O R I G I N A L A R T I C L E
Subchronic administration of riparin III
induces antidepressive-like effects and
increases BDNF levels in the mouse
hippocampus
Auriana S. Vasconcelos
a*
, Iris C.M. Oliveira
a, Laura T.M. Vidal
a,
Gabriel C. Rodrigues
a, Stanley J.C. Gutierrez
b, Jos
e M. Barbosa-Filho
b,
Silv
^
ania M.M. Vasconcelos
a, Marta M. de Franc
ß
a Fonteles
a,
Danielle M. Gaspar
a, Francisca C.F. de Sousa
aaDepartment of Physiology and Pharmacology, Medicine College, Federal University of Ceara, Rua Cel, Nunes de Melo
1127, 60430-270 Fortaleza, Ceara, Brazil
bLaboratory of Pharmaceutics Technology, Federal University of Paraiba, Caixa Postal 5009, 58051-970
Jo~ao Pessoa, Paraiba PB, Brazil
Keywords
Aniba riparia, BDNF, corticosterone, depression, riparin III
Received 16 March 2015; revised 16 March 2015; accepted 31 March 2015
*Correspondence and reprints: auriana_vasconcelos@hotmail. com
A B S T R A C T
Riparin III (Rip III) is an alcamide isolated from Aniba riparia that has presented effects of antidepressant and anxiolytic activities in acute stress behavioral models. The trial’s goal was to investigate the activity of Rip III in mice exposed to cortico-sterone-induced chronic depression model. Swiss female mice, 22–25 g, were dis-tributed in following experimental groups: control group (vehicle1: saline containing 0.1% dimethyl sulfoxide and 0.1% Tween-80, SC+ vehicle 2: distilled water emulsified with 2% Tween-80, PO); stressed group (corticosterone, 20 mg/kg, SC, + vehicle 2, orally); Rip III group (50 mg/kg, orally); and fluvoxamine (Flu) group (50 mg/kg, orally). The mice were exposed to the behavioral tests, and poste-riorly, Brain-derived neurotrophic factor protein levels were assessed in hippocampal samples. Statistical analysis of the data was performed by one-wayANOVA, followed by Newman–Keuls test. Both administrations of Rip III and Flu significantly reduced the immobility time in tail suspension and forced swimming tests after 21 days without affecting locomotor function. There was also an increase in BDNF protein levels in the mice hippocampus. These findings further support the hypothesis that Rip III could be a new pharmacological target for the treatment of mood disorders.
I N T R O D U C T I O N
Major depression (MD) is a serious mood disorder that has been the leading cause of disability worldwide [1] and affects people off all backgrounds, ages, and gen-ders [2]. There were over 298 billion cases of MD glob-ally at any point in time in 2010, with the highest proportion of cases occurring between 25 and 34 years, with higher prevalence in females (5.5%) compared to males (3.3%) in 2010 [3]. The female gender presents a greater risk of developing depression. Furthermore, it is considered to be a risk factor for
treatment-resistant depression [4,5]. Therefore, recent preclinical studies in depression have been conducted favoring the use of female animals [2,6,7].
The etiology of the disease is multifactorial, and some theories, such as the theory of psychosocial stress and stress hormones, attempt to explain the phenome-non. The link between stress and depression was ini-tially thought from observations of hyperactivity of the hypothalamic–pituitary–adrenal (HPA) axis, elevated
cortisol levels and disrupted cortisol rhythmicity in depressed patients [8,9]. Studies indicate that hyperac-tivity of HPA axis in this illness can be induced by the
Fundam
e
ntal &
Clinical
P
h
a
rm
a
co
lo
impairment of the feedback inhibition mechanisms [10,11]. The normal cortisol suppression response is absent in about half of the most severely depressed patients [12].
In rodents, several findings have indicated that repeated corticosterone (Cort) injections induce neuro-chemical aspects of depression, including effects on hip-pocampal neurogenesis [13,14] and brain monoamine metabolism [15]. Also, enhancement of depression-like behavior has been verified. These findings come from assays such as forced swimming test and tail suspen-sion test [7,16]. It was possible to conclude that this animal model is valid for the study of the etiology of depression and the effectiveness of antidepressant drugs [17]. In addition, it has been suggested as an animal model of treatment-resistant depression, and approxi-mately one-third of patients did not have an adequate clinical response after being treated with several differ-ent medications [18].
In this context, natural products have great potential as a source of new drugs [19]. Recently, anxiolytic-and antidepressant-like properties have been demon-strated in acute animal models through the use of ripa-rin III (Rip III), an alcamide isolated from the plant Aniba riparia[20,21].
Previous studies have shown that Rip III presents activity on the central nervous system, including anx-iolytic, antidepressive, hypnotic sedative, and anticon-vulsant effects [20–22]. After the aforementioned findings were described, the antidepressant effect of Rip III was better analyzed to discover its mechanism of action. With the use of specific antagonists, it was observed that Rip III showed antidepressant effects in the forced swimming test. These effects seem to be related to the modulation of the alpha 1 and alpha 2 noradrenergic systems, the serotonergic system, and the D2 dopaminergic system [21,22]. No changes were detected in the open field test [21,22].
In this study, the antidepressant-like effect of Rip III treatment was further evaluated in a female mice model of depression induced by Cort. Thus, our goal was to use the Cort-induced animal model of depres-sion in female mice to determine the antidepressant effects of the Rip III.
M A T E R I A L A N D M E T H O D S
Animals
Female Swiss mice (22–25 g) were used in each experi-ment and housed at a controlled temperature of
231 °C with a 12-h dark/light cycle. Moreover, the mice were given ad libitum access to water and food. Prior to an oral gavage procedure, food was made unavailable in a period of 4 h, being returned 20 min after drug administration. The animals were treated in accordance with the current laws and the National Institute of Health Guide for the Care and Use of Labo-ratory Animals. All efforts were made to minimize the suffering and to reduce the number of animals used in the experiments. The study was approved by the ethics committee with protocol 13/2014.
Drugs
All drugs were administered in a volume of 0.1 mL/ 10 g body weight. Cort (20 mg/kg, subcutaneous (SC), Sigma, Brazil, Ceara, Fortaleza) was dissolved in a sal-ine solution containing 0.1% dimethyl sulfoxide and 0.1% Tween-80 (Sigma). Flu (50 mg/kg, PO, ABOTT) was diluted in distilled water. Rip III [50 mg/kg,per os (PO)] was dissolved in 2% Tween 80 and diluted in dis-tilled water. Rip III was isolated from the green fruits of Aniba riparia [20,21]. This substance was deposited in the Bank of Standards of Natural and Synthetic Products of the Laboratorio de Tecnologia Far-mac^eutica of the Universidade Federal da Paraıba, and for its use in this work, it was repurified by thin-layer chromatography [20,21]. Figure 1 presents the molec-ular structure of Rip III.
Experimental procedure
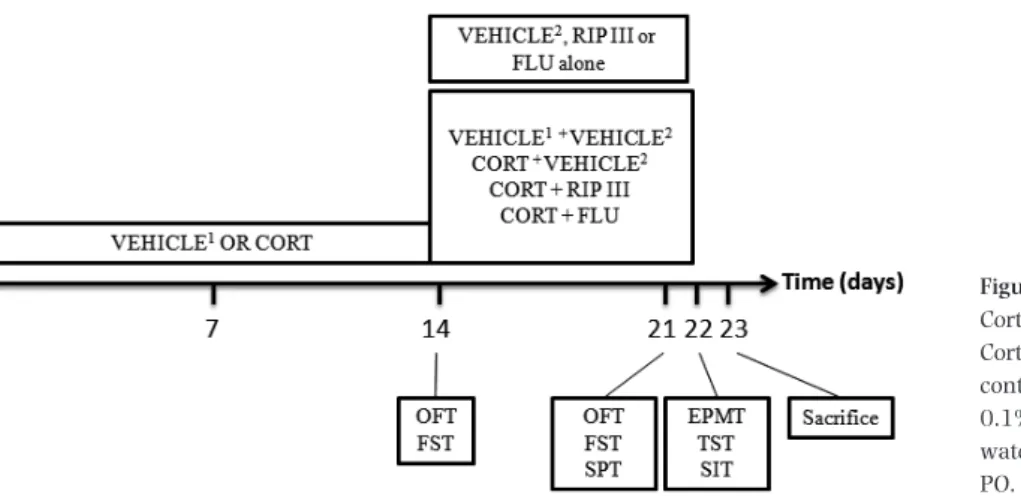
The animals were divided in five experimental groups (n =12 animals/group, on average): control group (vehicle 1: saline containing 0.1% dimethyl sulfoxide and 0.1% Tween-80, SC, during 14 or 21 days and vehi-cle 2: distilled water emulsified with 2% Tween-80, PO, for 8 days), stressed group (Cort, 20 mg/kg, SC, during 14 days or 21 days), Rip III group (50 mg/kg, PO, dur-ing 8 days), and Flu group (50 mg/kg, PO, for 8 days).
An overview of the experimental design is depicted in Figure 2. In the reversal model, we simulated the
MeO
NH
O OH
HO
subchronic treatment of depressive-like episodes [7]. Briefly, each animal group received one daily SC injec-tion of Cort 20 mg/kg or vehicle 1, for 22 days. From the 14th day of treatment onward, animals of the trea-ted groups also received a daily PO administration of vehicle 2, Rip III (50 mg/kg), or Flu (50 mg/kg) for 8 days, with a 1-h interval between treatments. Fur-thermore, Rip III (50 mg/kg) and Flu (50 mg/kg) were administered PO, each drug separately, for eight con-secutive days to evaluate whether these substances were capable of increasing the locomotor activity of healthy animals by themselves. Flu was used as the standard antidepressant because, among selective sero-tonin reuptake inhibitors, it has proven effective in sev-eral studies in monotherapy, specifically without the need to be combined with an antipsychotic drug, and it has shown high response rate in clinical use [23– 27]. Behavioral determinations were registered at the 14th, 21st, and 22nd days of treatment 1 h after the last drug administration.
Behavioral tests Open field test
The open field test (OFT) area was made of acrylic (i.e., transparent walls and black floor, 30 930915 cm) and divided into nine squares of equal area. The OFT was used to evaluate the exploratory activity of the mouse [28]. Each mouse was placed in the center of the arena, and the number of squares crossed with all four paws (i.e., locomotor activity) over a 6-min period was recorded. The parameters observed included the following: number of squares crossed (i.e., locomotor activity), number of grooming events (i.e., body clean-ing with paws, pickclean-ing at the body and pubis with mouth, and face-washing actions), and number of
rearing events (i.e., the animal standing on its hind legs or with its forearm against the wall of the observa-tion cage or in the free air). Before introducing each animal to the area, the arena was cleaned with 5% alcohol to eliminate possible bias due to odors that could remain on the surfaces from the previous ani-mals.
Forced swimming test
Mice were individually forced to swim in an open cylindrical container (diameter, 22 cm; height, 40 cm) that contained 20 cm of water held at 251 °C. The total time during which the mouse remained immobile during a 5-min period was recorded. Immobility was defined as the animal floating in the water without struggling and making only minimal movements nec-essary to keep its head above the water. An increase in the duration of immobility is indicative of depressed-like behavior [29].
Tail suspension test
For the TST, each mouse was suspended by the tail on the edge of a shelf placed 58 cm above a table top. The mouse was secured in place via adhesive tape placed approximately 1 cm from the tip of the tail. The time during which the mouse remained immobile over a 6-min period was recorded. As previously described, each animal underwent to this test only once [30].
Sucrose preference test
The test was performed as described previously [31], with minor modifications. Before the test, the mice were trained to adapt to sucrose solution (2%, w/v) by placing two bottles of sucrose solution in each cage for 18 h; then, one of the bottles was replaced with water
for 18 h. The beginning of the test started with the onset of the dark (active) phase of animals’ cycle. No previous food or water deprivation was applied before the test. Other conditions of the test were applied as described elsewhere [31]. After 18 h, the volumes of consumed sucrose solution and water were recorded and the sucrose preference was calculated by the fol-lowing formula:
Sucrose preference
¼ Sucrose consumption
Water consumption + Sucrose consumption100:
Social interaction test
The testing apparatus consisted of a 60940 cm Plexi-glas box divided into three chambers. Mice were able to move between chambers through a small opening (696 cm) in the dividers. An iron cage in each of the two side chambers contained the probe mice. Test mice were placed in the center chamber. Mice were allowed 5 min of exploration time in the box, after which an unfamiliar, same-sex probe mouse from the same experimental group was placed in one of two restrain-ing cages [32]. The time spent in each of the three chambers was measured, and social preference was defined as follows: (% time spent in the social chamber)
(% time spent in the opposite chamber).
Elevated plus maze test
The EPM for mice consisted of two perpendicular open arms (3095 cm) and two perpendicular closed arms (3095 925 cm). The open and closed arms were connected by a central platform (595 cm). The
plat-form and the lateral walls of the closed arms were made of transparent acrylic. The floor was made of black acrylic. The maze was 45 cm above the floor. After the respective treatment, each animal was placed at the center of the EPM with its nose in the direction of one of the closed arms and was observed for 5 min according to the following parameters: the number of entries in the open arms (NEOA) and closed arms (NECA) and the time spent within each of them (i.e., the time of permanence). The data were expressed as percentages. Anxiolytic compounds reduce the animal’s natural aversion to the open arms and promote the exploration thereof. On the other hand, the forced or voluntary passages of the animal into the open arms of the EPM are associated with hormonal and behavioral changes indicative of increased anxiety [33].
BDNF analysis
The animals were killed 23 days after the beginning of the experiments by decapitation. The skulls were removed, and hippocampi were dissected and stored in a freezer at 80°C for posterior biochemical analysis. BDNF levels in hippocampi were measured by anti-BDNF sandwich-ELISA, according to the manufacturer’s instructions (Chemicon, Billerica, MA, USA). Briefly, mouse hippocampi were homogenized in phosphate-buf-fered saline (PBS) with 1 mMphenylmethylsulfonyl fluo-ride (PMSF) and 1 mM ethylene glycol tetraaceticacid (EGTA). Microtiter plates (96-well flat-bottom) were coated for 24 h with the samples prepared at 1 : 2 in a diluent and the standard curve ranged from 7.8 to 500 pg/mL of BDNF. The plates were then washed four times with sample diluent. A monoclonal anti-BDNF rab-bit antibody diluted 1 : 1000 in sample diluent was then added to each well and incubated for 3 h at room tem-perature. After washing, a peroxidase-conjugated anti-rabbit antibody (diluted 1 : 1000) was added to each well and incubated at room temperature for 1 h. After the addition of streptavidin-enzyme, substrate, and stop solution, the amount of BDNF was determined by absor-bance in 450 nm and expressed as pg per g wet tissue protein. The standard curve demonstrates a direct rela-tionship between optical density and BDNF concentra-tion. Total protein was measured by Lowry’s method using bovine serum albumin as a standard [34].
Statistical analyses
All experimental results are expressed as the mean standard error of the mean (SEM). Statistical analyses were performed using one-way analysis of variance (ANOVA). In all cases, Student–Newman–Keuls test was applied forpost hoc comparisons. Results were considered significant atP <0.05.
R E S U L T S
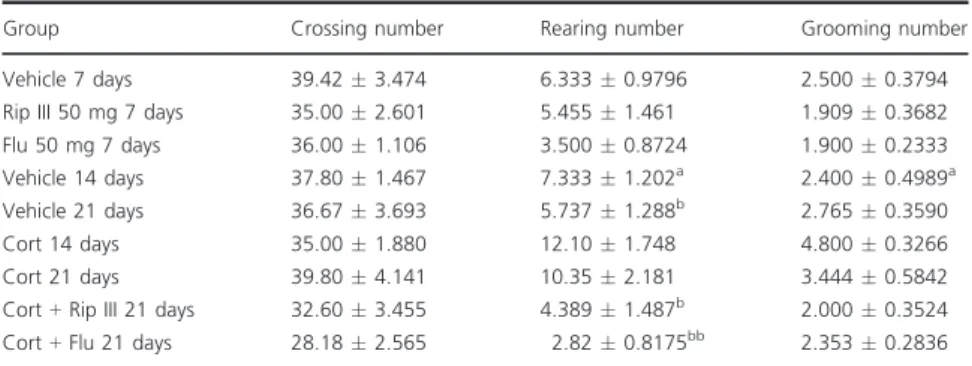
It was also possible to note that the administration of Rip III, Flu, or vehicle does not alter the animals motors parameters of the OFT, as noticed inTable I.
On the other hand, Cort injections for 21 days signif-icantly decreased the four parameters analyzed in the EPM test: number of entries in the open arms (NEOA), percentage of entries into open arms (PEOA), time of permanence in the open arms (TPOA), and percentage of time of permanence in the open arms (PTOA) as compared to the control. It is possible to observe in Table II that these parameters were normalized by the administration of both Rip III and Flu for eight consec-utive days.
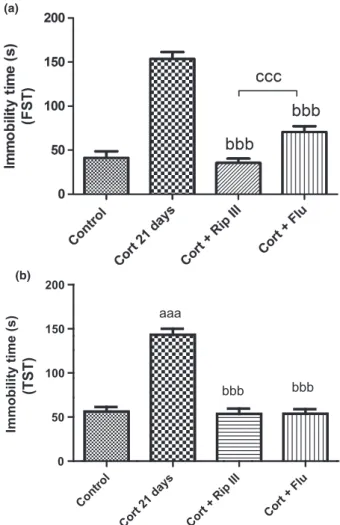
Figures 3 and 4show that repeated Cort injection for 14 and 21 days significantly increased the immobility time for mice in the FST (control: 41.187.498; Cort 14-treated group: 123.8 9.702,P <0.001; Cort 21-treated group: 153.5 7.940, P <0.001) and TST (control: 56.335.060; Cort 21-treated group: 143.36.813, P<0.001). This increased immobility indicates depressive-like behavior in mice. In contrast, the administration of Flu or Rip III for 7 days avoided the increase of this parameter in the groups of animals stressed by Cort.
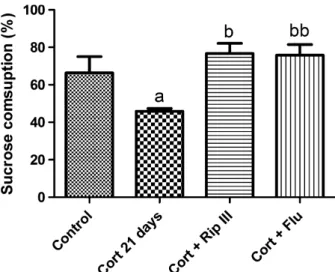
In the evaluation of the sucrose preference parame-ter (Figure 5), Cort 21-treated group had a lower
sucrose preference compared with the control, Rip III, and Flu groups. In addition, treatment with Rip III and Flu led to recovery of sucrose preference in Cort-exposed mice (control: 63.978.61; Cort: 46.311.508; Cort+Rip III: 76.795.431; Cort+Flu: 81.47 5.652,P< 0.001).
Our data revealed that Cort administration signifi-cantly decreased the percentage for social preference when compared with vehicle-treated animals. Further-more, Rip III and Flu reversed Cort-induced decrements in the percentage of social preference, as described in Figure 6 (control: 79.506.859; Cort 21-treated group: 56.548.393, P <0.05; Cort+Rip III: 78.132.301, P<0.05; Cort+Flu: 84.52 4.083, P <0.05).
Cort administration significantly decreased BDNF lev-els (Figure 7). They were slightly recovered by Rip III and Flu administration (control: 468.73.22; Cort: 168.721.37; Cort+Rip III: 186.716.91; Cort+Flu: 260.9 13.55,P< 0.05).
D I S C U S S I O N
The present study demonstrated that the administra-tion of Rip III was capable of reversing the neurochem-ical and behavioral alterations induced by repeated
Group Crossing number Rearing number Grooming number Vehicle 7 days 39.423.474 6.3330.9796 2.5000.3794 Rip III 50 mg 7 days 35.002.601 5.4551.461 1.9090.3682 Flu 50 mg 7 days 36.001.106 3.5000.8724 1.9000.2333 Vehicle 14 days 37.801.467 7.3331.202a 2.400
0.4989a
Vehicle 21 days 36.673.693 5.7371.288b 2.765
0.3590 Cort 14 days 35.001.880 12.101.748 4.8000.3266 Cort 21 days 39.804.141 10.352.181 3.4440.5842 Cort+Rip III 21 days 32.603.455 4.3891.487b 2.000
0.3524 Cort+Flu 21 days 28.182.565 2.820.8175bb 2.353
0.2836 Significant values:a
P<0.05 vs.CORT 14 days;b
P<0.05 andbb
P<0.01 vs.CORT 21 days.
Table IDrugs were administered separately and in combination. Results are expressed as the meanSEM of
n=10–15 animals/group. Statistical analysis was determined by one-way ANOVAfollowed by Student–Newman– Keuls test.
Group NEOA PEOA TOA PTPO Vehicle 21 days 7.0000.5641 40.422.215 56.584.195 35.551.887 Cort 21 days 4.4170.4840a 26.512.098aaa 36.463.831aa 21.912.195aa
Cort+Rip III 21 days 6.8330.5198b 41.80
2.756bbb 66.94
5.378bbb 38.21
2.647bbb
Cort+Flu 21 days 7.5000.7923bb 39.47
1.881bbb 62.92
3.892bb 31.29
2.179bb
Significant values: bP
<0.05, bbP
<0.01 and bbbP
<0.001 vs. Cort 21-treated group and aP <0.05,
aa
P<0.01 andaaa
P<0.001 vs.vehicle 21-treated group.
administration of Cort. It is was possible to verify that subchronic treatment with Rip III and Flu on stressed mice increased immobility time in the forced swimming and tail suspension tests while it maintained locomotor function, normalized the number of rearing events (a type of stereotyped behavior), normalized parameters of anxiety, increased the percentages of social interaction, increased the percentage of sucrose preference, and normalized the levels of hypothalamic BDNF.
These findings are in agreement with data from sci-entific literature, which support anxiolytic and antide-pressant actions for Rip III in basic studies [20,21]. A previous study using models of acute depression veri-fied normalization of depressive behavior and an addi-tional increase in the monoamines levels [22]. The same study concluded that Rip III was able to increase the levels of monoamines NA, 5-HT and DA in the stri-atum and prefrontal cortex, and NA and 5-HT in the hippocampus, while decreasing their metabolites in the striatum and prefrontal cortex [22].
Regarding anti-immobility effects, Detke et al. [35] indicated that antidepressant drugs that enhance noradrenergic neurotransmission increase climbing behavior, whereas the enhancement of serotonergic neurotransmission increases swimming time in the forced swimming test. Likewise, our findings indicate that Rip III (and Flu) consistently reduced immobility time in mice.
The efficacy of the most widely used antidepressants is limited by their theoretical reliance on the monoamine
hypothesis. Nevertheless, depression is a disease that is too intricate to be explained by the monoamine hypoth-esis alone. Thus, new therapeutic agents, based on theo-ries other than those focused on monoamines, are garnering increasing interest for the treatment of depression. Possible candidates are agents that may intervene in the damaging process of oxidative/nitrosa-tive stress and that increase the levels of BDNF [36,37].
The neurotrophin hypothesis of depression suggests that there is a reduction in BDNF levels in depressive individuals, and that the reversal of this situation could Figure 3 Values are expressed as the meanSEM (n=10–15).
Statistical analysis was determined by one-wayANOVAfollowed by Student–Newman–Keuls test. Significant values:aaaP<0.001 vs. vehicle-treated group andbP
<0.05 vs. CORT 14-treated group.
(a)
(b)
Control
Cort 21 days Cort + Rip III Cort + Flu
0 50 100 150 200
bbb bbb aaa
Immobility time (s)
(TST)
Figure 4Effect of subchronic administration Rip III (50 mg/kg) and Flu (50 mg/kg) on the immobility time in FST (a) and in TST (b) in a chronic Cort-induced depression mouse model. Results are expressed as the meanSEM ofn=10–15 animals/group. Statistical analysis was determined by one-wayANOVAfollowed by Student–Newman–Keuls test. Significant values:bbbP
<0.001 vs. Cort 21-treated group andccc
be involved with the production of antidepressant action [38]. It was observed in acute and chronic ani-mal models that the reduced levels of BDNF brain pre-dispose depression [39]. Still regarding chronic stress,
corticosterone and other stress factors have been reported to decrease neurogenesis in the dentate gyrus (DG) [40–45]. Many studies have suggested that sig-naling of BDNF-TrkB receptor is required for the nor-mal action of antidepressant drugs [46,47]. Our results showed that administration of corticosterone reduced BDNF levels in the hippocampus, and that the adminis-tration of Rip III (50 mg/kg) and Flu (50 mg/kg) aids in the reversal of this situation. The first classes of antidepressants aimed at restoring monoamine neuro-transmitter’s levels while not considering other factors. However, it was observed that the treatment with antidepressants seems to increase neurotrophic factors, recovering normal values as well as cell proliferation and survival, which are probably associated with DG neurogenesis [48,49].
In the present study, the beneficial effects of Rip III on Cort-induced behavioral alterations is evidenced by the reduction of anhedonia in SPT and the increased percentage of social interaction in the SIT.
Anhedonia, loss of interest on pleasure, while in association with depressed mood, is a necessary crite-rion for the diagnosis of depression [50,51]. It is related to dysfunction in the brain reward system [52]. There is no definite and specific pharmacological approach to the treatment of anhedonia in depression so far, and even though it is clear that antidepressants treat depressive symptoms as a whole [53], anhedonia remains a difficult symptom to treat. Also, first-line pharmacotherapies are not always sufficient to correct Figure 5 Effect of subchronic administration Rip III (50 mg/kg)
and Flu (50 mg/kg) on the percentage of sucrose consumption of animals in a chronic Cort-induced depression mouse model. Results are expressed as the meanSEM ofn=10–15 animals/ group. Statistical analysis was determined by one-wayANOVA followed by Student–Newman–Keuls test. Significant values:
a
P<0.05 vs. control group.bP<0.05;bbP<0.01 vs. CORT 21-treated group.
Control
Cort
Cort + Rip III Cort + F lu
0 20 40 60 80
100 b
b
a
% Social preference
Figure 6 Results are expressed as the meanSEM ofn=10–15 animals/group. Statistical analysis was determined by one-way
ANOVAfollowed by Student–Newman–Keuls test. Significant values:aP
<0.05 vs. control group;bP
<0.05 vs.CORT 21-treated group.
Control
Cort
Cort + III Cort + Flu
0 100 200 300 400 500
b
bb
a
BDNF pg/g
Figure 7 Statistical analysis was determined by one-wayANOVA followed by Student–Newman–Keuls test. Significant values:
a
the aforementioned situation [54,55]. Our results obtained from SPT are promising.
The experiments also showed that animals treated with Flu and Rip III spent more time in social housing. The features of social interaction are associated exten-sively with depression due to its relative unique rela-tion to anhedonia [56–59]. This is due to social interaction interfering with a vast range of social encounters, which serve as sources of pleasure [59].
The animal model of depression used in this study has also been appointed as a supposed model of psy-chotic depression and resistant depression [17,60]. It is interesting to notice that antidepressant-type effects triggered by the administration of Rip III are similar to those of Flu, which has shown high response rate in cases of psychotic MD [26]. This experimental evidence opens perspectives for further studies that may lead to future therapeutic uses of Rip III in psychotic or resis-tant depression.
C O N C L U S I O N
Our study therefore provides experimental evidence and opens perspectives for further studies that may lead to future therapeutic use of Rip III in the treat-ment of depression associated with anxiety. However, subsequent and better detailed studies are needed to confirm the mechanisms involved in the effects pro-duced by Rip III.
A C K N O W L E D G E M E N T S
This study was supported in part by grants from Con-selho Nacional de Desenvolvimento Cientıfico e Tec-nologico (CNPq-Brazil), Coordenacß~ao de Aperfeicßoamento de Pessoal de Nıvel Superior (CAPES-Brazil) and Fundacß~ao Cearense de Apoio a Pesquisa (FUNCAP-Ceara-Brazil).
A B B R E V I A T I O N S
ANOVA– analysis of variance Cort – corticosterone
EPM – elevated plus maze test Flu – fluvoxamine
FST – forced swimming test
HPA – hypothalamic–pituitary–adrenal
NECA – number of entries in the closed arms NEOA – number of entries in the open arms OFT – open field test
PEOA – percentage of entries into open arms PO –per os
PTOA – percentage of time of permanence in the open arms
Rip III – riparin III
TPOA – time of permanence in the open arms TST – tail suspension test
R E F E R E N C E S
1 World Health Organization. Depression. Fact sheet N°369. October 2012. http://www.who.int/mediacentre/factsheets/ fs369/en/.
2 Ye L., Hu Z., Du G. et al. Antidepressant-like effects of the extract fromCimicifuga foetidaL. J. Ethnopharmacol. (2012) 144683–691.
3 Ferrari A.J., Charlson F.J., Norman R.E. et al. The epidemiological modeling of major depressive disorder: application for the global burden of disease study (2010) PLoS One. (2013)81–14.
4 Rai D., Zitko P., Jones K., Lynch J., Araya R. Country- and individual-level socioeconomic determinants of depression: multilevel cross-national comparison. Br. J. Psychiatry (2013) 24195–203.
5 Kornstein S.G., Schneider R.K. Clinical features of treatment resistant depression. J. Clin. Psychiatry (2001)1618–25. 6 Carrier N., Kabbaj M. Sex differences in the antidepressant-like
effects of ketamine. Neuropharmacology (2013)193–8. 7 Silva M.C.C., Sousa C.N.S., Sampaio L.R.L. et al.
Augmentation therapy with alpha-lipoic acid and desvenlafaxine: a future target for treatment of depression? Naunyn-Schmiedeberg’s Arch. Pharmacol. (2013)386685– 695.
8 Dinan L. Phytoecdysteroids inKochia scoparia(burning bush). J. Chromatogr. A (1994)65869–76.
9 Reus V.I.R., Miner C. Evidence for physiological effects of hypercortisolemia in psychiatric patients. Psychiatry Res. (1985)1447–56.
10 Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology (2000)23477–501. 11 Pariante C.M., Miller A.H. Glucocorticoid receptors in major
depression: relevance to pathophysiology and treatment. Biol. Psychiatry (2001)49391–404.
12 Carroll B.J., Cassidy F., Naftollowtiz D. et al. Pathophysiology of hypercortisolism in depression. Acta Psychiatr. Scand. Suppl. (2007)43390–103.
13 Pitta S., Augustine B.B., Kasala E.R., Sulakhiya K.,
Ravindranath V., Lahkar M. Honokiol reverses depressive-like behavior and decrease in brain BDNF levels induced by chronic corticosterone injections in mice. Pharmacognosy J. (2013)5211–215.
15 Inoue T., Koyama T. Effects of acute and chronic administration of high dose corticosterone and
dexamethasone on regional brain dopamine and serotonin metabolism in rats. Prog. Neuropsychopharmacol. Biol. Psychiatry (1996)20147–156.
16 Ago Y., Yano K., Araki R. et al. Metabotropic glutamate 2/3 receptor antagonists improve behavioral and prefrontal dopaminergic alterations in the chronic corticosterone-induced depression model in mice. Neuropharmacology (2013)6529–38.
17 Sterner E.Y., Kalynchuk L.E. Behavioral and neurobiological consequences of prolonged glucocorticoid exposure in rats: relevance to depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry (2010)34777–790.
18 National Institute of Mental Health (NIMH). Questions and Answers about the NIMH Sequenced Treatment Alternatives to Relieve Depression (STAR*D) Study—All Medication Levels (2006).
19 Carlson E.E. Natural products as chemical probes. ACS Chem. Biol. (2010)5639–653.
20 Melo, C.T.V. Investigacß~ao do efeito antidepressivo da riparia III: alteracß~oes comportamentais, neuroquımicas e avaliacß~ao do estresse oxidativo. 2012. Tese (doutorado).–Universidade Federal do Ceara, Centro de Ci^encias da Saude, Faculdade de Medicina, Departamento de Fisiologia e Farmacologia, Programa de Pos-Graduacß~ao em Farmacologia, Doutorado em Farmacologia, Fortaleza, 2012.
21 Sousa F.C., Melo C.T., Monteiro A.P. et al. Antianxiety and antidepressant effects of riparin III fromAniba riparia(Nees) Mez (Lauraceae) in mice. Pharmacol. Biochem. Behav. (2004) 7827–33.
22 Melo C.T., de Carvalho A.M., Moura B.A. et al. Evidence for the involvement of the serotonergic, noradrenergic, and dopaminergic systems in the antidepressant-like action of riparin III obtained fromAniba riparia(Nees) Mez (Lauraceae) in mice. Fundam. Clin. Pharmacol. (2013)27104–112. 23 Kroessler D. Relative efficacy rates for therapies of delusional
depression. Convul. Ther. (1985)1173–182.
24 Zanardi R., Perez J., Smeraldi E. Fluvoxamine alone in the treatment of delusional depression. Eur.
Neuropsychopharmacol. (1996)6126.
25 Zanardi R., Franchini L., Gasperini M., Perez J., Smeraldi E. Selective serotonin reuptake inhibitors alone and in association with pindolol in the treatment of delusional depression. Eur. Neuropsychopharmacol. (1998)8598–599. 26 Gatti F., Bellini L., Gasperini M., Perez J., Zanardi R., Smeraldi
E. Fluvoxamine alone in the treatment of delusional depression. Am. J. Psychiatry (1996)153414–416. 27 Furuse T., Hashimoto K. Fluvoxamine for aripiprazole-associated
akathisia in patients with schizophrenia: a potential role of sigma-1 receptors. Ann. Gen. Psychiatry. (2010)69–11. 28 Archer J. Tests for emotionality in rats and mice: a review.
Anim. Behav. (1973)21205–235.
29 Porsolt R.D., Bertin A., Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch. Int. Pharmacodyn. Ther. (1977)229327–336.
30 Steru L., Chermat R., Thierry B. et al. Tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (1985)5367–370.
31 Strekalova T., Steinbuch H.W.M. Measuring behavior in mice with chronic stress depression paradigm. Prog.
Neuropsychopharmacol. Biol. Psychiatry (2010)34348–361. 32 Radyushkin K., Hammerschimidt K., Boretius S. et al.
Neuroligin-3 deficient mice: model of a monogenic heritable form of autism with an olfactory deficit. Genes Brain Behav. (2009)8416–425.
33 Lister R.G. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (1987)92180–185. 34 Frey B.N., Andreazza A.C., Cereser K.M.M. et al. Effects of
mood stabilizers on hippocampus BDNF levels in an animal model of mania. Life Sci. (2006)79281–286.
35 Detke M.J., Rickels M., Lucki I. Active behavior in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (1995)12166–172.
36 Pae C.U. Monocyte chemoattractant protein-1 (MCP1) may play a role in depression. Prog. Neuropsychopharmacol. Biol. Psychiatry (2008)32313–314.
37 Lee S., Lee S., Han C., Patkar A.A., Masan P.S., Pae C. Oxidative/nitrosative stress and antidepressants: targets for novel antidepressants. Prog. Neuropsychopharmacol. Biol. Psychiatry (2013)46224–235.
38 Duman R.S., Monteggia L.M. A neurotrophic model for stress-related mood disorders. Biol. Psychiatry (2006)591116– 1127.
39 Karege F., Perret G., Bondolfi G., Schwald M. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psych. Res. (2002)15143–148. 40 Cameron H.A., McKay R.D. Restoring production of
hippocam-pal neurons in old age. Nat. Neurosci. (1999)2894–897. 41 Tanapat P., Hastings N.B., Rydel T.A., Galea L.A., Gould E.
Exposure to fox odor inhibits cell proliferation in the hippocampus of adult rats via an adrenal hormone-dependent mechanism. J. Comp. Neurol. (2001)437496–504. 42 Mattson M.P., Duan W., Wan R., Guo Z. Prophylactic
activa-tion of neuroprotective stressor response pathways by dietary and behavioral manipulations. NeuroRx (2004)1111–116. 43 Mirescu C., Gould E. Stress and adult neurogenesis.
Hippocampus (2006)16233–238.
44 Pawluski J.L., Brummelte S., Barha C.K., Crozier T.M., Galea L.A. Effects of steroid hormones on neurogenesis in the hippocampus of the adult female rodent during the estrous cycle, pregnancy, lactation and aging. Front.
Neuroendocrinol. (2009)30343–357.
45 Cherng C.G., Lin P.S., Chuang J.Y. et al. Presence of conspecifics and their odor-impregnated objects reverse stressor-decreased neurogenesis in mouse dentate gyrus. J. Neurochem. (2010)1121138–1146.
47 Castren E., V~oikar V., Rantam€aki T. Role of neurotrophic factors in depression. Curr. Opin. Pharmacol. (2007)718–21. 48 Schmidt H.D., Duman R.S. The role of neurotrophic factors in
adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behav. Pharmacol. (2007)18391–418.
49 David D.J., Samuels B.A., Rainer Q. et al. Neurogenesis-dependent and -inNeurogenesis-dependent effects of fluoxetine in an animal model of anxiety/depression. Neuron (2009)62479–493. 50 Kendler K.S., Mun~oz R.A., Murphy G. The development of the
Feighner criteria: a historical perspective. Am. J. Psychiatry (2010)167134–142.
51 Gaillard R., Gourion D., Liorca P.M. L’anhedonie dans la depression. L’Encephale (2013)39296–305.
52 Keedwell P.A., Andrew C., Williams S.C.R., Brammer M.J., Phillips M.L. The neural correlates of anhedonia in major depressive disorder. Biol. Psychiatry (2005)58843–853. 53 Di Giannantonio M., Martinotti G. Anhedonia and major
depression: the role of agomelatine. Eur. Neuropsychopharmacol. (2012)22505–510.
54 Mccabe C., Cowen P.J., Harmer C.J. Neural representation of reward in recovered depressed patients. Psychopharmacology (2009)205667–677.
55 Franco-Chaves J.A., Mateus C.F., Luckenbaugh D.A., Martinez P.E., Mallinger A.G., Zarate C.A. Jr Combining a dopamine agonist and selective serotonin reuptake inhibitor for the treatment of depression: a double-blind, randomized pilot study. J. Affect. Disord. (2013)149319–325.
56 Alden L.E., Taylor C.T., Mellings T.M.J.B., Laposa J.M. Social anxiety and the interpretation of positive social events. J. Anxiety Disord. (2008)22577–590.
57 Brown E.J., Turovsky J., Heimberg R.G., Juster H.R., Brown T.A., Barlow D.H. Validation of the social interaction anxiety scale and the social phobia scale across the anxiety disorders. Psychol. Assess. (1997)921–27.
58 Hughes A.A., Heimberg R.G., Coles M.E., Gibb B.E., Liebowitz M.R., Scheneier F.R. Relations of the factors of the tripartite model of anxiety and depression to types of social anxiety. Behav. Res. Ther. (2006)441629–1641.
59 Kashdan T.B. Social anxiety spectrum and diminished positive experiences: theoretical synthesis and meta-analysis. Clin. Psychol. Rev. (2007)27348–365.
![Figure 1 The image depicts the chemical structure of Riparin III, an alcamide isolated from the Aniba riparia plant [21].](https://thumb-eu.123doks.com/thumbv2/123dok_br/15267116.540524/2.892.497.758.883.990/figure-depicts-chemical-structure-riparin-alcamide-isolated-riparia.webp)