w ww . e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
Uliginosin
B,
a
natural
phloroglucinol
derivative
with
antidepressant-like
activity,
increases
Na
+
,K
+
-ATPase
activity
in
mice
cerebral
cortex
Ana
C.
Stein
a,
Liz
G.
Müller
a,
Andréa
G.K.
Ferreira
b,
Andressa
Braga
a,
Andresa
H.
Betti
a,
Fernanda
B.
Centurião
a,
Emilene
B.
Scherer
b,
Janaína
Kolling
b,
Gilsane
L.
von
Poser
a,
Angela
T.S.
Wyse
b,
Stela
M.K.
Rates
a,∗aProgramadePós-graduac¸ãoemCiênciasFarmacêuticas,UniversidadeFederaldoRioGrandedoSul,PortoAlegre,RS,Brazil
bLaboratóriodeNeuroprotec¸ãoeDoenc¸asMetabólicas,DepartamentodeBioquímica,UniversidadeFederaldoRioGrandedoSul,PortoAlegre,RS,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received23December2015 Accepted12April2016 Availableonline21June2016
Keywords:
UliginosinB
Antidepressant-likeactivity Na+,K+-ATPase
Veratrine
Voltage-gatedsodiumchannel
a
b
s
t
r
a
c
t
UliginosinB,aphloroglucinolisolatedfromHypericumpolyanthemumKlotzschexReichardt, Hyperi-caceae,hasantidepressant-likeeffectintheforcedswimmingtestinrodentsandinhibitsmonoamines neuronalreuptakewithoutbindingtotheirneuronalcarriers.StudiesshowedtheinvolvementofNa+,K+
-ATPasebrainactivityindepressivedisorders,aswellasthedependenceofneuronalmonoaminetransport fromNa+gradientgeneratedbyNa+,K+-ATPase.ThisstudyaimedatevaluatingtheeffectofuliginosinBon
Na+,K+-ATPaseactivityinmicecerebralcortexandhippocampus(1and3hafterthelastadministration)
aswellastheinfluenceofveratrine,aNa+channelopener,ontheantidepressant-likeeffectofuliginosin
B.Miceweretreated(p.o.)withuliginosinBsingle(10mg/kg)orrepeateddoses(10mg/kg/day,3days). Acuteadministrationreducedtheimmobilityintheforcedswimmingtestandtailsuspensiontestand increasedNa+,K+-ATPaseactivityincerebralcortex1haftertreating,whereastherepeatedtreatment
inducedtheantidepressant-likeeffectandincreasedtheNa+,K+-ATPaseactivityatbothtimesevaluated.
Nonetreatmentaffectedthehippocampusenzymeactivity.Veratrinepretreatmentpreventeduliginosin Bantidepressant-likeeffectintheforcedswimmingtest,suggestingtheinvolvementofNa+balance
reg-ulationonthiseffect.Altogether,thesedataindicatethatuliginosinBreducesthemonoamineuptakeby alteringNa+gradient.
©2016SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Thisisanopen accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Basedonthewell-knownefficacyofHypericumperforatum(St. John’swortherb)forthetreatmentofmildtomoderatedepression
(Linde, 2009)ourgroup hasbeenstudying chemical and
phar-macologicalfeaturesofSouthBrazilianHypericumspecies(Daudt
etal.,2000;Ferrazetal.,2002;Viana,2007;Vianaetal.,2003,2005,
2006,2008;Steinetal.,2012).HypericumpolyanthemumKlotzsch
exReichardt,Hypericaceae,extractshaveshownantinociceptive
(Vianaetal.,2003;Haasetal.,2010)andantidepressant-likeeffects
inratsandmice(Steinetal.,2012).Itsmajorchemicalconstituents
∗ Correspondingauthor.
E-mail:stela.rates@ufrgs.br(S.M.Rates).
arethreebenzopyrans,namedHP1(6-isobutyryl-5,7-dimethoxy-2, 2-dimethyl-benzopyran),HP2 (hydroxy-6-isobutyryl-5-methoxy-2,2-dimethyl-benzopyran) and HP3 (5-hydroxy-6-isobutyryl-7-methoxy-2,2-dimethyl-benzopyran), and a phloroglucinol derivative,uliginosinB(VonPoseretal.,2006).UliginosinB(1) hasa dimericstructure consistingofphloroglucinolandfilicinic acidmoieties(Rochaetal.,1995;Nöretal.,2004;Duarteetal., 2014), and seems tobe responsible for theantidepressant-like effects observedin animalsbehavioral tests(Stein etal.,2012). Thiscompound showedantidepressant-like effectin theforced swimmingtestinmice,whichwaspreventedbytheimpairmentof themonoaminergicneurotransmissioninvivo;italsoinhibitedthe sinaptossomaluptakeofdopamine,noradrenalineandserotonin, butinterestinglyitdidnotinteractwiththeirrespectivesiteon neuronalcarriers(Steinetal.,2012).Thesefindingssuggestthat
http://dx.doi.org/10.1016/j.bjp.2016.04.005
uliginosinBactsbyadistinctmechanismthantheclassical antide-pressantdrugs,whichactbyblockingmonoaminetransporters.
O
O
OH
OH HO
O
O
OH
1
Monoaminetransportersarelocatedintheplasmamembrane andaredrivenbytheelectrochemicalgradientofNa+generatedby
Na+,K+-ATPase(NelsonandLill,1994).Ithasbeenshownthatsome
antidepressants,suchasamitriptyline,nortriptyline,imipramine anddesipramine,inhibittheNa+,K+-ATPaseactivity(Carfagnaand
Muhoberac,1993;Sanganahallietal.,2000;ViolaandArnaiz,2007).
On theotherhand, dopamine,noradrenalineand serotoninare abletostimulatetheactivityof this enzyme(Violaand Arnaiz,
2007).
Na+,K+-ATPaseactivityisessentialtothebrainnormalfunction,
sincetheflowofNa+andK+ionsacrossthemembraneisnecessary
toneuronalexcitability,regulationofosmoticbalance,cellvolume andintracellulartransportofmoleculeslinkedtotheco-transport ofNa+,suchasglucose,aminoacidsandneurotransmitters(Kaplan,
2002;Jorgensen etal.,2003).Severalexperimentalstudieshave
describedtheinvolvementofNa+,K+-ATPasebrainactivityinthe
depressivedisorders (Zanattaet al.,2001; Gamaro etal., 2003;
Vasconcellosetal.,2005;Ackeretal.,2009).Maniaandbipolar
depressionhavebeenassociatedwithincreasedintracellularNa+
concentrations(LiandEl-Mallakh,2004).Ratsexposedtochronic variablestress(CVS)developeddespair-likeendophenotypesand haddecreasedhippocampalandamygdalarNa+,K+-ATPase
activ-ity (Crema et al., 2010).The antidepressant fluoxetine and the
moodstabilizerlithiumsimultaneouslypreventeddespairinduced by CVS and prevented the decrease in Na+,K+-ATPase activity
(Vasconcellosetal.,2005).
The observation that several neuroactive drugs act on Na+
channelsalsoindicatestheimportanceofNa+gradient inbrain
disordersneurobiology.Sodiumchannelsarevoltage-dependent transmembraneproteinsresponsiblefortheincreaseof permeabil-itytosodium,whichinitiatesandpropagatestheactionpotential inexcitablecells(CestèleandCatterall,2000;Bourinetal.,2009). TheNa+channelsaretargetstodifferentclassesofdrugssuchas
anticonvulsants,localanestheticsandantiarrhythmics(Rasgdale etal.,1996).Someanticonvulsants(carbamazepine,lamotrigine, phenytoin,topiramate, valproatesodium) arealsousedtotreat bipolardisorder(Reinaresetal.,2012)andlamotriginehasbeen usedspeciallytotreatbipolardepression(Lengetal.,2013).
Inthiscontext,theaimofthepresentstudywastoevaluate theeffectoftheacuteandsub-acuteadministrationofuliginosin B on Na+,K+-ATPase activity in mice cerebral cortex and
hip-pocampusandtheinvolvementofsodiumchannelsinuliginosin Bantidepressant-likeeffect.
Materialsandmethods
Plantmaterial
HypericumpolyanthemumKlotzschexReichardt,Hypericaceae aerial parts were collected at Cac¸apavado Sul, in the state of Rio Grandedo Sul–Brazil(October, 2008).The voucher speci-mensweredepositedattheherbariumoftheFederalUniversity
ofRioGrandedoSul(ICNBordignon,3118Herbáriodo Departa-mentodeBotânica–InstitutodeBiociências–UFRGS).Theplant collectionwasauthorizedbyIBAMA(InstitutoBrasileirodoMeio AmbienteedosRecursosNaturaisRenováveis)(n◦003/2008;
Pro-tocol02000.001717/2008–60).
Preparationofextract
H.polyanthemum lipophilic extractwas obtainedfromdried andpowderedplantmaterial(300g)extractedwithcyclohexane (plant/solventratio1:10,w/v)bystaticmacerationfor48h.The extractwasevaporatedtodrynessunderreducedpressureat45◦C
toeliminatethesolvent,yieldingafreesolventextracttermedPOL (3.5%).Then,theextractwastreatedwithacetonetoremovethe waxes,accordingtoRochaetal.(1994),producing aninsoluble residue,whichwaseliminatedbyfilteringwithpaperfilter.
CharacterizationoftheextractbyHPLCanduliginosinBisolation
TheextractwasdissolvedinHPLCgrademethanol(2mg/ml), filtered(0.22mporesize,Merck)andanalyzedbyhigh
perfor-manceliquidchromatographyWaterHPLCsystem(Milford,MA, USA).UliginosinB(1)determinationwascarriedoutwithan iso-craticsolventcondition(95%CH3CN,5%H2O,0.01%TFA)througha
WatersNova-PackC18column(4m,3.9mm×150mm)adapted toaguardcolumnWatersNova-PackC1860A(3.9mm×20mm), flowrateof1mlmin−1andUVdetectionat220nm,accordingto
themethodpreviouslydescribed(Nunesetal.,2009).UliginosinB concentrationwasdeterminedas16%,isolatedbymeansof prepar-ativethinlayerandcolumnchromatographyandfinallyidentified by1H–13CNMRasdescribedelsewhere(Rochaetal.,1995;Ferraz
etal.,2002;Nöretal.,2004).
Animals
BehavioralandbiochemicaltestswerecarriedoutwithmaleCF1 mice(25–30g)purchasedfromFundac¸ãoEstadualdeProduc¸ãoe PesquisaemSaúde,RS(Brazil).Theanimalswerehousedbyfive in plastic cages(17cm×28cm×13cm)and werekeptunder a 12hlight/darkcycle(lightsonat7a.m.)atconstanttemperature of23±1◦Cwithfreeaccesstostandardcertifiedrodentdietand
tapwater.AllexperimentalprotocolswereapprovedbyThe Ani-malCareLocalEthicalCommittee(CEUAUFRGS;Protocol18518), and performedaccording toBrazilian law(Brazil,2008), which areincompliancewiththeEuropeanCommunitiesCouncil Direc-tiveof24November1986(86/609/EEC)andInternationalGuiding PrinciplesforBiomedicalResearchInvolvingAnimals(Bankowski,
1985).
Experimentaldesign
Mice(n=8pergroup)weretreatedbygavage(10ml/kg)with uliginosinB(1)acutely(10mg/kg,p.o.)orsub-acutely(10mg/kg,
p.o.,onceaday,during3days),basedonpreviousresultsofour group(Vianaetal.,2008).Controlgroupswereperformedforboth treatmentregimens and theanimalswere treatedwithvehicle (saline+polisorbate802.0%).Differentgroupswereusedfor bio-chemicalandbehavioralexperiments(tailsuspensiontest,TST– andforcedswimmingtest,FST).
Time-courseof uliginosinBantidepressant-like effectonthe TST and FST was evaluated by testing independent groups of micewhich were acutelyor repeatedly treated withuliginosin B(10mg/kg,p.o.)orvehicle.Theanimalswereevaluatedinthe behavioral assays only once after treatment. Measurement of Na+,K+-ATPase activitywasperformedusing animals thatwere
repeatedlytreated)wereeuthanizedbydecapitation1or3hafter receivingthelastdrugadministrationandbrainstructureswere removedimmediately.
Inanothersetofexperiments,weinvestigatedthepossible con-tributionofsodiumchannelstouliginosinBantidepressant-like effect.Weevaluatedtheinfluenceofthepre-treatmentwith ver-atrine,aNa+channelopener,ontheFST.Initially,dose-response
experimentswereperformedintheFSTandlocomotoractivityin ordertodeterminethedoseofveratrinetobeused:veratrine(0.06, 0.125and0.5mg/kg,i.p.)wasadministered45minbeforethetest. Thesubeffectivedosewasdefinedasthedosenotabletoreduce immobilityintheFSTand withnoeffectonlocomotoractivity
(Centuriãoetal.,2014).TheassociationofveratrineanduliginosinB
consistedofthepretreatmentwithveratrine(0.06mg/kg)or vehi-cle,i.p.,45minbeforetheFST,plus uliginosinBorvehicle,p.o., 30minbeforethetest.
Assays
Tissuepreparation
Cerebralcortexandhippocampuswerehomogenizedin10 vol-umes(1:10, w/v) of0.32mMsucrose solutioncontaining5mM HEPESand1mMEDTA,pH7.5.Thehomogenateswerecentrifuged at 1000×g for 10min and thesupernatants wereremoved for Na+,K+-ATPaseactivitydetermination.
Na+,K+-ATPaseactivityassay
ThereactionmixtureforNa+,K+-ATPaseassaycontained5mM
MgCl2,80mMNaCl,20mMKCl,and40mMTris–HCl,pH7.4,ina
finalvolumeof200l.After10minofpre-incubationat37◦C,the
reactionwasinitiatedbytheadditionofATPtoafinalconcentration of3mM,andincubatedfor20min.Controlswerecarriedoutunder thesameconditionswiththeadditionof1mMouabain.Na+,K+
-ATPaseactivitywascalculatedbythedifferencebetweenthetwo assaysaccordingtothemethoddescribedbyWyseetal.(2000). Releasedinorganicphosphate(Pi)wasmeasuredbythemethodof
Chametal.(1986).Specificactivityoftheenzymewasexpressed
asnmolPireleasedperminpermgofprotein.
Proteindetermination
ProteinconcentrationwasdeterminedbytheBradfordmethod
(1976)usingbovineserumalbuminasstandard.
Behavioralexperiments
Tailsuspensiontest(TST)
TheTSTwasconductedaccordingtoSteruetal.(1985)with minormodifications(Mülleretal.,2012).Micewereadaptedto thelaboratoryconditions1hbeforetheexperiment.Animalswere suspendedbytail60cmabovethefloorusingadhesivetape(1cm fromthetipoftheend)inadimlightroom.Immobilitytimewas recorded(inseconds)byablindtotreatmentobserverduring6min. Micewereconsideredimmobilewhen theyhungpassivelyand completelymotionless.
Forcedswimmingtest(FST)
TheFSTwascarriedoutaccordingtoPorsoltetal.(1978)with minormodificationsstandardizedandvalidatedinourlaboratory
(Vianaetal.,2005).Micewereadaptedtothelaboratoryconditions
1hbeforebeingexposedtotheFST.Theanimalswere individu-allyforcedtoswiminacylinderpool(10cmdiameter,13cmhigh, waterat22±1◦C)andthetotaltimeofimmobilityduring6min
wasscored(inseconds).Immobilitytimewasrecordedwhenthe mouseremainedfloatingmotionlessor makingonlythe move-mentsnecessarytokeepitsheadabovewater.
Locomotoractivity
The spontaneous locomotor activity was performed in the open-field (Viana et al., 2005). Forty-five minutes after the administration, mice were placed in a transparent acrylic box measuring45cm×30cm×30cmwithadarkbottomdividedinto 24 equal quadrants. They were evaluated during 10min, after 5minhabituation.Thenumberofcrossingswasrecordedbyan observerblindedtotreatments.
Statisticalanalysis
Data wereexpressedasmean+SEMof themean.Data from biochemicalanalysiswereanalyzedbyStudent’sttest.Behavioral experimentsdatawereanalyzedbyone-wayortwo-wayANOVA followedbyStudent–Newman–Keuls.Differenceswereconsidered statisticallysignificantatp<0.05.Thestatisticalprocedureswere performedusing theSigmaStatsoftware 2.03 (JandelScientific Corporation).
Results
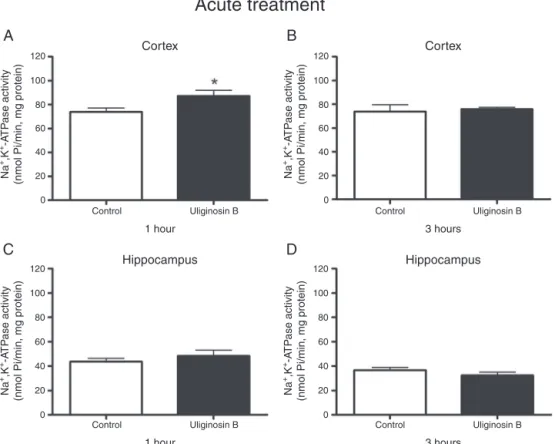
AcuteadministrationofuliginosinBincreasedNa+,K+-ATPase
activityincerebralcortex1haftertreatment[t(9)=2.447,p<0.05] (Fig.1A),butnotafter3h[t(8)=0.323,p=0.755](Fig.1B).Inthe hippocampus,Na+,K+-ATPaseactivitywasnotalteredatbothtimes
studied:1h[t(9)=0.898,p=0.393](Fig.1C)and3h[t(8)=1.155,
p=0.281](Fig.1D).
Sub-acute administration of uliginosin B increased Na+,K+
-ATPaseactivityincerebralcortex1hafterthelastadministration [t(10)=3.300,p<0.01](Fig.2A),aswellas3hafter[t(9)=2.518,
p<0.05](Fig.2B).Noalterationswereverifiedinthehippocampus atthetwodifferenttimes:1h[t(10)=1.494,p=0.166](Fig.2C)and 3h[t(8)=0.788,p=0.449](Fig.2D).
TheacuteadministrationofuliginosinB(10mg/kg,p.o.)inthe TSTandFSTareshowninFig.3.One-wayANOVAshoweda signifi-canteffectofuliginosinBadministrationintheTST[F(2,25)=4.411;
p<0.05]; posthoc analysis indicated a reduction onthe immo-bility time at 1h(p<0.05), but not at 3hafter administration (p=0.079) when compared to the control group (Fig. 3A).The sameeffectwasobservedwhenanimalsweresubmittedtotheFST [F(2,30)=11.136;p<0.001]:posthocanalysisindicatedasignificant decreaseintheimmobilitytime1hafteradministration(p=0.076), butnotafter3h(p>0.05)(Fig.3B).
The effect of sub-acute administration of uliginosin B (10mg/kg/day,p.o.)isshowninFig.3C.One-wayANOVArevealed a significanteffect of uliginosinB[F(2,21)=6.360; p<0.01] and
posthocanalysisindicatedasignificantanti-immobilityeffectat 1h(p<0.01)and3h(p<0.05)afterthelastadministrationwhen comparedtocontrolgroup.
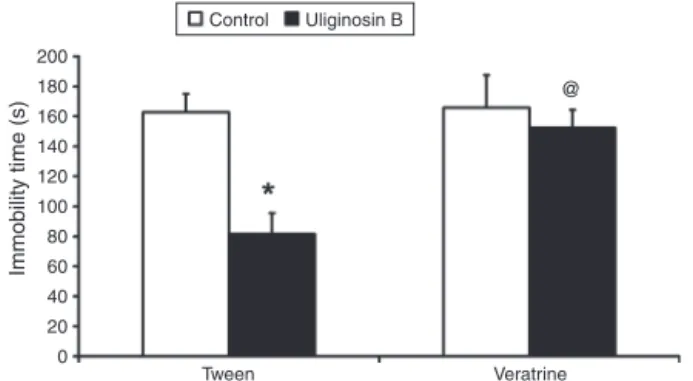
Theanti-immobilityeffectofuliginosinB(10mg/kg,p.o.)was prevented by thepretreatment withveratrine (0.06mg/kg, i.p.) [two-way ANOVA: Fpre-treatment×treatment(1,31)=6,205, p=0.019]
(Fig.4).Theco-administrationof veratrineanduliginosinBhas noeffectwhencomparedtotheuliginosinBandvehicle(Tween), thusveratrineaffecttheantidepressant-likeeffectofuliginosinB inmouseFST.
Discussion
Inthepresentstudy,wedemonstratedthat uliginosinB, the mainphloroglucinolderivativefromH.polyanthemum,increases activity of Na+,K+-ATPase in mice cerebral cortex, but not in
120
100
80
60
40
20
0
Control Uliginosin B
1 hour
Na
+,K +-A
TP
ase activity
(nmol Pi/min, mg protein)
Acute treatment
Cortex
A
C
D
B
120
100
80
60
40
20
0
Control Uliginosin B
1 hour
Na
+,K +-A
TP
ase activity
(nmol Pi/min, mg protein)
Hippocampus
120
100
80
60
40
20
0
Control Uliginosin B
3 hours
Na
+,K +-A
TP
ase activity
(nmol Pi/min, mg protein)
Hippocampus
120
100
80
60
40
20
0
Control Uliginosin B
3 hours
Na
+,K +-A
TP
ase activity
(nmol Pi/min, mg protein)
Cortex
Fig.1.EffectoftheacuteadministrationofuliginosinBonbrainNa+,K+-ATPaseactivity.MicewereacutelytreatedwithuliginosinB(10mg/kg,p.o.)andeuthanizedby
decapitation1or3hafterthelastadministration;immediatelycerebralcortexandhippocampuswereremovedtomeasureenzymeactivity.Studentttest;valuesare expressedasmean+SEM(n=5–6).DifferencefromControl*p<0.05.
120
100
80
60
40
20
0
Control Uliginosin B
1 hour
Na
+,K +-A
TP
ase activity
(nmol Pi/min, mg protein)
Cortex
A
120
100
80
60
40
20
0
Control Uliginosin B
1 hour
Na
+,K +-A
TP
ase activity
(nmol Pi/min, mg protein)
Hippocampus
Sub acute treatment
C
120
100
80
60
40
20
0
Control Uliginosin B
3 hours
Na
+,K +-A
TP
ase activity
(nmol Pi/min, mg protein)
Cortex
B
120
100
80
60
40
20
0
Control Uliginosin B
3 hours
Na
+,K +-A
TP
ase activity
(nmol Pi/min, mg protein)
Hippocampus
D
Fig.2.Effectofthesub-acutetreatmentofuliginosinBonbrainNa+,K+-ATPaseactivity.MicewererepeatedlytreatedwithuliginosinB(10mg/kg/day,3days,p.o.)and
250
200
150
100
50
0
Control
Immobility time (s)
1 hour
Uliginosin B (10 mg/kg, p.o)
TST
FST
FST
3 hours
250
200
150
100
50
0
Control
Immobility time (s)
1 hour
Uliginosin B (10 mg/kg, p.o)
3 hours
250
C
B
A
200
150
100
50
0
Control
Immobility time (s)
1 hour
Uliginosin B (10 mg/kg/day, 3 days, p.o)
3 hours
Fig.3.Effectoftheacuteandsub-acutetreatmentofuliginosinB(10mg/kg,p.o.) ontheimmobilitytime.MicewereacutelytreatedwithuliginosinBandsubmitted totest,1or3hafteradministration:tailsuspensiontest(TST,panelA)andforced swimmingtest(FST,panelB);miceweretreatedfor3days,onceadayandsubmitted toFST1or3hafteradministration(FST,panelC).One-wayANOVAfollowedby Student–Newman–Keuls;valuesareexpressedasmean+SEM(n=8–10).Difference fromControl*p<0.05,**p<0.01.
FSTsuggestingthattheuliginosinBeffectis,atleastinpart,due to its action on Na+,K+-ATPase. Furthermore, the pretreatment
withveratrine,aNa+channelopener,preventedtheuliginosinB
antidepressant-likeeffect,reinforcingthattheactivityofuliginosin BinvolvestheregulationofNa+balance.
Depressionis definedclinically asa pathological complexof psychological,neuroendocrineandsomaticsymptomsthatcannot be reproduced in animals. However, in mice, specific measur-ablebehaviors’canbeassayedsuchasFSTandTSTwhicharea
200 180 160 140 120 100 80 60 40 20 0
Tween
Immobility time (s)
Control Uliginosin B
Veratrine
@
Fig.4.Effectofthepretreatmentofmicewithveratrine(0.06,mg/kgi.p.)on theanti-immobilityeffectofuliginosinB(10mg/kg,p.o.)intheFST. Indepen-dentgroupsofmicewerepretreated(i.p.)withvehicle(saline+polisorbate80 2%=tween)andtreated(p.o.)withvehicle(Tween–Controlgroup)oruliginosin B(Tween–UliginosinBgroup);orpretreated(i.p.)withveratrineandtreated(p.o.) withvehicle(Veratrine–Controlgroup)oruliginosinB(Veratrine–UliginosinB group).Valuesexpressedasmean+SEM(n=8–10).Two-wayANOVAfollowedby Student–Newman–Keuls.*DifferencefromTween–UliginosinBversusTween– Control,p<0.05.@DifferencefromTween–UliginosinBversusVeratrine– Uligi-nosinB,p<0.05.
goodscreeningtoolswithgoodreliabilityandpredictivevalidity
(Petit-Demouliereetal.,2005;Cryanetal.,2005;Castagnéetal.,
2009).TheeffectofuliginosinBin reducingimmobilitytimein theTSTandFSTreinforcesourpreviousresultsthathavealready demonstrated theantidepressant-likeeffectof this phlorogluci-nol derivative(Steinet al.,2012).In addition,in this studywe movedonthepossiblemodeofactionofuliginosinBby study-ing its effecton theenzyme Na+,K+-ATPase. Clinical (Goldstein
et al., 2006, 2009; Tochigi et al., 2008) and preclinical studies
(Ackeretal.,2009;Gamaroetal.,2003;Vasconcellosetal.,2005;
Cremaetal.,2010;Kirshenbaumetal.,2011)reportedthatNa+,K+
-ATPaseisdiminishedindepressivedisorders.Gamaroetal.(2003)
demonstrated thatthe Na+,K+-ATPaseactivitydecreased in rats
hippocampus subjected to chronic stress model of depression, effectthatwasreversedbytherepeatedtreatmentwithfluoxetine andlithium(Vasconcellosetal.,2005),whereastricyclic antide-pressantsinhibitedtheenzymeactivity(Sanganahallietal.,2000). Although theanimalswere not subjectedto anydepression model, the resultsshowed that uliginosin B increased Na+,K+
-ATPase activity in cerebral cortex about 18% 1h after the last administration,butitdidnotaltertheenzymeactivityafter3h. Inthesub-acutetreatment, uliginosinBwasabletoincreasein 20%theenzymeactivity1and3hafterthelastadministration, suggesting a longstandingeffectof therepeatedtreatment (for 3days).ThesefindingsareconsistentwithZanattaetal.(2001), whodemonstratedthatthechronicadministrationoffluoxetine (14days)increasestheenzymeactivity,factthatcancontributeto fluoxetinetherapeuticefficacy.
Ontheotherhand,noneoftheuliginosinBtreatmentregimens (acuteand sub-acute)havechanged theenzymeactivityin the hippocampus.Morphologicalandneurochemicalalterationshave beenreportedinthehippocampusofdepressedpatients(Sheline et al.,2003).Thus, thelackof theeffect ofuliginosin Bonthe Na+,K+-ATPaseactivityinmicehippocampusmaybeduethefact
thattheanimalswerenotsubmittedtoanyexperimentalmodel ofchronicdepression.Thisfindingalsosuggestsaselectiveeffect ofuliginisonBoncorticalNa+,K+-ATPase,whichcouldbe
particu-larlyrelevant,sincethisbrainstructureisinvolvedindepression neurobiology(Price andDrevets,2010)and postmortem studies have demonstratedalterations onNa+,K+-ATPaseactivityin the
thecortex,butnotinthehippocampus,ofanimalstreatedwith valepotriates.Wespeculatethatthisprofilecouldberelatedtothe irregulardistributionofNa+,K+-ATPaseisoenzymes.Three
␣
iso-formsareabundantinthebrain:␣1,expressedbyneuronsandglia; ␣2,predominantinglia;and␣3,neuronal(McGrailetal.,1991).
Tochigietal.(2008)studiedthegeneexpressionpatternin
post-mortembrainsofsubjectswithmajordepressionandfoundthat Na+,K+-ATPase␣3geneexpressionisdecreasedinprefrontal
cor-texofsubjectswithmajorandbipolardepression.Anotherstudy withNa+,K+-ATPase␣3heterozygousmiceshowedareductionof
15%in neuronal Na+,K+-ATPase activity.We speculate that this
profilecouldbe related tothe irregulardistribution of Na+,K+
-ATPaseisoenzymes.Three␣isoformsareabundantinthebrain: ␣1,expressedbyneuronsandglia;␣2,predominantinglia;and ␣3, neuronal (McGrail et al., 1991).Tochigi et al. (2008)
stud-iedthegeneexpressionpatterninpostmortembrainsofsubjects withmajor depression and found that Na+,K+-ATPase ␣3 gene
expressionisdecreasedinprefrontalcortexofsubjectswithmajor and bipolar depression. Another study withNa+,K+-ATPase
␣3
heterozygousmiceshowedareductionof15%inneuronalNa+,K+
-ATPaseactivity.Furthermore,theseanimals werevulnerableto developincreased depression-like endophenotypesina chronic variablestressmodel(Kirshenbaumetal.,2011).Thesedata sug-gestthatNa+,K+-ATPase␣3 incerebralcortexcouldbea target
tonewantidepressantdrugsandtostudythepathophysiologyof depressivedisorders.
The pre-treatment with veratrine prevented the anti-immobility effect of uliginosin B in the FST, effect that is in linewiththeliterature,whichdemonstratedasimilarprofileto hyperbrasilolB (Centurião et al., 2014), hyperforin (Codagnone etal.,2007)andlamotrigine(Calabreseetal.,2008;Pricaetal.,
2008;Bourinetal.,2009).Arecentstudyfromourgroupshowed
thathyperbrasilolB,anatural dimericphloroglucinolderivative fromH.caprifoliatum,alsohaditsanti-immobilityeffectprevented byveratrine,anditwasabletoincreaseNa+,K+-ATPaseactivityin
micehippocampus,butnotcerebralcortex(Centuriãoetal.,2014). Hyperforin,aphloroglucinolderivativefromH.perforatum,which is an European species worldwide used for depression, seems to exert its antidepressant action by mechanisms dependent onNa+ channels,withoutaltering theactivityofNa+,K+-ATPase
(Chatterjee et al., 1998a,b).In addition, hyperforin inhibitsthe
reuptake of monoamines without bindingto theirtransporters
(Wonnemann et al., 2000). Several authors demonstrated the
presenceofacarrier-mediatedmonoaminetransportmechanism, responsible for the entrance of dopamine, norepinephrine and serotonininthenerveterminal,accompaniedbyNa+ions(Xhaard
etal.,2008;Kristensenetal.,2011)mechanismabolishedinthe
absenceofNa+ (Krueger,1990;Guetal.,1994;Pifletal.,1997).
Lamotrigine, in turn, is an anti-epiletic agent used at bipolar depression tomaintenance treatment.It acts bystabilizing the presynapticmembranethroughtheblockadeofvoltage-gatedNa+
channels(Codagnone etal.,2007).Vitezicet al.(2008) demon-stratedapartialprotectiveeffectoflamotrigineontheinhibition ofNa+,K+-ATPaseactivityinducedbykainicacidinratsprefrontal
cortex and hippocampus. Also, Southam et al. (1998) demon-stratedinvitrothatlamotrigineinhibitsthereuptakeofserotonin, norepinephrine and dopamine, reinforcing the involvement of sodiumgradient(Xhaardetal.,2008).
ConsideringtheeffectofuliginosinBontheactivityofNa+,K+
-ATPaseand onNa+ channelstogether withpreviousstudies of
ourgroupdemonstratingthatuliginosinBinhibitsthereuptake ofmonoaminesinadifferentmannerfrommostantidepressants
(Steinetal.,2012)wecanspeculatethatuliginosinBreducesthe
monoamineuptakebyalteringNa+gradient.
Inconclusion,thepresentstudyrepresentsonestepaheadinthe elucidationofthemechanismofactionoftheantidepressant-like
activityofuliginosinB,anaturalphloroglucinolderivative, suggest-ingthatinvolvementofuliginosinBinregulationofNa+balance
mayoccurthroughincreasedofNa+,K+-ATPaseactivity.The
reg-ulatorymechanisminvolvingNa+mayberegardedanimportant
property for antidepressant-like activity of this phloroglucinol derivative.
Ethicaldisclosures
Protectionofhumanandanimalsubjects. Theauthorsdeclare
thattheallexperimentalprotocolswereapprovedbyTheAnimal CareLocalEthicalCommittee(CEUAUFRGS;Protocol18518),and performedaccordingtoBrazilianlaw(Brazil,2008),whicharein compliancewiththeEuropeanCommunitiesCouncilDirectiveof 24November1986(86/609/EEC)andInternationalGuiding Princi-plesforBiomedicalResearchInvolvingAnimals(Bankowski,1985). Alleffortsweremadetominimizeanimalsuffering,toreducethe numberofanimalsused,andtoutilizealternativestoinvivo tech-niques.
Confidentialityofdata. Theauthorsdeclarethatnopatientdata appearinthisarticle.
Righttoprivacyandinformedconsent. Theauthorsdeclarethat nopatientdataappearinthisarticle.
Authors’contributions
ACSandLGMcontributedinrunningthelaboratorywork, chro-matographicanalysis,analysisofthedataanddraftedthepaper. AGKF,AB,AHB,FBC,EBS,JK,contributedtobiologicalstudies.GLVP contributedincollectingplantsampleandidentification,chemical analysesandcriticalreadingofthemanuscript.ATSWandSMKR plannedthestudy,supervisedthelaboratoryworkandcontributed tocriticalreadingofthemanuscript.Alltheauthorshavereadthe finalmanuscriptandapprovedthesubmission.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgments
ThisworkwassupportedbyCAPES-COFECUB[grantnumber 656/09],CNPq fellowshipsand Programa de Pós-graduac¸ãoem CiênciasFarmacêuticas(PPGCF-UFRGS).
References
Acker,C.,Luchese,C.,Prigol,M.,Nogueira,C.W.,2009.Antidepressant-likeeffectofa diphenyldiselenideonratsexposedtomalation:involvementofNa+,K+-ATPase
activity.Neurosci.Lett.455,168–172.
Bankowski,Z.,1985.CIOMS.CouncilforInternationalOrganizationsofMedical SciencesInternationalGuidingPrinciplesforBiomedicalResearchInvolving Ani-mals.
Bourin,M.,Chenu,F.,Hascoët,M.,2009.Theroleofsodiumchannelsinthe mech-anismofactionofantidepressantsandmoodstabilizers.Curr.DrugTargets10, 1052–1060.
Bradford,M.M.,1976.Arapidandsensitivemethodforthequantitationof micro-gramquantitiesofprotein-die-binding.Anal.Biochem.72,248–254.
Brazil.CongressoNacional.Lei11794;Brasília,8deoutubrode2008.
Calabrese,J.R.,Huffman,R.F.,White,R.L.,Edwards,S.,Thompson,T.R.,Ascher,J.A., Monaghan,E.T.,Leadbetter,R.A.,2008.Lamotrigineintheacutetreatmentof bipolardepression:resultsoffivedouble-blind,placebocontrolledclinicaltrials. BipolarDisord.10,323–333.
Castagné,V.,Porsolt,R.D.,Moser,P.,2009.Useoflatencytoimmobilityimproves detectionofantidepressant-likeactivityinthebehavioraldespairtestinthe mouse.Eur.J.Pharmacol.616,128–133.
Centurião,F.B.,Sakamoto,S.,Stein,A.C.,Müller,L.G.,Chagas, P.M.,VonPoser, G., Nogueira, C.W., Rates, S.M.K., 2014. The antidepressant-like effect of hyperbrasilolB, a natural dimeric phloroglucinol derivative, is prevented byveratrine,asensitive-voltageNa+ channelopener.Eur.J.Med.Plants4,
1268–1281.
Cestèle,S.,Catterall,W.A.,2000.Molecularmechanismsofneurotoxinactionon voltage-gatedsodiumchannels.Biochimie82,883–892.
Cham,K.M.,Delfert,D.,Junger,K.D.,1986.AdirectcolorimetricassayforCa2+
-stimulatedactivity.Anal.Biochem.220,375–380.
Chatterjee,S.S.,Nöldner,M.,Koch,E.,Erdelmeier,C.,1998a.Antidepressantactivity ofHypericumperforatumandhyperforin:theneglectedpossibility. Pharma-copsychiatry31,7–15.
Chatterjee,S.S.,Bhattacharya,S.K.,Wonnemann,M.,Singer,A.,Müller,W.E.,1998b.
HyperforinasapossibleantidepressantcomponentofHypericumextracts.Life Sci.63,499–510.
Codagnone,F.T.,Consoni,A.L.S.,Rodrigues,M.A.B.F.,Andreatini,V.R.,2007.Veratrine blocksthelamotrigine-inducedswimmingincreaseandimmobilitydecreasein themodifiedforcedswimmingtest.Prog.Neuropsychopharmacol.Biol. Psychi-atry31,1307–1311.
Crema,L.,Schlabitz,M.,Tagliari,B.,Cunha,A.,Simão,F.,Krolow,R.,Pettenuzzo,L., Salbego,C.,Vendite,D.,Wyse,A.T.S.,Dalmaz,C.,2010.Na+,K+-ATPaseactivityis
reducedinamygdalaofratswithchronicstress-inducedanxiety-likebehavior. Neurochem.Res.35,1787–1795.
Cryan,J.F.,Mombereau,C.,Vassout,A.,2005.Thetailsuspensiontestasamodefor assessingantidepressantactivity:reviewofpharmacologicalandgeneticstudies inmice.Neurosci.Biobehav.Rev.29,571–625.
Daudt,R.,vonPoser,G.L.,Neves,G.,Rates,S.M.K.,2000.Screeningforthe antidepres-santactivityofsomespeciesofHypericumfromSouthBrazil.Phytother.Res.15, 344–346.
Duarte,M.O.,Lunardelli,S.,Kiekow,C.J.,Stein,A.C.,Müller,L.,Stolz,E.D.,Rates,S.M.K., Gosmann,G.,2014.Phloroglucinolderivativespresentanantidepressant-like effectinthemicetailsuspensiontest.Nat.Prod.Commun.9,671–674.
Ferraz,A.B.F.,Schripsema,J.,Pohlmann,A.R.,vonPoser,G.L.,2002.UliginosinBfrom HypericummyrianthumCham.&Schltdl.Biochem.Syst.Ecol.30,989–991.
Gamaro,G.D.,Streck,E.L.,Matté,C.,Prediger,M.E.,Wyse,A.T.S.,Dalmaz,C.,2003.
ReductionofhippocampalNa+,K+-ATPaseactivityinratssubjectedtoan
exper-imentalmodelofdepression.Neurochem.Res.28,1339–1344.
Goldstein,I.,Levy,T.,Galili,D.,Ovadia,H.,Yirmiya,R.,Rosem,H.,Lichtstein,D., 2006.InvolvementofNa+,K+-ATPaseandendogenousdigitalis-likecompounds
indepressivedisorders.Biol.Psychiatry60,491–499.
Goldstein,I.,Lerer,E.,Laiba,E.,Mallet,J.,Muyahed,M.,Laurent,C.,Rosen,H.,Ebstein, R.P.,Lichtstein,D.,2009.Associationbetweensodium-and-potassium-activated adenosinetriphosphatase␣isoformsandbipolardisorders.Biol.Psychiatry65, 985–991.
Gu,H.,Wall,S.C.,Rudnick,G.,1994.Stableexpressionofbiogenicaminetransporters revealsdifferencesininhibitorssensitivity,kineticsandiondependence.J.Biol. Chem.269,7124–7130.
Haas,J.S., Viana,A.F.,Heckler,A.P.M.,vonPoser,G.L.,Rates,S.M.K.,2010.The antinociceptiveeffectofabenzopyran(HP1)isolatedfromHypericum polyanthe-muminmicehot-platetestisblockedbynaloxone.PlantaMed.76,1419–1423.
Jorgensen,P.L.,Hakansson,K.O.,Karlish,S.J.D.,2003.Structureandmechanismof Na+,K+-ATPase:functionalsitesandtheirinteractions.Annu.Rev.Physiol.65,
817–849.
Kaplan,J.H.,2002.BiochemistryofNa+,K+-ATPase.Annu.Rev.Biochem.71,511–535.
Kirshenbaum, G.S., Saltzman, K., Rose, B., Petersen, J., Vilsen, B., Roder, J.C., 2011.DecreasedneuronalNa+,K+-ATPaseactivityinAtp1a3heterozygousmice
increasessusceptibilitytodepression-likeendophenotypesbychronicvariable stress.GenesBrain.Behav.10,542–550.
Kristensen,A.S.,Andersen,J.,Jorgensen,T.N.,Sorensen,L.,Eriksen,J.,Loland,C.J., Stromgaard,K.,Gether,U.,2011.SLC6neurotransmittertransporters:structure, function,andregulation.Pharmacol.Rev.63,585–640.
Krueger,B.K.,1990.Kineticsandblockofdopamineuptakeinsynaptosomesfrom ratcaudatenucleus.J.Neurochem.55,260–267.
Leng,Y.,Fessler,E.B.,Chuang,D.M.,2013.Neuroprotectiveeffectsofthemood stabilizer lamotrigine againstglutamateexcitotoxicity: roles of chromatin remodellingandBcl-2induction.Int.J.Neuropsychopharmacol.16,607–620.
Li,R.,El-Mallakh,R.S.,2004. Differentialresponseofbipolarand normal con-trollymphoblastoidcellsodiumpumptoethacrynicacid.J.Affect.Disord.80, 11–17.
Linde,K.,2009.St.John’sWort–anoverview.Forsch.Komplementmed.16,146–155.
McGrail,K.M.,Phillips,J.M.,Sweadner,K.J.,1991.Immunofluorescentlocalizationof threeNa+,K+-ATPaseisozymesintheratcentralnervoussystem:bothneurons
andgliacanexpressmorethanoneNa+,K+-ATPase.J.Neurosci.11,381–391.
Müller,L.G.,Salles,L.A.,Stein,A.C.,Betti,A.H.,Sakamoto,S.,Cassel,E.,Vargas,R.F., vonPoser,G.L.,Rates,S.M.K.,2012.Antidepressant-likeeffectofValeriana gle-chomifoliaMeyer(Valerianaceae)inmice.Prog.Neuropsychopharmacol.Biol. Psychiatry36,101–109.
Müller,L.G.,Salles,L.,Lins,H.A.,Feijó,P.R.,Cassel,E.,Varga,R.,vonPoser,G.L.,Noël, F.,Quintas,L.E.,Rates,S.M.,2015.EffectsofdienevalepotriatesfromValeriana glechomifoliaonNa+/K+-ATPaseactivityinthecortexandhippocampusofmice.
PlantaMed.81,200–207.
Nelson,N.,Lill,H.,1994.Portersandneurotransmittertransporters.J.Exp.Biol.196, 213–228.
Nör,C.,Albring,D.,Ferraz,A.B.F.,Schripsema,J.,Pires,V.,Sonnet,P.,Guillaume, D., vonPoser, G.L.,2004. Phloroglucinol derivativesfrom four Hypericum species belonging to theTrigynobrathys section. Biochem. Syst. Ecol. 32, 517–519.
Nunes,J.M.,Pinhatti,A.V.,vonPoser,G.L.,Rech,S.B.,2009.Promotiveeffectsof long-termfertilizationongrowthoftissueculture-derivedHypericumpolyanthemum plantsduringacclimatization.Ind.CropsProd.30,329–332.
Petit-Demouliere, B., Chenu, F., Bourin, M., 2005. Forced swimming test in mice:areviewofantidepressantactivity.Psychopharmacology(Berlin)177, 245–255.
Pifl,A.,Drobny,H.,Reither,H.,Singer,E.A.,1997.IntroductionbylowNa+orCl−of
cocainesensitivecarrier-mediateeffluxofaminesfromcellstransfectedwith clonedhumancatecholaminetransporters.Br.J.Pharmacol.121,205–212.
Porsolt,R.D.,Anton,G.,Blavet,N.,Jafre,M.,1978.Behavioraldespairinrats:anew modelsensitivetoantidepressanttreatments.Eur.J.Pharmacol.47,379–391.
Prica,C.,Hascoet,M.,Bourin,M.,2008.Antidepressant-likeeffectoflamotrigineis reversedbyveratrine:apossibleroleofsodiumchannelsinbipolardepression. Behav.BrainRes.191,49–54.
Price,J.L.,Drevets,W.C.,2010.Neurocircuitryofmooddisorders. Neuropsychophar-macology35,192–216.
Rasgdale,D.S.,Mcphee,J.C.,Scheuer,T.,Catterall,W.A.,1996.Commonmolecular determinantsoflocalanesthetic,antiarrhythmic,andanticonvulsantblockof voltage-gatedNa+channels.Proc.Natl.Acad.Sci.U.S.A.93,9270–9275.
Reinares,M.,Rosa,A.R.,Franco,C.,Goikolea,J.M.,Fountoulakis,K.,Siamouli,M., Gonda,X.,Frangou,S.,Vieta,E.,2012.Asystematicreviewontheroleof anticonvulsantsinthetreatmentofacutebipolardepression.Int.J. Neuropsy-chopharmacol.10,1–12.
Rocha, L., Marston,A., Kaplan, M.A.C., Stoeckli-Evans, H., Thull, U.,Testa, B., Hostettmann, K., 1994. An antifungal gamma-pyrone and xanthones wit monoamineoxidaseinhibitoryactivityfromHypericumbrasiliense. Phytochem-istry36,1381–1385.
Rocha,L.,Marston,A.,Potterat,O.,Kaplan,M.A.C.,Stoeckli-Evans,H.,Hostettmann, K., 1995. Antibacterial phloroglucinols and flavonoids from Hypericum brasiliense.Phytochemistry40,1447–1452.
Sanganahalli,B.G.,Joshi,P.G.,Joshi,N.B.,2000.Differentialeffectsoftricyclic antide-pressantdrugsonmembranedynamics–afluorescencespectroscopicstudy. LifeSci.68,81–90.
Sheline,Y.I.,Gado,M.H.,Kraemer,H.C.,2003.Untreateddepressionandhippocampal volumeloss.Am.J.Psychiatry160,1516–1518.
Southam, E.,Kirkby,D.,Higgins,G.A.,Hagan, R.M.,1998. Lamotrigineinhibits monoamineuptakeinvitroandmodulates5-hydroxytryptamineuptakeinrats. Eur.J.Pharmacol.358,19–24.
Stein,A.C.,Viana,A.F.,Müller,L.G.,Nunes,J.M.,Stolz,E.D.,DoRego,J.C.,Constentin, J.,vonPoser,G.L.,Rates,S.M.K.,2012.UliginosinB,aphloroglucinol deriva-tivefromHypericumpolyanthemum:apromisingnewmolecularpatternforthe developmentofantidepressantdrugs.Behav.Brain.Res.228,66–73.
Steru,L.,Chermat,R.,Thierry,B.,Simon,P.,1985.Thetailsuspensiontest:anew methodforscreeningantidepressantsinmice.Psychopharmacology(Berlin)85, 367–370.
Tochigi,M.,Iwamoto,K.,Bundo,M.,Sasaki,T.,Kato,N.,Kato,T.,2008.Gene expres-sionprofiling ofmajordepression andsuicidein theprefrontalcortexof postmortembrains.Neurosci.Res.60,184–191.
Vasconcellos,A.P.S.,Zugno,A.I.,DosSantos,A.H.,Nietto,F.B.,Crema,L.M.,Gonc¸alves, M.,Franzon,R.,Wyse,A.T.S.,Rocha,E.R.,Dalmaz,C.,2005.Na+,K+-ATPase
activ-ityisreducedinhippocampusofratssubmittedtoanexperimentalmodelof depression:effectofchroniclithiumtreatmentandpossibleinvolvementin learningdeficits.Neurobiol.Learn.Mem.84,102–110.
Viana,A.F.,(PhDThesis)2007.Estudodemoléculaspotencialmenteantidepressivas eanalgésicasdeespéciesdeHypericumnativasdoRS.PortoAlegre. Universi-dadeFederaldoRioGrandedoSul/UniversitédeRouen,220p.
Viana,A.F.,Heckler,A.P.,Fenner,R.,Rates,S.M.K.,2003.Antinociceptiveactivityof HypericumcaprifoliatumandHypericumpolyanthemum(Guttiferae).Braz.J.Med. Biol.Res.36,631–634.
Viana,A.F.,DoRego,J.C.,vonPoser,G.,Ferraz,A.,Heckler,A.P.,Constentin,J.,Rates, S.M.K.,2005.Theantidepressant-likeeffectofHypericumcaprifoliatumCham &Schlecht(Guttiferae)onforcedswimmingtestresultsfromaninhibitionof neuronalmonoamineuptake.Neuropharmacology49,1042–1052.
Viana, A., Do Rego, J.C., Munari, L., Dourmap, N., Heckler,A.P., Dalla Costa, T.,von Poser,G.L.,Costentin,J.,Rates,S.M.K., 2006.Hypericum caprifolia-tum (Guttiferae)Cham. &Schltdl.: a speciesnative to SouthBrazil with antidepressant-likeactivity.Fundam.Clin.Pharmacol.20,507–514.
Viana,A.F.,Rates,S.M.K.,Naudin,B.,Janin,F.,Costentin,J.,DoRego,J.C.,2008.
Effectsofacuteor3-daytreatmentsofHypericumcaprifoliatumCham.&Schltdt. (Guttifereae)extractoroftwoestablishedantidepressantsonbasaland stress-inducedincreaseinserumandbraincorticosteronelevels.J.Psychopharmacol. 22,681–690.
Viola,M.S.,Arnaiz,G.R.L.,2007.BrainNa+,K+-ATPaseisoforms:different
hypothala-musandmesencephalonresponsetoacutedesipraminetreatment.LifeSci.81, 228–233.
Vitezic,D.,Pelcic,J.M.,Zupan,E.,Vitezic,M.,Ljulicic,D.,Simonic,A.,2008.Na+,
K+-ATPaseactivityinthebrainoftheratswithkainicacid-inducedseizures:
influenceoflamotrigine.Psychiatr.Danub.20,270–277.
Wonnemann,M.S.,Singer,M.S.,Müller,W.E.,2000.Inhibitionofsynaptosomal uptakeofhyperforin, amajor constituent ofSt. John’sWort: the roleof amiloridesensitivesodiumconductivepathways.Neuropsychopharmacology 23,188–197.
Wyse,A.T.S.,Streck,E.L.,Worm,P.,Wajner,A.,Ritter,F.,Netto,C.A.,2000. Precog-nitionpreventstheinhibitionofNa+,K+-ATPaseactivityafterbrainischemia.
Neurochem.Res.25,917–975.
Xhaard,H.,Backström,V.,Denessiouk,K.,Johnson,M.S.,2008.Coordinationof Na(+)bymonoamineligandsindopamine,norepinephrine,andserotonin trans-porters.J.Chem.Inf.Model.48,1423–1437.