!
Universidade de Aveiro Departamento de Química 2015
Marta Cecília
BIOREMEDIAÇÃO DE FOSFATOS EM ÁGUA
Carvalho Martins
SALGADA – ABORDAGEM PARA EFLUENTES
MARINHOS DE AQUACULTURA DE
RECIRCULAÇÃO
Dissertação apresentada à Universidade de Aveiro para cumprimento dos requisitos necessários à obtenção do grau de Mestre em Biotecnologia, ramo de Biotecnologia Industrial e Ambiental, realizada sob a orientação científica da Doutora Eduarda Santos, Professora Auxiliar do Departamento de Química da Universidade de Aveiro e da Doutora Catarina Marques, do Departamento de Biologia da Universidade de Aveiro
" "
!
!
Universidade de Aveiro Departamento de Química 2015
Marta Cecília
BIOREMEDIATION OF PHOSPHATES
Carvalho Martins
IN SEAWATER - APPROACH FOR
RECIRCULATING MARINE AQUACULTURE
EFFLUENTS
" "
!
!
" "
!
!
o júri
presidente Doutor Jorge Manuel Alexandre Saraiva
Investigador Auxiliar do Departamento de Química da Universidade de Aveiro
Prof. Doutora AnaCatarinaSousa
investigadora pós-doc do Centro de Investigação em Ciências da Saúde (CICS), Universidade da Beira Interior
Doutora Catarina R. Marques
" "
!
!
agradecimentos Agradeço à Professora Eduarda e à Doutora Catarina, pelo acompanhamento e conhecimento científico transmitido e pela dedicação com que orientaram o meu trabalho ao longo deste percurso.
À Engenheira Célia Miranda, à Engenheira Maria João Bastos e à Dra. Manuela Marques pela ajuda, disponibilidade e prontidão nas análises de caracterização físico-química do material.
" "
!
!
palavras-chave bioadsorção, biomateriais, bioprecipitação, calcinações, efluentes de aquacultura, métodos de remediação, resíduos de conchas de ostra, remoção de fosfato
resumo As atividades realizadas em Aquacultura Marinha de Recirculação (RAS) levam à constante produção de efluentes, sendo a maior parte reutilizados/recirculados ou reciclados depois de submetidos a diferentes métodos de tratamento/remediação, ou parcialmente lançadas em corpos de água vizinhos (DWW). Os fosfatos, em particular, são normalmente acumulados em altas concentrações em DWW, tanto porque a sua monitorização não é obrigatória para a produção de peixe, uma vez que não é um parâmetro limitante, e também porque não há nenhum tratamento específico até agora desenvolvido para removê-los, em especial no que diz respeito a efluentes de água salgada. Como tal, este trabalho aborda duas questões científicas principais. Uma delas diz respeito à compreensão dos métodos de (bio)remediação aplicados aos efluentes produzidos em RAS marinhos, identificando as suas vantagens, desvantagens e lacunas relativas à sua exploração nos efluentes de água salgada. A segunda é o desenvolvimento de um método novo, inovador e eficiente para o tratamento de efluentes de água salgada que potencialmente preenchem as lacunas identificadas nos tratamentos convencionais. Desse modo, os objetivos desta tese são: (i) rever os tratamentos convencionais aplicados para os principais contaminantes nos efluentes RAS marinhos, com especial incidência sobre as abordagens de biorremediação já realizados para fosfatos; (ii) caracterizar e avaliar o potencial de resíduos de concha de ostra colhidos na Ria de Aveiro como um agente de biorremediação de fosfatos em água salgada artificial, mediante diferentes fatores (e.g., pré-tratamento das conchas de ostra por calcinação, tamanho de partícula, concentração de adsorvente). Apesar das conchas de ostras já terem sido testadas na remoção de fósforo (P) em água doce, o seu potencial de biosorção de P em água salgada, tanto quanto eu estou ciente, ainda não foi avaliado. Os resultados demonstraram que as conchas naturais (NOS) são compostas principalmente por carbonatos, sendo estes praticamente convertidos em cal (CaO) após calcinação. Tal pré-tratamento permitiu a obtenção de um material mais reativo (COS) para a remoção de P, uma vez que se observou maiores percentagens de remoção e capacidade de adsorção. Frações de menor tamanho, tanto para NOS e COS, aumentaram a remoção de P. Os modelos cinéticos mostraram que NOS ajusta-se, simultaneamente, aos modelos de Elovich e de Difusão Intrapartícula, o que sugere que a remoção de P é um processo controlado tanto quimicamente como pela taxa de difusão. A percentagem de remoção de P em COS não é controlada por difusão e o modelo Elovich foi o que melhor se ajustou para a remoção de fosfato. Este trabalho demonstrou que os resíduos de conchas de ostra, quer NOS ou COS, podem ser usados como biosorventes na remoção de fosfato em água salgada. Desse modo, este biomaterial pode sustentar uma estratégia de biorremediação económica e amiga do ambiente, com potencial para aplicação em RAS de água salgada.
" "
!
!
keywords aquaculture wastewaters, bioadsorption, biomaterials, bioprecipitation, calcinations, oyster-shell waste, phosphate removal, remediation methods
abstract Marine Recirculating Aquaculture Systems (RAS) produce great volume of wastewater, which may be reutilized/recirculated or reutilized after undergoing different treatment/remediation methods, or partly discharged into neighbour water-bodies (DWW). Phosphates, in particular, are usually accumulated at high concentrations in DWW, both because its monitoring is not compulsory for fish production since it is not a limiting parameter, and also because there is no specific treatment so far developed to remove them, especially in what concerns saltwater effluents. As such, this work addresses two main scientific questions. One of them regards the understanding of the actual (bio)remediation methods applied to effluents produced in marine RAS, by identifying their advantages, drawbacks and gaps concerning their exploitation in saltwater effluents. The second one is the development of a new, innovative and efficient method for the treatment of saltwater effluents that potentially fulfil the gaps identified in the conventional treatments. Thereby, the aims of this thesis are: (i) to revise the conventional treatments targeting major contaminants in marine RAS effluents, with a particular focus on the bioremediation approaches already conducted for phosphates; (ii) to characterize and evaluate the potential of oyster-shell waste collected in Ria de Aveiro as a bioremediation agent of phosphates spiked into artificial saltwater, over different influencing factors (e.g., oyster-shell pre-treatment through calcination, particle size, adsorbent concentration). Despite the use of oyster-shells for phosphorous (P) removal has already been applied in freshwater, its biosorptive potential for P in saltwater was never evaluated, as far as I am aware. The results herein generated showed that NOS is mainly composed by carbonates, which are almost completely converted into lime (CaO) after calcination (COS). Such pre-treatment allowed obtaining a more reactive material for P removal, since higher removal percentages and adsorption capacity was observed for COS. Smaller particle size fractions for both NOS and COS samples also increased P removal. Kinetic models showed that NOS adsorption followed, simultaneously, Elovich and Intraparticle Difusion kinetic models, suggesting that P removal is both a diffusional and chemically rate-controlled process. The percentage of P removal by COS was not controlled by Intraparticle Diffusion and the Elovich model was the kinetic model that best fitted phosphate removal. This work demonstrated that waste oyster-shells, either NOS or COS, could be used as an effective biosorbent for P removal from seawater. Thereby, this biomaterial can sustain a cost-effective and eco-friendly bioremediation strategy with potential application in marine RAS.
" "
!
!
! 1
Table of Contents
I. INTRODUCTION 8
1.1. Aquaculture: definition, production and culture methods 8
1.1.2. Recirculating Aquaculture System (RAS) 15
1.2 Objectives and structure of the thesis 18
1.3. References 19
!
II. REVIEW: REMEDIATION METHODS OF EFFLUENTS OF MARINE
RECIRCULATING AQUACULTURE SYSTEMS 21
2.1. Contaminants in RAS effluents 21
2.2. Remediation methods for contaminant removal from RAS effluents 23
2.2.1 Recirculating wastewater 23
a. Solid waste removal 23
b. Oils/Lipids 26
c. Nitrogenous compounds 27
d. Phosphorus 31
d. Pathogens 32
2.2.2 Discharged wastewater 33
2.3. Digging into the “bio” of phosphate remediation in RAS effluents 35
2.3.1. Live organisms 35
2.3.2. Biological materials 37
2.4. References 39
!
III. EFFICIENCY OF SHELL WASTES FOR THE REMOVAL OF PHOSPHATES FROM ARTIFICIAL SEAWATER – AN APPROACH TO MARINE RECIRCULATING
AQUACULTURE SYSTEMS 53
3.1. Introduction 53
3.2. Materials and Methods 55
3.2.1. Processing, pre-treatment and characterization of oyster-shells 55
3.2.2. Experimental setup 56 3.2.3. Analytical methods 57 3.2.4. Data analysis 58 a. Removal equations 58 b. Kinetic models 58 c. Adsorption isotherms 60
d. Statistics and software 61
3.3. Results and Discussion 61
3.3.1. Chemical characterization of the crushed oyster shells 61
3.3.2. The effect of pre-treatment 65
!
!
! 2
3.3.3.1. Kinetics of phosphorus removal 73
a. Kinetics of phosphorus removal by NOS 76
b. Kinetics of phosphorus removal by COS 78
3.3.4. The effect of P concentration on its removal 79
3.3.5. Adsorption isotherms 80
3.3.6. The effect of adsorbent concentration on P-removal 82
3.3.7. Biosorbent characterization after P removal assays 83
3.4. Conclusions 85
3.5. Supplementary Information 86
3.6. References 87
!
IV. FINAL REMARKS 92
! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! !
!
!
! 3
Abreviations
AMW - Artificial Marine WaterAnammox - Anaerobic ammonium oxidation
AOS - calcined oyster-shell under argon atmosphere COD - Chemical oxygen demand
BOD - Biological/biochemical oxygen demand COS – calcined oyster-shells under air atmosphere DW - Distilled Water
DWW – Discharged Wastewater EU – European Union
EPS - Extracellular Polymeric Substances IMTA – Integrated Multi-Trophic Aquaculture LCFA - Long Chain Fatty Acids
MT – Metric Tonne N – Nitrogen N2O - Nitrous Oxide NH3-N – Ammonia Nitrogen NO2-N - Nitrite nitrogen NO3-N - Nitrate nitrogen
NOS – Natural Oyster-Shells P – Phosphorus
PFO – Pseudo First-Order
PO43--P -ortho-phosphate phosphorus
PSO – Pseudo Second-Order
RAS – Recirculating Aquaculture System RSE - Residual Standard Error
RWW – Recirculating Wastewater SBR - Sequencing Batch Reactor TAN - total ammonium nitrogen TGA – Thermogravimetric Analysis TN – Total Nitrogen
TP – Total Phosphorus UV – Ultraviolet
! 4
List of Figures
Figure 1.1. Schematic representation of some aquaculture systems. RAS - Recirculating
Aquaculture System. 8
Figure 1.2. Aquaculture production (in tonnes) by European countries from 2004 to 2013 (FEAP,
2014). 9
!
Figure 1.3. Average consumption of fishery and aquaculture products (kg/inhabitant/year) by European countries in 2010 (EC, 2014). PT - Portugal, ES - Spain, LT - Lithuania, FI - Finland, FR - France, MT - Malta, SE - Sweden, LU - Luxembourg, BE - Belgium, IT - Italy, LV - Latvia, NL - The Netherlands, EU28 - Europe, DK - Denmark, IE - Ireland, CY - Cyprus, EL - Greece and UK - United Kingdom. 10 !
Figure 1.4. Portugal aquaculture production (in tonnes) by species from 2004 to 2013 (FEAP,
2014). 10
Figure 1.5. Inland and mariculture production (million tonnes) in the world from 1980 to 2012
(FAO, 2014). 11
Figure 1.6. Different aquaculture classifications. 12
Figure 1.7."Representations of some water-based production systems usually directly deployed in
the environment. 13
Figure 1.8. Diagram representing the simplified process of a Recirculating Aquaculture System. 15 Figure 2.1. Foam fractionator for organic matter and fine particles removal 25 Figure 2.2. Nitrification and denitrification processes and images of two biofilter designs. Adapted
from Schreier et al. (2010). 29
Figure 2.3. Illustration of a) an UV light treatment in Recirculating Aquaculture Systems, and b) a
typical ozone generator. 32
!
!
! 5
Figure 2.4. Sequencing batch reactor process stages: fill, react, settle, decant and idle (Boopathy et
al., 2005). 34
!
Figure 3.1 – Thermogravimetric (TGA) (expressed as % weight loss) and differential thermal (DTA) analyses of natural oyster-shell (fraction F0.5) under air atmosphere 62 Figure 3.2 – XRD patterns for NOS (natural shell, F<0.125) and COS (calcinated oyster-shell under air atmosphere, F0.5) samples. CPS – counts per second. The circles highlight the peaks of calcite (CaCO3; !), quartz (SiO2; !) and lime (CaO; !) 63
Figure 3.3. Comparison of removal percentage (R), adsorption capacity (q) and phosphate concentration in saltwater solution (C) for argon-calcined (AOS), air-atmosphere calcined (COS) and natural oyster-shells not subjected to calcination (NOS). Experimental conditions: samples fraction F0.125, 30 minutes exposure, initial P concentration of 12 mg L-1 saltwater, adsorbent
concentration of 1 g L-1. 66
Figure 3.4. Assessment of phosphorus removal by NOS and COS: (a,d) fractions removal percentage; (b,e) adsorption capacity (q) and (c,f) solution pH. Exposure time 0-120 minutes; initial P concentration 12 mg L-1; adsorbent concentration 1 g L-1 for COS and 50 g L-1 for NOS. 69 !
Figure 3.5. Plots of the Elovich model and Intraparticle Diffusion model for NOS analysed with
average values from 0 to 120 min and from 0 to 75 minutes. 77
Figure 3.6. Plots of the PSO (a) and Elovich (b) models for COS 78 !
Figure 3.7. Removal percentage in NOS and COS over time for initial concentrations of 0.5, 1.5, 3 and 6 mg P L-1. Experimental conditions: adsorbent concentrations of 1g L-1 for COS (F0.5) and 50
g L-1 for NOS (F<0.125) with exporure times of 30 and 480 minutes, respectively. 79
Figure 3.8. Adsorption isotherms of phosphate in NOS. The same conditions as “The effect of initial P concentration” with the exposure time of 8h were used. 80 Figure 3.9. Effect of five adsorbent concentrations in phosphate removal (R) and (b) solution pH; using COS F1 during 15 minutes of exposure to a [P]i of 12 mg L-1. 82
!
!
! 6
Figure 3.10 – XRD patterns for NOS (natural shell; F<0.125) and COS (calcinated oyster-shell under air atmosphere; F0.5) after phosphate (12 mg L-1) removal from artificial saltwater. CPS – counts per second. The circles highlight the peaks of calcite (CaCO3; !), quartz (SiO2; !) and
!
!
! 7
List of Tables
Table 2.1. Concentrations and major types of contaminants usually detected in saltwater effluents after an aquaculture production cycle, in several types of aquaculture including RAS, andmaximum values admissible for RAS and for discharge. 22
!
Table 2.2. Average concentration (mg/L) of ammonium (NH4+-N), nitrite (NO2--N) and nitrate
(NO3--N) before and after different remediation techniques in saltwater RAS producing different
fish species. 28
!
Table 2.3. Average concentration (mg/L) of phosphate (PO43--P) before and after different
remediation techniques in saltwater RAS. 31
!
Table 3.1 – Chemical composition (mg g-1) of crushed oyster-shells determined by ICP-MS, before
(NOS and COS) and after (NOS+P and COS+P) phosphate removal experiments 64 Table 3.2 - CHN-S analysis of the natural oyster-shells (NOS, F<0.125) and oyster-shells
calcination performed in air atmosphere (COS, F0.5). 65
Table 3.3. Comparison of the phosphate removal percentage (R), adsorption capacity (q) and assay
parameters of other studies using oyster-shells. 70
!
Table 3.4. Parameters of the adsorption kinetic models onto NOS, when data is analysed using average values including all time exposure points assessed (a. 0 – 120 minutes) or only shorter
equilibration times (b. 0 - 75 minutes). 74
!
Table 3.5. Parameters of the adsorption kinetic models onto COS, when data is analysed using average values of several exposure time points (0 - 30 minutes) 75 !
Table 3.6. Equilibrium isotherm model parameters for Langmuir and Freundlich isotherm models applied to NOS. The same conditions as “The effect of initial P concentration” with the exposure
" "
_________________________________________"
I. Introduction
" " " " " " " " " " "" "
! ! ! 8
I. Introduction
____________________________________________________________! ! ! !1.1. Aquaculture: definition, production and culture methods
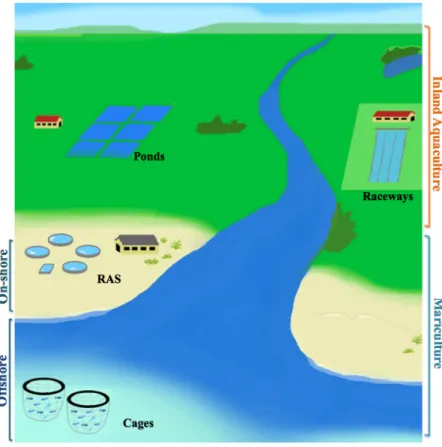
Aquaculture has been defined as the farming of fish and other aquatic organisms by applying a flexible and adaptable set of technologies, systems and species (Sugunan et al., 2007). It can generally be classified as inland aquaculture or mariculture (cf. Figure 1.6). Inland aquaculture is usually freshwater-based, but some uses saline water in inland areas,
i.e. non-coastal areas. Mariculture includes production operations in the sea/offshore,
intertidal and on-shore zones (FAO, 2014) (Figure 1.1). Some authors, however, consider mariculture as every system using saltwater.
!
Figure 1.1. Schematic representation of some aquaculture systems. RAS - Recirculating Aquaculture System.!
!
!
! 9
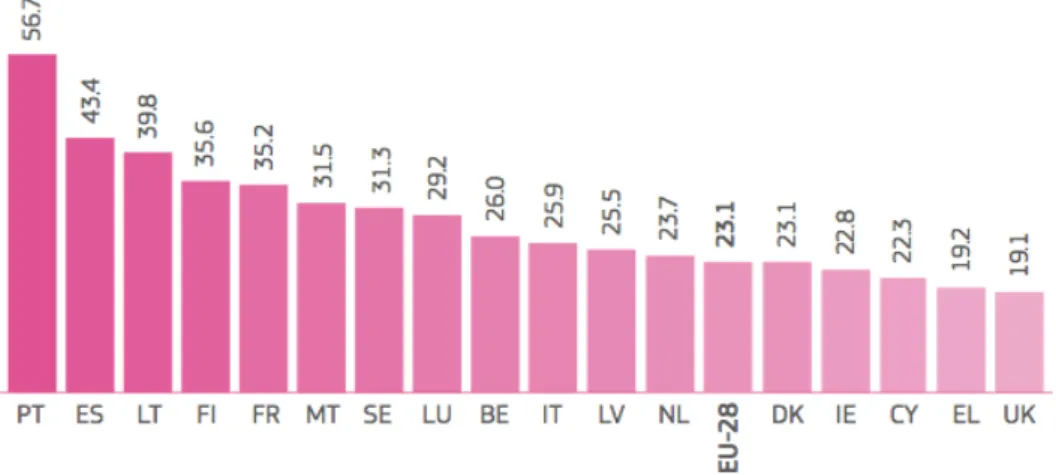
Aquaculture production has been rising in Europe in the last years (Figure 1.2). Great differences are noticeable between European countries, not only related with the production density, but also regarding the consumption of fishery and aquaculture products (Figure 1.3). The highest consumption was recorded for Portugal, comparatively to the average observed for 28 European Member States (EU-28, cf. Figure 1.3) or to the worldwide consumption (18.9 kg/inhabitant/year) (EC, 2014). Overall, farmed products represent 24% of the total European fishery consumption (EUMOFA, 2014). In Portugal, the main fish species produced are the turbot and sea bream (Figure 1.4), which have been a consistent selling product of economic importance (Cassamo, 2012).
Figure 1.2. Aquaculture production (in tonnes) by European countries from 2004 to 2013 (FEAP, 2014).
!
!
! 10
Figure 1.3. Average consumption of fishery and aquaculture products (kg/inhabitant/year) by European countries in 2010 (EC, 2014). PT - Portugal, ES - Spain, LT - Lithuania, FI - Finland, FR - France, MT - Malta, SE - Sweden, LU - Luxembourg, BE - Belgium, IT - Italy, LV - Latvia, NL - The Netherlands, EU28 - Europe, DK - Denmark, IE - Ireland, CY - Cyprus, EL - Greece and UK - United Kingdom.
! !
Figure 1.4. Portugal aquaculture production (in tonnes) by species from 2004 to 2013 (FEAP, 2014).
The leading aquaculture-producing countries worldwide rely essentially on inland aquaculture of finfish (FAO, 2014), though mariculture production has been rising in the last years (Figure 1.5). This is because the mariculture-farmed finfish includes more valuable species than freshwater aquaculture (FAO, 2014). In the European Union, however, the number of marine aquaculture farms is 50,300, whilst inland aquaculture occurs only in 11,500 farms (Rana & Choo, 2002).
!
!
! 11
"
Figure 1.5. Inland and mariculture production (million tonnes) in the world from 1980 to 2012 (FAO, 2014).
! ! !
Aquaculture can be characterized in a number of different ways depending on the environment location of the culture (i.e., inland aquaculture versus mariculture), the type of production system used, the production intensity and the rearing operation designs (Figure 1.6.). Regarding the production system, it can be considered either land- or water-based systems (EP, 2014). The land-water-based systems are localized inland and use water captures from a different source. These can be categorized as
- ponds: mostly earthen structures with 0.5-1.5 m of depth, covered by waterproof membrane or soil to limit water leakage and a water exchange at > 5-30% per day. - tanks: made of polythene, polypropylene, concrete or other materials; can sustain water volumes of 1m3 to several hundred m3; usually circular or as long rectangles (raceways); with a water exchange of 0.5-4 L/hour.
Figure 1.6. Different aquaculture classifications.
!
Aquaculture classification according to:
Localization Mariculture Inland Aquaculture Production system Land-based Ponds Tanks Water-based Floating
Cages, Rafts and Long-lines Fixed Bouchot' and Trestles Bottom culture Intensity of production Extensive Semi-intensive Intensive Hyper-intensive
Rearing operacional design
Open systems
Semi-closed systems
Closed systems
The water-based systems (Figure 1.7) comprehend either floating or static constructions located in a natural water body. They involve:
- cages: generally based on a secured floating collar with a net bag suspended below where the fish is cultured. The mesh size depends on the size of fish. Volumes can go from 100 to over 20.000 m3.
- rafts: floating platforms usually made of wood to support ropes that hang down in the water, upon which mussels or other bivalves can be attached.
- longlines: long horizontal ropes fixed to the shore and each end is supported in the water with floats. They also have vertical ropes to support shellfish production but are cheaper than rafts.
- bottom culture: no containment structures, just management practices used for mussels, scallops, clams and others.
- bouchot’: wooden poles with rope twisted around and placed in intertidal areas for mussel attachment.
- trestles: plastic mesh bags or trays containing oysters or other shellfish fixed with steel to sands in intertidal zones.
- lines: suspended between fixed poles and used for seaweed cultivation, or to suspend baskets for shellfish.
Trestles
Figure 1.7. Representations of some water-based production systems usually directly deployed in the environment. Images obtained from: http://www.thefishsite.com/articles/contents/09-05-12ProductionCycle.gif,
http://www.fao.org/fi/figis/culturespecies/data/assets/images/mytilus/blue_mussel_cycle.jpg,,
http://www.ecasatoolbox.org.uk/the-toolbox/indicator-groups/How%20to%20assess%20an%20existing %20and%20a%20new%20(planned)%20site/DSCN3274.JPG/view
!
!
! 14
Aquaculture can also be characterized according to the production intensity, which may range from less (extensive) to more intensive production systems (Edwards, 1993; Tidwell, 2012):
- Extensive: no feed added, low stocking density and low water exchange.
- Semi-intensive: partial feed added, medium stocking density and medium water exchange.
- Intensive: pelleted feed added, medium to high stocking densities, high water exchange, use of mechanical aeration.
- Hyper-intensive: feed added, very high stocking densities and very high water exchange & O2 supplementation.
Most aquaculture plants rely on earthen ponds, operating at a wide range of intensities. For examples, the smaller ponds with less than 500 m2, which are extensively stocked and fertilized (i.e., extensive and semi-intensive production), can promote the stability of small-scale systems for household use and local marketing. Depending on the stocking density and the food source, cages may fall within the extensive, semi-intensive or intensive categories (Masser, 2012). The highest stocking densities are accomplished in hyperintensive systems, which may include cages, but also raceways and recirculating aquaculture system (RAS), explained in detailed later.
The intensity of production is also associated with the rearing operational designs that range from open, to semi-closed and closed systems. Open systems rely on natural water movement (i.e., tides) to transport waste products away and bring renewed water. These aquaculture systems are developed in natural environments (e.g., lake, rivers, sea/offshore), namely through the implementation of extensive production conditions. In semi-closed systems, the production units are manmade, but still rely on the same level of human labour and water flow factors as those of open systems. Here raceways and ponds are the major production methods. Closed systems (e.g., RAS), in turn, reuse water and the operators control most environmental variables in the system (Tidwell, 2012).
For certain open systems, the production intensity is a practical indicator of potential local environmental impacts. Still other parameters like the conditions of the site and management techniques can contribute to reduce the possible environmental negative effects of this type of aquaculture systems. However, the production intensity in closed
!
!
! 15
systems is not necessarily an indicator of its potential environmental impact, e.g., a hyper-intensive farm using recirculation technology may in part be environmental friendly due to its lower exchanges with the neighbouring environment compared to other systems (EC, 2012).
1.1.2. Recirculating Aquaculture System (RAS)
! !
RAS are a land-based fish farming that treat and re-use wastewater from the tank(s) where the fish is produced, while simultaneously reducing wastes through several filtration stages. Thus, water can be recycled back into the system without needing a great percentage of water renewal (Figure 1.8) (Timmons & Ebeling, 2010). In the literature, the terms recycling and reuse/recirculation are often misled or used without a clear distinction. Recycling is technically a form of reusing, but it refers to the use of wastewater from the tanks without undergoing a treatment process. If the wastewater is treated and used again, then it is considered a reuse or recirculation system.
Figure 1.8. Diagram representing the simplified process of a Recirculating Aquaculture System.
Image adapted from: http://www.water-proved.de/de/aquakultur/Bilder/Schemata/ Banner_Kreislaufanlage_1400px_web.jpg
!
!
! 16
Overall, RAS systems have been exploited for the hyper-intensive production of many different fish species at any size and life stage. RAS production is generally difficult to evaluate since the available statistics are limited. However, European RAS production has been increasing considerably both in volume and species diversity (Martins et al., 2010). In Portugal, it was estimated a production of 10,000,000 fingerlings (fingerling heads/year) in 2005 of freshwater and marine species, being the third highest value observed for European countries. Additionally, Portuguese RAS grow-out production went from 110 to 112 (MT/year) from 2008 to 2009, being turbot, seabass and sole the major marine species produced (Martins et al., 2010).
Comparatively to RAS, the conventional aquaculture systems (e.g., outdoor ponds and cages) are not sustainable in the long term run, not only due to environmental concerns, but also because of their limitation to assure the safest products to the consumers, free from chemicals and metals, freshly raised and local products (Ebeling & Timmons, 2012). In fact, RAS offer many advantages over these systems by:
● reducing water (e.g., saltwater RAS with almost no freshwater inputs compared to traditional production systems) and land usage (RAS use less than 1% of land than ponds and can be explored in areas with poor food production potential, e.g., deserts) (van Rijn, 2013, Zhang et al., 2013)
● reducing shipping costs and transport-related fossil-fuel emissions (i.e., CO2) (Martins et al. 2010)
● enhancing fish farming by improving culture conditions, feeding efficiency and stocking densities (Ebeling and Timmons, 2012)
● reducing impacts on marine ecosystems (e.g., diseases, pollutants) through effluent treatment, while avoiding concerns related with the exploration of coastal water areas by using on-shore tank systems (Goldberg et al., 2001)
● producing by-products (e.g., N and P contents used as fertilizers) for varied applications (e.g., agriculture, cosmetics, feeds, industry) (Klinger & Naylor, 2012).
Notwithstanding, RAS also present disadvantages, despite the availability of solutions to overcome them:
!
!
! 17
● the high cost of feed, labour and operations (Klinger & Naylor, 2012) can be offset by the construction of large-scale (>50 MT/year) that would increase labour, processing and infrastructures
● the high start-up costs could be counteracted by increasing stocking densities and productions (Martins et al., 2010)
● the significant energy requirements (Klinger & Naylor, 2012) could be fulfilled by the use of "green" energy sources (e.g., solar and wind)
● the potential accumulation of contaminants, fish mortality and disease outbreak from water re-use, and feed efficiency can be surpassed by improving N and P removal [e.g., by altering feed inputs, optimizing beneficial bacterial growth (Klinger & Naylor, 2012), cost-effective bioremediation solutions that could provide value-added products]
● the tight monitoring of water quality and waste management can be achieved through a meticulous design and management of RAS, which involves an efficient feed utilization, incorporation of treatment methods of recirculating water or discharged wastewater, as to reduce waste production (van Rijn, 2013).
In spite of these drawbacks, RAS were partially developed to answer the increasingly established environmental regulations at the European [e.g., Water Framework Directive (EC, 2000); Waste Directive (EC, 2008); Environmental Liability Directive (EC, 2004)] and national [e.g., Lei da Água (AR, 2005), law by decree 236/98 establishing criteria and thresholds for water quality according to its uses (MA, 1998)] levels, which dictate land and/or water use, protection and/or management (Martins et al., 2010).
Under this context, the efficient remediation of wastewater resulting from RAS activities is, hence, an extremely relevant aspect. Before going further, it is important to clarify the two types of effluents or wastewater normally produced in a RAS system. Considering that RAS usually replenish less than 5-20% of its water volume per day (Summerfelt & Vinci, 2008; EP, 2014) to account for evaporation, solids removal and other losses (USEPA, 2002), it means that most wastewater (RWW – recirculating wastewater) from the production tanks is treated and recycled back to the system. The remaining wastewater effluents are discharged (DWW – discharged wastewater) after being remediated. !
!
!
! 18
1.2 Objectives and structure of the thesis
! !
This study encloses two main goals. The first one was to revise the available literature regarding the remediation methods usually performed to remove or transform the contaminants present in marine RAS effluents, also focusing on the bioremediation approaches applied to phosphates. The second goal intended to explore the bioremediation potential of a biogenic, biodegradable and nontoxic biosorbent based on oyster-shell waste, to remove phosphates from artificial seawater. In order to achieve this objective different influencing factors were evaluated to select the optimal conditions for P bioremediation: shell waste pre-treatment, biosorbent concentration, particle size and equilibration time.
The proposed aims were fulfilled along the following sections: i) the first section comprised the introduction of the thesis.
ii) in the second section it is presented a revision of the literature on marine RAS and (bio)remediation methods applied for reducing the presence of different contaminants usually detected in RAS wastewaters, entitled Review: Remediation methods of Effluents of
Marine Recirculating Aquaculture Systems;
iii) the third section entitled Efficiency of shell wastes for the removal of phosphates from
artificial seawater – an approach to marine recirculating aquaculture systems presents the
outcomes obtained in the different experimental trials conducted under laboratory conditions. It was prepared in the form of a manuscript to be submitted to a peer-review journal;
!
!
! 19
1.3. References
!
AR, Assembleia da República (2005). Lei 58/2005. 29 de Dezembro. Diário Da República
Cassamo A. (2012). Aquicultura em Portugal, produção intensiva de pregado (psetta maxima). Dissertação de mestrado em medicina veterinária - Universidade técnica de Lisboa Faculdade de Medicina Veterinária 125
EC, European Council (2000). Directive 2000/60/EC. 23 October 2000. The European Parliament And The Council.
EC, European Council (2004). Directive 2004/35/CE. 21 April 2004. The European Parliament And The Council.
EC, European Council (2008). Directive 2008/98/EC. 19 November 2008. The European Parliament And The Council.
EC, European Commission (2012). Guidance on Aquaculture and Natura 2000. Sustainable aquaculture activities in the context of the Natura 2000 Network, 89
EC, European Commission (2014). Facts and figures on the Common Fisheries Policy. Luxemburg Publication Office of the European Union 48 pp doi: 10.2771/35745
Edwards, P. (1993). Environmental Issues in Integrated Agriculture-Aquaculture and Wastewater Fed Systems. IN: Pullin, R.S.V., Rosenthal, H., & Maclean, J.L. (Eds) Environment and Aquaculture in Developing Countries. Worldfish. 139-170.
EP, European Parliament (EP), (2014). The Long-Term Economic and Ecologic Impact of Larger Sustainable Aquaculture. Directorate-general for internal policies- policy department b: structural and cohesion policies
EUMOFA, European Market Observatory for Fisheries and Aquaculture Products (2014). The EU Fish Market, 2014 Edition, EU-Directorate General for Maritime Affairs and Fisheries.
Federation of European Aquaculture Producers (FEAP) (2014). European Aquaculture production Report 2004-2013.
Food and Agriculture Organization (FAO). (2014). The State of World Fisheries and Aquaculture 2014. Rome. 223 pp.
Goldberg, R.J., Elliot, M.S. & Naylor, R.L. (2001). Marine Aquaculture in the United States: Environmental Impacts & Policy Options. Pew Oceans Commission. Arlington, Virginia, 33p.
Klinger, D. & Naylor, R. (2012). Searching for Solutions in Aquaculture: Charting a Sustainable Course. Annual Review of Environment and Resources, 37(1), 247–276. doi:10.1146/annurev-environ-021111-161531
Martins, C. I. M., Eding, E. H., Verdegem, M. C. J., Heinsbroek, L. T. N., Schneider, O., Blancheton, J. P., d’Orbcastel, E. R., (2010). New developments in recirculating aquaculture systems in Europe: A
!
!
! 20
perspective on environmental sustainability. Aquacultural Engineering, 43(3), 83–93. doi:10.1016/j.aquaeng.2010.09.002
Masser, M.P. (2012.) Cage Culture in Freshwater and Protected Marine Areas. In: Aquaculture Production Systems. Ed. J.H. Tidwell. Wiley-Blackwell. Oxford, United Kingdom pp 119-134
Ministério do Ambiente (MA) (1998). Decreto-Lei 236/98, 1 Agosto. Diário da República. Governo da República Portuguesa.
Rana K. & Choo P.S. (2002). women in fisheries in the European Union. Global Symposium on Women in Fisheries: Sixth Asian Fisheries Forum
Sugunan, V.V., Welcomme, R.L., Béné, C, Brummett R.E. & Beveridge M.C.M. (2007). Inland Fisheries and Aquaculture. Water for Food, Water for Life: A Comprehensive Assessment of Water Management in Agriculture. London: Earthscan, and Colombo: International Water Management Institute. 459-483
Summerfet S. & Vinci, B. (2001). Best Waste Management Practises for Recirculation Systems. 2001 Aquacultural Waste Management Symposium. Virginia Sea Grant, Publication Number VSG-02-01 Tidwell, J. H, (2012). Characterization and Categories of Aquaculture Production Systems. John Wiley &
Sons, Inc. 1st Edition. Aquaculture Production Systems, pp 64
Timmons, M.B. & Ebeling, J.M. (2010). Recirculating Aquaculture, 2nd Edition. Cayuga Aqua Ventures LLC, pp 2-882
USEPA, US. Environmental Protection Agency (2002). Development Document for Proposed Effluent Limitations Guidelines and Standards for the Concentrated Aquatic Animal Production Industry Point Source Category, EPA-821-R-02-016
van Rijn, J. (2013). Waste treatment in recirculating aquaculture systems. Aquacultural Engineering, 53, 49– 56. doi:10.1016/j.aquaeng.2012.11.010
Zhang, X., Spanjers, H., van Lier, J.B., (2013). Potentials and limitations of biomethane and phosphorus recovery from sludges of brackish/marine aquaculture recirculation systems: a review. Journal Environmental Management. 131, 44–54.
Zhang, X., Hu, J., Spanjers, H., & van Lier, J. B. (2014). Performance of inorganic coagulants in treatment of backwash waters from a brackish aquaculture recirculation system and digestibility of salty sludge. Aquacultural Engineering, 61, 9–16. doi:10.1016/j.aquaeng.2014.05.005
Zhu, S., Chen, S., (2001). Effects of organic carbon on nitrification rate in fixed film biofilters. Aquacult. Eng 25, 1–13.
Zhuang X., Han Z., Bai Z., Zhuang G., Shim H., (2010). Progress in decontamination by halophilic microorganisms in saline wastewater and soil. Environmental Pollution, 158, 1119–112.
! ! !
!
" "
" " " " " " " " " " " " " " " " " " " "
"
"
"
"
"
"
"
"
________________________________________________________________________"
"
II. Review: Remediation methods of Effluents of
Marine Recirculating Aquaculture Systems
" " " " " " "
" "
!
!
! 21
II. Review: Remediation methods of Effluents of Marine
Recirculating Aquaculture Systems
____________________________________________________"As most marine Recirculating Aquaculture Systems (RAS) are set close to the coast, wastewater discharge into the sea or even in neighbour streams is still the most usual practice. While in marine RAS with online wastewater treatment such discharge results in minor environmental impact, the same does not happen for discharges from RAS with little or no wastewater treatment (i.e., recirculation aquaculture operations that do not follow environmental regulations). This may cause profound impacts into the receiving environment (van Rijn, 2013), particularly if we consider the accumulation of contaminants that may still persist after wastewater treatment whenever applied. In this regard, water quality control in RAS together with the improvement of available remediation measures is a crucial part for a successful, eco-friendly and cost-effective operation. Therefore, in the next sections will be highlighted the contaminants usually detected in RAS effluents and which methods are usually applied for their reclamation.
!
2.1. Contaminants in RAS effluents
Uneaten feed, excreted metabolites from cultured organisms, pharmaceutical residues and water disinfectant agents are common residues in RAS wastewater after each culture cycle. As a result, many contaminants enter the system and have been detected in saltwater RAS effluents (cf. Table 2.1). Some of them are not considered a threat for the aquaculture production and receiving environment (e.g., nutrients, organic matter), unless they reach concentrations above the available regulatory levels. Regarding the receiving environment, in particular, if high accumulation of contaminants or negative activities occur, then direct (e.g., algal blooms triggered by nutrient surplus) or indirect (e.g., pH or dissolved organic carbon can affect the toxicity of other compounds in water) impacts are likely to take place. More specifically, Goldberg et al. (2001) considered five types of potential effects ranging from biological pollution, contaminated fish used in fish feeds, organic pollution/eutrophication, chemical pollution to habitat modification.
Table 2.1. Concentrations and major types of contaminants usually detected in saltwater effluents after an aquaculture production cycle, in several types of aquaculture including RAS, and maximum values admissible for RAS and for discharge.
na – not available.
cfu (colony-forming unit); MPN (Most Probable Number); TN (Total Nitrogen); TP (Total Phosphorus).
* TOC (Total Organic Carbon); † Recommended by Losordo et al. (1998) a Sharrer et al. (2007) b Zhang et al. (2014) c Carballeira et al. (2012) d Decreto-Lei 236/98, 1 Agosto e Decreto-Lei 152/97, 19 Junho ! Solids (mg/L) Oils/ Lipids (mg/L) Organic Matter (mg C* /L) Nitrogenous Compounds (mg/L) Phosphorus (mg TP/L) Pathogens TN NH4+-N NO2--N NO3--N Concentration in saltwater effluents production 754 - 1732 a na 37 - 107 b 50.7 - 67 a na na na 19.2 - 38.7 a 7.4 x 106 - 2.8 x 107 cfu/mL a 108 – 259 b 2.36 - 8.04 c 9.8 – 12.7 b 14 – 39 c Maximum values admissible RAS † na na na na na 0.2–5.0 <1000 na na Bathing water d na na na na na na na na MPN/100mL10 000 Wastewater discharges 60 d 15 d na 10 – 15 e 10 d na 50 d 10 d 1 – 2 e na
!
!
! 23
In order to prevent unbalanced environmental scenarios, marine RAS wastewater treatment systems must have into consideration these problems, as to maximize efficiency and meet regulatory demands. In this context, the remediation procedures performed in RAS for the removal of the main concerning contaminants (cf. Table 2.1) from the RWWand/or DWW may target all or part of the following major steps (Tucker & Hargreaves, 2008):
● solid waste removal,
● nutrient removal/transformation, ● bacteria/pathogens sterilization, ● carbon dioxide removal, ● dissolved O2 supplementation.
A review on the methods applied for major classes of contaminants in RAS effluents is developed along the next sections. For matters of clarity, this is performed separately for RWW and DWW.
2.2. Remediation methods for contaminant removal from RAS effluents
2.2.1 Recirculating wastewater a. Solid waste removal
Solid waste consist of colloid and suspended solid particles of different sizes, which may include nutrients and organic matter namely derived from fish excretions and feed residues, as well as some pathogens or other non-cultured organisms. Solids removal is one of the most critical processes in RAS, since they affect the efficiency of all other downstream unit processes (Ebeling & Timmons, 2012). For that, solid particles, are firstly concentrated into a sludge, i.e., the concentrated biosolid (Zhang et al., 2013). This may be generally done by settling or mechanical filtration, being this latter process performed through, for example, a
!
!
! 24
microscreen filter.
The microscreen filters act as a sieve, retaining suspended particles larger than their mesh size (Ebeling & Timmons, 2012). They are widely used in large-scale RAS designs as the primary means for the removal of solids (Cripps & Bergheim, 2000) and have numerous advantages, like easy installation and operation, interchangeable screens and varied mesh sizes (usually 60 to 100µm) (Malone, 2013). Despite this, screen filters require high maintenance, capital and operation costs (Ebeling & Timmons, 2012). Because of reduced efficiency, they create sludge with relatively low concentration of solids (Piedrahita, 2003). Besides, the small-suspended solids are not efficiently removed and tend to accumulate afterwards (van Rijn, 1996; Malone, 2013).
Sludge thickening for settling/capture of large solids in basins or ponds, geotextile bags or belt filters and membrane reactors are also applied in RAS. Notwithstanding, settling/sedimentation methods are not recommended as the only path for solids removal from the RWW in RAS, because they indeed provide low efficiency in retrieving fine solids. Moreover, they require more makeup water than screen filters (Piedrahita, 2003). Therefore, fine particles (<100 µm) are usually removed by foam fractionation (Timmons & Ebeling, 2010; Riche et al., 2012; Malone, 2013).
Foam fractionators (also referred as protein skimmers) (Figure 2.1), which can be applied after mechanical filtration or before oxygenation (cf. Figure 1.8), involve the adsorption of surfactants in the form of dissolved and fine particles onto bubble surfaces (Losordo et al., 1998). As bubbles rise through the tube they collect dissolved materials and fine particles from the RWW, because of the chemical properties of both surfactant and air bubbles. Briefly, surfactants have a hydrophilic and a hydrophobic end; the hydrophobic end goes into the air bubble leaving the hydrophilic end in contact with the water, if this end is negatively charged it attracts positively charged materials in the water column, and these positively charged materials in turn attract negatively charged particles (Timmons & Ebeling, 2010). Bubbles attract, therefore, both negative and positive fine and dissolved solids (and even bacteria) and when they reach the surface they are removed. It is a simple and inexpensive method (Timmons, 1994) that complements the solids removal methods via filtration or settling (Tucker & Hargreaves, 2008). Additionally, foam fractionators can be
!
!
! 25
used for ozone injection, making them both a solid removal and a disinfection device (ozonation) all in one unit. They work better in saltwater than in freshwater, because in saline water small bubbles are easily formed and foam production is enhanced, since the surface tension in seawater is higher than in freshwater (Timmons & Ebeling, 2010). Thus, foam fractionators are widely established in marine RAS (Malone, 2013; Timmons & Ebeling, 2010). Their efficiency is dependent on bubble diameter, the chemical properties of the contaminants, the air-to-water ratio and the presence of lipids in the water (which derive from fish oils in fish feeds and generally reduce foam formation).
Figure 2.1. Foam fractionator for organic matter and fine particles removal. Image credits:
http://www.alibaba.com/product-detail/Fish-Farm-Aquaculture-Aquarium-Foam-Fractionator_1905444291.html
Ozone, besides its germicidal activity, it is highly effective in the oxidation of nitrites to nitrates dissolved in water, which results in an improved filtration and removal of colloids and suspended matter (Schroeder et al., 2011). For all these reasons, ozone treatments have been widely applied (Gonçalves & Gagnon, 2011; Schroeder et al., 2011). However, there are several disadvantages to its usage. Besides being an expensive method, this technique may lead to the formation of ozone residues that can be lethal to fish even at small concentrations, depending on the species and their life stage (Timmons & Ebeling, 2010). An example is the formation of biocidal oxidants like bromates [e.g., hypobromous acid (HOBr) and
!
!
! 26
hypobromite ions (OBr-)] through the reaction of ozone with bromide ions present in saltwater (Malone, 2013). These bromates are toxic to fish and shellfish; which makes this technique problematic in aquaculture production. Fine suspended material is also removed by biological oxidation, which takes place in most RAS within the nitrifying filters discussed later (van Rijn, 1996). ! ! ! b. Oils/Lipids !
Despite being hugely overlooked, fish oil from fish feeds is one of the major problems to deal in the treatment of RAS effluents, which due to its high organic loading, makes disposal of oily wastewater a challenging environmental concern. Portuguese admissible values of fat for the discharge of wastewater are 15 mg/L (Table 2.1). However, quantitative data on the lipidic content of aquaculture wastewater and its remediation is still deeply overlooked.
The removal of oils/lipids from RWW is usually carried out through a combination of physical, chemical and biological approaches (Elleuch et al., 2013). Coagulation–flocculation is commonly performed by adding compounds (e.g., ferric chloride) to wastewater as to destabilize the colloidal materials and allow the small particles to agglomerate into larger settable flocs, and therefore can be employed in the solid waste removal phase. Coagulation– flocculation process may be used as a pre-treatment, enhancing the biodegradability of the compounds before the application of a biological treatment. In what concerns biological methods, microorganisms can be applied to degrade oils.
Lipase is an enzyme produced by different fungi, bacteria and yeast species that can transform lipids into glycerol and fatty acids (Bhumibhamon et al., 2002; Azhdarpoor et al., 2014). The problem is that, fatty acids can inhibit microbial activity because they tend to attach to the cellular membrane, making it less permeable and, therefore, preventing their further degradation. One reported example is the long chain fatty acids (LCFA) that are produced during hydrolysis of triacylglycerol lipids. Oils high in triacylglycerol, such as fish
!
!
! 27
oil (95-99% of the lipids therein contained; Torstensen & Tocher, 2010), show low biodegradation efficiency, because of the LCFA antimicrobial activity (Elleuch et al., 2013) which tends to result in the accumulation of LCFA.
Besides the fact that lipase-producing bacteria are considerably ubiquitous, their use on the treatment of oily wastewater can be a quite effective technique if the low permeability issue is solved. Therefore, a combination of coagulation–flocculation process with biological treatments turns out to be more successful than the use of these processes alone (Elleuch et al., 2013). But the necessity for two techniques is not ideal, hence new and sustainable alternatives should be seek.
c. Nitrogenous compounds
!
Total ammonia-nitrogen (TAN) is a by-product of fish metabolism and is produced during bacterial decomposition of solid organic waste (e.g., fish feeds); it consists of un-ionized ammonia (NH3) and ionized ammonia (NH4+); posteriorly this ammonia can be oxidized to form nitrite-nitrogen (NO2-) and nitrate-nitrogen (NO3-) (Losordo et al., 1998). NH3 and NO2− are toxic to fish at low quantities (0.1 and 2 mg/L, respectively), but they can tolerate fairly high levels of nitrate (>500 mg NO3−/L). However, long-term exposure to nitrate can be harmful to some fish (van Bussel et al., 2012). Therefore, these compounds are also targeted in RAS RWW treatment. Table 2.2 shows the average removal of N compounds in saltwater RAS RWW and DWW after being subjected to different remediation methods.
!
!
! 28
Table 2.2. Average concentration (mg/L) of ammonium (NH4+-N), nitrite (NO2--N) and nitrate (NO3-
-N) before and after different remediation techniques in saltwater RAS producing different fish species.
nd - not determined.
N compounds and part of the dissolved organic matter are commonly removed by biofilters (Yang et al,. 2001). In general, biofilters are compartments that include a substrate/medium for microbial attachment and growth, or a section that allows suspended microbial growth for aerobic and anaerobic microbial elimination of wastes (Schreier et al., 2010). The media used in the biofilters must be inert and non-biodegradable (e.g., sand, plastic or ceramic materials) (Ebeling & Timmons, 2012). These biofilters perform nitrification and denitrification by exposing the wastewater to beneficial bacteria. Nitrification is a two-stage process (Figure 2.2). Firstly, nitrifying bacteria (e.g., Nitrosomonas sp. and Nitrosococcus sp.) oxidize ammonia to nitrite. Secondly, Nitrospira sp. oxidizes nitrite to nitrate (Gutierrez-Wing & Malone, 2006; Schreier et al., 2010). Many systems rely on a variety of different microorganisms to accomplish denitrification, i.e., the conversion of nitrate into nitrogen gas (N2) under anaerobiosis (Schreier et al., 2010; Chavez-Crooker & Obreque-Contreras, 2010; van Rijn et al., 2006).
!
!
! 29
Figure 2.2. Nitrification and denitrification processes and images of two biofilter designs.Adapted from Schreier et al. (2010). Image credits: https://www.youtube.com/watch?v=y-XcK0opWKY,
http://www.ecotao.co.za/html/aquaculturerecirculationsystem.html !
!
There are several types of biofilters frequently used in saltwater RAS. Two of them are: the microbead biofilters and the moving bed bioreactors (Figure 2.2). Microbead biofilters have been long used mainly because of their simplicity, low cost and negligible ecological footprint. They operate with small plastic beads that float in the system, which gives a high specific surface area of contact, what favours microorganism-contaminant contact. For these reasons, this type of biofilter is usually preferred to other designs. Its main detriment is the inherently dependence on supplementary addition of O2 to the water flow (i.e., they do not have a O2 pumping system incorporated) to maintain aerobic conditions for the microorganisms in the biofilm. Therefore, under low influent dissolved O2, anaerobic
!
!
! 30
conditions will be generated within the biofilter, leading to inadequate nutrient removal due to microbial loss (Ebeling & Timmons, 2012).
More recently, moving bed bioreactors have been introduced and are already the most competitive of all the biofilter designs (Ebeling & Timmons, 2012). The media is in suspension and is mechanically aerated, which provides effective mixing of microorganisms with the effluent (Ebeling & Timmons, 2012), thus contributing to RWW aeration and degasification later required. The main advantage of this system is the already integrated O2 addition system, and the simultaneous provision of carbon dioxide stripping. However, this technique provides low specific surface area and the application of a substrate with higher surface area requires more expensive filters. In contrast, microbead biofilters, like previously said, use substrates with high specific surface area, hence triggering lower costs and space requirements (Ebeling & Timmons, 2012).
Notwithstanding, the biofiltersapplied to treat saline effluents, as oppose to freshwater, may not be effective to remove N compounds because of the inhibitory effect of salinity on nitrifying and denitrifying bacteria (cf. Yang et al., 1995, Sakairi et al., 1996, Glass and Silverstein, 1999, Sánchez et al., 2004) and sometimes concentrations in the effluents surpass admissible values (Table 2.1).
A good performance of a marine RAS is strongly dependent on biofiltration efficiency. Because of the salinity levels, biofilters still are not completely adequate in removing N compounds from saltwater RWW. Data regarding microbial ecology of these nitrification/denitrification reactors in RAS is still limited and the microbial community in biofilters is difficult to control, which becomes the main cause of the limitations in the biofiltration phase (Martins et al., 2010).
!
!
! 31
d. Phosphorus
!
Besides N, the excess of phosphorus (P) also greatly contributes for the eutrophication of aquatic systems. The removal of P is a serious concern that has not yet been sufficiently solved (Vohla et al., 2011) (Table 2.3).
!
Table 2.3. Average concentration (mg/L) of phosphate (PO43--P) before and after different remediation
techniques in saltwater RAS.
nd - not determined.
Phosphorous has been removed from RWW by biofilters and retention ponds techniques. Sharrer et al. (2007) using a membrane biological reactor obtained a total phosphorus removal of 96.1% in freshwater, but with increasing salinities this rate was reduced until it reached 65.2% at 32 of salinity of TP. Hussenot et al. (1998) using a retention lagoon removed 25-52% of phosphates and acknowledge it as a low efficient removal method for orthophosphates.
The efficiency and cost effectiveness of phosphate removal is one of the difficulties in RAS and quite overlooked, despite some authors obtained acceptable removal after RWW treatment (Table 2.3). However, some still don’t reach admissible concentration for discharge (Table 2.1). Phosphate is usually neglected for RWW, which could lead to accumulation In DWW. Yet, RAS do not have a specific phosphate removal system in freshwater and saltwater RAS.
!
!
! 32
d. Pathogens
To kill bacterial, viral, fungal and protozoan pathogens, UV light and ozone gas (Figures 2.3) are usually employed before water re-enters the system (Schroeder et al., 2011; Gonçalves & Gagnon, 2011). Foam fractionators, besides removing fine solids, can also sterilize the effluent, alone or coupled with UV and ozone treatment.
UV lights emit wavelengths that are deadly to microorganisms and the disinfection rates are normally proportional to the light intensity. If properly sized, UV radiation is effective. However, the light penetration in the water requires that RAS effluents are not very turbid as to assure effectiveness. Furthermore, UV light bulbs have to be routinely replaced every few months (Malone, 2013). The second method mainly used for disinfection is ozone gas. Ozone is a very powerful oxidant rapidly absorbed by most recirculating waters. Ozone destroys the remaining dissolved organic matter, bacteria, viruses and protozoan organisms. The main advantages and downsides of ozone treatment have already been previously mentioned in solids waste removal. Although the combined use of ozone and UV treatments is particularly effective in pathogen removal (Martins et al., 2010), it becomes expensive and quite demanding in operational terms.
a)# #b)# #
Figure 2.3. Illustration of a) an UV light treatment in Recirculating Aquaculture Systems, and b) a
typical ozone generator. Image credits:
http://www.sunderlandmarine.com/australia/insurance/aquaculture/image-gallery,
!
!
! 33
2.2.2 Discharged wastewater
RAS effluents that are not recycled back into the recirculation loop (i.e., DWW) may be directed to a wastewater treatment plant or, alternatively, be discharged into a receiving water body, after being (or not) subjected to some treatment methods (Tucker & Hargreaves, 2008). The main difference between the remediation procedures for DWW and RWW in RAS is that the latter requires fast acting techniques that guarantee an efficient aquaculture production and safe products. Therefore, more time-consuming (and sometimes cheaper) methods can usually be applied to remediate DWW. At the end, the aim is to achieve good quality emission levels as established in national legislation (e.g., Lay by decree 236/98) in what concerns nutrients (phosphate, nitrates), dissolved organic matter, biological/biochemical oxygen demand (BOD), chemical oxygen demand (COD), and total suspended solids.
A common preliminary treatment approach for both RWW and DWW concerns the removal of suspended solids, which is often made in sedimentation basins (USEPA, 2002b). Settling basins are simple and of low maintenance, and many of the contaminants of concern in RAS can be partly removed with the captured solids, such as P and N compounds and disinfectants. However, these basins require large areas and regular cleaning of accumulated solids (Timmons & Ebeling, 2010). Besides, they present reduced efficiency in removing small-sized particles without chemical addition (USEPA, 2002b). Whenever the levels of nutrients like phosphatesand nitrates are not in compliance with the regulated levels, further treatment of DWW can be developed in hydroponics (Gorder, 2001) and/or constructed wetlands (USEPA, 2002a) (cf. following section). Aquaponics (i.e., combination of RAS with hydroponics; Klinger & Naylor, 2012) and constructed wetlands are still mainly applied in freshwater aquaculture effluent treatment (Webb et al., 2012), being their application in saltwater quite disregarded. Aquaponics could still be used in the treatment of effluents from RAS mariculture, as far as a commercially valuable halophytic plants, micro- or macroalgae, capable of growing in saline wastewater, are employed (Webb et al., 2012). Such approach would benefit from the continuous nutrient-rich discharges from RAS culture tanks (USEPA, 2002a, Klinger & Naylor, 2012), while helping reducing nutrient concentrations towards the safe release of DWW in receiving waters.
!
!
! 34
Another approach to reduce the contaminant burden in RAS DWW is based on constructed wetlands. These are man-made or engineered ponds that treat wastewater through physical, chemical and biological processes (USEPA, 2002a). Overall, they are a relatively simple and low cost technology that uses the ecological interactions of aquatic plants, detritus, microbes and fauna from wetland ecosystems to capture and transform contaminants (e.g., nutrients) from wastewaters (Lymbery et al., 2006; Sim et al., 2008). As a result, constructed wetlands have been already implemented for the treatment of different types of effluents (e.g., municipal or domestic sewage, industrial and agricultural wastewater) (Webb et al., 2012), but they offer potential for the remediation of saltwater RAS effluents if halotolerant organisms are used (e.g., halophytes; Webb et al., 2012). In marine RAS there are only a few studies on operations integrating constructed wetlands and its saline effluent treatment (Lymbery et al., 2006; Webb et al., 2012; Jesus et al., 2014). However, Jesus et al. (2014) using a simulated constructed wetland reported nutrient removal efficiencies from the DWW in compliance with Portuguese regulations.
Large-scale RAS could economically benefit from their own waste treatment facility, such as a Sequencing Batch Reactor (SBR) (Gorder, 2001). This procedure has several steps (Figure 2.4), where both solid and dissolved wastes (e.g., nitrates and phosphates) are treated in a single container. In the aerobic phase, carbon oxidation and nitrification are accomplished, and in the anaerobic phase denitrification occurs (Fontenot et al., 2007). SBR allows the treatment of RAS wastewater for posterior discharge into adjacent waterways.
Figure 2.4. Sequencing batch reactor process stages: fill, react, settle, decant and idle (Boopathy et al., 2005).
!
!
! 35
2.3. Digging into the “bio” of phosphate remediation in RAS effluents
!
Phosphate is one of the contaminants that is not yet satisfactorily, cost-effectively, environmental-friendly and efficiently removed/transformed with the currently applied remediation methods (Martins et al., 2010), because high concentrations can be detected in DWW, which may cause impact in the marine environments. Besides, it has not been pointed out as a strong limiting factor in fish production, what conversely does not push towards the development of new and efficient (bio)remediation measures. Notwithstanding, the levels of phosphorus usually surpass the regulated levels in RAS DWW (Trepanier et al., 2002; Martins et al., 2009a; Martins et al., 2010). In a closed aquaculture system, it has been shown that phosphate can be accumulated in concentrations as high as 19.5 mg P/L (Martins et al., 2009a), and after effluent treatment P concentrations were not always below the admissible values for discharge (Table 2.1). Thereby, the release of P-enriched effluents into adjacent coastal areas may ultimately trigger the development of eutrophication scenarios, which are quite harmful for aquatic life (Trepanier et al., 2002). Also, focusing the remediation of RAS DWW only on the reduction of N compounds is not enough to preclude those scenarios (Schindler et al., 2008).
Novel and promising approaches for eco-friendly wastewater treatment in saltwater RAS are greatly welcomed, and they could involve bioremediation strategies using live organisms and/or biological materials produced by them. Overall, bioremediation refers to the application of biological systems, metabolites or materials to destroy, transform and/or immobilize organic and inorganic contaminants (Gadd, 2010).
!
2.3.1. Live organisms
! !
The metabolic activities of live organisms can be exploited in bioremediation strategies (Fomina & Gadd, 2014) that may bear on, for example, biosorption,