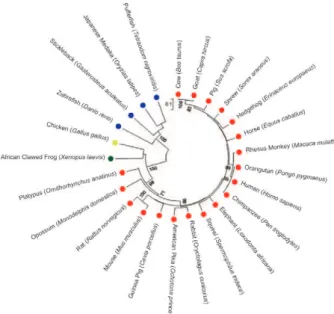
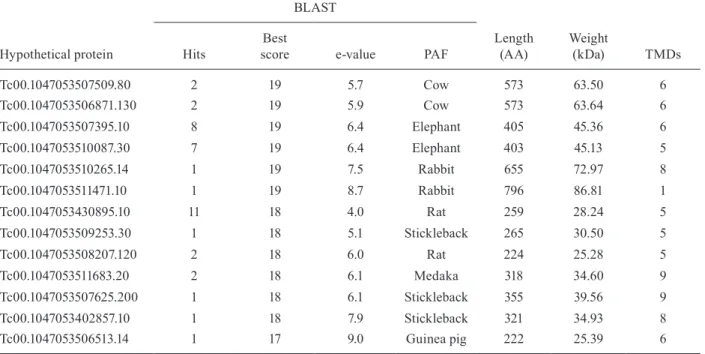
957
online | memorias.ioc.fiocruz.br
Mem Inst Oswaldo Cruz, Rio de Janeiro, Vol. 106(8): 957-967, December 2011
Search for a platelet-activating factor receptor in the
Trypanosoma cruzi
proteome: a potential target for Chagas disease chemotherapy
Daniel Fábio Kawano
1/
+, Vinicius Barreto da Silva
1, Daniel Macedo de Melo Jorge
2,
Carlos Henrique Tomich de Paula da Silva
1, Ivone Carvalho
11