w w w . r b h h . o r g
Revista
Brasileira
de
Hematologia
e
Hemoterapia
Brazilian
Journal
of
Hematology
and
Hemotherapy
Original
article
Evaluation
of
erythrocyte
and
reticulocyte
parameters
as
indicative
of
iron
deficiency
in
patients
with
anemia
of
chronic
disease
Ana
Beatriz
Barbosa
Torino,
Maria
de
Fátima
Pererira
Gilberti,
Edvilson
da
Costa,
Gisélia
Aparecida
Freire
de
Lima,
Helena
Zerlotti
Wolf
Grotto
∗UniversidadeEstadualdeCampinas(UNICAMP),Campinas,SP,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received25March2014 Accepted24July2014
Availableonline17February2015
Keywords:
Automation
Anemia,iron-deficiency Erythrocyteindices Reticulocytes Erythropoiesis
a
b
s
t
r
a
c
t
Objective:Theaimofthisstudywastoevaluatetheeffectivenessofmatureredcelland retic-ulocyteparameterstoidentifythreeconditions:irondeficiencyanemia,anemiaofchronic disease,andanemiaofchronicdiseaseassociatedwithabsoluteirondeficiency.
Methods:Peripheralbloodcellsfrom117adultpatientswithanemiawereclassified accord-ingtoironstatus,inflammation,andhemoglobinopathiesas:irondeficiencyanemia(n=42), anemiaofchronicdisease(n=28),anemiaofchronicdiseaseassociatedwithirondeficiency anemia(n=22),andheterozygous-thalassemia(n=25).Thepercentageofmicrocytic eryth-rocytes,hypochromicerythrocytes,andthelevelsofhemoglobininbothreticulocytesand matureredcellsweredetermined.Receiveroperatingcharacteristicanalysiswasusedto evaluatetheaccuracyoftheparametersindifferentiatinganemia.
Results:Therewasnodifferencebetweenthegroupsofirondeficiencyandanemiaofchronic diseaseassociatedwithabsoluteirondeficiencyforanyoftheparameters.Thepercentage ofhypochromicerythrocyteswasthebestparametertoidentifyabsoluteirondeficiency inpatientswithanemiaofchronicdisease(areaundercurve=0.785;95%confidence inter-val:0.661–0.909withsensitivityof72.7%,andspecificityof70.4%;cut-offvalue1.8%).The formulamicrocyticerythrocytecountminushypochromicerythrocytecountwasvery accu-ratetodifferentiateirondeficiencyanemiafromheterozygous-thalassemia(areaunder curve=0.977;95%confidenceinterval:0.950–1.005withasensitivityof96.2%,andspecificity of92.7%;cut-offvalue13.8).
Conclusion: Theerythrocyteandreticulocyteindicesaremoderatelygoodtoidentifyabsolute irondeficiencyinpatientswithanemiaofchronicdisease.
©2015Associac¸ãoBrasileiradeHematologia,HemoterapiaeTerapiaCelular.Published byElsevierEditoraLtda.Allrightsreserved.
∗ Correspondingauthor at:DepartamentodePatologiaClínica,Faculdadede CiênciasMédicas,UniversidadeEstadualdeCampinas
(UNICAMP),13083-970Campinas,SP,Brazil.
E-mailaddress:lenagrotto@gmail.com(H.Z.W.Grotto).
http://dx.doi.org/10.1016/j.bjhh.2015.02.004
Introduction
New automated blood cell analyzers can provide informa-tionaboutindividualcellcharacteristics,suchashemoglobin contentofreticulocytes,hemoglobincontentofmature eryth-rocytes, and percentages of microcytic erythrocytes and hypochromic cells. These parameters have been used in thediagnosisofirondeficiencyanemia(IDA),-thalassemia (-Thal),1–3andanemiaofchronicdisease(ACD).4,5The
differ-entiationbetweenthesethreeconditionsisveryimportantas theclinicalapproachisdifferentineachparticulardiagnosis. Asreticulocyteshaveanormallifespanofoneortwodays, information concerning the hemoglobin content of young red cells is a good indication of iron availability and an earlymarkerofiron-deficiencyerythropoiesis.6 Reticulocyte
hemoglobin equivalent (Ret-He) reflects real-time informa-tiononthesynthesisbyyoungerythrocytesinbonemarrow. Otheravailableparametersarethepercentageofredcellswith Hbcontentequivalenttoorlessthan17pg(%HypoHe),and thepercentageofredcellswithavolumelessthan60fL(% MicroR),1whichcorrespondstoasub-populationofmature
redcellsexhibitingevidenceofinsufficientironcontent. A mathematical formula using %MicroR and %HypoHe (MHe) proposed by Urrechaga et al.7 tested discriminant
indices in healthy individuals, -Thal and IDA patients. A sensitivityof97.4%andspecificityof97.1%wasreportedin differentiating-ThalfrommildIDA.
Anemiaassociatedwithchronicinflammation,infection ormalignancyisthemostcommonanemiainhospitalized patients.Althoughstainableironispresentinbonemarrow, elevatedlevelsofinflammatorycytokinesinterferein erythro-poiesisleadingtoahyporegenerativeanemiaanddefective ironincorporationintoerythrocyteprogenitors.Reduced con-centrationsofcirculatingironandnormalorincreasediron storescharacterizethestateoffunctionalirondeficiency.8
Anemia of inflammation can be associated with abso-luteirondeficiency (ACDcombi) generallyinpatients with inflammatorydiseaseandchronicbloodloss.Differentiation betweenACDandACDcombiisclinicallyimportant,butinthe clinicalpractice,differentiationisdifficultusingconventional biomarkers such as ferritin concentration and transferrin saturation.9Thesolubletransferrinreceptor/logferritinratio
maybeusefulindistinguishingACDfromACDcombi.10
Theaimofthisstudywastoanalyzetheeffectivenessof newlaboratoryparametersrelatedtomatureredbloodcells andreticulocytestodifferentiatethreeconditionsrelatedto irondeficiency:IDA,ACDandACDcombi.Moreover,the per-formanceoftheseparameterswastestedtodistinguishIDA from-Thal,twocommoncausesofmicrocyticanemia.
Methods
ThisprojectwasapprovedbytheEthicsCommitteeofthe Fac-ultyofMedicalSciences,UniversidadeEstadualdeCampinas (UNICAMP),SãoPaulo,Brazil.Allsampleswereselectedfrom routinecollectionsandinformedconsentwaswaived.
Peripheral blood samples from 117 adult patients with anemia(Hb<12.0g/dLforwomenandHb<14.0g/dLformen)
wereselectedfromroutineworkload.Bloodanalysishadbeen requested bygeneralpractitioners, ingeneraltoinvestigate anemia.
Patients were classified according to iron status analy-sis(commercialkitsfrom RocheDiagnosticsGermany):IDA when serum iron (SI) levels were <45mg/dL for men and <30mg/dLforwomen;percentagetransferrinsaturation(%TS) <15%;serum ferritin(SF) <30g/Lformenand<13g/Lfor women.
PatientswereclassifiedasACDwhenSIlevelswere nor-malorreduced(40–160mg/dLand30–160mg/dLformenand women, respectively), %TS normal or decreased (30–50%), normal orhigh SF (30–400g/Land 13–150g/formen and women,respectively)andC-reactiveprotein(C-RP)>5mg/dL (Tina-QuantC-ReactiveProtein,RocheDiagnostics,Germany). Soluble transferrin receptor (sTfR) levels (Roche Diag-nostics, Germany) were measured in all samples, and the sTfR/logferritinratiowasusedtoidentifyirondeficiencyin patientswithACD.PatientswithACDhavingasTfR/log fer-ritinratio>2.06orsTfR>3.71g/mL(cut-offvaluesindicative ofirondeficiency inourlaboratory)were classifiedasACD combi.
Twenty-sixpatientshadthediagnosisof-Thalaccording tothehemoglobinA2leveldeterminedbyhighperformance liquidchromatography(HPLC-VariantII–HemoglobinTesting System,Bio-RadLaboratories,Inc.,CA,USA).
Patientswith-Thalassociatedwithotherkindsofanemia, patientswithreticulocytosisorpancytopenia,individualswho hadreceivedtransfusionswithinthepreviousthreemonths, andpatientsonironreplacementtherapywereexcludedfrom thestudy.
Acontrolgroup(CG)wascompoundedbyhealthy individ-ualswithnoclinicalsignsorsymptomsofdisease,including acute inflammatory/infectious conditions, normal hemato-logic findings, and C-RP<5mg/L. Healthy individuals were studentsorlaboratorystaff,allofwhomdonatedblood sam-plesonavoluntarybasis.
Determinationoferythrocyteandreticulocyteparameters wasperformedusingtheSysmexXE-5000automated hema-tologicalanalyzer(Sysmex,Kobe,Japan),whichprovidesthe followingparameters:Ret-He,%MicroR,hemoglobincontent oferythrocytesobtainedfromtheopticalcountingofredblood cells(RBC-He),and%HypoHe.TheMHeindexwascalculated as:%MIcroR-%HypoHe.7
Mann–Whitney test was applied to compare groups. Receiver operatorcharacteristicanalysis(ROC) wasusedto evaluate the accuracy of the parameters to differentiate betweentypesofanemia. Thelevelofsignificancewas set forap-value<0.05.DatawereanalyzedusingtheSPSS statis-ticsprogramforWindows(version13.0.SPSSInc.Chicago,IL, USA).
Results
Accordingtoadoptedcriteria, individualswereclassifiedas IDA (42 patients), -Thal (25 individuals), ACD combi (22 patients),ACD(28patients)andCG(54individuals).
Table1–Demographiccharacteristicsandhematologicaldataofpatientsandthecontrolgroup.
Parameter IDA
(n=42)
-Thal (n=25)
ACDcombi (n=22)
ACD (n=28)
CG (n=54)
Age(years) 42.0a,b
(16.0–82.0)
54.0a
(15.0–76.0)
65.6b
(20.0–81.0)
51.0 (17.0–82.0)
41.0 (16.0–75.0)
Gendermale/female(%) 26.2/73.8 66.7/33.3 42.9/57.1 63.6/36.4 35.8/62.3
Hb(g/dL) 10.1
(6.2–13.6)
12.0 (6.9–14.7)
9.9 (7.3–12.2)
9.5 (6.6–12.8)
13.7 (12.0–16.1)
MCV(fl) 75.2
(61.2–79.6)
65.7 (58.4–70.8)
74.4 (58.5–88.9)
81.1 (61.4–95.2)
87.9 (80.5–94.8)
MCH(pg) 22.5
(17.0–26.1)
20.4 (18.4–24.4)
24.2 (18.4–28.9)
26.8 (19.6–32.0)
29.1 (25.4–32.2)
RDW(%) 16.4
(13.4–21.0)
16.3 (14.0–19.0)
16.6 (13.7–28.2)
15.5 (13.3–25.6)
13.2 (12.0–15.5)
Valuesasmedians,minimumandmaximum.
IDA:irondeficiencyanemia:-Thal:heterozygousbeta-thalassemia;ACDcombi:anemiaofchronicdiseaseassociatedwithabsoluteiron deficiency;ACD:anemiaofchronicdisease;CG:controlgroup;Hb:hemoglobin;MCV:meancellvolume;MCH:meancellhemoglobin;RDW:red celldistributionwidth.
a p-value=0.030.
b p-value=0.023.
Table2–Biochemicaldata.
Parameters IDA
(n=42)
-Thal (n=25)
ACDcombi (n=22)
ACD (n=28)
CG (n=54)
SI (mg/dL) 24.5
(7.0–73.0)
94.0 (49.0–224.0)
23.5 (12.0–99.0)
28.0 (9.0–98.0)
100.0 (49.0–185.0)
TS(%) 6.1
(1.5–15.5)
31.9 (22.4–68.2)
9.2 (4.5–25.7)
11.6 (3.0–42.2)
29.5 (17.6–50.1)
SF(g/L) 8.2
(3.2–23.4)
224.5 (66.8–1619.0)
254.5 (27.8–2000.0)
163.7 (42.1–2000.0)
59.6 (19.7–407.9)
sTfR(g/mL) 9.4
(1.4–28.8)
7.4 (2.6–25.5)
7.0 (3.5–22.4)
2.7 (0.5–6.2)
2.4 (1.6–3.8) sTfR/logferritin 10.4
(1.9–40.9)
2.6 (1.3–10.9)
3.4 (1.3–9.6)
1.1 (0.2–3.8)
1.3 (0.8–2.2)
Valuesasmedians,minimumandmaximum.
IDA:irondeficiencyanemia:-Thal:heterozygousbeta-thalassemia;ACDcombi:anemiaofchronicdiseaseassociatedwithabsoluteiron deficiency;ACD:anemiaofchronicdisease;CG:controlgroup;SI:serumiron,TS:transferrinsaturation;SF:serumferritin;sTfR:soluble transferrinreceptor.
theironstatusmeasurementsusedtoclassifythepatientsin differentgroups.
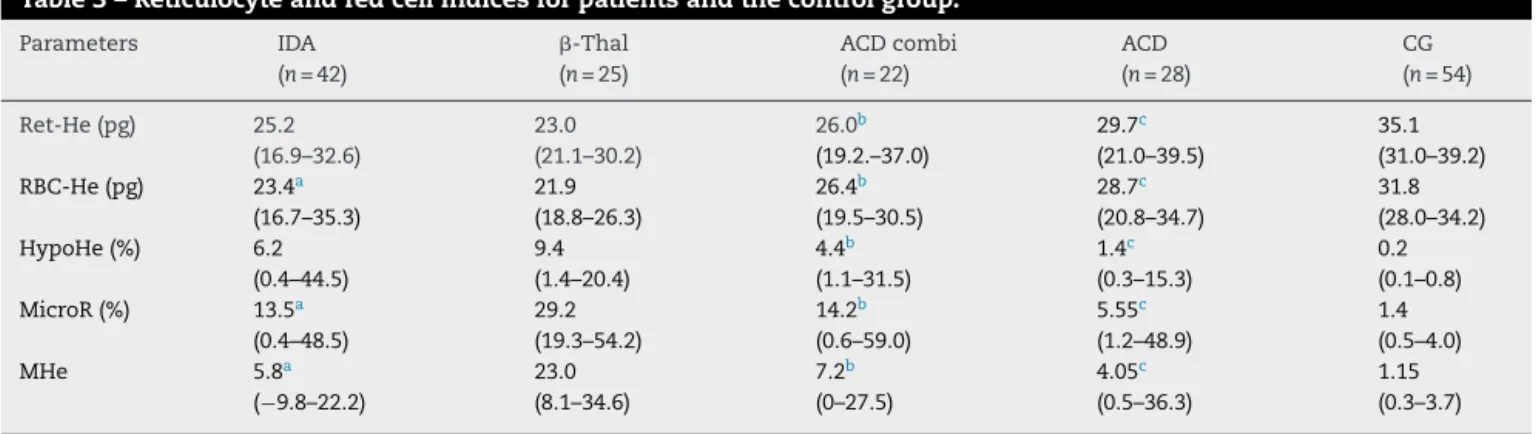
Asexpected the -Thal grouphad the highest %MicroR (Table3).The%HypoHewashigherinthe-Thalgroup com-paredtoothergroups exceptforIDA patients. However,as themicrocyticcellsweremoreabundantinthe-Thalgroup, whenthesetwoparameterswereassociatedintheMHeindex, thisdifferencebecamemoreevidentandstatistically signifi-cant.
TheMann–Whitneytestidentifiednodifferencebetween the IDA and ACDcombi groups inrespectto any parame-ter.WhentheACDandACDcombigroupswerecompared, theRBC-HeandRet-HeweresignificantlylowerinACDcombi patients(p-value=0.016andp-value=0.003,respectively). Fur-thermore, the %MicroR and %HypoHe were significantly higher in ACD combi patients, as was the MHe index (p -value=0.001,p-value=0.003andp-value=0.014,respectively). Although the ACD group had a sTFR/log ferritin ratio below the cut-off indicative of iron deficiency, the Ret-He and RBC-He values were significantly lower than the CG
whereasthe%HypoHe,%MicroRandMHeindexwerehigher (p-value<0.001forallcomparisons).
ThebesttestfordifferentiatingIDAfrom-Thalwasthe MHe index (areaunder curve [AUC]:0.977; 95% confidence interval[95/%CI]0.950–1.005).Acut-offof13.8wasvery sen-sitive (96.2%) and specific (92.7%) to identify IDA patients. The parameter %MicroR was effective (AUC 0.886: CI: 95% 0.810–0.963) to detect iron deficiency with sensitivity and specificityforvalues<25.0%of84.6%and78.0%,respectively.
The ROC curve for %HypoHewas the mosteffective to differentiatebetweenACDandACDcombialthoughwitha moderateAUC(0.785:CI:95%0.661–0.909),sensitivity72.7%, andspecificity71.4%(cut-off1.8%).
Table3–Reticulocyteandredcellindicesforpatientsandthecontrolgroup.
Parameters IDA
(n=42)
-Thal (n=25)
ACDcombi (n=22)
ACD (n=28)
CG (n=54)
Ret-He(pg) 25.2 (16.9–32.6)
23.0 (21.1–30.2)
26.0b
(19.2.–37.0)
29.7c
(21.0–39.5)
35.1 (31.0–39.2) RBC-He(pg) 23.4a
(16.7–35.3)
21.9 (18.8–26.3)
26.4b
(19.5–30.5)
28.7c
(20.8–34.7)
31.8 (28.0–34.2) HypoHe(%) 6.2
(0.4–44.5)
9.4 (1.4–20.4)
4.4b
(1.1–31.5)
1.4c
(0.3–15.3)
0.2 (0.1–0.8) MicroR(%) 13.5a
(0.4–48.5)
29.2 (19.3–54.2)
14.2b
(0.6–59.0)
5.55c
(1.2–48.9)
1.4 (0.5–4.0)
MHe 5.8a
(−9.8–22.2)
23.0 (8.1–34.6)
7.2b
(0–27.5)
4.05c
(0.5–36.3)
1.15 (0.3–3.7)
Valuesasmedians,minimumandmaximum. Mann–Whitneytestwasappliedtocomparegroups.
IDA:irondeficiencyanemia:-Thal:heterozygousbeta-thalassemia;ACDcombi:anemiaofchronicdiseaseassociatedwithabsoluteiron deficiency;ACD:anemiaofchronicdisease;CG:controlgroup;Ret-He:reticulocytehemoglobincontent;RBC-He:redbloodcellhemoglobin content;HypoHe:percentageofhypochromicerythrocytes;MicroR:percentageofhypochromicerythrocytes;MHe:MicroR-HypoHeindex. a p-value<0.01(IDA×-Thal).
b p-value≤0.01(ACD×ACDcombi).
c p-value<0.001(ACD×CG).
Thecapacityofthe teststo discriminateIDA from ACD combiwasnotsatisfactoryandtheAUCwaslessthan0.700 foralltheseparameters.
Discussion
Thediagnosticaccuracyofreticulocyteparametershasbeen tested by many authors, especially to diagnose iron defi-ciencyinpatientssubmittedtodialysis.6,10,11Measurement
ofthereticulocytecontentishelpfulindetectingearlystages ofirondeficiencypriortothe developmentofanemia.6,12,13
Areduction inreticulocyte hemoglobin hasbeen observed in other conditions besides iron deficiency, such as in hemoglobinopathies.14,15
Theeffectivenessofreticulocyteparameterstodiagnose IDAandACDwasreportedinotherresearch.2,8,16Inarecent
study with geriatric patients, the authors concluded that Ret-Heisno betterthanclassic indices,suchasmean cell hemoglobinandmeancellhemoglobinconcentration,in dif-ferentiatingbetweenIDAandACD.5Ourresultsshowedthat,
althoughtheRet-Hevaluewaslower inIDAthan ACD,the accuracy of the test to distinguish both types of anemia wasmoderate,andlesseffectivecomparedto%HypoHe.The potentialofRet-Hewasdemonstratedinastudywithpatients withchronicrheumaticdiseaseandanemia.Thepredictive valueofRetHewas testedinresponsetooralirontherapy and,accordingtotheauthors,thefindingssupporttherole ofRet-Heasamarkerforironresponsiveness.17
Additionalextendedredbloodcellparametersbesides Ret-Hehavebeen tested, suchas%MicroR,and %HypoHe,and indicesgeneratedbycombiningthem.18Theresultsregarding
the differentiation betweenIDA and -Thal are promising, althoughtheoptimumcut-offvariesaccordingtothe popu-lationstudiedandthecriteriaadoptedtoclassifyanemia.18,19
TheMHeindexwasfirstproposedandtestedbyUrrechaga etal.16Theperformanceofthisindexwasbetter(sensitivity
98.0%andspecificity95.9)thanotherpublishedindices.The
authorssuggestthatsampleswithMHevalues>11.5canbe chosenforfurtheranalysistoconfirmthediagnosisof tha-lassemia;thesedataarecoincidentwithourresults.TheMHe indexwasthebestparametertodiscriminateIDAfrom-Thal, althoughthecut-offvalueusedwasdifferentfromthevalue describedbyUrrechagaetal.16probablybecausepatientswere
notstratifiedaccordingtotheseverityofanemia.
As far as we know, no other reports exist about the efficiency of expanded erythrocyte parameters in identify-ing patientswith anemia ofinflammation associatedwith absolute iron deficiency. The clinical usefulness of the determinationof%Hypohaslongbeenrecognizedin differ-entiatingbetweeniron-deficientandiron-sufficientpatients withchronickidneydiseasereceivingerythropoietin stimu-latingagents.20
ThecurrentstudyappliedthesTfR/logferritinratioto iden-tifyirondeficiencyinpatientswithACD.Measurementofthe sTfRisconsideredagoodindicatoroffunctionalironstatus, asitdoesnotsufferanyinfluenceofsystemicinflammation unlike the SI, transferrin,and ferritin levels.21,22 Thus, the
transferrinreceptorsynthesisisstimulatedwhenthereisa reductionoffunctionalironasshownbytheresultsofthis study.IrondeficiencyischaracterizedbyanincreaseinsTfR levelsandlowferritinvalues,whileinanemiaof inflamma-tion,transferrinreceptorlevelsareslightlyaffectedwhereas serumferritinincreasesgreatly.23Intheclinicalpracticethis
erythropoiesis,but thedisturbanceof theiron metabolism infunctionalirondeficiencywaslessremarkablethaninthe associationofACDwithIDA.
Inpractical terms the incorporation ofnewcell indices can speed up the diagnosis of IDA, -Thal and ACD, and consequentlytargetmorequicklyandmorepreciselythe sub-sequentconfirmatoryexamsinordertointroduceappropriate treatment. On the other hand,the difficulty inidentifying absoluteirondeficiencyinpatientswithinflammatory con-ditionsremains.Therefore,thechallengepersists,andother studiesareneededtoidentifyaparameterwithclinical deci-sionvalue.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
r
e
f
e
r
e
n
c
e
s
1. UrrechagaE,BorqueL,EscaneroJF.Erytrocyteand
reticulocyteparametersinirondeficiencyandthalassemia.J ClinLabAnal.2011;25(3):223–8.
2. CanalsC,RemachaAF,SardáMP,PiazueloJM,RoyoT,Romero A.ClinicalutilityofthenewSysmexXE-2100parameter– reticulocytehemoglobinequivalent–inthediagnosisof anemia.Haematologica.2005;90(8):1133–4.
3. SudmannAA,PiehlerA,UrdalP.Reticulocytehemoglobin equivalenttodetectthalassemiaandthalassemic hemoglobinvariants.IntJLabHematol.2012;34(6):605–13.
4. MarkovicM,Majkic-SinghN,IgnjatovicS,SinghS. Reticulocytehaemoglobincontentvs.solubletransferrin receptorandferritinindexinirondeficiencyanemia accompaniedwithinflammation.IntJLabHematol. 2007;29(5):341–6.
5. JoostenE,LioenP,BrusselmansC,IndevuystC,BoeckxN.Is analysisofthereticulocytehaemoglobinequivalentauseful testfordiagnosisofirondeficiencyanemiaingeriatric patients?EurJIntMed.2013;24(1):63–6.
6. BrugnaraC,SchillerB,MoranJ.Reticulocytehemoglobin equivalent(RetHe)andassessmentofiron-deficientstates. ClinLabHaematol.2006;28(5):303–8.
7. UrrechagaE,BorqueL,EscaneroJF.Theroleofautomated measurementofredcellsubpopulationsontheSysmexXE 5000analyzerinthedifferentialdiagnosisofmicrocytic anemia.IntJLabHematol.2011;33(1):30–6.
8. ThomasC,ThomasL.Anemiaofchronicdisease:pathology andlaboratorydiagnosis.LabHematol.2005;11(1):14–23.
9. MastA.Theclinicalutilityofperipheralbloodtestsinthe diagnosisofirondeficiencyanemia.Bloodline.2001;1:7–9.
10.TheurlI,AgnerE,TheurlM,NairzM,SeiferM,SchrollA,etal. Regulationofironhomeostasisinanemiaofchronicdisease andirondeficiencyanemiadiagnosisandtherapeutic implications.Blood.2009;113(21):5277–86.
11.FishbaneS,ShapiroW,DuktaP,ValenzuelaOF,FaubertJ.A randomizedtrialofirondeficiencytestingstrategiesin hemodialysispatients.KidneyInt.2001;60(6):
2406–11.
12.WaradyBA,KauszA,LernerG,BrewerED,ChadhaV,Brugnara C,etal.Irontherapyinthepediatrichemodialysis
population.PediatrNephrol.2004;19(6):655–61.
13.BrugnaraC.Reticulocytecellularindices:anewapproachin thediagnosisofanemiasandmonitoringoferythropoietic function.CritRevClinLabSci.2000;37(2):93–130.
14.StoffmanN,BrugnaraC,WoodsER.Analgorithmusing reticulocytehemoglobincontent(CHr)measurementin screeningadolescentsforirondeficiency.JAdolescHealth. 2005;36(6):529.
15.ChouliarasGL,StamoulakatouA,TsiftisG,PerissakiG, PremetisE,LycopoulouL.Age,betathalassaemiatrait,and iron-deficiencyanaemiasignificantlyaffectreticulocyte indicesinpre-schoolchildren.EurJPediatr.
2010;169(9):1097–104.
16.UrrechagaE,BorgesL,EscaneroJF.Analysisofreticulocyte parametersontheSysmexXE5000andLH750analyzersin thediagnosisofineficienteerythropoiesis.IntJLabHematol. 2011;33(1):37–44.
17.vanSantenS,deMastQ,OostingJD,vanEdeA,SwinkelsDW, vanderVenAJ.Hematologicalparameterspredictinga responsetooraltherapyinchronicinflammation. Haematologica.2014;99:e171–3.
18.UrrechagaE,BorqueL,EscaneroJF.Potentialutilityofthenew SysmexXE5000redbloodcellextendedparametersinthe studyofdisordersofironmetabolism.ClinChemLabMed. 2009;47(11):1411–6.
19.SchoorlM,SchoorlM,LinssenJ,VillanuevaMM,NoGuerraJA, MaritnezPH,etal.Efficacyofadvanceddiscriminant algorithmsforscreeningoniron-deficiencyanemiaand -thalassemiatrait:amulticentricevaluation.AmJClin Pathol.2012;138(2):300–4.
20.TessitoreN,SoleroGP,LippiG,BassiA,FacciniGB,BedognaV, etal.Theroleofironstatusmarkersinpredictingresponseto intravenousironinhaemodialysispatientsonmaintance erythropoietin.NephrolDialTransplant.2001;16(7): 1416–23.
21.PunnonenK,IrjalaK,RajamakiA.Serumtransferrinreceptor anditsratiotoserumferritininthediagnosisofiron deficiency.Blood.1997;89(3):1052–7.
22.MalopeBI,MacPhailAP,AlbertsM,HissDC.Theratioof serumtransferrinandserumferritininthediagnosisofiron status.BrJHaematol.2001;115(1):84–9.