2017 | Lavras | Editora UFLA | www.editora.ufla.br | www.scielo.br/cagro
http://dx.doi.org/10.1590/1413-70542017413000117
REVIEW
Portable X-ray fluorescence (pXRF) applications in tropical Soil Science
Aplicações da fluorescência de raios-X portátil (pXRF) na Ciência do Solo tropicalBruno Teixeira Ribeiro1*, Sérgio Henrique Godinho Silva1, Elen Alvarenga Silva1, Luiz Roberto Guimarães Guilherme1
1Universidade Federal de Lavras/UFLA, Departamento de Ciência do Solo/DCS, Lavras, MG, Brasil
*Corresponding author: brunoribeiro@dcs.ufla.br
Received in March 10, 2017 and approved in May 26, 2017
ABSTRACT
The X-ray fluorescence (XRF) is an analytical technique for determination of elemental composition of different materials. In soils, the XRF has many pedological, environmental and agronomic applications, mainly after the emergence of portable equipments (pXRF). This technique has been recently adopted and successfully used for soil characterization worldwide, but very rare works have been carried out in soils of developing countries. The soil characterization includes the complete elemental composition determination (nutrients, trace and rare-earth elements) and allows estimating some soil physical and chemical properties. In Brazil, this technique is still incipient, mainly the use of pXRF, however, it can greatly contribute to soil characterization in-field or in-lab conditions and also replacing methods of soil analyses considered non-environmentally friendly. This review summarizes the XRF technique including principles and the main applications of pXRF in soils highlighting its potential for tropical Soil Science.
Index terms: Soil analyses; soil morphometrics; soil characterization.
RESUMO
Fluorescência de raios-X (FRX) é uma técnica analítica para determinação da composição elementar de diferentes materiais. Em solos, a FRX apresenta muitas aplicações pedológicas, ambientais e agronômicas, principalmente após a emergência de equipamentos portáteis (pXRF). Essa técnica tem sido utilizada com sucessso no mundo todo para caracterização do solo, entretanto, são raros os trabalhos em solos de países em desenvolvimento. A caracterização do solo inclui a determinação completa da composição elementar (nutrientes, elementos-traço e terras-raras) e permite a estimativa de atributos químicos e físicos do solo. No Brasil, a FRX é ainda incipiente, principalmente o uso do pXRF, entretanto, essa técnica pode contribuir grandemente para a caracterização do solo no campo, em condições laboratoriais e, também, substituindo alguns métodos de análise do solo considerados não prejudicial ao ambiente. Esta revisão sumariza a técnica de FRX incluindo princípios e as principais aplicações do pXRF, destacando seu potencial
de uso na Ciência do Solo tropical.
Termos para indexação: Análise do solo; morfometria do solo; caracterização do solo.
INTRODUCTION
Historically, in Soil Science the analyses of soil physical, chemical and mineralogical properties are characterized by using a great amount of chemical reagents, being time-consuming, costly and, in most of cases, non-environmentally friendly methods since they generate chemical residues. However, soil analyses are required for more detailed soil characterization and, hence, development of several soil-related studies.
The X-ray fluorescence (XRF) is a technique capable of identifying and quantifying elemental composition of several solid materials with the use of X-rays, allowing for a chemical characterization of the
analyzed material and correlation with other properties (Gazley; Fisher, 2014; Weindorf et al., 2014a). Atoms that compose the structure of the analyzed materials are reached by high energy X-rays, promoting a dislodgement of electrons from their original orbit. As the electrons return to their original orbit, they emit energy in fluorescence form that is characteristic of each element. Thus, the elements present in the analyzed materials can be identified (Weindorf et al., 2014a).
Ciência e Agrotecnologia 41(3):245-254, May/Jun. 2017
246 RIBEIRO, B. T. et al.
materials (Radu; Diamond, 2009; Zhu; Weindorf; Zhang, 2011; Bastos; Melquiades; Biasi, 2012; Weindorf et al., 2012a; Weindorf et al., 2012b Weindorf et al., 2014a; Weindorf et al., 2014b; Stockmann et al., 2016a, 2016b).
Worldwide, the pXRF has been recently adopted and successfully used for pedological, agronomic and environmental purposes (Weindorf et al., 2014a), showing adequate correlations to other standard laboratory methods (Weindorf et al., 2012b). In USA, the US Environmental Protection Agency (US-EPA, 2007) recognized the pXRF for the determination of elemental concentrations in soils and sediments (method 6200) and the USDA established the protocol method for using it (Soil Survey Staff, 2014). More recently, Weindorf and Chakraborty (2016) published the chapter Portable X-ray fluorescence spectrometry analysis of soils in the Methods of Soil Analysis by the Soil Science Society of America. A complete historical description about XRF and pXRF can be found in Weindorf et al. (2014a).
In Brazil, the use of pXRF equipments for in-field or in-lab soil characterization is still incipient and needs further studies in order to assess the effects of different tropical soil conditions on pXRF results, such as soil moisture, particle size distribution, structure, mineralogy and organic matter content. One of the available works with pXRF in Brazil is that of Terra et al. (2014), who checked the accuracy of pXRF using some certified reference standard materials for soil characterization purposes and found significant and good correlation (R2 >
0.90) for K, Ca, Mn, Fe and Si. However, until now, pXRF has not been applied in natural conditions to Brazilian soils characterization. Wastowski et al. (2010), assessed the soil elemental composition using energy dispersive X-ray fluorescence spectrometry (EDXRF). This technique was sensible to detect the effect of different land use and management. Simabuco and Nascimento Filho (1994) used EDXRF in a Red Latosol and Red Yellow Argisol under influence of vinasse. A complete elemental composition distribution down soil profile was obtained. Using XRF technique it was possible to study the pedogeochemical processes and mobility of trace and rare-earth elements in a lithochronosequence of soils from Lavras, Minas Gerais State, Brazil (Lacerda; Andrade; Quéméneur, 2002).
For the following 30 years, Brazil has a great challenge to mapping its territory in a large-scale (1:25,000) through the Brazilian Soils National Program – PronaSolos (Embrapa, 2016). Undoubtedly, the pXRF can substantially contribute to rapid, accurate, low cost, in-field and environmentally
friendly analyses of soils, giving insights of their properties after tests with Brazilian soils have been carried out.
This review summarizes pXRF technique including principles and some pedological, agronomical and environmental applications, highlighting the opportunities for tropical soils characterization.
PRINCIPLES OF X-RAY FLUORESCENCE ANALYSES
X-ray fluorescence (XRF) spectroscopy is a technique that has been widely used in industrial activities that require rapid analytical routines to control the quality of products (Santos et al., 2013). According to Weindorf et al. (2014a), currently XRF spectroscopy is the most applied X-ray technique in environmental sciences, mining, chemistry, metallurgy, archeology, Soil Science and agronomy. XRF offers some advantages over other methods of determining chemical elements, as it is inexpensive, fast, requires minimal or no sample preparation, does not generate an environmental liability and is non-destructive, besides determining simultaneously or sequentially the concentration of several chemical elements (Weindorf et al., 2014a).
The XRF is based on the measurement of the intensities of X-ray emissions characteristic of each chemical element that makes up the sample. For this emission to occur, the sample must receive enough energy to excite the electrons of the elements that compose the sample. The technique requires that species be irradiated with very energetic photons and the excitation of the atom happens when it is struck by particles such as electrons, protons or ions produced in particle accelerators, electromagnetic waves or through X-ray tubes of Ta/ Au, Rh or Ag (Kalnicky and Singhvi, 2001; Gazley and Fisher, 2014; Sharma et al., 2014; Weindorf et al., 2014a), this latter being the most common process.
247
Ciência e Agrotecnologia 41(3):245-254, May/Jun. 2017
E Kα1 Kβ1 Lα1 Lβ1 E Kα1 Kβ1 Lα1 Lβ1 E Kα1 Kβ1 Lα1 Lβ1 Mα1 Mβ1
Be 0.108 --- --- --- Se 11.224 12.497 1.379 1.419 Tb 44.482 50.385 6.273 6.975 1.240 1.269
B 0.183 --- --- --- Br 11.924 13.292 1.481 1.526 Dy 45.999 52.113 6.498 7.248 1.293 1.325
C 0.277 --- --- --- Kr 12.648 14.112 1.585 1.636 Ho 47.547 53.877 6.720 7.526 1.348 1.383 N 0.392 --- --- --- Rb 13.396 14.961 1.692 1.751 Er 49.128 55.674 6.949 7.811 1.404 1.448 O 0.525 --- --- --- Sr 14.165 15.835 1.806 1.871 Tm 50.742 57.505 7.180 8.102 1.462 1.503
F 0.677 --- --- --- Y 14.958 16.739 1.924 1.998 Yb 52.388 59.382 7.416 8.402 1.526 1.573 Ne 0.849 --- --- --- Zr 15.775 17.668 2.044 2.126 Lu 54.070 61.290 7.655 8.710 1.580 1.630 Na 1.040 --- --- --- Nb 16.615 18.625 2.169 2.260 Hf 55.790 63.244 7.899 9.023 1.646 1.700 Mg 1.254 1.302 --- --- Mo 17.480 19.606 2.292 2.394 Ta 57.535 65.222 8.146 9.343 1.712 1.770 Al 1.486 1.557 --- --- Tc 18.367 20.626 2.423 2.535 W 59.318 67.244 8.398 9.672 1.775 1.838
Si 1.740 1.837 --- --- Ru 19.279 21.656 2.558 2.683 Re 61.141 69.309 8.652 10.010 1.843 1.906
P 2.010 2.139 --- --- Rh 20.216 22.724 2.697 2.834 Os 63.000 71.414 8.911 10.354 1.907 1.978
S 2.309 2.465 --- --- Pd 21.177 23.818 2.838 2.990 Ir 64.896 73.560 9.175 10.708 1.980 2.052
Cl 2.622 2.812 --- --- Ag 22.163 24.941 2.983 3.150 Pt 66.831 75.570 9.442 11.071 2.050 2.127
Ar 2.958 3.190 --- --- Cd 23.173 26.093 3.133 3.315 Au 68.806 77.982 9.713 11.443 2.123 2.203 K 3.314 3.590 --- --- In 24.210 27.275 3.286 3.487 Hg 70.818 80.255 9.989 11.824 2.195 2.281
Ca 3.692 4.013 0.341 0.345 Sn 25.271 28.485 3.444 3.663 TI 72.872 82.573 10.269 12.213 2.271 2.363
Sc 4.093 4.464 0.395 0.400 Sb 26.359 29.725 3.604 3.842 Pb 74.970 84.939 10.551 12.614 2.342 2.444
Ti 4.512 4.933 0.452 0.458 Te 27.473 30.993 3.768 4.029 Bi 77.107 87.349 10.839 13.023 2.423 2.526
V 4.953 5.428 0.510 0.518 I 28.612 32.294 3.938 4.221 Po 79.291 89.803 11.131 13.446 2.499 2.614
Cr 5.415 5.947 0.572 0.582 Xe 29.775 33.620 4.110 4.418 At 81.516 92.304 11.427 13.876 2.577 2.699 Mn 5.900 6.492 0.637 0.648 Cs 30.973 34.982 4.285 4.619 Rn 83.785 94.866 11.727 14.315 2.654 2.784
Fe 6.405 7.059 0.705 0.718 Ba 32.194 36.378 4.466 4.828 Fr 86.106 97.474 12.031 14.771 2.732 2.868
Co 6.931 7.649 0.775 0.790 La 33.442 37.797 4.647 5.038 Ra 88.478 100.130 12.339 15.236 2.806 2.949 Ni 7.480 8.267 0.849 0.866 Ce 34.720 39.256 4.839 5.262 Ac 90.884 102.846 12.652 15.713 2.900 3.051 Cu 8.046 8.904 0.928 0.947 Pr 36.027 40.749 5.035 5.492 Th 93.351 105.605 12.968 16.202 2.996 3.149
Zn 8.637 9.570 1.012 1.035 Nd 37.361 42.272 5.228 5.719 Pa 95.868 108.427 13.291 16.703 3.082 3.240 Ga 9.251 10.267 1.098 1.125 Pm 38.725 43.827 5.432 5.961 U 98.440 111.303 13.614 17.220 3.171 3.336
Ge 9.886 10.982 1.188 1.218 Sm 40.118 45.414 5.633 6.201 Np 101.059 114.234 13.946 17.751 3.250 3.435
As 10.543 11.726 1.282 1.317 Eu 41.542 47.038 5.849 6.458 Pu 103.734 117.228 14.282 18.296 3.339 3.534
Gd 42.996 48.695 6.053 6.708 Am 106.472 120.284 14.620 18.856 3.438 3.646
Ciência e Agrotecnologia 41(3):245-254, May/Jun. 2017
248 RIBEIRO, B. T. et al.
Kα1 and Kα2 represent the electron transition energy from shell LII to K and from LIII to K, respectively (Figure 1). Kβ1 corresponds to electron transition from MIII shell to K. Lα1 and Lα2 energies correspond to the transition from MV to LIII and from MIV to LIII, respectively, and Lβ1 corresponds to the transition from MIV to LII shell. Different manufactured pXRF and their descriptions were summarized and can be found in Weindorf et al. (2014a).
The X-ray technique is widely used in the world in various fields of science. X-ray diffraction (XRD), widely used in Soil Science, uses the dispersion technique and determines the crystalline composition of minerals in soils and sediments, whereas XRF is an example of an X-ray absorption/emission technique used for determine the chemical composition of the sample (Kalnicky; Singhvi, 2001; Gazley; Fisher, 2014; Weindorf et al., 2014a). There are two types of XRF available today: wavelength dispersion X-ray fluorescence (WDXRF) and energy dispersive X-ray fluorescence (EDXRF).
Until 1970’s, the XRF analyses were performed by using WDXRF equipments. This technique is based on Braggs’s Law and needs a synchronized and precise system between the diffractor crystal and the detector. There are some advantages of WDXRF compared to EDXRF, such as: higher spectral resolution, optimum wide range determination, and the measurement of light elements (atomic number smaller than 13, such as C, N, O, F, and Na). However, there are also some disadvantages of WDXRF: lower detection efficiency and
higher power X-ray sources (Kalnicky; Singhvi, 2001; Weindorf et al., 2014a).
From the development of superconducting detectors, which are able to discriminate near-spectral lines of energies (Kα and Kβ), emerged the energy dispersive X-ray fluorescence (EDXRF) (Kalnicky; Singhvi, 2001; Weindorf et al., 2014a). These high-resolution detectors can produce electronic pulses proportional to X-ray energies. The most used detectors are the silicon drift detectors (SDD) and the semiconductors Si (Li), Ge (Li) and Ge hyperpure. When using these detectors it is not required the synchronous movement of the diffraction crystal and detector, therefore, there are no moving parts. The EDXRF system has the advantage of producing smaller equipment since it uses low-power X-ray tubes and/or radioactive sources of excitation. Some disadvantages of the EDXRF compared to WDXRF are: low efficiency for low energy X-rays, and EDXRF is not recommended for detection of light elements.
For several decades, XRF has been used in many laboratory analyses, but the development of portable X-ray fluorescence (pXRF) equipments using EDXRF contributed for a wide range of scientific investigations. For example, the pXRF became a useful tool to identify contaminated areas, allowing the determination of total elemental composition in-situ easily and rapidly (Peinado et al., 2010; Weindorf et al., 2012a, 2012b, 2014a; Hu et al., 2014). Some ways to use the pXRF equipments and applications in Soil Science are described below.
Figure 1: Shell electron distribution of Fe and scheme showing the excitation of electrons when subjected to high
HOW CAN THE pXRF EQUIPMENT BE USED?
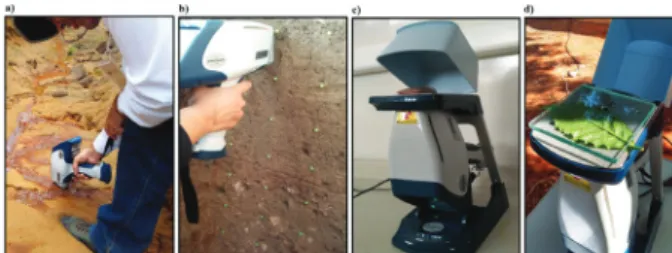
The pXRF equipments can be easily used in field or lab conditions. For in-field measurements, soil characterization can be performed on surface or directly in the soil profile (Figures 2a and 2b). In lab conditions, normally, it has been used for analyses of disturbed soil samples (e.g., dry soil samples passed through a 2 mm sieve), clay or other size fractions (Figure 2c). Also, it is possible to scan on the surface of undisturbed soil core samples. Normally, each measurement takes up to 60 s, although the time of scanning can be defined by the user. The elemental composition of plant leaves can be also determined by using pXRF equiments (Figure 2d).
element being analyzed. Bastos, Melquiades and Biasi (2012) proposed a procedure for decreasing the moisture influence on soil analyses by pXRF. They utilized the background radiation as a parameter for low energies and concluded that, after this correction, the obtained results were satisfactory. These findings emphasize that, correcting the moisture effect, the field-use of the pXRF is feasible. Kalnicky and Singhvi (2001) suggested drying the soil samples if the water content is greater than 0.20 g g-1.
b) Particle size fraction: When a soil sample is grounded and sieved, the particle size distribution obtained may influence the results. For smaller soil particles, the XRF intensity is increased due to smaller incident angles (Maruyama et al., 2008). Then, for fine soil particles it may be expected a higher elemental concentration. For in-lab determination, it is recommended to use air-dried soil samples passed through a 2 mm sieve (Laiho; Perämäki, 2005). The pXRF data obtained in field conditions (e.g., directly in the soil profile) can significantly differ from the results obtained using disturbed soil samples in lab conditions. Stockmann et al. (2016a) quantified the elemental composition at different depths in three soil profiles in-field and in-lab conditions. Mainly for K, Fe, Mn and Ti, the concentration obtained directly in the soil profile was lower than when it was used ground samples. c) Spectral interference: Some elements may have their spectral lines overlapped, such as: S and Mo; Cl and Rh; As and Pb; V and Cr; Fe and Co. For example, the As Kα peak (10.543 keV) is close to the Pb Lα peak (10.551 keV). Much attention must be driven to adjacent elements (Z-1 or Z+1), which show similar excitation energies (see Table 1). Also, a given element may absorb or scatter the fluorescence of another element. The energy emitted by one element may excite another (Kalnicky; Singhvi, 2001).
d) Scanning through polyethylene plastic bags: Polyethylene plastic films may absorb part of incident X-rays energy (see Figure 2c). So, if soil samples are packed into thin plastic bags and scanned by pXRF the results may be influenced (see Figure 2c). To avoid this interference it is recommended to use 0.2 mil Mylar or polypropylene X-ray film (Kalnicky; Singhvi, 2001). e) Scanned area and penetration depth of X-rays: Normally, the area scanned by pXRF is about 1 cm2 and the
penetration from 2 to 5 mm (Kalnicky; Singhvi, 2001). So, the heterogeneity (e.g., particle size distribution, mineralogy, organic matter content) of the total soil volume will affect the results. For example, Hangen and Vieten (2016) found that simple soil pore length influenced on pXRF results. Figure 2: Details of different uses of pXRF equipments:
a) directly on soil surface; b) down the soil profile; c) using disturbed dry soil samples packed into plastic bags (see below factors affecting the XRF results); and d) scanning a coffee plant leaf. Photos from authors.
FACTORS AFFECTING THE XRF RESULTS Some factors may affect the XRF results and must be strongly taken into account, as summarized below:
Ciência e Agrotecnologia 41(3):245-254, May/Jun. 2017
250 RIBEIRO, B. T. et al.
APPLICATIONS OF pXRF FOR TROPICAL SOIL CHARACTERIZATION
In recent years, diverse works have been published in several research areas, such as geology, archeology, and paleontology, whereas regarding soil characterization for varying purposes not too many works are found worldwide. In this review we emphasize the potential application of pXRF in soils for environmental, pedological and agronomic applications, as an effort to provide insights and stimulate future tests mainly in tropical countries with lack of detailed soil information.
Environmental applications
For environmental purposes, the determination of heavy metals and rare-earth elements in different materials can be greatly favored by the use of pXRF (Kalnicky; Singhvi, 2001), and this method can help in-field recognition and fast decision making. Traditionally, metal analyses for environmental studies require acid digestion in microwave oven and subsequent determination by ICP-OES or atomic absorption spectrophotometer (USEPA 3051A method). For instance, in a mining area in Ireland, high correlation was observed between the As, Cu, Pb and Zn concentrations determined by pXRF and those obtained through atomic absorption spectrophotometer after digestion in aqua regia (Radu; Diamond, 2009). Rouillon and Taylor (2016), studying soils contaminated with heavy metals and comparing element data obtained from both pXRF and inductively coupled plasma atomic emission spectroscopy (ICP-AES) with certified reference materials, found that pXRF provided adequate results for Ti, Fe, Cu, Zn, Mn, Cr, Sr, Cd, and Pb after careful sample preparation and pXRF calibration. These findings stimulate similar environmental studies in tropical conditions.
Pedological applications
Considering that pXRF is capable of obtaining the total content of many elements in soils and other materials, diverse elements distribution can be accessed by scanning the soil profile in the field, providing adequate results. Weindorf et al. (2012a) showed the applicability of using pXRF in 10 soil pedons as a manner to quantitatively differentiate soil horizons of alluvial soils from Lousiana (USA) based on differences of elemental concentration, clay content and laboratory analyses results.
For the purpose of this work, aiming to illustrate the potential of pXRF also in tropical conditions, we created a graphic showing the varying Fe contents in depth and among soil profiles obtained by pXRF scanning (Figure 3).
This element was chosen since Fe has an important role in soils, influencing soil aggregation, and, hence, soil structure, porosity, bulk density, etc. (Kämpf; Marques; Curi, 2012), adsorption of anions (anion exchange capacity), such as phosphate in more weathered soils (Motta et al., 2002), indicative of pedogenic processes that occurred in soil, reflected by the color associated to the presence of Fe oxides (hematite and goethite) (Resende et al., 2014), drainage conditions (Schaetzl; Anderson, 2005), soil parent material, etc. It can be noticed in Figure 3 that pXRF could differentiate Fe contents in depth within a profile and among soil profiles rapidly and at low cost. Spatialization of such information to the entire soil profile, as illustrated in Hartemink (2015), can also be performed using interpolation techniques and may be of great importance in future works involving the so-called digital soil morphometrics (Hartemink; Minasny, 2014).
Figure 3: Fe content (%) down soil profiles. LV – Red Latosol; LVdf – Dystroferric Red Latosol; OX – Haplic Organosol; GX – Haplic Gleysol; LVA – Red-Yellow Latosol; PA – Yellow Argisol; PV – Red Argisol; CX – Haplic Cambisol; NV – Red Nitosol. Approximate taxonomic correspondence between Brazilian System of Soil Classification (Embrapa, 2013) and Soil Taxonomy (Soil Survey Staff, 2014): Latosol = Oxisol; Organosol = Histosol; Gleysol = Acquent; Argisol = Ultisol; Cambisol = Inceptisol; and Nitosol = Oxisol.
in the laboratory, and some differences in the results were found. In this sense, Weindorf et al. (2015) used visible near-infrared diffuse reflectance (VisNIR DRS) and pXRF to investigate differences in soil parent material from soils of three countries and pXRF was more efficient in capturing soil properties variability.
Hseu et al. (2016)compared Ni and Cr contents in soil profiles derived from serpentinite obtained by pXRF and aqua regia digestion followed by ICP-AES and an adequate correlation between the methods was found, although there were slightly differences between the absolute values obtained by each method. Aldabaa et al. (2015), studying soil salinity, evaluated the feasibility of predicting electrical conductivity from VisNIR DRS, pXRF and remote sensing data and found adequate results by combining the three sources of data to make predictions.
Most works using pXRF for soil characterization regards mineral soils and few reports of element contents have been made evaluating the effect of organic carbon on pXRF results. Among those works, Shand e Wendler (2014) evaluated element concentration through pXRF and ICP spectrometry after aqua regia digestion in topsoil samples collected in Scotland, with a wide range of organic carbon content, and noticed that specific pXRF calibration for bog soils should be necessary.
In Amazonia, Söderström et al. (2016) studied the effectiveness of proximal sensors used in the field, such as pXRF and a sensor that obtains both the electric conductivity and magnetic susceptibility, to predict, separately and in combination, cation exchange capacity, soil organic carbon, A horizon depth and P content in Amazonian dark earths. The authors found that combining data of the sensors promoted better predictions, but pXRF and the magnetic susceptibility were considered the most powerful single predictors, in this order.
Regarding soil survey, classification and digital mapping perspectives, Silva et al. (2016) employed pXRF elemental data in addition to soil magnetic susceptibility and high resolution digital terrain models (slope gradient, topographic wetness index, etc.) to aid in distinguishing four types of Latosols (Oxisols) according to their chemical properties related to parent material, and mapping their spatial distribution over the study area, in Minas Gerais (Brazil). The authors found that pXRF could capture differences of element contents among soils and, in association with magnetic susceptibility, these data had greater contribution to soil classes differentiation than the high resolution digital terrain models used.
Among soil analyses performed in the laboratory in Brazil, one widely used for several purposes is the sulfuric acid digestion analysis (Embrapa, 1997; Embrapa, 2011). This analysis consists of determining contents of Fe2O3, TiO2, Al2O3,SiO2, P2O5, and MnO. Based on preliminary tests, soil weathering indexes can be calculated, such as Ki (Ki = SiO2/Al2O3) and Kr (Kr = SiO2/(Al2O3 + Fe2O3)), in which greater values are expected to be found in low weathered soils (Birkeland, 1999). In addition, Fe2O3 content in soils is a key value for classification purposes in the Brazilian System of Soil Classification (Embrapa, 2013).
Despite of the variety of inferences allowed by results of sulfuric acid digestion analysis, it is time-consuming, costly, only performed by very few laboratories in Brazil and it generates large amounts of chemical residues that require careful handling and treatment prior to final disposal. For the purpose of this review and aiming to furnish some application examples, we conducted comparisons of pXRF elemental data with Fe2O3 results from sulfuric acid digestion analysis. A Cambisol (Inceptisol) profile located at Federal University of Lavras campus, Minas Gerais, Brazil, was scanned using a pXRF (Figure 4) and the results were compared to sulfuric acid digestion results. For the A horizon, the Fe2O3 content was equal to that obtained by sulfuric acid digestion analysis, although for B and C horizons the Fe2O3 content by pXRF underestimated the results of the conventional method. Further investigations are required for better evaluation of these results.
Agronomic applications
Several soil physical and chemical properties have been predicted based on pXRF elemental data solely or in association with other variables, such as remote and proximal sensors data, laboratory results of soil samples, geology information, etc. Here we compiled published works on this nature aiming to draw attention to the pXRF potential of use.
Particle size distribution can be estimated based on the results of pXRF, taking into account that there is a characteristic chemical and mineralogical composition of each size fraction (sand, silt, clay) (Zhu; Weindorf; Zhang, 2011). These authors found a significant correlation between Fe and clay content (R2 = 0.94) in soils of the state
Ciência e Agrotecnologia 41(3):245-254, May/Jun. 2017
252 RIBEIRO, B. T. et al.
Weindorf et al. (2013) satisfactorily determined the calcium sulphate content in soils using pXRF. This can be especially interesting in the monitoring and management of many areas in Brazil with gypsum application for reducing the exchangeable Al3+ contents in depth of cultivated soils.
Additionally, another work showed that, by means of multiple linear regression, cation exchange capacity could be adequately estimated from the results of the pXRF, using 450 soil samples from California and Nebraska, USA (Sharma et al., 2015). Similarly, Sharma et al. (2014) obtained a significant correlation between soil pH and the elemental composition of soil samples measured by pXRF, since low soil pH values are associated with elements such as Al, and high pH values are associated with elements such as Ca and Mg.
Another possibility of using pXRF is the direct measurement of the elemental composition of plant tissues (leaves) and grains by using pXRF (McLaren; Guppy; Tighe, 2012; Paltridge et al., 2012). Towet, Shephred and Drake (2015) evaluated the composition of leaves of several plants by using pXRF and found a high correlation (R2 > 0.90) for Mg, P, S,
K, Ca and Mn contents with those obtained by the traditional acid digestion followed by determination by ICP-OES.
CONCLUDING REMARKS AND FURTHER RECOMMENDATIONS FOR TROPICAL
REGIONS
This review intended to draw attention to the potential uses of pXRF for works of varying nature. This
equipment has been recently adopted by Soil Science community and, thus, additional tests should be performed under varying tropical conditions to better understand and extrapolate its applicability.
Under tropical conditions, especially in developing countries, pXRF may contribute to increase and gather soils information in a rapid, low cost and environmentally friendly way. Thus, future projects aiming to characterize soils both spatially and locally may have in pXRF a practical and useful tool.
DISCLAIMER
In this review we do not endorse any pXRF manufacturer or model over another.
ACKNOWLEDGMENTS
The authors would like to thank Prof. Nilton Curi (Department of Soil Science of the Federal University of Lavras) for motivating us to elaborate this review and for his precious suggestions and corrections that greatly improved the quality of the manuscript. Also, we thank the following Brazilian funding agencies: CNPq, Fapemig and Capes.
REFERENCES
ALDABAA, A. A. A. et al. Combination of proximal and remote sensing methods for rapid soil salinity quantification. Geoderma, 239:34-46, 2015.
Figure 4: Cambisol (Inceptisol) profile located at Federal University of Lavras campus, Minas Gerais State, Brazil: a) and b) scanning detail of the soil profile; c) comparison between Fe2O3 content estimated by pXRF and that
obtained after sulfuric acid digestion. Error bars indicate the standard deviation (n = 13 for A horizon; n = 10 for B horizon; and n = 26 for C horizon). Red lines indicate the Fe2O3 content obtained by sulfuric acid digestion method
BASTOS, R. O.; MELQUIADES, F. L.; BIASI, E. V. Correction for the effect of soil moisture on in situ XRF analysis using low-energy background. X-Ray Spectrometry, 41:304-307, 2012.
BIRKELAND, P. W. Soils and Geomorphology. 3ª Ed. Oxford University Press, New York, 1999. 448p.
EMPRESA BRASILEIRA DE PESQUISA AGROPECUÁRIA - EMBRAPA. Manual de métodos de análises de solos. 1ª Ed. Embrapa Solos, Rio de Janeiro, 1997. 212p.
EMPRESA BRASILEIRA DE PESQUISA AGROPECUÁRIA – EMBRAPA. Manual de métodos de análises de solos. 2ª Ed. Embrapa Solos, Rio de Janeiro, 2011. 230p.
EMPRESA BRASILEIRA DE PESQUISA AGROPECUÁRIA - EMBRAPA. Sistema Brasileiro de Classificação de Solos. 3ª Ed. Embrapa, Brasília, 2013. 353p.
EMPRESA BRASILEIRA DE PESQUISA AGROPECUÁRIA - EMBRAPA. Programa Nacional de Solos do Brasil (PronaSolos). 1ª Ed. Embrapa Solos, Rio de Janeiro, 2016. 53p.
GAZLEY, M. F.; FISHER, L. A. A review of the reliability and validity of portable X-ray fluorescence spectrometry (pXRF) data. In: GAZLEY, M. F.; FISHER, L. A. Mineral Resource and Ore Reserve Estimation – The AusIMM Guide to Good Practice. 2ª Ed. p.69-82, 2014.
HANGEN, E.; VIETEN, F. Influence of soil pore length upon portable X-ray fluorescence spectrometer measurments of elements in soils. Water, Air and Soil Pollution, 227:1-7, 2016.
HARTEMINK, A. E. New tools for pedologists: Digital soil morphometrics. Soil Horizons, 56:1-2, 2015.
HARTEMINK, A. E.; MINASNY, B. Towards digital soil morphometrics. Geoderma, 230-231:305-317, 2014.
HSEU, Z. et al. Portable X-ray fluorescence (pXRF) for determining Cr and Ni contents of serpentine soils in the field Zeng-Yei. In: HARTEMINK, A. E.; MINASNY, B. (Org.). Digital Soil Morphometrics. Springer International Publishing, 2016. p.37-50.
HU, W. et al. Metals analysis of agricultural soils via portable x-ray fluorescence spectrometry. Bulletim Environmenal Contamination Toxicology, 92, 2014.
KALNICKY, D.; SINGHVI, R. Field portable XRF analysis of environmental samples. Journal of Hazardous Materials, 83:93-122, 2001.
KÄMPF, N.; MARQUES, J. J.; CURI, N. Mineralogia de solos brasileiros. In: KER, J. C. et al. (Eds). Pedologia – Fundamentos. SBCS, Viçosa, 2012, p.81-146.
LACERDA, M. P. C.; ANDRADE, H.; QUÉMÉNEUR, J. J. G. Pedogeoquímica em perfis de alteração na região de Lavras (MG). II – Elementos menores e elementos terras-raras. Revista Brasileira de Ciência do Solo, 26:87-102, 2002. LAIHO, J. V. P.; PERÄMÄKI, P. Evaluation of portable X-ray
fluorescence (PXRF) sample preparation methods. Special Paper of the Geological Survey of Finland, 73-82, 2005.
McLAREN, T. I.; GUPPY, C. N.; TIGHE, M. K. A rapid and nondestructive plant nutrient analysis using portable X-ray fluorescence. Soil Science Society of America Journal, 76:1446-1453, 2012.
MARUYAMA, Y. et al. Laboratory experiments of particle size effect in X-ray fluorescence and implications to remote X-ray spectrometry of lunar regolith surface. Earth Planet Space, 60:293-297, 2008.
MOTTA, P. E. F. et al. Adsorção e formas de fósforo em Latossolos: Influência da mineralogia e histórico de uso. Revista Brasileira de Ciência do Solo, 26:349-359, 2002.
PALTRIDGE, N. G. et al. Energy-dispersive x-ray fluorescence spectrometry as a tool for zinc, iron and selenium analysis in whole grain wheat. Plant Soil, 361:261-269, 2012. PEINADO, F. M. et al. A rapid field procedure for screening trace
elements in polluted soil using portable X-ray fluorescence (pXRF). Geoderma, 159:76-82, 2010.
RADU, T.; DIAMOND, D. Comparison of soil pollution concentrations determined using AAS and portable XRF techniques. Journal of Hazardous Materials, 171:1168-1171, 2009.
RESENDE, M. et al. Pedologia: base para distinção de ambientes. 6ª Ed. Editora UFLA, Lavras, 2014. 378p. ROUILLON, M.; TAYLOR, M. P. Can field portable X-ray
fluorescence (pXRF) produce high quality data for application in environmental contamination research? Environmental Pollution. 214:255-264, 2016.
SAHRAOUI, H.; HACHICHA, M. Effect of soil moisture on trace
elements concentrations. Journal of Fundamental
Applied Science, 9:468-484, 2017.
SANTOS, E. S. et al. Espectrometria de fluorescência de raios-X na determinação de espécies químicas. Enciclopedia Biosfera, 9:3413-3432, 2013.
Ciência e Agrotecnologia 41(3):245-254, May/Jun. 2017
254 RIBEIRO, B. T. et al.
SHAND, C. A.; WENDLER, R. Portable X-ray fluorescence analysis of mineral and organic soils and the influence of organic matter. Journal of Geochemical Exploration. 43:31-42, 2014.
SHARMA, A. et al. Characterizing soils via portable x-ray fluorescence spectrometer: 3. Soil reaction (pH). Geoderma, 232-234:141-147, 2014.
SHARMA, A. et al. Characterizing soils via portable x-ray fluorescence spectrometer: 4. Cation exchange capacity (CEC). Geoderma, 239-240:130-134, 2015.
SILVA, S. H. G. et al. Proximal sensing and digital terrain models applied to digital soil mapping and modeling of Brazilian Latosols (Oxisols). Remote Sensing, 8:614-635, 2016.
SIMABUCO, S. M.; NASCIMENTO FILHO, V. F. Study on vinasse dynamics in soil using energy dispersive X-ray fluorescence with radioisotopic excitation. Scientia Agricola, 51:207-215, 1994.
SÖDERSTRÖM, M. et al. Sensor mapping of Amazonian Dark Earths in deforested croplands. Geoderma, 281:58-68, 2016.
SOIL SURVEY STAFF, 2014. Soil Survey Field and Laboratory Methods Manual. Soil Survey Investigations Report No. 51. Version 2.0. USDA-NRCS National Soil Survey Center, Lincoln, NE. Available in: <http://www.nrcs.usda.gov/wps/portal/ nrcs/detail/soils/research/report/?cid=nrcs142p2_053371>. Access in: December 2016.
STOCKMANN, U. et al. Utilizing portable X-ray fluorescence spectrometry for in-field investigation of pedogenesis. Catena, 139:220-231, 2016a.
STOCKMANN, U.; JANG, H. J.; MINASNY, B.; McBRATNEY, A. The effect of soil moisture and texture of Fe concentration using portable X-ray fluorescence spectrometers. In: HARTEMINK, A. E.; MINASNY, B. (Eds.). Digital Soil Morphometrics. Springer International Publishing, 2016b. p.63-71.
TERRA, J. et al. Análise multielementar de solos: Uma proposta envolvendo equipamento portátil de fluorescência de raios-X. Semina, 35:207-214, 2014.
TOWET, E. K.; SHEPHRED, K. D.; DRAKE, B. L. Plant elemental composition and portable X-ray fluorescence (pXRF)
spectroscopy: Quantification under different analytical parameters. X-Ray Spectrometry, 45:117-124, 2015.
US ENVIRONMENTAL PROTECTION AGENCY, 2007. Method 6200: Field portable X-ray fluorescence spectrometry for the determination of elemental concentrations in soil and sediment. In: Test Methods For Evaluating Solid Waste, US Environmental Protection Agency, Washington, DC, USA. Available in: <http://www.epa.gov/osw/hazard/ testmethods/sw846/online/6_series.htm>. Access in: December 2016.
WASTOWSKI, A. D. et al. Caracterização dos níveis de elementos químicos em solo, submetido a diferentes sistemas de uso e manejo, utilizando espectrofotometria de fluorescência de raios-X por energia dispersiva (EDXRF). Química Nova, 33:1449-1452, 2010.
WEINDORF, D. C.; BAKR, N.; ZHU, Y. Advances in portable X-ray fluorescence (pXRF) for environmental, pedological, and agronomic applications. Advances in Agronomy, 128:1-45, 2014a.
WEINDORF, D. C. et al. Influence of ice on soil elemental characterization via portable X-ray fluorescence spectrometry. Pedosphere, 24:1-12, 2014b.
WEINDORF, D. C.; CHAKRABORTY, S. Portable X-ray fluorescence spectrometry analysis of soils. Methods of Soil Analysis, v.1, n.1, 2016.
WEINDORF, D. C. et al. Lithologic discontinuity assessment in soils via portable X-ray fluorescence spectrometry and visible near-infrared diffuse reflectance spectroscopy. Soil Science Society of America Journal, 79:1704-1716, 2015.
WEINDORF, D. C. et al. Direct soil gypsum quantification via portable x-ray fluorescence spectrometry. Soil Science Society of America Journal, 77:2071-2077, 2013. WEINDORF, D. C. et al. Enhanced pedon horizonation using
portable X-ray fluorescence spectrometry. Soil Science Society of America Journal, 76:522-531, 2012a.
WEINDORF, D. C. et al. Characterizing soils via portable x-ray fluorescence spectrometer: 2. Spodic and Albic horizons. Geoderma, 189-190:268-277, 2012b.