness performedbytheinvestigatorateachvisittotheresearch center;aswellasthefrequencyofadverseevents.
Statisticalmethods
To calculate NIS at baseline, the mean values of valid measuresofthescoreforeachpatientinthesevendays pre-cedingtheRVwereused.TocalculateNISafterfourweeks, themeanvaluesofthefourthweekoftreatmentwereused, consideringthedatarecordedinthediary.
In the present study, the main efficacy measure was the NIS, a combined score calculated by the sum of individual scores of three nasal symptoms (nasal conges-tion/obstruction,runnynose,andsneezing).Eachsymptom wasindividuallygraded from0(nosymptom)to3(severe symptoms),andthetotalNIScanrangefrom0to9.Although theTNSS scoreismore frequentlyused,theNISisa valid scaleandhasbeenusedinseveralstudiestoassessthe effi-cacyofintranasalcorticosteroidsinseasonalandperennial rhinitis.16---19
Fortheperprotocol(PP)population,theresultsobtained fornasalcongestion (obstruction),runnynose, and sneez-ing areshown as individualsymptoms and asa combined score(NIS).Inadditiontothesethreesymptomsthat con-stitute theNIS, patients hadtograde in their diaries the other twosymptoms (one:nasal pruritus, andtwo: pruri-tus,lacrimation,andocularredness),whichwereanalyzed individually.
No imputation of missing data was taken. Continuous variablesweresummarizedbymeansofvariation(minimum andmaximum),mean,standarddeviation(SD),median,and interquartile range (IQR, 25th percentile, and 75th per-centile).Categoricalvariablesweredescribedbymeansof relativefrequencies.
When comparing both groups, parametric or non-parametric methods were used, according to the distribution pattern of the outcome variables. The Kolmogorov---Smirnov test with Lilliefors correction was usedtoassess the pattern ofdistribution of theoutcome variablesinthesample.
Continuousvariableswithnormaldistributionwere com-paredusingt-tests,whilenon-normallydistributedvariables were compared using the Mann---Whitney non-parametric
test. Categorical variables were compared using the chi-squaredorFisher’sexacttest,accordingtothenumberof individuals.Forthemultivariateanalysisoftheproportions ofpatientswithreductionof≥0.55inNIStotalscoreover timeinbothgroups,theANOVAtestwithrepeatedmeasures wasused,withGreenhouse---Geissercorrection.
Two-tailed levels of significance of 5% were used as indicative of statistical differences between groups. For declaration of non-inferiority of the investigational drug comparedtothecontroldrug, theupperlimit of the95% CIforthedifferencebetweentheproportionsofpatientsin bothgroupswithreductionof≥0.55inthetotalNISscore (controldrugminusinvestigationaldrug)afterfourweeks oftreatment should beup to13.7% inabsolute values.If non-inferioritywasdemonstrated,asuperioritytestwould beperformed,withatwo-tailedlevelof5%asindicativeof significantdifferencebetweengroups.
Analyses were performed using Microsoft Excel soft-ware(Microsoft Office2007) fordescriptive analyses,and Rstatistical software(v.2.13.1)andMedCalc(v.11.3.3.0, Mariakerke,Belgium),forinferentialanalyzes.
Samplesizedetermination
Basedontheliterature,the determinationofsample size inthenon-inferioritydesignofthestudyconsideredtheNIS scoreafterfourweeksofequal treatmentinboth groups. Consideringaone-tailedalphaerrorof2.5%andastatistical powerof80%forthestudytofindthemaximumdifferenceof 13.7%orlessbetweenthecontrolandinvestigationalarms (limitfornon-inferiority),164patientswouldbeincludedin eacharmofthestudy.Assumingthattherewouldbealoss offollowupofapproximately15%,thestudywouldinclude 193patientsineacharm,i.e.,atotalof386patients.
A non-inferioritymargin (M) of 13.7% wasdetermined. Thedeterminationofthenon-inferioritymarginwasbased onthemaximumacceptabledifferencebetween experimen-talandcontrolgroups,asjudgedbythespecialists,inorder toassurefortheexperimentalgrouptheretentionofa min-imumof the beneficial effectof treatment in relation to placebo or absence of medication. In addition, the non-inferioritymarginchosenforthisstudyisinaccordancewith theEuropeanMedicinesAgency’sguidelinesandother pub-licationsthatrecommendtheuseofmarginsbetween10% and20%,dependingonthetherapeuticfield.
Results
Studypopulation
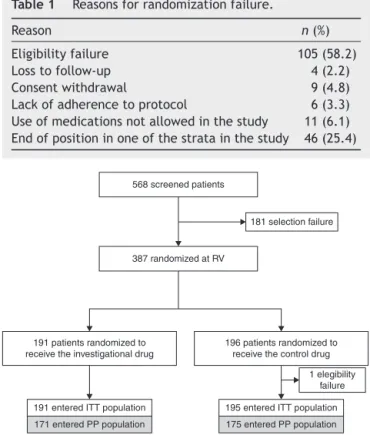
BetweenMayandSeptember2012,568subjectswithmild, moderate, or severe persistent allergic rhinitis, classified accordingtotheARIAcriteria,wereincludedinthestudy, and387ofthemwererandomizedtoreceiveoneofthestudy drugs.The reasonsfor randomizationfailureareshown in Table1.
Table1 Reasonsforrandomizationfailure.
Reason n(%)
Eligibilityfailure 105(58.2)
Losstofollow-up 4(2.2)
Consentwithdrawal 9(4.8)
Lackofadherencetoprotocol 6(3.3)
Useofmedicationsnotallowedinthestudy 11(6.1)
Endofpositioninoneofthestratainthestudy 46(25.4)
568 screened patients
181 selection failure
387 randomized at RV
191 patients randomized to receive the investigational drug
196 patients randomized to receive the control drug
1 elegibility failure
195 entered ITT population 175 entered PP population 171 entered PP population
191 entered ITT population
Figure1 Flowofpatients inthestudy (ITT,intent totreat population; PP, per-protocol population; RV, randomization visit).
Allofthe387randomizedpatientsreceivedatleastone doseofthestudymedicationandenteredthesafetysample. Onerandomizedsubjectviolatedaneligibilitycriterionand thestudywasdiscontinuedatthetimetheviolationbecame known.Thus,thissubjectwasexcludedfromthe intention-to-treat(ITT)andperprotocol(PP)subgroups.
Fortyof the386subjectsin theITTpopulation didnot enter the PP population due to: lack of data for evalua-tionoftheprimaryendpoint(n=23);useofmedicationnot allowedinthestudy(n=10);oruseofhigherdosesofrescue medicationbetweenweekoneandweekfourofthestudy (n=7).TheITTpopulationwasthereforecomposedof386 patients,thePPpopulationof346patients,andthesafety populationof386patients.
Seventy-seven of 386 randomized patients were pre-maturely discontinuedfromthe studydue toconcomitant disease(n=4),lackofadherencetoprotocolortreatment (n=19),losstofollowup(n=24),consentwithdrawal(n=3), toxicityoradverse events(n=2)oruseofmedicationsnot allowedintheprotocolduringthestudy(n=24).
The demographic characteristics of patients are pre-sentedinTable2.
Efficacyendpoint
SymptomsofallergicrhinitiswereevaluatedbyNISscore, whichincludedtheevaluationofindividualsymptomssuch asnasalcongestion/obstruction,runnynose,andsneezing. Inadditiontothesesymptoms,patientshadtogradeintheir
Table2 DemographiccharacteristicsoftheITTandPPsubgroups.
Characteristic ITTpopulation(n=386) PPPopulation(n=346)
Investigational drug
(n=191)
Controldrug (n=195)
Investigational drug
(n=171)
Controldrug (n=175)
Gender,n(%)
Women 119(62.30) 117(60.00) 107(62.57) 108(61.71)
Men 72(37.70) 78(40.00) 64(37.43) 67(38.29)
Age,years(mean±SD) 27.74±12.51 31.04±14.41 28.04±12.81 31.15±14.42
Range 12.19---70.38 12.01---67.54 12.19---70.38 12.00---67.54
Median(IQR) 25.12
(17.60---34.39)
28.79 (18.68---41.35)
25.39 (17.69---34.94)
28.79 (19.01---41.40)
Ethnicity,n(%)
White 112(58.64) 120(61.54) 99(57.89) 109(62.29)
Mixed 32(16.75) 33(16.92) 28(16.37) 27(15.43)
African-American 42(21.99) 37(18.97) 39(22.81) 34(19.43)
Asian 5(2.62) 5(2.56) 5(2.92) 5(2.86)
BMI,km/m2(mean±SD) 24.96±5.39 25.02±5.07 24.65±5.36 25.03±4.84
Range 15.27---44.31 15.27---45.92 15.27---44.31 15.27---39.81
Median(IQR) 24.13
(20.76---28.41)
24.61 (21.90---27.29)
23.72 (20.55---28.13)
24.64 (21.95---27.36)
Historyofsmoking;n(%)
Non-smokers 177(92.67) 181(92.82) 158(92.40) 162(92.57)
Ex-smokersa 14(7.33) 14(7.18) 13(7.60) 13(7.43)
SD,standarddeviation;BMI,bodymassindex; NIS,nasalindex score;IQR,interquartile range;ITT,intentto treatpopulation;PP, per-protocolpopulation.
2.5
Mean symptom score (PP)
2
1.5
1
0.5
0
Investigational Control
Beginning
Investigational Control Investigational Control
After 4 weeks
Symptom score
Nasal obstruction
2.00
1.83 1.78
1.73
1.32
1.91
1.82 1.83
1.71
1.21 1.33 1.02 1.03 1.02
0.85
1.23
1.00
1.04
0.93
0.85 0.85 0.60 0.79 0.73
0.57
0.84
0.76 0.74 0.66 0.5
Rhinorrhea Sneezing Nasal pruritus Ocular pruritus, lacrimation and conjuntival hyperemia
After 12 weeks
Figure2 Symptomscore.
diariestheothertwosymptoms:nasalpruritus;andpruritus, lacrimation,andocularredness;whichwereanalyzed indi-vidually. There wasnosignificant difference between the groups,exceptfornasalobstruction:accordingtothedata recordedinthediary,thescorefornasalobstructionat base-linewassignificantlyhigherintheinvestigationalarmthan inthecontrolarm(Fig.2).
Theefficacyprimaryanalysiswasperformedby evaluat-ingthenon-inferiorityoftheinvestigationaldrugcompared tothecontroldrug.Table3showsthecomparisonbetween the successrates in the twotreatment groups in PP pop-ulation. It can be observed that 76.6% of patients in the investigationalarmand80%inthecontrolarmhada reduc-tion of at least 0.55 in NIS score after four weeks of treatmentandthattherewasnostatisticallysignificant dif-ferencebetweengroups(p=0.586).
The95%CIforthedifferencebetweenthesuccessrates (investigationaldrugandcontroldrug)afterfourweeksof treatment,obtainedbyproportionsequalitytestwith conti-nuitycorrection,was−5.87to12.65.Fig.3showstheresults graphically,withtheupperlimitoftheCI(12.7%)belowthe non-inferioritymargin(M=13.7%).Thus,thenon-inferiority oftheinvestigationaldrugcomparedtothecontroldrugwas demonstrated.
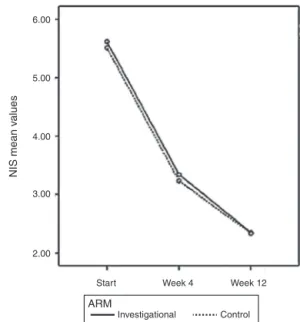
AsobservedinthePPpopulation,therewasasignificant effectof treatmenttimeonthe valuesofNIS (p<0.0001) andnosignificantdifferencebetweengroups (p=0.624)in theITT population.Thevaluesof NISscoreinthe investi-gationalarmwere5.61±1.52;3.34±2.06;and2.34±1.91 beforetreatmentandafterfourand12weeksoftreatment,
13.7%
12.65% 0
–5.86% Favors investigational drug
Favors control drug
95% CI for the difference between the succes rates (control - investigational)
Figure3 Primary analysis of efficacy in thePP population (n=346).
respectively. The group treated with the control drug showed a NIS score of 5.51±1.36; 3.24±2.07; and 2.34±1.91beforetreatment andafterfourand12weeks oftreatment,respectively(Fig.4).
Useofrescuemedication
Afterthebeginningofthetreatment,54.3%ofthepatients whoreceivedthecontroldrugand45.71%ofthose receiv-ingtheinvestigationaldrugusedebastineduringthestudy treatment.Therewasnosignificantdifferencebetweenthe treatmentarms.
Table3 Comparisonoftheproportionsofpatientswithareductionofatleast0.55inNISscoreafterfourweeksoftreatment inbothgroups,PPpopulation(n=346).
Reductionofatleast0.55inNISscoreafter4weeksoftreatment Investigationaldrug,n(%) Controldrug,n(%) pa (n=171) (n=175)
Yes 131(76.6) 140(80.0) 0.586
No 40(23.4) 35(20.0)
PP,per-protocolpopulation;NIS,nasalindexscore.
6.00
5.00
4.00
3.00
2.00
Start Week 4
NIS mean v
alues
Week 12
ARM
Control Investigational
Figure4 TotalNISscoreovertimebasedonpatientdiary,PP population(n=308).
Safetyresults
Table4shows thelevelof serumcortisolinthebeginning ofthestudy(SV)andafterfourweeksoftreatment(FV)in bothtreatment groups,considering thesafetypopulation. Thecomparisonofmediansshowednosignificantdifference betweengroupsinthebeginningorintheendoftreatment. In the safety population, 235 patients presented non-seriousadverseeventsduringthestudy.Inthecontrolgroup, 119of191patients(62.30%)hadanadverseevent,andinthe investigationalarm,116of196patients(59.2%)presented anadverseevent(p=0.530).Withtheexceptionofepistaxis andmyalgia,morefrequentinthecontrolarm,and abnor-malelectrocardiogram,morefrequentintheinvestigational arm,therewasnosignificantdifferencebetweengroupsin thefrequencyofadverseevents.Themostfrequentadverse eventsinbothgroupswerebackpain,cough,dysmenorrhea, headache,andnasopharyngitis.
Therewasareportofasingleseriousadverseevent dur-ingthestudy,classifiedasperianalabscess,whichoccurred inthecontrolarmandwasunrelatedtothestudy medica-tion.Therewerenoreportsofdeathduringthestudy.
Discussion
Allergic rhinitis is an inflammatory disease of the nasal mucosawithhighprevalenceworldwide---whichhasbeen increasinginrecentyears,enhancingtheinterestof differ-entmedicalfieldsandofpublichealthinthesubject.
Inpersistentrhinitis,nasalobstructionisoftenthe pre-dominantsymptom.Inthesecases,corticosteroids arethe drugs of first choice and may or may not be associated with antihistamine/decongestant,20 constituting the only drug class that promotes significant improvement of all symptoms,suchaspruritus,sneezing,rhinorrhea,andnasal congestion.8
The update of the ARIA clinical recommendations1 following the approach taken by GRADE (grading of rec-ommendations assessment, development, and evaluation) indicatestheuseofintranasalcorticosteroidsforthe treat-mentofallergicrhinitisinadultsandalsosuggeststheiruse inchildren.Thisdecisionindicatesthehighvalueofthe effi-cacyofintranasalcorticosteroidsandthelowvalueoftheir possibleadverseevents.8
Mometasone furoate is a potent corticosteroid, as demonstrated by receptor affinity, and it has a lower bioavailability compared to other formulations.21 High power and low availability confer efficacy and safety to mometasonefuroate.22
Theefficacyprimaryanalysis,performedconsideringthe primaryendpoint in the PP population,demonstrated the non-inferiorityoftheinvestigationaldruginrelationtothe controldrugfor thetreatmentofpersistentallergic rhini-tisofanyintensity,sincetheupperlimitofthe95%CIfor thedifferencebetweenthesuccessratesafterfourweeks oftreatment(12.7%)wasbelowthenon-inferioritymargin defined(13.7%).
Moststudiesonintranasalcorticosteroidsinpatientswith mildtomoderatepersistentallergicrhinitisidentifiedinthe literature evaluate nasal symptoms using the TNSS scale, whichincludesnasalobstruction,sneezing,rhinorrhea,and pruritus. These symptoms are individually ranked from 0 (nosymptoms)to3(severesymptoms)andthetotalscore ranges,therefore,from0to12.Adecreaseofatleast0.55 in this score can be considered clinically significant. The presentstudyusedascalewhichincludesonlynasal obstruc-tion, rhinorrhea, and sneezing as symptoms, also ranked from0(nosymptoms)to3(severesymptoms),rangingfrom
Table4 LevelofserumcortisolinSVandFV(mg/dL)inthesafetypopulation(n=387).
Cortisol Investigationaldrug Controldrug pa
SV,g/dL n=191 n=196
Range 0.29---40.11 2.26---38.51
Mean±SD 11.97±7.27 11.91±6.52
Median(IQR) 10.30(6.70---15.40) 10.44(7.34---15.20) 0.736
FV,g/dL n=168 n=171
Range 2.90---37.87 1.0---39.48
Mean±SD 13.64±7.05 13.17±6.60
Median(IQR) 12.10(8.84---17.01) 11.90(8.70---15.91) 0.747
SD,standarddeviation;IQR,interquartilerange;SV,screeningvisit;FV,finalvisit.
0to9.Thus,theuseofthevalue0.55inthepresentstudy canbeconsideredconservative.
Mostindividualswithallergicrhinitishaveocular symp-toms--- abouttwo-thirdsofasthmapatientshavesymptoms ofallergic rhinoconjunctivitis.23 However,the diagnosisof allergic conjunctivitis is undervalued by physicians. Ocu-lar symptoms associated with allergic rhinitis are caused by the direct contactof the allergen withthe conjuncti-valmucosa,andnasal-ocular reflexeswould constitutean indirectpathway,dependingonhistaminerelease,sinceit isblockedwithtopicalintranasalantihistamine. Evidence-basedclinicalresearchshowsthatintranasalcorticosteroids promote relief of nasal and ocular symptoms, but the mechanism howintranasal corticosteroids improve ocular symptoms remains unknown. ARIA guidelines recommend theuseofintranasalcorticosteroidsfortreatmentofallergic rhinoconjunctivitis.8,24
Regarding security, few numerical differences were observed between groups in some of the analyses, andit seems that there is not a pattern that allows asserting whichofthe treatmentisless toxic.Some adverseevents wererecordedinonlyoneofthestudygroups.Foradverse events that were recorded in both groups and could be comparedtothefrequency,nostatisticallysignificant differ-encesbetweentreatmentswerefound,exceptforepistaxis and myalgia --- more frequent in the control arm --- and abnormalelectrocardiogram--- morecommoninthe inves-tigational arm. However, it is important tonote that the adverseeventswerenotrelatedtothedrugsassessedinthis study.Themainadverseeventsofintranasalcorticosteroids arerare,evenwhentheuseisextended.22
Measurementofplasmacortisolinthemorningis sensi-tiveenoughtodeterminesystemicactivityofcorticosteroids on the adrenal gland, although other tests might be used with greater accuracy for this purpose.21 A recent review, includingmore than 20 studies and6000 patients treated with mometasone furoate, found no effect on thehypothalamic-pituitaryaxis.25 Furthermore,theuseof mometasonefuroate for 12months didnotcauseatrophy andmetaplasia of thenasal mucosa,representing further evidenceofitssafety,evenwithprolongeduse.26
Althoughfrequentlyseenasatrivial,fleeting,orminor illness,allergicrhinitis cansignificantlyimpairthequality oflifeofpatients,affectingschoollearningandworkplace productivity.27,28Thisisbecausesymptomssuchassneezing inbursts, nasalpruritus,andnasalcongestion canleadto fatigue,difficultyinconcentrating andlearning,headache and,insomecases,sleepdisorderssuchasapnea.29---31
Conclusion
Mometasone furoate is a safe and effective drug for the treatment of persistent allergic rhinitis. The study demonstratednon-inferiorityinefficacyandsafetyof inves-tigationaldrugcomparedtothecontroldrug,validatingthe proposed development asan option for the treatment of patientswithmild,moderate,orseverepersistentallergic rhinitis.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
References
1.BousquetJ,KhaltaevN,CruzAA,DenburgJ,FokkensWJ,Toqias A,etal.Allergicrhinitisanditsimpactonasthma(ARIA)2008 update.Allergy.2008;Suppl.86:8---160.
2.PlautM,ValentineMD.Clinicalpractice:allergicrhinitis.NEngl JMed.2005;353:1934---44.
3.IbiapinaCda.C., SarinhoES,CamargosPA, AndradeCR, Cruz Filho AA. Allergicrhinitis:epidemiologicalaspects, diagnosis andtreatment.JBrasPneumol.2008;34:230---40.
4.DykewiczMS,FinemanS.Executivesummaryofjointtaskforce practiceparametersondiagnosisandmanagementofrhinitis. AnnAllergyAsthmaImmunol.1998;81:463---8.
5.LimaRG,PastorinoAC,CasagrandeRR,SoleD,LeoneC,Jacob CM. Prevalenceof asthma,rhinitisand eczemain 6---7years oldstudentsfromthewesterndistrictsofSãoPaulocity,using thestandardizedquestionnaireofthe‘‘InternationalStudyof AsthmaandAllergiesinChildhood’’(ISAAC)-phaseIIIB.Clinics. 2007;62:225---34.
6.III Consenso brasileiro sobrerinites. Braz J Otorhinolaringol. 2012;75:6---51.
7.KayAB.Allergyandallergicdiseases:secondoftwoparts.N EnglJMed.2001;344:109---13.
8.Brozek JL, Bousquet J, Baena-Cagnani CE, Bonini S, Canon-icaGW,Casale TB,et al. Allergicrhinitisand its impacton ashtma(ARIA)guidelines:2010revision.JAllergyClinImmunol. 2010;126:466---76.
9.BousquetJ,ReidJ,vanWeelC,BaenaCagnaniC,CanonicaGW, DemolyP,etal.Allergicrhinitismanagementpocketreference 2008.Allergy.2008;63:990---6.
10.Weiner JM,Abramson MJ,Puy RM.Intranasal corticosteroids versus oral H1 receptor antagonists in allergic rhinitis: systematic review of randomised controlled trials. BMJ. 1998;317:1624---9.
11.YanezA,RodrigoGJ.Intranasalcorticosteroidsversustopical H1receptorantagonistsforthetreatmentofallergicrhinitis: a systematic reviewwith meta-analysis.Ann Allergy Asthma Immunol.2002;89:479---84.
12.TaramarcazP,GibsonPG.Intranasalcorticosteroidsforasthma controlinpeoplewithcoexistingasthmaandrhinitis.Cochrane DatabaseSystRev.2003;4:CD003570.
13.PenagosM,Compalati E,TarantiniF,Baena-CagnaniCE, Pas-salacquaG,CanonicaGW.Efficacyofmometasonefuroatenasal spray in the treatment of allergic rhinitis: meta-analysis of randomized, double-blind,placebo-controlled,clinicaltrials. Allergy.2008;63:1280---91.
14.Baldwin CM, Scott LJ. Mometasone furoate: a review of its intranasaluseinallergicrhinitis.Drugs.2008;68:1723---39. 15.BarnesML,Vaidyanathan S,WilliamsonPA,Lipworth BJ.The
minimalclinicallyimportantdifferenceinallergicrhinitis.Clin ExpAllergy.2010;40:242---50.
16.MunkZM,GrossGN,HampelFCJr,RatnerPH.Preseasonal,once dailytriamcinoloneacetonidenasalaerosolforseasonalallergic rhinitis.AnnAllergyAsthmaImmunol.1997;78:325---31. 17.MeltzerEO.Clinicalandantiinflammatoryeffectsofintranasal
budesonideaqueouspumpsprayinthetreatmentofperennial allergicrhinitis.AnnAllergyAsthmaImmunol.1998;81:128---34. 18.Creticos P, Fireman P, Settipane G, Bernstein D, Casale T, SchwartzH,RhinocortAquaStudyGroup.Intranasalbudesonide aqueouspumpspray(RhinocortAqua)forthetreatmentof sea-sonalallergicrhinitis.AllergyAsthmaProc.1998;19:285---94. 19.BendeM,CarrilloT,Vona I,daCastel-BrancoMG,ArhedenL.
A randomized comparison of the effects of budesonide and mometasonefuroateaqueousnasalspraysonnasalpeakflow rate andsymptoms inperennialallergicrhinitis. AnnAllergy AsthmaImmunol.2002;88:617---23.
21.DerendorfH,MeltzerEO.Molecularandclinicalpharmacology ofintranasalcorticosteroids:clinicalandtherapeutic implica-tions.Allergy.2008;63:1292---300.
22.PettyDA,BlaissMS.Intranasalcorticosteroidstopical charac-teristics: sideeffects,formulation,and volume.AmJRhinol Allergy.2013;27:510---3.
23.Chong Neto HJ, Rosario NA, Westphal GLC, Riedi CA, San-tos HLBS. Allergic conjunctivitis in asthmatic children: as common as underreported. Ann Allergy Asthma Immunol. 2010;105:399---400.
24.LightmanS,ScaddingGK.Shouldintranasalcorticosteroidsbe usedforthetreatmentofocularsymptomsofallergic rhinocon-junctivitis?Areviewoftheirefficacyandsafetyprofile.IntArch AllergyImmunol.2012;158:317---25.
25.Hochhaus G. Pharmacokinetic/pharmacodynamic profile of mometasonefuroatenasalspray:potentialeffectsonclinical safetyandefficacy.ClinTher.2008;30:1---13.
26.MinshallE,GhaffarO,CameronL,O’Brien F,QuinnH, Rowe-Jones J,et al. Assessmentbynasal biopsy oflong-termuse ofmometasonefuroateaqueousnasalspray(Nasonex)inthe
treatmentofperennialrhinitis.Otolaryngol HeadNeckSurg. 1998;118:648---54.
27.Allergies in Latin America: a landmark survey of nasal allergy sufferers; 2010. Available from: http://www. meyallergiesinamerica.com[October2013].
28.MeltzerEO,BlaissMS,DereberyMJ,MahrTA,GordonBR,Sheth KK,etal.Burdenofallergicrhinitis:resultsfromthepediatric allergiesinAmericasurvey.JAllergyClinImmunol.2009;124 Suppl3:S43---70.
29.BlaissMS,AllergicRhinitisinSchoolchildrenConsensusGroup. Allergic rhinitis and impairment issues in schoolchildren: a consensusreport.CurrMedResOpin.2004;20:1937---52. 30.LavieP,GertnerR,ZomerJ,PodoshinL.Breathingdisordersin
sleepassociatedwith‘‘microarousals’’inpatientswithallergic rhinitis.AcataOtolaryngol.1981;92:529---33.