w w w . s b f g n o s i a . o r g . b r / r e v i s t a
Review
Article
Horse
chestnut
–
efficacy
and
safety
in
chronic
venous
insufficiency:
an
overview
Marlena
Dudek-Makuch
∗,
El ˙zbieta
Studzi ´nska-Sroka
DepartmentofPharmacognosy,PoznanUniversityofMedicalSciences,Pozna´n,Polanda
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received14January2015 Accepted6May2015 Availableonline29June2015
Keywords: Aescin
Chronicvenousinsufficiency Horsechestnutseeds
a
b
s
t
r
a
c
t
Theextractfromhorsechestnutseeds(AesculushippocastanumL.,Sapindaceae),standardisedforthe contentofaescin,isusedasthetreatmentforchronicvenousinsufficiency.Ithasanti-inflammatoryand anti-oedematouspropertiesandindicatesapositiveeffectonthevenoustone,rheologicalproperties, andbloodcoagulability.Themechanismofhorsechestnutseedextract/aescinactivitywasproposedon thebasisofinvitroandinvivostudies,anditseffectivenesswasdocumentedwithnumerousrandomised clinicaltrials.Theresultsofthestudieshaveproventhathorsechestnutseedextractnotonlysignificantly improvessubjectivesymptomsinpatientswithchronicvenousinsufficiencylikecalfspasm,legpain, pruritus,fatigue,butitalsoreducedlegvolume,theankleandcalfcircumference.Thepreparations con-taininghorsechestnutseedextractareverypopularandtheyhavesimilareffectivenessascompression therapyandapreparationwithO-(-hydroxyethyl)-rutosides.Moreover,horsechestnutseedextract hasbeenproventobesafeandverywelltolerated.Thestudywastopresenttheresultsofthestudies thathavebeenconductedsofarandthathaveconfirmedtheeffectivenessofuseofhorsechestnutseed extractoraescinasthetreatmentforchronicvenousinsufficiency.
©2015SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Allrightsreserved.
Introduction
Chronic venous insufficiency (CVI) is a disorder affecting approximately25%oftheEuropeancommunity,womenin par-ticular(Piechaletal.,2005).CVIiscausedbyinbornoracquired anomaliesinthefunctioningofthevenoussystem,resultingfrom primarydefectsinaveinwallandvalvestructureaswellas insuf-ficiencythereof,andbyfactorsinfluencingtheweakenedtension andstructurethereof,suchashormonalchanges,pregnancy, obe-sity,limitedactivity,workinginasittingorstandingposition,and oralcontraceptives(Sudoł-Szopi ´nskaetal.,2006).Asaresultofthe weakenedvasculartensionandstructure,bloodcongestion, vascu-larbedoverflow,andhypoxiaoccur;inconsequence,mitochondrial oxidativephosphorylationisinhibited,andthecontentof adeno-sinetriphosphate(ATP)islowered.AdecreaseinATPcontentin endotheliumcellsinducesaseriesofcellularmodifications,such asanincreaseincytosolic calciumconcentration,thereleaseof inflammatorymediators,suchasprostaglandins(Michielsetal., 1993), and the platelet-activating factor (PAF) (Arnould et al., 1993)whichresultintherecruitment,activationandadhesionof
∗ Correspondingauthor.
E-mail:mbdudek@ump.edu.pl(M.Dudek-Makuch).
polymorphonuclearneutrophils(Arnouldetal.,1996).Leukocytes adheringtothevascular wallreleasephospholipase A2
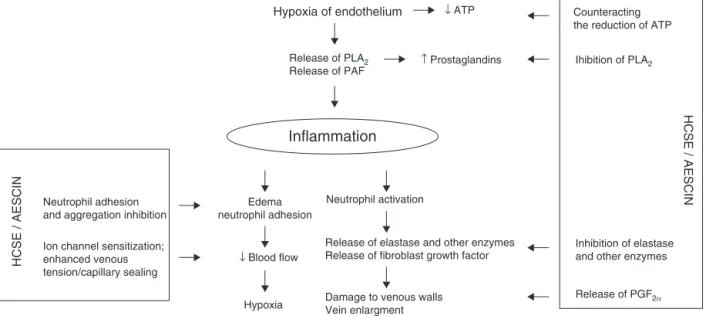
respon-siblefor productionof inflammationprecursors,toxicoxidative metabolites,andlysosomalenzymes(elastase,collagenase).They alsoleadtotheincreasedactivityofhyaluronidasethatdegrades hyaluronicacid, themajorconstituentof capillaryendothelium. Theincreasedactivityofotherenzymesbeingcomponentsof vas-cularwalls,i.e.-N-acetylglucosaminidase, -glucuronidaseand arylosulphatase,responsiblefordegradationofproteoglycans,has alsobeenobservedinchronicvenousinsufficiency.Degradation of hyaluronic acidand proteoglicans resultsin violation of the integrity ofblood vesselwalls, increasedcapillarypermeability, andfragility(ESCOP,2003).Inthecourseofaninflammatory reac-tion,histamineandserotonin–theenzymesaffectingtheincrease in capillary permeability – are released, too. The effect of the above-mentionedprocessesismigrationofleukocytesoutsidethe vascular walls,exacerbation oftheinflammation, occurrenceof oedema,andpathologicalchangesinveins(Fig.1).
Medicinesofplantoriginplayasignificantroleinthe pharma-cologicaltreatmentofCVI.Themostpopularonesincludethehorse chestnutseedextract oraescinisolated fromitand flavonoids: diosmin,hesperidin,rutin,anditsderivative–troxerutin.
Theaimofthestudywastopresenttheresultsof the stud-iesthathavebeenconductedsofarandthathaveconfirmedthe
http://dx.doi.org/10.1016/j.bjp.2015.05.009
Neutrophil adhesion and aggregation inhibition
Ion channel sensitization; enhanced venous tension/capillary sealing
Hypoxia
HCSE / AESCIN
HCSE / AESCIN
Inflammation
↓ Blood flow Edema neutrophil adhesion
Neutrophil activation
Counteracting the reduction of ATP
Release of elastase and other enzymes Release of fibroblast growth factor
Damage to venous walls Vein enlargment
Release of PGF2α
Ihibition of PLA2
Release of PLA2
Release of PAF
↑ Prostaglandins
Hypoxia of endothelium ↓ ATP
Inhibition of elastase and other enzymes
Fig.1.MechanismsofHCSE/aescinactivityinchronicvenousinsufficiency.ModifiedbasedonSirtori(2001).
effectivenessofuseofhorsechestnutseedextract(HCSE)oraescin asthetreatmentforCVI.
Materialandmethods
Inclusionandexclusioncriteria
Onlyrandomised,controlledtrialstestingtheefficacyoforal preparationscontaining HCSE as the only active component (a mono-preparation) withthe placeboor reference therapy were included.TrialsassessingHCSEasoneofseveralactivecomponents inacombinationpreparationorasapartofthecombination treat-mentwereexcluded.Incaseofpreparationsusedexternally,dueto alackofmono-preparations,thecombinationspreparationswere alsoincluded.Inallthetrialstheextractwasstandardisedtoaescin. The studies included if participants were patients withCVI (internaluse),andCVI,SVT,andfootulcersasaresultofdiabetes complications(externaluse).Thestudiesusingclinicaloutcome measureswereincluded,whereasthestudiesfocusingexclusively onphysiologicalparameterswereexcluded.
ThefollowingelectronicEnglishdatabasesweresearched:Ovid Medline, Pubmed and The Cochrane Library, from 1976 up to December2014.Theyweresearchedbytitleandabstractusing thefollowingsearchterms:Aesculushippocastanum,Hippocastani semen,horsechestnutseeds,aescin,HCSE,CVI.Handsearcheswere alsoconductedforpublicationsnotstored inthedatabases(e.g. webpages).
Referencelistsofallthearticlesweresearchedforfurther pub-lications.Fortheselectionofthemanuscripts,twoindependent investigators(MDM,ESS)firstassessedallthetitlesandabstracts andthencarriedoutfulltextanalysesofthepublications,against predefinedinclusioncriteria.
Nineteentrialsmettheabove-mentionedinclusioncriteria.Of these,ninewereplacebo-controlled;twocomparedHCSEagainst the reference treatment with compression stockings and the
placebo(Diehmetal.,1996;DiehmandSchmidt,2001);fourwere controlledagainst referencemedicationwith O--hydroxyethyl rutosides(HR)(Erdlen,1989;KalbfleischandPfalzgraf,1989;Erler, 1991;Rehnetal.,1996),andonewascontrolledagainstmedication withpycnogenol(Koch,2002).Threetrialsrelatedtopreparations usedexternallywererandomised,buttheirefficacywasnot com-paredwiththeplacebo.
Chemicalconstituents
Horsechestnutseedsarerichinsaponins(3–5%),overthirtyof whichhavebeenisolatedandidentified.Themaincompoundis aescin– amixtureofacylatedtriterpeneglycosides.Three frac-tionsofaescin,denotedascrypto-, ␣-, and-aescinhavebeen described inthe literature.Cryptoaescin containsC-28-O-acetyl saponins,and-aescincontainsC-22-O-acetylsaponins,whereas
TheeffectivenessofHCSEandaescinasthetreatmentforchronic venousinsufficiencyresultsfromtheanti-oedematousand anti-inflammatoryactivityaswellastheirinfluenceonthetensionof bloodvesselwalls.Thebiologicalactivityofbothhorsechestnut seedextract(HCSE)standardisedforthecontentofaescinand iso-latedaescinhasbeenconfirmedbynumerousinvitro,invivo,and theclinicalstudies(PittlerandErnst,2012).Theresultsfromthe studieshavebeenpresentedbelowandsummarisedin chronolog-icalorderinTables1–3.
Anti-inflammatoryandanti-oedematousactivity
Ithasbeenconfirmedthataescinismainlyresponsibleforthe anti-inflammatoryandanti-oedematousactivityofthehorse chest-nutseedextract(Table1).Theresearchresultshaveshownthat thecompleteextracthasbeenaround100timesmoreeffectiveas thetreatmentforinflammationandlymphoedemainratsthanthe extractfromwhichaescinwasremoved(GuillaumeandPadioleau, 1994).
Themechanismoftheanti-inflammatoryandanti-oedematous activityofHCSEandaescinismulti-directionalandhasbeen pro-posedonthebasisofresultsofinvitroandinvivostudies(Fig.1). ItwasdemonstratedthataescincounteractedATPreductionand anincreaseintheactivityofphospholipaseA2responsibleforthe
releaseofprecursorsofinflammatorymediators(Arnouldetal., 1996;Sirtori,2001).Moreover,aescinreducedneutrophiladhesion andaggregation,whichwasshowninexvivostudiesonanisolated humanumbilicalvein.Incubationthereofinhypoxicconditionsin anaescinsolutionorwithoutitprovedthataescininhibited adhe-sionofneutrophilstothevascularendothelium,activatedthem, andproducedleukotrieneB4andthesuperoxideanion(Bougelet etal.,1998).Inanexperimentalmodelofratpleurisy,HCSEreduced plasmaextravasationandleukocytemigrationtothepleuralcavity aswellasthereleaseofinflammatorymediators,whichresultedin inhibitionofinflammationandoedema(GuillaumeandPadioleau, 1994).Ininvitrostudies,theaescinrestrainedhyaluronidase activ-ityby93%,whichresultedininhibitionofpermeabilityandthe lossofplasmafromthecells ofvascularendotheliumand, asa consequence,inadecreaseintheoccurrenceofoedema(Facino etal.,1995).Inaddition,aescindecreasedtheactivityoflysosomal enzymes,whichshiftedtheproteoglicansynthesis-breakdown bal-ancetowardssynthesisthereof.Invivostudiesshowedthataescin administeredintraperitoneallytoratsforthreeweekssignificantly reduceddegradationofmucopolysaccharidesintheconnective tis-sue(thexiphoidprocess)(Panigati,1992).AescinandHCSEarealso characterisedbytheantihistaminicandantiserotoninactivity.It wasdemonstrated thatHCSEadministeredorallytorats dimin-ishedorinhibitedexcessivepermeabilityofskincapillariescaused bypreviousadministrationofhistamineandserotonin(Guillaume andPadioleau,1994).ThelaterresearchprovedthataescinIb(2), IIa(3),andIIb(4)hadtheantihistaminicandanti-serotonin activ-itywhileaescinIa(1)mainlyinhibitedhistamine(Matsudaetal., 1997).Theanti-exudativeactivityofaescinisalsoconnectedwith selectivesensitisation ofvascular smooth muscles toCa2+ions,
whichleadstotheincreasedtensionandsealingthereofand,as a result, toa decreasein theinflammation caused by vascular endotheliumhypoxia. Results of in vitro studies confirmedthe above.Aescincausedconcentration-dependentcontractionrings ofinferiorvena cavafrommaleratsincubatedinnormalKrebs. InCa2+−freeKrebstherewasessentiallynocontractiontoaescin,
butinaescin-treatedveinsincubatedinCa2+freeKrebs,stepwise
additionofextracellularCaCl2causedcorrespondingincreasesin
contraction(RaffettoandKhalil,2011).
TheeffectivenessofaescinandHCSEinternalapplication(p.o.,
i.v., i.p., s.c.) to prevent the formation of oedema was mainly
demonstratedinmodelsofinflammationthatreproducethe ini-tialexudativephases,suchasoedemaoflaboratoryanimalpaws inducedbyarangeofirritatingagents(albuminfromchickenegg white(Girergetal.,1961)dextran(Damasetal.,1976),cotton pel-let,carrageenin(Ceboetal.,1976;Matsudaetal.,1997),bradykinin, compound48/80,chloroform(Matsudaetal.,1997)),inserous peri-tonitiscausedbyinjectionsofformalininratsorcarrageeninin mice(Sirtori,2001).
Effectonvenoustensionandbloodflow
TheeffectivenessofHCSEandaescinasthetreatmentforchronic venousinsufficiencyisalsoconnectedwiththeinfluenceonthe tensionofveins(Table2).Asaresultofanincreaseinthetension ofveinwalls,thebloodflowthroughthevesselsisacceleratedand venousoutflowimproves.Theconsequenceisthatvenousblood congestiondecreases,which,inturn,enhancesmicrocirculationas erythrocytedispersionincreasesandtissueoxygenationimproves, theflowthroughvasavasorumaccelerates,thetimeofleukocyte migrationthroughthebloodvesselsand,therefore,thechancefor activationthereofinitiatingthecascadeofaninflammatory reac-tionislowered.
ItwasshownthatHCSEandaescinaffectedvenoustensionby increasingproductionofprostaglandinF2 (PGF2),which
partici-patesinregulatingthecontractileactionofveins,andinhibitingthe catabolismofvenoustissuemucopolysaccharides(Sirtori,2001). Theincreasedvenoustensionmayalsobeconnectedwiththeeffect ofHCSEonserotonin5-HT2Areceptors,whichwasdemonstrated
ininvitrostudiesonanisolatedveinandartery.Theirincubation inHCSEcausedacontraction,whereasinasimultaneously con-ductedexperimenttheirpre-incubationinasolutionofketanserin, anantagonistfor5-HTA2receptors,didnotshowanycontraction
afterHCSE.Thisprovedthatthecontractionmechanismwas con-nectedwithstimulationof5-HTA2receptors(Felixssonetal.,2010).
Theimprovementofbloodflowinvesselsbytheextractfromhorse chestnutseedswasalsoassociatedwithitseffectonbloodrheology, bydecreasingviscosityinparticular(Klemm,1982).Theinfluence ofaescinandHCSEontheincreaseinvenoustensionwasconfirmed ininvitrostudiesonisolatedveinsofvariousanimalsandhuman veinsaswellasininvivostudies(Annonietal.,1979;Felixsson etal.,2010;GuillaumeandPadioleau,1994;Lochsetal.,1974).
Clinicalstudies
Evaluation of the effectiveness of HCSE-containing prepara-tionswasperformedonpatientswithCVI;onlytwoclinicaltrials involved healthy volunteers (Table3). These wererandomised, double-blind,placebo-controlledstudies,partofwhichwas con-ductedbythecross-overmethodwhereeachpatienttookboth HCSEandtheplaceboinseparatetreatmentcycleswhiletherest were parallel studies in which patientswere divided into two groups–takingeitherHCSEortheplacebo.
Inthestudiesadailydoseof600mgofHCSE(whichcorresponds to100mgofaescin/day)wasadministeredmostfrequently,mainly intwodoses;thepatientstookthemedicinefor2–16weeks.
Intheresearchcarriedoutbetween1976and1978,a0–3scale wasusedtoevaluatethedegreeofintensityofthecomplaints typ-icalforCVI.Asignificantreductioninthesymptomswasobserved inpatientsadministeredHCSEincomparisonwiththosetakingthe
placebo(Friederichetal.,1978;NeissandBöhm,1976).
Table1
Studiesofanti-inflammatoryandantioedemaactivityofHCSEandaescin.
Extracts/compounds Dose/routeofadministration Model/effect Authors
Invitro
Aescin 300M Inhibitionofactivityofhyaluronidaseby93%(IC50=150mM), respectivelylessatlowerconcentrations
Facinoetal.(1995)
Aescin (Reparil)
100–750ng/ml Humanumbilicalveinendothelialcellsactivatedbyhypoxia, inhibitionofdecreaseinATPcontent(EC50=260ng/ml),inhibition ofactivationofphospholipaseA2(EC50=90ng/ml),inhibitionof adhesionofneutrophils(EC50=90ng/ml)
Arnouldetal.(1996)
Aescin 10−9–10−4M Inringsofinferiorvenacavafrommaleratssegmentsincubatedin
normalKrebs(2.5mMCa2+),aescincaused
concentration-dependentcontraction(max104.3mg/mgtissueat 10−4M;48.3%ofcontrolKClcontraction).InCa2+freeKrebs,there
wasessentiallynocontractiontoaescin.Inaescin-treatedveins incubatedinCa2+freeKrebs,stepwiseadditionofextracellular CaCl2causedcorrespondingincreasesincontraction(max80.0%of controlat2.5mM)
RaffettoandKhalil (2011)
Invivo
Aescin i.v.;0.2–2.5mg/kg Reductionofoedemaofratpawinducedbyovalbumin Girergetal.(1961) Aescin s.c.;4mg/kg Reductionofdextraninducedratpawoedema Damasetal.(1976) Fractionofsaponins i.v.–3.75mg/kg
p.o.–7.5mg/kg
Inhibitionofhindratpawsinflammationinducedbycarrageenan, analgesicactivity
Ceboetal.(1976)
Aescin (Reparili.v.form) (exvivo)
250ng/ml(0.22M) Inhibitionofneutrophiladhesiontohypoxicendothelium, activationandreleaseoffreeradicals
Bougeletetal.(1998)
Aescin i.p.;1mg/kg/day(3weeks) Reductionofdegradationofmucopolysaccharidesinrat connectivetissues(xiphoidcartilage);studiesof sulphomucopolysaccharidesmarkedS35
Panigati(1992)
HCSE (70%aescin) (Veinotonyl75)
p.o.;200–400mg/kg i.v.;1–10mg/kg
Lymphaticoedemainrats;reductionofplasmaticextravasation, leukocytesmigration,releaseofinflammatorymediators,leading toinhibitionofinflammation
Guillaumeand Padioleau(1994)
p.o.;50–300mg/kg i.v.;2.5–5mg/kg
Reductionofcapillaryhyperpermeabilityinducedbychloroformin rabbits
p.o.;150–400mg/kg (60minbeforethe administrationofhistamine andserotonin)
Reductionofcapillaryhyperpermeabilityinducedbyserotoninor histamineinrats(maximumeffectatadoseof200mg/kg)
p.o.;50–400mg/kg Increaseinskincapillaryresistanceinguineapigsfeda scorbutogenicdietfor3weeks
AescinsIa(1),Ib (2),IIaandIIb
p.o.;50–200mg/kg Dose-dependentinhibitionofincreasedvascularpermeability inducedbysubcutaneousadministrationofaceticacidtomice, andhistamine(aescinIa,Ib,IIa,IIb)andserotonin(aescinIb,IIa, IIb)torats
Matsudaetal.(1997)
Inhibitionofhindpawoedemainducedbycarrageeninatfirst phaseinrats(aescinIa,Ib,IIaandIIb)
Dose-dependentinhibitionofscratchingbehaviourinducedby subcutaneousinjectionofcompound48/80inmice(aescinIb,IIa andIIb,aescinIa–theweakestactivity,onlyatamaximumdose)
Table2
StudiesofvenotonicactivityofHCSEandaescin.
Extracts/compounds Dose/routeof
administration
Model/effect/routeofapplication Authors
Invitro HCSE Aescin
0.2mg/ml 0.1mg/ml
Increasedtensionofcowveinandhumansaphenous veinincubatedintestedsolutions,theeffectlastedfor 3h
Lochsetal.(1974)
Aescin 1ng/ml–1mg/ml Increasedtensionofhumansaphenousveinincubated intestedsolutions,thepharmacologicaleffect comparabletoserotoninanddihydroergotamine, significantlystrongerthanacetylcholineand vasopressin
Annonietal.(1979)
HCSE (70%aescin)
0.05–0.5mg/ml (0.5–5×10−4)
Increasedtensionofdogsaphenousveinincubatedin testedsolutions
Guillaumeand Padioleau(1994) 25–50mg Increasedtensionofperfuseddogsaphenousvein
HCSE 1mg/ml InhibitionofaggregationofADP-inducedhuman platelets
Felixssonetal.(2010)
0.1–10mg/ml Contractionofisolatedveinandarteryincubatedin HCSEsolution,previousincubationinketanserin solution(10−6and10−5M)resultedininhibitionof
contractionafterHCSE Invivo
HCSE (70%aescin)
i.v.;50mg Increasedvenouspressure,venousandlymphatic flowsinfemoraldogvein
Table3
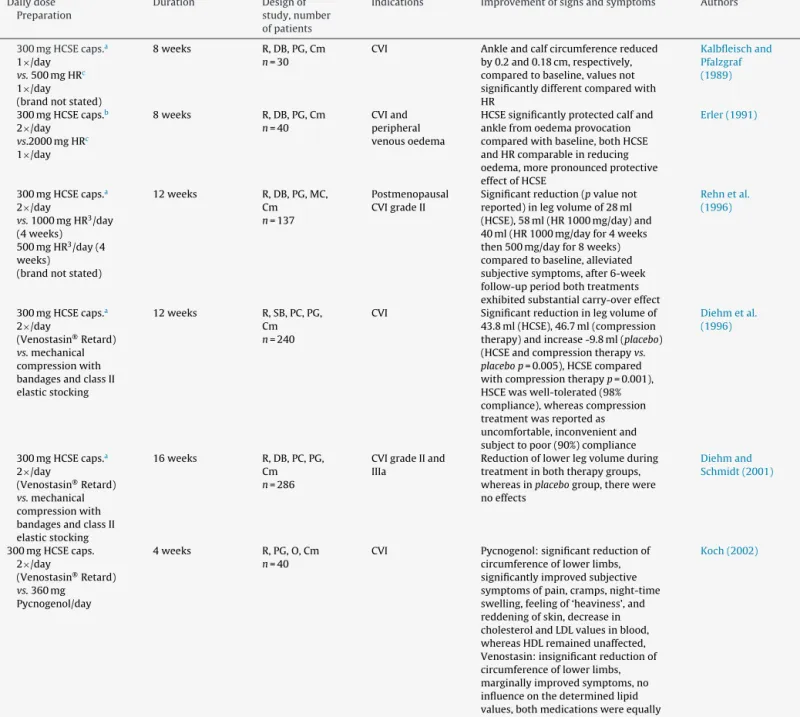
ClinicaltrialsofefficacyofHSCEinCVI.
Dailydose Preparation
Duration Designof
study,number ofpatients
Indications Improvementofsignsandsymptoms Authors
HCSE–internaluse 300mgHCSEcaps.a 2×/day
(brandnotstated)
20days R,DB,PC,PG, CO n=226
CVI,varicosis Significantalleviation(p<0.05)ofleg pain,oedemaandpruritusin participantstreatedwithHCSE comparedtoplacebo
Neissand Böhm(1976)
300mgHCSEcaps.a 2×/day
(brandnotstated)
20days R,DB,PC,CO n=95
CVI,varicosis Significantreduction(p<0.05)ofcalf spasm,pain,fatigue,andtenseness comparedtoplacebo
Friederichetal. (1978)
300mgHCSEcaps.a 2×/day
Venostasin®Retard
8weeks R,DB,PC,PG n=74
CVI Reductionoflegvolumeof16.5ml comparedto3.8mlreductionwith placebo,reductionofformationof oedema(21.0ml)withHCSEand increase(0.2ml)withplaceboduring oedema-provokingperiod
Lohretal. (1986)
2×300mgHCSE caps.a1
×/day Venostasin®Retard
4weeks R,DB,PC,CO n=22
CVI Significantreductions(p<0.01)of capillaryfiltrationcoefficientby22% comparedtoplacebo
Bisleretal. (1986)
300mgHCSEcaps.a 2×/day
(brandnotstated)
4weeks R,DB,PC,PG n=39
CVIgradeIorII Significantreduction(p<0.001)inleg volume(78ml)comparedtoincrease withplacebo(34ml),significant reduction(p<0.01)incalfandfoot circumference,significantreductionin subjectiveparameters(pain,tiredness, tension,andpruritusinlegs)compared toplacebo
Rudofskyetal. (1986)
300mgHCSEcaps.a 2×/day
(brandnotstated)
20days R,DB,PC,PG n=28
CVI Significantreduction(p<0.05)of 0.8cminlegcircumferenceand oedemaatanklecomparedwith placebo,alleviationofsubjective symptoms(p<0.05)
Pilz(1990)
300mgHCSEcaps.a 2×/day
Venostasin®Retard
20days R,DB,PC,CO n=52
Pregnant womenwith oedemadueto CVI
Significantreduction(p<0.01)infoot volumebeforeandafteroedema provocationandgreaterresistanceto oedemaprovocationcomparedto placebo,reductioninfoot circumferenceandsubjective symptomscomparedtoplacebo
Steinerand Hillemanns (1990)
300mgHCSEcaps.a 2×/day
Venostasin®Retard
2weeks R,DB,PC,CO n=20
CVI,varicosis during pregnancy
Significantreduction(p=0.009)inleg volume(114mlinpatientswith varicoseveinsand126mlinpatients withCVI)andsubjectivesymptoms (p<0.05)comparedtoplacebo
Steiner(1991)
300mgHCSEcaps.b 2×/day
(brandnotstated)
6weeks R,DB,PC,PG n=39
Venous oedemain chronicdeep vein incompetence (CVIgradeII)
Significantreduction(p<0.01)inleg volumebyanaverageof84ml comparedwithbaseline,while reductionof4mlwithplacebo, alleviationofsubjectivesymptoms (feelingsofheaviness,tension,fatigue, andparesthesia)inlegs,itchingnot helped
Diehmetal. (1992)
HCSE–externaluse
Essevengel 4weeks R
n=30
SVT Alleviationofsubjectivesymptoms, decreaseinbodytemperature
DeSanctisetal. (2001) Essevengel
1×/day
4weeks R
n=22
CVI,acute lowerleg ulcers
Improvedbloodflowparametersand microcirculation,alleviationof subjectivesymptoms
Cesaroneetal. (2001a)
Essevengel 4weeks R,DB
n=15
Footulcersasa resultof diabetes complications
Improvementofmicrocirculation, reductionofskinulcers
Cesaroneetal. (2001b)and Incandelaetal. (2001)
HCSEvs.referencemedications 300mgHCSEcaps.a 2×/day
vs.reference medication (probablyrutosides, brandnotstated)
4weeks R,DB,PG,Cm n=30
CVI Significantlyreduced(pvaluenot reported)anklecircumferenceby 0.4cmandimprovedsubjective symptomscomparedwithbaseline, anklecircumferencereducedby0.4cm (rutosides)
Table3(Continued)
Dailydose Preparation
Duration Designof
study,number ofpatients
Indications Improvementofsignsandsymptoms Authors
300mgHCSEcaps.a 1×/day
vs.500mgHRc 1×/day (brandnotstated)
8weeks R,DB,PG,Cm n=30
CVI Ankleandcalfcircumferencereduced by0.2and0.18cm,respectively, comparedtobaseline,valuesnot significantlydifferentcomparedwith HR
Kalbfleischand Pfalzgraf (1989)
300mgHCSEcaps.b 2×/day
vs.2000mgHRc 1×/day
8weeks R,DB,PG,Cm n=40
CVIand peripheral venousoedema
HCSEsignificantlyprotectedcalfand anklefromoedemaprovocation comparedwithbaseline,bothHCSE andHRcomparableinreducing oedema,morepronouncedprotective effectofHCSE
Erler(1991)
300mgHCSEcaps.a 2×/day
vs.1000mgHR3/day (4weeks) 500mgHR3/day(4 weeks)
(brandnotstated)
12weeks R,DB,PG,MC, Cm n=137
Postmenopausal CVIgradeII
Significantreduction(pvaluenot reported)inlegvolumeof28ml (HCSE),58ml(HR1000mg/day)and 40ml(HR1000mg/dayfor4weeks then500mg/dayfor8weeks) comparedtobaseline,alleviated subjectivesymptoms,after6-week follow-upperiodbothtreatments exhibitedsubstantialcarry-overeffect
Rehnetal. (1996)
300mgHCSEcaps.a 2×/day
(Venostasin®Retard)
vs.mechanical compressionwith bandagesandclassII elasticstocking
12weeks R,SB,PC,PG, Cm n=240
CVI Significantreductioninlegvolumeof 43.8ml(HCSE),46.7ml(compression therapy)andincrease-9.8ml(placebo) (HCSEandcompressiontherapyvs. placebop=0.005),HCSEcompared withcompressiontherapyp=0.001), HSCEwaswell-tolerated(98% compliance),whereascompression treatmentwasreportedas uncomfortable,inconvenientand subjecttopoor(90%)compliance
Diehmetal. (1996)
300mgHCSEcaps.a 2×/day
(Venostasin®Retard)
vs.mechanical compressionwith bandagesandclassII elasticstocking
16weeks R,DB,PC,PG, Cm n=286
CVIgradeIIand IIIa
Reductionoflowerlegvolumeduring treatmentinboththerapygroups, whereasinplacebogroup,therewere noeffects
Diehmand Schmidt(2001)
300mgHCSEcaps. 2×/day
(Venostasin®Retard)
vs.360mg Pycnogenol/day
4weeks R,PG,O,Cm n=40
CVI Pycnogenol:significantreductionof circumferenceoflowerlimbs, significantlyimprovedsubjective symptomsofpain,cramps,night-time swelling,feelingof‘heaviness’,and reddeningofskin,decreasein cholesterolandLDLvaluesinblood, whereasHDLremainedunaffected, Venostasin:insignificantreductionof circumferenceoflowerlimbs, marginallyimprovedsymptoms,no influenceonthedeterminedlipid values,bothmedicationswereequally welltolerated
Koch(2002)
Essavengel:Hippocastaniseminisextractumspissum,83.5mg/gaescin;Hyperianumnatricum,0.42mg/g.
CVI,chronicvenousinsufficiency;Cm,comparison;CO,cross-over;DB,double-blind;MC,multicentre;O,open;PC,placebo-controlled;PG,parallelgroup;R,randomised; SB,singleblind.
aHCSEcapsulesstandardisedto50mgaescineach. b HCSEcapsulesstandardisedto75mgaescineach. c O-(-Hydroxyethyl)-rutosides.
1986;Pilz,1990)wereadditionallyappliedtomeasurethe effec-tivenessofHCSE-containingpreparations.
The research involved people with diagnosed CVI, varicose veins,includingthosedevelopedduringpregnancy.Itwasshown thatthelegvolumeconsiderablydecreasedinthegroupstaking HCSE,whereasinthepatientsadministeredtheplaceboitlowered onlyslightlyornotatall(Diehmetal.,1992;Lohretal.,1986; Rudofskyetal.,1986;SteinerandHillemanns,1990).Alleviationof thesubjectivecomplaintsandanimprovementinthegeneral phys-icalactivitywasobservedinthepatientstakingHCSE,incontrast tothosetakingtheplacebo.
TheeffectofHCSEoncapillaryfiltrationandtheintravascular volumeofthelowerlimbveinwerealsoassessedinpatientswith CVI.Threehoursafteradministrationofthepreparation,the cap-illaryfiltrationincreasedinsignificantlyinthepatientstakingthe
placebowhileinthosetakingHCSEitdecreased.Adecreaseinthe intravascularvolume(−5%)wasnotconsiderable,whichproved
thattheeffectivenessofHCSEresultedfromtheimpactonthe abil-itytoreducecapillarypermeabilitytoalargerextentthanfromthe influenceontheveintension(Bisleretal.,1986).
UsingbothclassIIelasticstockingsandHCSEcausedasignificant decreaseinlegvolumewhileinthecaseoftheplacebolegvolume slightlyincreased.HCSEandcompressiontherapywerebothwell toleratedandnoseriouscomplicationswerereported.Theresults suggestedthatuseofelasticstockingsaswellasHCSEensured sim-ilareffectivenessintreatingpatientswithoedemacausedbyCVI (Diehmetal.,1996;DiehmandSchmidt,2001).
Theresearchthataimedatacomparisonoftheeffectivenessof HCSEwiththat ofO-(-hydroxyethyl)-rutosides(HR)wasdone, too.HRshowedsimilareffectiveness toHCSE (reductioninthe ankle and calf circumference, a leg volume reduction) (Erdlen, 1989; Erler, 1991; Kalbfleisch and Pfalzgraf,1989; Rehn et al., 1996).Intheotherstudies,theefficacyofHCSEandpycnogenol wascompared.Pycnogenolsignificantlyreducedthecircumference ofthelowerlimbs,improvedsubjectivesymptomsanddecreased cholesterol,andLDLvaluesintheblood,whereasHDLremained unaffected.HCSEonlymoderatelybutnotsignificantlyreducedthe circumferenceofthelowerlimbs,marginallyimprovedsymptoms andhadnoinfluenceonthedeterminedlipidvalues.Pycnogenol wasfoundtobemoreefficaciousthanHCSEforthetreatmentof CVI(Koch,2002).
Theeffectivenessoflocalapplicationofapreparationcontaining HCSEwithanadditionofheparin(Essavengel)wasalsoevaluated. Alleviationofthesymptomsandafallofskintemperaturewere observedinpatientswithsuperficialveinthrombosis(SVT)(De Sanctisetal.,2001)whileinthosewithCVIandacutelowerleg ulcersasignificantimprovementinthebloodflowand microcir-culationparameterswasfound(Cesaroneetal.,2001a).Improved microcirculationandaconsiderabledecreaseinskinulcerswithin thefootwerealsoobservedafterapplicationofthispreparationin diabetics(Cesaroneetal.,2001b;Incandelaetal.,2001).
TheeffectofHCSEonpedaloedemainpeopletravellingon long-haulflightswasassessed(n=19;double-blindtrials).Theresults demonstratedlesserfootoedemainpeopleprophylacticallytaking 600mgofHCSEbeforetheflight(MarshallandDormandy,1987). Otherstudiesonhealthyvolunteers(n=12;double-blind,placebo -controlledtrial)showedthatstandardisedHCSEadministeredonce atadoseof600mgdecreasedvascularvolumeandthecapillary filtrationrate(Pauschinger,1987).
Pharmacokinetics
A few of the reports on the pharmacokinetic and pharma-coavailability of aescin were published. The conducted studies allowedtocomparethebioavailabilityofaescin(50mgcontained in240–290mgofHCSE)inaprolongedrelease formulationand otherpharmaceuticalformulationsafteroraladministration.Ina singledoseexperimentCmaxwas3.2–9.8ng/mlwhileinarepeated
doseexperimentCmaxwas6.5–16.7ng/ml.Inbothofthediscussed
clinicalstudiesthedifferencesintheconcentrationsofaescininthe blood/plasmawereobserved(Loewetal.,2000).
Furthermore,afteroraladministrationofanaescinsolution,the absolutebioavailabilitywaslowanddeterminedasonly1.5%.This factwasprobablyduetothefirstpasseffect(metabolismand bil-iaryexcretion).TherelativebioavailabilityofaescinfromaHCSE (Venostasin retard®)was 100%compared to anaescinsolution
(EMEA,2012).
Thepharmacokineticsofaescin,afterintravenous administra-tion, corresponded to an open three compartment model. The eliminationshalf-timesof5mgaescin(infusionrate:718g/min) were:t0.5␣–6.6min;t0.5–1.74handt0.5␥–14.36h.Moreover, thedistributionvolume was100.9l,bindingtoplasma proteins was84%,totalplasmaandrenalclearanceswere21.8ml/minand 1.7ml/minrespectively.Following120hafterthedose administra-tion,aescinexcretedintheurine(EMEA,2012).
Side-effects,toxicity
Preclinicaldata
Weakgenotoxicactivityof acommercialdryextract aswell asfluidextractsofhorsechestnutseedswasdemonstratedinthe AmestestonstrainsofSalmonellatyphimuriumTA98(EMEA,2012; ESCOP,2003).
HCSE studies on rats and rabbits (100 and 300mg/kgbw) showed no teratogenic activity. A fall in the average body weight of the foetus was only observed after application of largedoses of HCSE. HCSE singledose toxicity studies (Venos-tatinretard® preparation,standardisedforthecontentof50mg
of aescin in 240–290mg extract) conducted on animals (mice, rats, guinea pigs, and rabbits) demonstrated greater toxicity of the extract when administered intravenously and intraperi-toneally (LD50 6.8–465mg/kgbw) thanafteroraladministration
(LD50 910–2600mg/kgbw). The LD50 values ranged from 3 to
17mg/kgbwafterintravenousandintraperitonealadministration ofaescintolaboratoryanimals(EMEA,2012).
ThestudiesofchronicoraltoxicityofHCSEwerecarriedouton dogs(20,40,80mg/kgbw;5days/week/3month)andrats(100, 200,400mg/kgbw;5days/week/3month).Theobtainedresults provedalackoftoxicityoftheadministeredextract.
Inthestudyofsub-acuteintravenoustoxicity,HCSEwas admin-isteredtoratsatdosesof9,30,90mg/kg/dayfor60days.Thedose of90mg/kgcauseddeathof8outof30animalsasearlyasinthe firstdaysofthestudy;thedosesof30and9mg/kgdidnotleadto anysignificantdisordersandweresafefortheanimals(Liehnetal., 1972).
Aescin,atadoseof2×50mg/kgbw,giventoyoung,32-dayold rats,didnotaffectfertility,andnephrotoxicitywasnotdetected either(EMEA,2012).
Alackofthenephrotoxicpropertiesofaescinwasalsoconfirmed inastudyontheinfluenceoffreeandalbumin-boundaescinon renaltubulartransportperformedonanisolated,artificially per-fusedfrogkidney.Itwasproventhatthelowharmfulnessresulted fromtheabilityofaescintobindwithplasmaproteins(50%),and theconcentrationoffreeaescinfilteredthroughthekidneywas consideredtoolowtobeabletohaveatoxiceffect(Barnesetal., 2007).
Clinicaldata
Theresultsofthreeclinicalstudiesprovedalackoftoxiceffects ofHCSEandaescinontherenalfunctionafteri.v.administration. Thestudiesinvolvedhealthyvolunteersorpatientswithimpaired renalfunction,bothadultsandchildren.Thekidneyfunctionwas monitoredeveryday;BUN,serumcreatinineandcreatinine clear-anceweredetermined,andtheurineanalysiswasperformed.It wasshownthataescindidnotcausedecreasedkidneyefficiencyin theindividualsfromtheexaminedgroups(EMEA,2012).
The research showed that use of HCSE was safe. Ailments connectedwithHCSEapplicationweretransientandoflittle inten-sity;gastrointestinaldisorders,dizziness,headaches,anditchwere observed(Barnesetal.,2007;Diehmetal.,1996;EMEA,2012;Neiss andBöhm,1976;Rehnetal.,1996;SteinerandHillemanns,1990).
Contra-indications,warnings
pharmacologicallyactiveconstituentsofA.hippocastanumshould beavoidedduringpregnancyandlactation(Barnesetal.,2007).
Despite the literature data describing interactions between preparationscontainingHCSEandothermedicinalagents, applica-tionoftheextractsimultaneouslywithothermedicines,especially thoseofsimilaroroppositeactivity,requirescaution.Itwasalso shownthataescinboundwithplasmaproteins;however,the influ-enceonbindingofotherdrugsoralackthereofwasnotconfirmed (Barnesetal.,2007;EMEA,2012).
Thereisnoinformationaboutoverdose,drugabuse,withdrawal, andreboundeffectortheimpactontheabilitytodriveoroperate machineryorimpairmentofmentalability.
Conclusions
CVIisacommondisorderofvenousvesselsaffectingmainlythe elderly.PreparationscontainingHCSE/aescinhavelongbeenused toalleviateboththesubjectivesymptoms,suchasthefeelingof tiredness,itching,cramps,paresthesia,andtheobjectiveones,i.e. decreasedlegvolume.HCSEstandardisedforthecontentofaescin oraescinisolatedfromtheextractareused.␣-Aescinisindustrially obtainedintheamorphousform,whichischaracterisedby bet-terbioavailabilityduetobettersolubility.Mostfrequently,600mg ofHCSE,whichcorrespondsto100mgofaescin,isapplieddaily. Randomisedclinicalstudiesshowedbeneficial effects of sucha treatmentafter2–16weeks,anditseffectivenesswassimilartothat ofcompressiontherapyoradministrationofhydroethylrutosideor pycnogenol.
Theactivityof HCSE is mainlyconnectedwith thepresence of aescin, which is the major active compound of the extract. Numerousinvitroandinvivostudiesenabledtoinvestigatethe mechanism of HCSE/aescin activity,which is mainly connected withanti-oedema,anti-inflammatory,vesselprotective,andthe venotonicactivity.
Moreover, preparations containing HCSE/aescin have been proventobesafe.Over40yearsofresearchintotheundesirable effectsofthehorse-chestnutseedextractappliedinternallyhave provedvery good tolerancethereof, and thesporadicailments, mainlygastrointestinaldisorders,havebeentransient.
Authors’contribution
MD-M participated in data collection and writing of the manuscript.Alltheauthorscontributedtothecriticalreadingand finaleditingofthemanuscript.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgements
ThisworkwassupportedbyaresearchprojectofNational Sci-enceCentre(NCN,Poland)(No.2012/07/N/NZ7/02246).Wewould liketoacknowledgeProf.DrHab.WiesławaBylka(Departmentof Pharmacognosy,PoznanUniversityofMedicalSciences,Poland)for herassistanceandhelpfulcommentsonthemanuscript.
References
Annoni,F.,Mauri,A.,Marincola,F.,Resele,L.F.,1979.Venotonicactivityofescinon thehumansaphenousvein.Arzneimittel-Forsch.29,672–675.
Arnould,T.,Janssens,D.,Michiels,C.,Remacle,J.,1996.Effectofaescineon hypoxia-inducedactivationofhumanendothelialcells.Eur.J.Pharmacol.315,227–233. Arnould,T.,Michiels,C.,Remacle,J.,1993.IncreasedPMNadherenceonendothelial cellsafterhypoxia:involvementofPAFCD18/CD11b,andICAM-1.Am.J.Physiol. 264,1102–1110.
Barnes,J.,Anderson,L.A.,Phillipson,J.D.,2007.HerbalMedicines,thirded. Pharma-ceuticalPress,London,Chicago,pp.363–366.
Bisler,H.,Pfeifer,R.,Klüken,N.,Pauschinger,P.,1986.Wirkungvon Roßkastanien-samenextraktaufdietranskapilläreFiltrationbeichronischvenöserInsuffizienz. Deut.Med.Wochenschr.111,1321–1399.
Bougelet,C.,Roland,I.H.,Ninane,N.,Arnould,T.,Remacle,J.,Michiels,C.,1998.Effect ofaescineonhypoxiainducedneutrophiladherencetoumbilicalvein endothe-lium.Eur.J.Pharmacol.345,89–95.
Cebo,B.,Krupinska, J.,Sobanski, H.,Mazur,J., Czarnecki,R., 1976.Własno´sci farmakologicznefrakcjisaponinowychotrzymanychzsurowcówkrajowych: SaponariaofficinalisPrimulaofficinalis,Aesculshippocastanum.Herba.Polon.22, 154–162.
Cesarone,M.R.,Incandela,L.,Belcaro,G.,DeSanctis,M.T.,Ricci,A.,Griffin,M.,2001a. Two-weektopicaltreatmentwithEssavengelinpatientswithdiabetic microan-giopathy–aplacebo-controlled,randomizedstudy.Angiology52(Suppl.3), 43–48.
Cesarone, M.R.,De Sanctis, M.T.,Incandela,L.,Belcaro, G.,Griffin, M.,2001b. Microvascularchangesinvenoushypertensionduetovaricoseveinsafter stan-dardizedapplicationofEssavengel–aplacebo-controlled,randomizedstudy. Angiology52(Suppl.3),11–16.
Damas,P.,Volon,G.,Damas,J.,1976.Surlàtionantioedemedeelèscine.Bull.Soc. Roy.Sci.Liège45,436–442.
DeSanctis,M.T.,Cesarone,M.R.,Incandela,L.,Belcaro,G.,Griffin,M.,2001.Treatment ofsuperficialveinthrombosiswithstandardizedapplicationofEssavengel–a placebo-controlled,randomizedstudy.Angiology52(Suppl.3),57–62. Diehm,C.,Schmidt,C.,2001.VenostasinretardgegenPlazeboundKompression
beiPatientenmitCVIII/IIIA.FinalStudyReport.KlingePharmaGmbHMunich, Germany.Reportedin:OttillingerBetal.BMCCardiovasc.Disord.1,5. Diehm,C.,Trampish,H.J.,Lange,S.,Schmidt,C.,1996.Comparisonoflegcompression
stockingandoralhorse-chestnutseedextracttherapyinpatientswithchronic venousinsufficiency.Lancet347,292–294.
Diehm,C.,Vollbrecht,D.,Amendt,K.,Comberg,H.U.,1992.Medicaledemaprotection –clinicalbenefitinpatientswithchronicdeepveinincompetence.VASA21, 188–192.
EMEA(EuropeanMedicinesAgency),2012.ScienceMedicinesHealth.EMEA,London http://www.ema.europa.eu(accessedNovember2014).
Erdlen,F.,1989.KlinischeWirksamkeitvonVenostasinretardim Doppelblindver-such.Med.Welt.40,994–996.
Erler,M.,1991.RoßkastaniensamenextraktbeiderTherapieperipherervenöser Ödeme–einklinischerTherapievergleich.Med.Welt42,593–596.
ESCOPMonographs,2003.TheScientificFoundationforHerbalMedicinalProducts, seconded.ThiemePublisher,Stuttgart,NewYork.
Facino,R.M.,Carini,M.,Stefani,R.,Aldini,G.,Saibene,L.,1995.Anti-Elastaseand anti-hyaluronidaseactivitiesofsaponinsandsapogeninsfromHederahelixAesculus hippocastanumandRuscusaculeatus:factorscontributingtotheirefficacyinthe treatmentofvenousinsufficiency.Arch.Pharm.328,720–724.
Felixsson,E.,Persson,I.A.-L.,Eriksson,A.C.,Persson,K.,2010.Horsechestnutextract contractsbovinevesselsandaffectshumanplateletaggregationthrough5-HT2A receptors:aninvitrostudy.Phytother.Res.24,1297–1301.
Friederich,H.C.,Vogelsberg,H.,Neiss,A.,1978.EinBeitragzurBewertungvonintern wirksamenVenenpharmaka.Z.Hautkrankheiten.53,369–374.
Girerg,R.J.,DiPascquale,G.,Steinetz,B.G.,1961.Theanti-edemapropertiesofaescin. Arch.Int.Pharmakodyn.133,127–137.
Guillaume,M.,Padioleau,F.,1994.Veinotoniceffect,vascularprotection, antiin-flammatoryandfreeradicalscavengingpropertiesofhorsechestnutextract. Arzneimittel-Forsch.44,25–35.
Incandela,L.,Belcaro,G.,Nicolaides,A.N.,Geroulakos,G.,Cesarone,M.R.,DeSanctis, M.T.,2001.MicrocirculationafterstandardizedapplicationofEssavengelon normalskin–aplacebo-controlled,randomizedstudy.Angiology52(Suppl.3), 5–10.
Kalbfleisch, W., Pfalzgraf, H.,1989. ÖdemprotektivaÄquipotente Dosierung– RoßkastaniensamenextraktundO--HydroxyethylrutosideimVergleich. Ther-apiewoche39,3703–3707.
Klemm, J.,1982. Strömungsgeschwindigkeit vonBlut invarikösen Venen der unterenExtremitäten.Munchen.Med.Wochen124,579–582.
Koch,R.,2002.ComparativestudyofVenostasinandPycnogenolinchronicvenous insufficiency.Phytother.Res.16(Suppl.1),1–5.
Liehn,H.D.,Franco,P.A.,Hampel,H.,Hofrichter,G.,1972.Atoxicologicalstudyof extractumHippocastanisemen(EHS).PanminervaMed.14,84–91.
Lochs,H.,Baumgartner,H.,Konzett,H.,1974.ZurBeeinflussungdesVenetonusdurch Rosskastanienextrakte.Arzneimittel-Forsch.24,1347–1350.
Loew,D.,Schrödter,A.,Schwankl,W.,März,R.W.,2000.Measurementofthe bioavailabilityofaescin-containingextracts.MethodsFind.Exp.Clin.Pharmacol. 22,537–542.
Lohr,E.,Garanin,G.,Jesau,P.,Fischer,H.,1986.ÖdempräventiveTherapiebei chro-nischerVeneninsuffizienzmitÖdemneigung.Munchen.Med.Wochen.128, 579–581.
Marshall,M.,Dormandy,J.A.,1987.Oedemaoflong-distanceflights.Phlebology2, 123–124.
Matsuda,H.,Li,Y.,Murakami,T.,Ninomiya,K.,Araki,N.,Yoshikawa,M., Yama-hara,J.,1997.AntiinflammatoryeffectsofescinsIa,Ib,IIa,andIIbfromhorse chestnuttheseedsofAesculushippocastanumL.Bioorg.Med.Chem.Lett.7, 1611–1616.
Neiss,A.,Böhm,C.,1976.ZumWirksamkeitsnachweisvon Roßkastaniensamenex-trakt beim varikösen Symptomenkomplex. Munchen. Med. Wochen. 118, 213–216.
Panigati,D.,1992.ThepharmacologyofescinasaponinofAesculushippocastanum
L.III.Pharmacokineticsandtoxicology.Boll.Chim.Farm.131,320–321. Pauschinger,P.,1987.KlinischexperimentelleUntersuchungenzurWirkungvon
RoßkastaniensamenextraktaufdietranskapilläreFiltrationunddasintravasale VolumenanPatientenmitchronischvenöserInsuffizienz.Phlebol.Proktol.16, 57–61.
Piechal,A.,Blecharz-Klin,K.,Widy-Tyszkiewicz,E.,2005.Kasztanowieczwyczajny (Aesculushippocastani)wewspółczesnejterapii.Przew.Lek.4,74–81. Pilz,E.,1990.ÖdemebeiVenenerkrankungen.Med.Welt41,1143–1144. Pittler,M.H.,Ernst, E., 2012. Horsechestnut seedextract for chronicvenous
insufficiency. Cochrane Database Syst. Rev., 11. Art. No.: CD003230. http://dx.doi.org/10.1002/14651858.CD003230.pub4.
Raffetto,J.D.,Khalil,R.A.,2011.Ca(2+)-dependentcontractionbythesaponoside escininratvenacava:implicationsinvenotonictreatmentofvaricoseveins.J. Vasc.Surg.54,489–496.
Rehn,D.,Unkauf,M.,Klein,P.,Jost,V.,Lücker,P.W.,1996.Vergleichder klinis-chenwirksamkeitundvertraglichkeitvonoxerutinundRosskastanien–extract beipatientenmitchronischervenoserInsuffizienz.Arzneimittel-Forsch.46, 483–487.
Rudofsky,G.,Neiss,A.,Otto,K.,Seibel,K.,1986.ÖdemprotektiveWirkungund klin-ischeWirksamkeitvonRoßkastaniensamenextraktimDoppeltblindversuch. Phlebol.Proktol.15,47–54.
Sirtori,C.R.,2001.Aescin:pharmacologypharmacokineticsandtherapeuticprofile. Pharmacol.Res.44,183–193.
Steiner,M.,Hillemanns,H.G.,1990.Venostasinretardinthemanagementofvenous problemsduringpregnancy.Phlebology5,41–44.
Steiner, M., 1991. Ausmaßder ödemprotektiven Wirkung von Roßkastanien-samenextrakt.VASA20(Suppl.33),217.