w ww.e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
Inhibition
of
cytochrome
P450
3A
enzyme
by
Millettia
aboensis:
its
effect
on
the
pharmacokinetic
properties
of
efavirenz
and
nevirapine
Sunday
O.
Nduka
a,∗,
Mathew
J.
Okonta
b,
Daniel
L.
Ajaghaku
c,
Kosisochi
C.
Amorha
b,
Chinwe
V.
Ukwe
baDepartmentofClinicalPharmacyandPharmacyManagement,NnamdiAzikiweUniversity,Awka,Nigeria bDepartmentofClinicalPharmacyandPharmacyManagement,UniversityofNigeria,Nsukka,Nigeria cDepartmentofPharmacologyandToxicology,NnamdiAzikiweUniversity,Awka,Nigeria
a
r
t
i
c
l
e
i
n
f
o
Articlehistory: Received19May2016 Accepted17October2016 Availableonline15December2016
Keywords: Bioavailability Herb–druginteraction CytochromeP4503A(CYP3A) Antiretrovirals
Enzymeinhibition
a
b
s
t
r
a
c
t
ThechronicandcomorbidnatureofHIVinfectionnecessitatetheuseofmultipledrugsincludingherbs torelievesymptomswithapossibleincreaseinherb–druginteractioncases.Thisstudywasdesigned toevaluatetheeffectofMillettiaaboensis(Hook.f.)Baker,Fabaceae,oncytochromeP4503A isoen-zymeandtheinfluenceofthiseffectonthebioavailabilityoftwoantiretroviralagents.Invitroeffectof ethanolextractofM.aboensisonintestinalandlivermicrosomesextractedfromfemaleratswasassessed usingerythromycin-N-demethylationassaymethodwhileinvivoeffectsweredeterminedby estimat-ingsimvastatinplasmaconcentrationsinrats.Theeffectoftheextractonpharmacokineticparameters oforallyadministeredefavirenz(25mg/kg)andnevirapine(20mg/kg)wasdeterminedinratsdivided intogroups(n=5).PlasmadrugconcentrationswereassayedusingHPLCandpharmacokinetic parame-tersdeterminedthroughanon-compartmentalanalysisasimplementedinWinNonlinpharmacokinetic program.TheextractinhibitedbothintestinalandlivermicrosomalcytochromeP4503Aisoenzyme activitiesinvitroandenhancedsimvastatinabsorptioninvivowithpossibleinhibitionofmetabolizing enzymesasindicatedbysignificant(p<0.05)increaseinmaximalconcentration,areaundercurveand meanresidenttimeofthedrug.However,furtherinvivointeractionstudiesinanimalmodeldidnot producesignificant(p>0.05)changesinthepharmacokineticparametersofefavirenzandnevirapine. HPLCfingerprintingindicatedthepresenceofquercetinandkaempferolintheextract.Thesefindings revealedM.aboensisasaninhibitorofcytochromeP4503Aenzymebut,withnosignificanteffectonthe bioavailabilityoforallyadministerednevirapineandefavirenz.
©2016SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Thisisanopen accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
PolypharmacyinHIVtreatmentandresultantdruginteractions
duetothenatureofthediseaseremainanimportantchallenge
inHIVtreatment(MachtingerandBangsberg,2013),inaddition
to challenges related to pooradherence and intersubject
vari-abilityinpharmacokinetics(Michaudetal.,2012).Interindividual
variabilitywithitscrucial roleintreatmentfailureortoxicityis
probably,mostlydrivenbygeneticandenvironmentalfactorssuch
asdrug–druginteractions,drug–foodinteractionsanddrug–herb
interactions(Michaudetal.,2012), withdrug–herb interactions
becomingmoreremarkablebecauseofthecurrentincreaseinthe
useofherbalmedicines.AreportbyRahmanandSinghal(2002)
estimated that about 65–80% of the population in developing
∗ Correspondingauthor.
E-mail:so.nduka@unizik.edu.ng(S.O.Nduka).
countriesrelyonherbalmedicinesastheprimarysourceof
treat-mentwithanestimatedannualexpenditureofover60billionUS
dollars(WHO,2003).Someherbalconstituentshavebeenshown
toaffectthebioavailabilityofco-administereddrugs(Kangetal.,
2009)throughvariousmechanismsincludingmodulationofdrug
metabolizingenzymes(Dudhatraetal.,2012).Drugmetabolizing
enzymesplaymajorroles inthebioavailabilityand elimination
oforallyadministered drugsin thebody.Hence,modulationof
theseenzymesbyherbalextractscouldleadtoalterationinthe
pharmacokineticpropertiesofco-administeredsubstrateagents,
ultimatelyaffectingtheiroveralltherapeuticoutcomes.
Theplant,Millettiaaboensis(Hook.f.)Baker,Fabaceae,is
consid-eredtobeanall-purposeplantinmostpartsofAfricabecauseof
themultiplicityofitsuseinethnomedicine(Banzouzietal.,2008).
Itis usedforthetreatmentof constipationinchildren, as
laxa-tive,for thetreatmentofcold andcatarrh,diarrhea,headaches,
dysentery, chicken pox and measles including respiratory
dif-ficulties (Harrison et al., 2011; Borokini and Omotayo, 2012;
http://dx.doi.org/10.1016/j.bjp.2016.10.008
Onyegeme-OkerentaandOkafor,2014)mostofwhichare
asso-ciatedwithHIV/AIDS. Hence,ourunpublishedsurvey foundan
increaseduseofthisplantbypeoplelivingwithHIV/AIDS.
Although the use of Highly Active Antiretroviral Therapy
(HAART)hasmadelong-termsuppressionofHIVareality,
pharma-cotherapyofthisdiseaseisstillassociatedwithsomechallengesas
someoftheagentsaresubstratesforcytochromeP450enzymes
predisposingthemtodruginteractionswithenzymemodulators.
Also,thedrugsareassociatedwithlotsofadversedrugeventsthat
mayresultinpoormedication.
Nevirapineandefavirenzbelongtothenon-nucleosidereverse
transcriptaseinhibitorclassofantiretroviralagentsapprovedfor
the treatment of HIV-1 infection. Although nevirapine is well
absorbedafteroraladministration,across-overcontrolledstudy
ineighthealthyadultmalesindicatedsignificanteffectsonmost
ofitspharmacokineticparameterswhenco-administeredwitha
bioenhancer(Kasibhattaand Naidu,2007).It undergoeshepatic
oxidation by cytochrome P450 isoforms namely CYP3A4 and
CYP2B6toyieldseveralmetabolites(RaffantiandHaas,2001).The
mostfrequentadverseeventsassociatedwithnevirapineare:rash
thatmayprogresstoStevens-Johnsonsyndromeandelevatedliver
enzymes(Fagotetal.,2001;DumondandKashuba,2009)andboth
aredosedependent.Amongtheantiretroviralagentsthatare
cur-rently inuse, nevirapine isthemostcommoncause ofserious,
clinicallyapparentacuteliverinjury(UnitedStatesNationalLibrary
ofMedicine).
Efavirenz, with a low oral bioavailability of approximately
40–45%andalonghalf-lifeof40–55h(DumondandKashuba,2009)
isalsometabolizedbyCYP3A4andCYP2B6enzymes(Veldkamp
etal.,2001).Itcrossesthebrainbloodbarrierattaining0.5–1.2%
ofthecorrespondingplasmaconcentrationinthecerebrospinal
fluidcausinghighratesofneuropsychiatricsideeffectsin>50%of
individuals(Veldkampetal.,2001).
Therefore,sincenevirapineandefavirenzarecytochromeP450
enzymesubstratesandaresusceptibletopharmacokinetic
interac-tionswithenzymemodulators,itisimperativetoclinicallyevaluate
someoftheherbsusedbyHIV/AIDSpatientsforpossible
interac-tionswiththedrugs.Hence,thisstudyevaluatedtheinvitroand
invivoeffects ofM.aboensisleafextract onliverandintestinal
cytochromeP4503Aisoenzymes.Italsoevaluatedthe
pretreat-menteffectoftheextractonthebioavailabilityoftwoantiretroviral
agents–nevirapineandefavirenzinaratmodel.
Materialsandmethods
Drugs
Simvastatintablets,Teva® (Teva,UnitedKingdom),and
dexa-methasoneinjection,Ecnudexainjection® (Yanzhou,China)were
purchasedfromaregisteredcommunitypharmacyinAwka,
Anam-brastate,Nigeria.Erythromycinpuresamplewaspurchasedfrom
CenturyPharmaceuticalsLtd,India.EfavirenzUSPandnevirapine
anhydrousUSPwerepurchasedfromAurobindoPharmaLtd,India.
KetoconazoleUSPwaspurchasedfromAartiDrugsLtd,India.
Nevi-rapinesyrup, Nevimune® (Cipla Ltd,India)and efavirenztablet
(StridesArcolabLtd,India)wereobtainedasgifts.
Animals
Albinorats(127–320g)ofeithersexwereobtainedfromthe
FacultyofVeterinaryMedicine,UniversityofNigeria,Nsukka.All
animals were allowed to acclimatize to the new environment
beforethecommencementoftheexperiment.Feedandwaterwere
freelyprovidedandtheanimalshousedaccordingtotheirsex.Full
ethicalapprovalforuseofanimalsubjectswasobtainedfromthe
AnimalResearchEthicsReviewBoardoftheUniversityofNigeria,
Nsukkaon28thJune2013.Allanimalexperimentswereconducted
inlinewithNIHguideontheuseandcareoflaboratoryanimals.
Plantmaterial
The leaves of Millettia aboensis (Hook. f.) Baker, Fabaceae,
NigerialocalnamesEdoawo,Ukperurumwesi,Mkpukpumanya,
were collected from Nsukka, Enugu State, Nigeria, identified
and authenticatedby ataxonomist, Mr.Alfred Oziokoof
Biore-source Development and Conservation Project Center, Nsukka,
Enugu State,Nigeria. Avoucher specimenwasdepositedin the
DepartmentofPharmacognosy,oftheFacultyofPharmaceutical
Sciences,NnamdiAzikiweUniversitywiththeherbariumnumber,
PCG/474/A/021.Collectedleavesweresubsequentlycleaned,
air-driedunderroomtemperatureandpulverized.Pulverizedleaves
(500g)werecoldmaceratedin70%aqueousethanolforoneweek
with intermittent shaking and changing of solvent every 48h.
Theresultingsolutionwasfilteredand thefiltrateconcentrated
usingrotaryevaporatorat40◦C.Theresultingextractwasproperly
labeledasEMAandstoredintherefrigeratorforfurtheruse.
ExvivoeffectoftheextractoncytochromeP4503A(CYP3A) isoenzyme
TheexvivoeffectsofEMAonCYP3Aactivityinintestinaland
livermicrosomeswerestudiedusing themethoddevelopedby
Wrightonetal.(1985)andvalidatedbyUmatheetal.(2008).The
method is based on the principle that erythromycin is rapidly
demethylated by cytochrome P4503A microsomalenzymes to
yielddes-N-methyl-erythromycinandformaldehydewhich
pro-duceayellowcolorwithNashreagent.
Afteranovernightfast,femaleratswereeuthanizedwith
pento-barbitoneandcarefullyexcisedthroughlongitudinalincisioninthe
abdomentoexposetheperitonealcavity.Liverwasperfusedinsitu
with10mlof0.1Mice-coldphosphatebufferedsaline(PBS,pH7.4)
andthen,isolated.Similarly,apieceofupperpartoftheintestine
(about20cmfromthestomach)wasalsoisolatedandwashedwith
PBSandthetissuesusedforfurtherprocessing.
Preparationofintestinalmicrosomes
ThemethodsvalidatedbyCotreauetal.(2003),Takemotoetal.
(2003)andUmatheetal.(2008)wereused.Thisinvolved
scrap-ping of the intestinal mucosa witha light plastic slip and the
scrapingsweremixedfollowedbysuccessiveincreasing
centrifu-gationusingrefrigeratedultracentrifuge(TGL-20M,China)inice
coldhistidine-sucrosebuffer(HSB,pH7.0)toobtainasupernatant.
Thesupernatants werecombinedand then,mixed with52mM
CaCl2(0.2mlof52mMCaCl2to1mlofthesupernatant),andthis
wasallowedtostandfor 20mintoprecipitate themicrosomes
followedbycentrifugationat20,000×gfor15mintoobtainthe
microsomalpelletwhichwassuspendedin0.5mlof0.1M
potas-siumphosphatebuffercontaining20%glycerolandstoredat−20◦C
untilneeded.
Preparationoflivermicrosomes
Liver microsomes were prepared using the methods of
Schenkmanand Cinti(1978) modifiedby Umatheetal. (2008).
Theisolatedliverwasmincedandhomogenizedin10ml0.25M
sucrose containing 10mM Tris–HCl (pH 7.4) and then
cen-trifuged at 600×g for 5min followed by 12,000×g for 10min
toobtainapost-mitochondrialsupernatant.Theseparated
aconcentrationof8mMwiththesupernatant.Thiswasthen
cen-trifuged at 20,000×g for 20min to obtain pellets which were
suspended in a mixture of 150mM KCl-10mM Tris–HCl, and
centrifugedat20,000×gfor20mintoobtainapink-colored
micro-somal pellet.This wassuspended in 0.5ml of 0.1M potassium
phosphatebuffercontaining20%glycerolandstoredat−20◦Cuntil
needed.
Erythromycin-N-demethylationassay
Themicrosomal erythromycin N-demethylation activitywas
performedasdescribedbyWrightonetal.(1985).Mixtureof
micro-somal suspension (0.1ml, 25%), erythromycin (0.1ml, 10mM),
magnesiumchloride(0.1ml,150mM)and potassiumphosphate
buffer(0.6ml, 50mM, pH7.25) was preparedin duplicate test
tubes.Thesetubeswerepre-incubatedfor3minat37◦Calongwith
theplantextractattwodifferentdoses(0.1ml,50and100g)and
fornegativeandpositivecontrolscontainingTween20(0.1ml,1%)
andketoconazole(0.1ml, 5M) respectively.Reactionbetween
these agents was initiated by adding NADPH (0.1ml, 10mM),
and terminated after 10min by adding ice-cold trichloroacetic
acid(0.5ml,12.5%,w/v)solution.Thetubeswerecentrifugedat
1740×gfor10mintoremoveproteins.To1mlofthesupernatant,
1ml offreshly prepared Nashreagent(2Mammonium acetate
(30g),0.05Mglacialaceticacid(0.4ml),and0.02Macetylacetone
(0.6ml))wereaddedandthenheatedinawaterbathat50◦Cwith
intermittentshakingfor30min.Aftercooling,theirabsorbances
werereadat412nm.TheerythromycinN-demethylationactivity
wascalculatedfromstandard(0–100Mformaldehyde)prepared
and ran under the same conditions. The CYP3A4 activity was
expressedasnMofformaldehydeobtainedpermilligramofprotein
permincalculatedfromtheequation;
CYP3Aactivity=AmountofCHOproduced(n/mol)
× 1
25mgprotein×
1
10min
InvivocytochromeP4503Aactivityassay
Invivo,CYP3Aactivitywasassessedusinganadaptedmethod
developedbyKanazuetal.(2004)andvalidatedbyUmatheetal.
(2008)inratmodels.Thismethodisbasedontheprinciplethat
theconcentrationofaCYP3Asubstrateagentwhenorally
admin-isteredwillinverselyreflectontheplasmaconcentrationofthe
agentonmodulationoftheenzyme.Simvastatinwasusedasthe
probesubstrateofchoiceintheexperimentaccordingtoHuetal.
(2007)and inlinewiththefindingsofFotietal.(2010).CYP3A
activitywasartificiallyinducedbydexamethasonepretreatment
sothattheinhibitoryeffectoftheextractwouldbetterreflecton
simvastatinlevel.
Extractanddrugadministration
Femalerats(20)weredividedintofourgroupsoffiveanimals
pergroup.Threegroupsreceiveddexamethasone(80mg/kgi.p.)
dailyinthreedivideddosesforthreeconsecutivedays,whileone
groupreceivedthevehicleandservedasdexamethasoneuntreated
controlgroup.After24hoflastdoseofdexamethasoneand
vehi-cletreatment,EMAatthedoseof400mg/kg[basedonestablished
LD50(Ajaghakuetal.,2012), andunpublishedED50results]was
administeredtoonegroup,whileketoconazole,5mg/kg(positive
control)wasorallyadministeredtoanothergroup.Theremaining
twogroupsreceivedthevehicle(1%Tween20).After1hofthe
lastsetofadministrations,simvastatin(20mg/kg,p.o.)was
admin-istered to all the groups and blood samples were collected in
heparinizedtubesat0.5,1,2,4,8,12and24hintervalpost
simvas-tatinadministration.Bloodsampleswereimmediatelycentrifuged
at1740×gfor10mintoobtainplasmawhichwerestoredat−20◦C
forfurtheranalysis.
SamplepreparationandHPLCanalysisofsimvastatin
Simvastatin in the prepared samples was analyzed by the
methodof Eggadiet al.(2013)withmodifications.Plasma
sam-pleswerepreparedbyadding10%trichloroaceticacidaboutthree
times the amount of the plasma and then, mixed to
precipi-tate protein. The mixture was left in a cool place for 15min
followedbycentrifugationat1700×gfor10mintoobtaina
super-natant which was filtered through a 0.45-m disk membrane
filter. The filtrate (10l) was injected into the HPLC and
ana-lyzedusingC18(4m,30mm×4.60mm)asthestationaryphase
andacetonitrile:water:orthophosphoricacidinrespectiveratiosof
65%:35%:0.1%(v/v)asthemobilephaseataflowrateof1ml/min
andaUVdetectionwavelengthof235nmwitharetentiontimeof
0.24min.Differentconcentrationsofpuresimvastatinwere
pre-paredtoyieldvaryingconcentrationswhichwerefilteredthrough
themembranefilterandusedtoobtainthestandardcurvefor
sim-vastatin.
In vivo interactionstudies
InvivoeffectofMillettiaaboensisextractonefavirenzand nevirapinepharmacokinetics
Albinoratsweredividedintofourgroups(n=5).Feedandwater
werewithdrawn12hbeforethecommencementofthe
experi-ment.Animalsingroup1and2receivedanoraldoseofefavirenz
alone (25mg/kg) and nevirapine alone (20mg/kg) respectively.
Group3receivedEMA(400mg/kg)1hbeforetheadministrationof
nevirapine(20mg/kg)andGroup4receivedEMA(400mg/kg)1h
priortoefavirenz(25mg/kg)administration.Bloodsampleswere
collectedfromtheanimalsacrossthegroupsthroughocularvein
punctureusingcapillarytubesintoEDTAtubesattimeintervalsof
0,0.5,1,2,4,8,and12h.Thebloodsampleswerecentrifugedat
1740×gfor10minforplasmaseparationandtheplasmafrozenat
−20◦Cuntilassayed.
HPLCanalysisofefavirenz
Plasma efavirenz concentrations were assayed using HPLC
accordingtothemethodsofSailajaetal.(2007).Chromatography
wasperformedwithC18(4m,30mm×4.60mm)analytical
col-umnand50:50acetonitrile–phosphatebuffer(pH3.5)asmobile
phaseatUVdetectionof247nmataflowrateof0.8ml/minand
retentiontimeof0.2min.
HPLCanalysisofnevirapine
Nevirapineconcentrationsintheplasmaweredetectedusing
theHPLCmethoddevelopedbyKumaretal.(2010).The
chromatog-raphywascarriedoutonaC18column(4m,30mm×4.60mm)
usingamixtureofammoniumacetatebuffer(pH4.0)and
acetoni-trile(85:15,v/v)asthemobilephaseat254nmUVdetectionata
flowrateof1.2ml/minandretentiontimeof0.17min.
Determinationofpharmacokineticparameters
Differentpharmacokineticparametersweredeterminedusing
anon-compartmentalmethodusingWinNonLinpharmacokinetic
CA).Thepharmacokineticparametersdeterminedincluded:Tmax,
Cmax,Clast,AUC,AUMC,MRT,t1/2,VzandCl.
Tmax timetakenfordrugstoattainmaximalplasma
concentration
Cmax maximaldrugplasmaconcentration
Clast lastmeasurabledrugplasmaconcentration
AUC areaundercurvefromthetimeofdosingtothetimeof thelastobservation
AUMC areaundermomentcurvefromthetimeofdosingto thetimeoflastmeasurableconcentration
MRT meanresidencetime
t1/2 terminalhalf-life
Vz volumeofdistributionbasedontheterminalphase
Cl totalbodyclearance
HPLCfingerprintanalysisofMillettiaaboensisextract
Theextract(EMA,2mg)wasreconstitutedwith2mlofHPLC
grademethanol.Themixturewassonicatedfor10minand
there-after centrifuged at 1740×g for 5min. The dissolved sample
(100g)wasmixedwith500lofHPLCgrademethanol.HPLC
analysiswas doneusing Dionex P580HPLC systemcoupledto
photodiodearraydetector(UVD340S,DionexSofttronGmbH,
Ger-many). Detectionwas at 235, 254 and 340nm. The separation
column(125mm×4mm;length×internaldiameter)wasprefilled
withEurospher-10C18(Knauer,Germany),andalineargradient
ofnanopurewater(adjustedtopH2bytheadditionofformic
acid).Methanol wasusedas eluent.Compoundswere detected
usingdiodearrayandidentifiedbasedonsimilaritywithdatain
theinbuiltlibrary.
Statisticalanalysis
Allresultswerepresentedasmean±SEM.Thedatawere
sub-jectedtot-testandone-wayanalysisofvariance(ANOVA)testwith
groupdifferencesdeterminedusingposthoc Dunnett’smultiple
comparisonstestusingSPSS.Resultswereconsideredstatistically
significantatp<0.05.
Results
ExvivoassessmentofCYP3Aactivity
EffectofMillettiaaboensisextractonintestinalmicrosomes
The resultof the ex vivo effects of the extract onintestinal
CYP3AactivityispresentedinFig.1.Fromtheresult,whereasthe
50gconcentrationsoftheextractcouldnotproducereductions
inintestinalCYP3Aactivitythe100gconcentrationachieveda
significantinhibitionoftheenzymeactivity(p=0.040)similarto
ketoconazoleat5M.
EffectofMillettiaaboensisextractonlivermicrosomes
TheextractshowedadirectdosedependentliverCYP3A
inhibi-tion(Fig.2).WhileonewayANOVAshowedsignificancedifference
withtheextract,posthocanalysisindicatedthattheextractwas
significantat100gbutnotat50g.
AssessmentofinvivoCYP3Aactivity
Thepharmacokineticparametersofsimvastatinadministered
toalbinoratswithEMAtobothdexamethasoneandvehicle
pre-treated and then, to simvastatin control groups are presented
in Table1.The plasmaconcentration–time graphsused forthe
estimation ofthepharmacokineticparameters arepresented in
Fig. 3. One way analysis of variance showed significant
dif-ference in all the parameters with further subgroup analysis
0.0 5.0 10.0 15.0 20.0 25.0 30.0 35.0
nM/mg protein
p
er min
Ethanol extract of M. aboensis
50 µg 100 µg Ketoconazole (5µM) Tween 20 (1%) ∗
∗
Fig.1.EffectofMillettiaaboensisextractonCYP3Aactivityinintestinalmicrosomes. *p<0.05comparedtothenegativecontrol.
(using LSD) indicating that pretreatment with dexamethasone
andvehicleresultedinasignificantdecreaseinCmax(p=0.005),
AUC (p=0.001), and AUMC (p=0.005) while t1/2, Vz (p=0.001)
of simvastatin and Ke were increased compared to the
non-dexamethasone treated control group. Pretreatment with EMA
significantly(p<0.05)increasedTmax,t1/2,AUC,AUMCandMRTof
simvastatinwithasignificantdecreaseinclearance(Cl)andvolume
ofdistributionwhiletheotherparameterswerenotsignificantly
changed compared to the dexamethasone and vehicle treated
group.
0 20 40 60 80 100 120
∗ ∗
nM/mg protein
p
er min
Ethanol extract of M. aboensis
50 µg
100 µg Ketoconazole (5µM) Tween 20 (1%)
Table1
ComparativeeffectofMillettiaaboensisextractonthepharmacokineticparametersofsingleoraldoseofsimvastatin.
Parameter Vehicle+SIMVA DX+EMA+SIMVA DX+KETO+SIMVA DX+vehicle+SIMVA
Tmax(h) 8.00±1.000 8.00±0.000a 4.00
±0.000 1.00±0.000
Cmax(g/ml) 5.92±0.013 3.38±0.017 5.42±0.014a 2.22±0.021b
AUC(g/ml/h) 83.3±0.487 69.6±0.636a 58.4±0.115a 29.5±0.345b
t1/2(h) 11±1.170 133±85.770a 10±0.547 26±2.375
Vz(ml/kg) 2.75±0.362 5.48±1.145a 3.75±0.809a 11.84±2.071b
Cl(ml/kg/h) 0.17±0.014 0.08±0.052a 0.26
±0.019a 0.31
±0.049 AUMC(g/ml/h2) 955.3
±4.151 842.2±10.498a 534.2
±6.030a 311.7
±4.881b
MRT(h) 11.47±0.107 12.07±0.406a 9.15
±0.184a 10.54
±0.423
Ke(1/h) 0.044±0.004 0.014±0.011 0.070±0.004a 0.027±0.002
DX,dexamethasone(80mg/kg,i.p.);SIMVA,simvastatin(20mg/kg,p.o.);EMA,ethanolextractofMillettiaaboensis(400mg/kg,p.o.);KETO,ketoconazole(5mg/kg).
ap<0.05comparedtoDX+vehicle+SIMVAgroup. b p<0.05comparedtovehicle+SIMVAgroup.
0 1 2 3 4 5 6 7
30 20
10 0
Simvastatin plasma conc. (µg/ml)
Time (h)
DX+EMA+SIMVA DX+KETO+SIMVA
DX+vehicle+SIMVA Vehicle+SIMVA
Fig.3.Plasmaconcentration–timecurveofsimvastatinadministeredwithethanol extractsofMillettiaaboensistodexamethasone/vehiclepretreatedrats.n=5 rep-resentedasmean±SEM;DX,dexamethasone(80mg/kg,i.p.);SIMVA,simvastatin (20mg/kg,p.o.);EMA,ethanolextractofM.aboensis(400mg/kg,p.o.);KETO, keto-conazole(5mg/kg).
Invivodruginteractionresults
InvivoeffectofMillettiaaboensisextractonefavirenz pharmacokinetics
Table2showsthepharmacokineticparametersobtainedfrom
rats following single oral administrations of efavirenz alone
and in the presence of EMA while Fig. 4 shows their plasma
Table2
ComparativeeffectofMillettiaaboensisextractonthepharmacokineticparameters ofsingleoraldoseofefavirenz.
Parameter EFValone EMA+EFV
Tmax(h) 2.67±0.667 2.00±0.000
Cmax(g/ml) 3.88±0.108 4.18±0.000
AUC(g/ml/h) 42.8±0.046 44.17±0.054
t1/2(h) 83.20±33.048 61.29±18.847
Vz(ml/kg) 5.06±2.317 4.90±1.970
Cl(ml/kg/h) 0.06±0.024 0.05±0.005
AUMC(g/ml/h2) 257.5±3.221 262.9±0.328
MRT(h) 6.01±0.034 5.95±0.003
Ke(1/h) 0.012±0.005 0.013±0.003
EMA,ethanolextractofM.aboensis(400mg/kg,p.o.);EFV,efavirenz(25mg/kg,p.o.).
concentration–timeprofiles.Theresultsshowedanon-significant increaseinCmaxfrom3.88±0.108g/mlinefavirenzonlygroup to4.18±0.000g/mlinEMApretreatedgroup.Areaundercurve showedlittleornochangeintheextractpretreatedgroup com-paredtothegroupthatreceivedonlydrug.AUMCincreasedfrom 257.5±3.221g/ml/h2whenefavirenzwasadministeredaloneto 262.9±0.328g/ml/h2 inthepresenceofEMA,though,not sig-nificant.Timetoreachmaximumconcentrations(Tmax)showeda non-significantdecreaseinthepresenceofEMAsimilartohalf-life (t1/2)andmeanresidenttime(MRT).Thevolumeofdistributionand clearancewerebothdecreased(p>0.05)inthepretreatedgroup comparedtothecontrol.
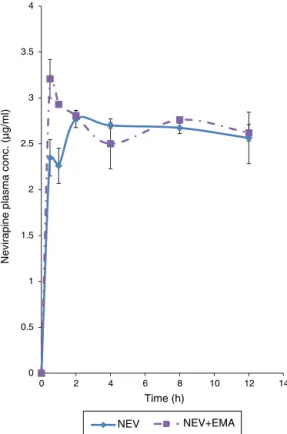
InvivoeffectofMillettiaaboensisextractonoralnevirapine pharmacokinetics
Table3showsthepharmacokineticparametersobtainedfrom
rats following oral administration of nevirapine alone and in
EMApretreatedratswiththeirplasmaconcentration–time
pro-filespresented in Fig. 5. From this table, it is evident that the
co-administrationofEMAextractinfluencedmostofthe
param-etersincludingTmax,Cmax,t1/2,andAUMChowever,withonlyt1/2
effectbeingsignificant(p=0.002).
0 0.5 1 1.5 2 2.5 3 3.5 4 4.5 5
15 10
5 0
Ef
avirenz plasma conc. (µg/ml)
Time (h)
EFV alone EMA+EFV
Table3
ComparativeeffectofMillettiaaboensisextractonthepharmacokineticparameters ofsingleoraldoseofnevirapine.
Parameter NVPalone EMA+NVP
Tmax(h) 2.17±1.014 0.67±0.167
Cmax(g/ml) 2.82±0.070 3.23±0.020
AUC(g/ml/h) 31.02±0.514 31.79±0.750
t1/2(h) 75.70±1.915 29.75±0.450a
Vz(ml/kg) 10.39±0.545 8.07±0.295
Cl(ml/kg/h) 0.06±0.045 0.10±0.046
AUMC(g/ml/h2) 186.99±4.659 192.62±1.201
MRT(h) 6.13±0.065 6.07±0.113
Ke(1/h) 0.014±0.002 0.034±0.001
EMA,ethanolextractofM.aboensis(400mg/kg,p.o.);NVP,nevirapine(20mg/kg, p.o.).
ap<0.05comparedtoNVPalone.
HPLCfingerprintresult
Thechromatogram(Fig.6)obtainedfromtheHPLCprofileof
EMAshows five major peaksnumbered A, B, C, D and E.Peak
numbersA andBcorrespondtoa simple phenolic derivative–
flavonoidglycosidesbasedontheirUVcurves.PeaknumberC(the
majorcompound)exhibitedaUVcurvetypicalofquercetin
gly-coside,suggestedbecausetheHPLC-UVabsorbancemaximawere
achievedat256and352nmwhichisacharacteristicspectrumof
quercetinglycoside.Furtherliquidchromatographic-electrospray
ionizationmassspectroscopicanalysisrevealedstrongpeaksthat
showedlossofhexosesugars.Theionpeakatm/z303.2wasanother
diagnosticindicationofquercetinaglycone.PeaknumbersDandE
exhibitedUVcurvestypicalofkaempferolglycosides.
Discussion
Thisstudy indicated that the ethanol extract of M.aboensis
exhibitedgoodcytochromeP450enzymeinhibitorypropertyboth
0 0.5 1 1.5 2 2.5 3 3.5 4
14 12 10 8 6 4 2 0
Nevirapine plasma conc. (µg/ml)
Time (h)
NEV NEV+EMA
Fig.5.Plasmaconcentration–timeprofileofnevirapineinethanolextractof Millet-tiaaboensispretreatedrats.n=5representedasmean±SEM;EMA,ethanolextract ofM.aboensis(400mg/kg,p.o.);NVP,nevirapine(20mg/kg,p.o.).
.000
750
500
250
–200
0.0 10.0 20.0 30.0 40.0 50.0 60
min AB E
C
D
Fig.6. HPLCfingerprintofethanolextractofMillettiaaboensis.(AandB) Sim-plephenolicderivatives:flavonoidglycosides;(C)quercetinglycoside;(DandE) kaempferoltypeglycosides.
invitroandinvivo.Furtherinteractionstudiestodeterminethe
effectoftheextractonefavirenzandnevirapinepharmacokinetics
demonstratedthattheextractdidnotshowsignificanteffecton
thepharmacokineticprofileofthesedrugs.
Drug interactions involving cytochrome P450 enzymes are
mostlymediatedthroughenzymeinhibitionorinduction
mecha-nisms.Enzymeinhibitionisanimmediatephenomenonandoccurs
more in practice. The therapeutic importance of enzyme
inhi-bitioncouldbeseenin practicewhen,theco-administrationof
aninhibitorwithatherapeuticagentleadtoanincreasein the
bioavailabilityoftheparentdrugoradecreaseintheelimination
ofcompoundswithresultantsubstanceaccumulationand
toxic-ity.Thisprocessis referredtoasa “bioenhancingeffect”.Many
herbalinhibitorsofcytochromeP450enzymesandbioenhancers
havebeenreported(Randhawa etal.,2011;Ndukaetal.,2013;
MazzariandPrieto,2014).
Theeffectof theethanolextract ofM.aboensis onintestinal
microsomes indicated variable concentrationeffects on
intesti-nalCYP3Aenzymesactivity.Theextractatalowerconcentration
(50g)actedasanactivatoroftheenzymeswhileinhibitoryeffect
wasevidentathigherconcentration(100g).Thisfindingmaybe
duetothenatureofenzymeinhibitionwherecompetitive
inhibi-tionofcomplexenzymesatlowinhibitorconcentrationsallowsa
shiftintheequilibriumtotheactivatedform.Thesmallintestine
isshowntobeinvolvedinfirstpassprocessessimilartotheliver,
though,toa muchsmallerdegree(Mitschkeetal.,2008).
How-ever,someresearchershavesuggestedthattheroleofintestinal
metabolismmaybegreaterthanhepaticmetabolismirrespective
ofthelowerquantitiesofCYPenzymesinthesmallintestine(Lin
etal.,1999;Paineetal.,1997).Similarly,theresultofthestudyon
thelivermicrosomesfurtherindicatedthattheextractofM.
aboen-sisexhibitedadosedependentliverCYP3Ainhibition.Thisresult,
like that of the intestinal microsomalinhibition, indicated the
possibilityofdruginteractionsoccurringwithco-administration
of theplantextractwithCYP3Asubstrate agents. Ketoconazole
servedasthereferencestandardinthisstudybecauseofits
estab-lishedinvitroandinvivoinhibitoryeffectsonCYP4503Aactivity
(Greenblattetal.,2011)whichwasalsoobservedinthisstudy.
The result of erythromycin-N-demethylation assay in the
intestinalandthelivermicrosomesindicatedthattheintestinal
microsomalCYP3Aactivitywasinhibitedtoagreaterextentwith
morevariableinhibitionthanlivermicrosomalactivitysimilarto
tothehigherconcentrationsofCYPenzymesinlivermicrosomes
comparedtointestinalmicrosomes(Linetal.,1999).
Theinvivo assaymethodis based ontheprinciple thatthe
suppressionofamajormetabolizingenzymeresponsibleforthe
metabolismof a substrate drug willlead toan increase in the
plasmaconcentrationofthesubstratedrugwhenorally
adminis-tered.Femaleratswereusedforthestudybecausedexamethasone
treated female rat livermicrosomes have been shown to have
propertiessimilartothoseofhumansandhence,ausefulmodel
for evaluatingdrug interactions involvingcytochrome P450 3A
enzymeinhibition(Kanazuetal.,2004).Simvastatinwaschosen
asthesubstrateofchoiceinlinewithstudiesbyHuetal.(2007).
Differentprobesubstratesareavailableforuseincluding
midazo-lam,nifedipine andtestosteroneamong otherswithmidazolam
beingmostlyused.However,astudybyFotietal.(2010)revealed
buspironeandsimvastatintoberelevantclinicallyasCYP3Aprobe
substrateswithsimilarorgreatersensitivitythanmidazolam.
ThesignificantincreasesinAUC,AUMCandslightincreasein
Cmaxofsimvastatinintheextracttreatedgroupwereindicative
ofenhanced absorptionandbioavailability ofthedrug.
Accord-ingto Jambhekarand Breen (2009), AUCand Cmax are used in
the measurement of extent of drug bioavailability which inter
aliaisdependentonfirstpasseffect.Therefore,thehighervalues
ofAUC,AUMCandMRTintheextract-treatedratswere
indica-tiveofenhancedsystemicavailabilityofsimvastatinandpossible
suppressionof CYP3Awhich is theenzyme responsiblefor the
metabolismofsimvastatinbothintheintestineandliver.
There-fore,theconcurrentuseofthisherbwithdrugsthataresubstrates
ofcytochromeP4503Amaybeexposing thepatient(s)todrug
interactions andsevere adverse drugeffects which mayimpair
adherence,patientsafetyandclinicaloutcomes.
TheAUC,AUMC,CmaxandTmaxareimportantpharmacokinetic
parametersusedtoassessthedegreeofabsorptionand
bioavail-abilityofdrugs(Labaune,1989;Proudfoot,1999;Jambhekarand
Breen,2009).Thenon-significantchangesseenwiththese
param-etersintheextractpretreatedgroupcomparedtothegroupsthat
receivedonlyefavirenzornevirapinewereindicativeoflittleor
noeffectonthebioavailabilityofthedrugsbytheextract.These
observationsmightberelatedtothepropertiesofthesedrugsand
theenzyme(s)involvedintheirmetabolism.Ithasbeen
demon-stratedthatintheabsenceofanimportantdetoxifyingsystemsuch
asCYP3A,organisms canstillmetabolizesomexenobioticsas a
resultofoverlyingsubstratespecificityofP450enzymesand
poten-tialactivationofalternativeenzymesinvolvedintheirmetabolism
(vanWaterschootandSchinkel,2011).Sinceefavirenzand
nevi-rapinearesubstratestobothCYP3A4and2B6enzymes,inhibition
ofCYP3Abytheextractmaynothavesignificantlyalteredtheir
bioavailabilitiesduetopossibleadaptationmechanismsthatmay
haveutilizedtheotherroute(s)ofdrugmetabolism.This
inhibi-tionmayalsohaveactivatedthealternativemetabolicpathway
(CYP2B6)similarto thereportsof vanWaterschoot in
midazo-lammetabolismin CYP3Aknockoutrats(vanWaterschoot and
Schinkel,2011).Moreover,someresearchershavereportedCYP2B6
asthemajorenzymeinvolvedinefavirenzmetabolism(Wardetal.,
2003).Thesignificantreductioninthehalf-lifeofnevirapinewhen
co-administeredwiththeextractsuggestspossibleeffectofthe
extractonnevirapinemetabolismwhichmayleadtonevirapine
accumulationandtoxicitywithtime.
Fingerprintingconstructionisanimportantqualitycontroltool
forherbalsampleswhichisacceptedbytheWorldHealth
Organi-zationandusedforidentifyingplantextracts’constituents(Ciesela,
2012).Theconstituentssuggestedtobepresentinourextractwere
mainlyofflavonoidoriginandmostknown‘bioenhancers’ofplants
originare mainly alkaloids, saponinsand flavonoids. Quercetin
foundincitrusfruitwasreportedtoachieveitsbioenhancing
activ-itythroughinhibitionofcytochromeP4503A4andP-glycoprotein
mechanisms(Randhawaetal.,2011).Ithasbeenfoundtoincrease
theAUCandCmaxofdiltiazem,tamoxifenanddigoxin(Randhawa
etal.,2011).
Themajorlimitationsofthisstudyweretheinabilityofthestudy
toestablishtheenzymekineticsofthestudiedextract;secondly,
the study neitherisolated nor characterized the different
con-stituentsintheextracttoevaluatetheireffectsontheisoenzymes
ofinterest;inaddition,thisstudyonlyfocusedoncytochromeP450
3Aenzymeswithoutconsideringtheotherisoenzymeswhichmay
alsobeinvolvedintheinteractions.
Conclusion
This study established in vitro and in vivo inhibition of
cytochromeP4503AenzymebyM.aboensiswithoutasignificant
effectonthedispositionofnevirapineandefavirenz.However,
pos-sibleaccumulationofnevirapinewithtimewasobserved.Although
nosignificantinteractionswereobservedbetweentheplantextract
andtheusedantiretroviralagents,co-administrationofthisplant
withdrugsthatdependsolelyonCYP3Afortheirmetabolismmay
leadtosignificantinteractions.Therefore,otherinteractionstudies
betweenthisplantextractandotherdrugsarerecommended.
Ethicaldisclosures
Protectionofhumanandanimalsubjects. Theauthorsdeclare
thattheproceduresfollowedwereinaccordancewiththe
regula-tionsoftherelevantclinicalresearchethicscommitteeandwith
thoseoftheCodeofEthicsoftheWorldMedicalAssociation
(Dec-larationofHelsinki).
Confidentialityofdata. Theauthorsdeclarethatnopatientdata
appearinthisarticle.
Righttoprivacyandinformedconsent. Theauthorsdeclarethat
nopatientdataappearinthisarticle.
Authorscontribution
SONandMJOdesignedthestudy.SONandDAwere
responsi-bleforexecutionoftheprojectandconducteddataanalysis.SON,
KCAandCVUdraftedthemanuscript.Alltheauthorsreviewedand
approvedthemanuscript.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgements
TheauthorsaregratefultoMrSebastianIgboemeofthe
Depart-mentofPharmacologyandToxicology,NnamdiAzikiweUniversity,
Awka,Nigeria,forhisassistanceandcontributionsinthelaboratory
procedures.We alsoappreciatethemanagement of Juhel
Nige-riaLtd,ChazmaxPharmaInd.Ltd,Nigeria andGauze Industries
andLaboratory,Nigeriafortheirassistancewithsomelaboratory
equipmentandprovisionofdrugs.
References
Ajaghaku,D.L.,Ilodigwe,E.E.,Obi,H.I.,Uzodimma,S.U.,2012.Toxicological evalua-tionofethanolleafextractofMilletiaaboensis(Hook.f)Baker.IJPIJ.Pharmacol. Toxicol.2,1–8.
Banzouzi,J.T.,Prost,A.,Rajemiarimiraho,M.,Ongoka,P.,2008.Traditionalusesof theAfricanMillettiaspecies(Fabaceae).Int.J.Bot.4,406–420.
Ciesela,L.,2012.Biologicalfingerprintingofherbalsamplesbymeansofliquid chro-matography.Chromatogr.Res.Int.,http://dx.doi.org/10.1155/2012/532418. Cotreau,M.M.,vonMoltke,L.L.,Beinfeld,M.C.,Greenblatt,D.J.,2003.Methodologies
tostudytheinductionofrathepaticandintestinalcytochromeP4503Aatthe mRNA,protein,andcatalyticactivitylevel.J.Pharmacol.Toxicol.Methods33, 45–55.
Dudhatra,G.B.,Mody,S.K.,Awale,M.M.,Patel,H.B.,Modi,C.M.,Kumar,A.,Kamani, D.R.,Chauhan,N.B.,2012.Comprehensivereviewonpharmacotherapeuticsof herbalbioenhancers.Sci.WorldJ.2012,1–33.
Dumond,J.B.,Kashuba,A.D.M.,2009.Pharmacotherapyofhumanimmunodeficiency virusinfection.In:Koda-Kimble,M.A.,Young,L.Y.,Alldredge,B.K.,Corelli,R.L., Guglielino,B.J.,Kradjan,W.A.,Williams,B.R.(Eds.),AppliedTherapeutics.The ClinicalUseofDrugs.,9thed.LippincottWilliamsandWilkins,NewYork,pp. 61-1-24.
Eggadi,V., Ponna,S.K.,Kankanala, S.R.,Sheshagiri, S.B.B.,Gaddam,S.R., 2013. DeterminationofsimvastatinanddiltiazeminratplasmabyHPLCand phar-macokineticstudies.Int.J.Pharm.Sci.Res.21,53–56.
Fagot,J.P.,Mockenhaupt,M.,Bouwes-Bavinck,J.N.,2001.Nevirapineandtheriskof Stevens-Johnsonsyndromeortoxicepidermalnecrolysis.AIDS15,1843–1848. Foti,R.S.,Rock,D.A.,Wienkers,L.C.,Wahlstrom,J.L.,2010.Selectionofalternative CYP3A4probesubstratesforclinicaldruginteractionstudiesusinginvitrodata andinvivosimulation.Am.Soc.Pharmacol.Exp.Ther.38,981–987.
Greenblatt,D.J.,Zhao,Y.,Venkatakrishnan,K.,Duan,S.X.,Harmatz,J.S.,Parent,S.J., Court,M.H.,vonMoltke,L.L.,2011.MechanismofcytochromeP4503Ainhibition byketoconazole.J.Pharm.Pharmacol.63,214–221.
Harrison,J.J.E.K.,Dankyi,E.,Kingsford-Adaboh,R.,Ishida,H.,2011.Insearchofnew leads:acloserlookatthetherapeuticpotentialoftheconstituentsof Millet-tiathonningii,Millettiapachycarpaandtheirstructuralanalogues.Int.J.Pharm. Pharm.Sci.3,71–81.
Hu,O.Y.,Hsiong,C.H.,Kuo,B.P.,Pao,L.,2007.CytochromeP4503Ainhibitorsand enhancers.UnitedStatesPatent,US7,169763B2.
Jambhekar,S.S.,Breen,P.J.,2009.BasicPharmaceutics:ExtravascularRoutesofDrugs Administration.PharmaceuticalPress,London.
Kanazu,T.,Yamaguchi,Y.,Okamura,N.,Baba,T.,Koike,M.,2004.Modelforthe drug–druginteractionresponsibleforCYP3Aenzymeinhibition.II: establish-mentandevaluationofdexamethasone-pretreatedfemalerats.Xenobiotica34, 403–413.
Kang,M.J.,Cho,J.Y.,Shim,B.H.,Kim,D.K.,Lee,J.,2009.Bioavailabilityenhancing activitiesofnaturalcompoundsfrommedicinalplants.J.Med.PlantsRes.3, 1204–1211.
Kasibhatta,R.,Naidu,M.U.R.,2007.Influenceofpiperineonthepharmacokineticsof nevirapineunderfastingconditions:arandomizedcrossoverplacebocontrolled study.DrugDev.Res.8,383–391.
Kumar,C.H.,Kumar,D.A.,Rao,J.V.L.N.S.,2010.AnewvalidatedRP-HPLCmethodfor thedeterminationofnevirapineinhumanplasma.Eur.J.Chem.7,821–826. Labaune,J.P.,1989.HandbookofPharmacokinetics.EllisHarwoodLtd.,Chichester. Lin,J.H.,Chiba,M.,Baillie,T.A.,1999.Istheroleofthesmallintestineinfirstpass
metabolismoveremphasized?Am.Soc.Pharmacol.Exp.Ther.51,135–157. Machtinger,E.L.,Bangsberg,D.R.,2013.AdherencetoHIVAntiretroviralTherapy.
HIVInSiteKnowledgeBaseChapter.UCSFCenterforHIVInformation. Mazzari,A.L.D.A.,Prieto,J.M.,2014.HerbalmedicinesinBrazil:pharmacokinetic
profileandpotentialherb–druginteractions.Front.Pharmacol.5,1–12. Michaud,V.,Bar-Magen,T.,Turgeon,J.,Flockhart,D.,Desta,Z.,Wainberg,M.A.,
2012.ThedualroleofpharmacogeneticsinHIVtreatment:mutationsand
polymorphisms regulating antiretroviral drug resistance and disposition. Pharm.Rev.64,803–833.
Mitschke, D., Reichel,A., Fricker, G., Moenning, U.,2008. Characterisation of cytochromeP450proteinexpressionalongtheentirelengthoftheintestine ofmaleandfemalerats.DrugMetab.Dispos.36,1039–1045.
Nduka,S.O.,Okonta,J.M.,Esimone,C.O.,2013.EffectsofZingiberofficinaleonthe plasmapharmacokineticsandlungpenetrationsofciprofloxacinandisoniazid. Am.J.Ther.20,501–513.
Onyegeme-Okerenta,B.M.,Okafor,U.A.,2014.Antimicrobialpropertiesofethanol leafextractofMillettiaaboensisonsomeselectedclinicalisolates.Univ.J.Plant Sci.2,97–101.
Paine,M.F.,Khalighi,M.,Fisher,J.M.,1997.Characterisationofinterintestinaland intraintestinalvariationsinhumanCYP3A-dependentmetabolism.J.Pharmacol. Exp.Ther.283,1552–1562.
Proudfoot,S.G.,1999.Factorsinfluencingbioavailability:factorsinfluencing absorp-tionfromthegastrointestinaltract.In:Aulton,M.E.(Ed.),Pharmaceutics:The ScienceofDosageFormDesigns.ChurchillLivingston,London,pp.135–137. Raffanti,S.,Haas,D.W.,2001.Antimicrobialagents:antiretroviralagents.In:
Hard-man,J.C.,Limbird,L.E.(Eds.),ThePharmacologicalBasisofTherapeutics.,10th ed.McGraw-Hill,US,pp.3–30.
Rahman,S.,Singhal,K.,2002.ProblemsinPharmacovigilanceofMedicinal Prod-uctsofHerbalOriginandMeanstoMinimizeThem.UppsalaReportsJanuary Supplement,Uppsala.
Randhawa,G.K.,Kullar,J.S.,Kumar,R.,2011.Bioenhancerfrommothernatureand theirapplicabilityinmodernmedicine.Int.J.Appl.BasicMed.Res.1,5–10. Sailaja,A.L.,Kumar,K.K.,Kumar,D.V.R.,Yugandhar,N.M.,Srinubabu,G.,2007.
Devel-opmentandvalidationofaliquidchromatographicmethodfordetermination ofefavirenzinhumanplasma.Chromatographia65,359–361.
Schenkman,J.B.,Cinti,D.L.,1978.Preparationofmicrosomeswithcalcium.Methods Enzymol.52,83–89.
Takemoto,K.,Yamazaki,H.,Tanaka,Y.,Nakajima,M.,Yokoi,T.,2003.Catalytic activ-itiesofcytochromeP450enzymesandUDP-glucuronosyltransferasesinvolved indrugmetabolisminratevertedsacsandintestinalmicrosomes.Xenobiotica 33,43–55.
Umathe,S.N.,Dixit,P.V.,Kumar,V.,Bansod,K.U.,Wanjari,M.M.,2008.Quercetin pretreatmentincreasesthebioavailabilityofpioglitazoneinrats:involvement ofCYP3Ainhibition.Biochem.Pharmacol.75,1670–1676.
vanWaterschoot,R.A.B.,Schinkel,A.H.,2011.Acriticalanalysisoftheinterplay betweencytochromeP4503AandP-glycoprotein:recentinsightfromknockout andtransgenicmice.Am.Soc.Pharmacol.Exp.Ther.63,390–408.
Veldkamp,A.I.,Harris,M.,Montaner,J.S.G.,2001.Thesteadystatepharmacokinetics ofefavirenzandnevirapinewhenusedincombinationinhuman immunodefi-ciencyvirustype1infectedpersons.J.Infect.Dis.184,37–42.
Ward,B.A.,Gorski,J.C.,Jones,D.R.,Hall,S.D.,Flockhart,D.A.,Desta,Z.,2003.The cytochromeP4502B6(CYP2B6)isthemaincatalystofefavirenzmetabolism: implicationforHIV/AIDStherapyandutilityofefavirenzassubstratemarkerof CYP2B6catalyticactivity.J.Pharmacol.Exp.Ther.306,287–300.
WHO, 2003. Traditional Medicine Facts Sheet. World Health Organisation, http://www.who.int/mediacentre/factsheets/2003/fs134/en/(accessedAugust 2015).