Corrosion behavior of as-cast binary Mg-Bi
alloys in Hank’s solution
*Wei-li Cheng
Male, born in 1982, Ph.D, Associate Professor. Research direction: high strength magnesium alloy and biomedical magnesium alloy.
E-mail: chengweili7@126.com
Received: 2015-07-09 Accepted: 2015-10-05 *Wei-li Cheng 1, 2
, Rui Huo 2
, Quan-wei Tian 2
, Liang Tian 2
, and Shou-fan Rong 3
1. Key Laboratory of Interface Science and Engineering in Advanced Materials, Ministry of Education, Taiyuan University of Technology, Taiyuan 030024, China;
2. School of Materials Science and Engineering, Taiyuan University of Technology, Taiyuan 030024, China; 3. School of Materials Science and Engineering, Jiamusi University, Jiamusi 154007, China
M
agnesium alloys have attracted considerable attention for biomaterial applications due to their similar Young’s modulus to natural bones, good biodegradability and bio-resorbability [1, 2]. However, the key obstacle hindering widespread application of Mg alloys as biomaterials is mainly related to poor corrosion resistance in body fluid, especially in the presence of such aggressive ions as chlorine [3, 4].One of the effective means of improving the corrosion resistance of Mg alloys is alloying [5, 6]. A great deal of previous investigations on Mg-Al [3, 7], Mg-Sn-Ca [8, 9], Mg-RE [2, 10], and several other alloying systems have been proposed to this aim. Nevertheless, in consideration of the bio-safety of biodegradable materials, the alloys above seem not to be excellent biomaterials. The element Al, well known as a neuro-toxicant, has been proposed to be related to neurological disorders and Alzheimer disease [11]. In addition, the administration of rare earth (RE) elements
Abstract:
Biodegradable Mg-xBi (x = 3, 6 and 9wt.%) alloys were fabricated by ingot casting, and the change of corrosion behavior of the alloys in the Hank's solution was analyzed with respect to the microstructure using optical micrograph (OM), X-ray diffraction (XRD), scanning electron microscope (SEM) equipped with an energy dispersive X-ray spectrometer (EDS), electrochemical and immersion tests. The results show that the microstructures of the as-cast Mg-Bi alloys mainly consisted of dendritic α-Mg grains and Mg3Bi2 phase in common, with the secondary dendrite arm spacing (SDAS) decreasing signiicantly from 41.2 μm to 25.4 μm and the fraction of Mg3Bi2 increasing from 3.1% to 10.7%. Furthermore, the corrosion rate increasing from 1.32 mm•a-1to 8.07 mm•a-1
as the Bi content was increased from 3wt.% to 9wt.%. The reduced corrosion resistance was mainly ascribed to the increasing fraction of the second phase particles, which bring positive effects on the development of pitting.
Key words:
magnesium; microstructure; electrochemical impedance spectroscopy (EIS); pitting corrosionCLC numbers: TG146.22 Document code: A Article ID: 1672-6421(2015)06-425-06
can lead to heapatotoxicity [10]. Our previous study [8] indicated that when the Ca content exceeds 1wt. % in Mg-8Sn-Ca based alloys, the coarse ternary CaMgSn phase is formed and leads to accelerated erosion rates, thereby reducing the corrosion resistance of the alloys. Moreover, as-cast Mg-5Ca (or higher) alloys are found to be very brittle at room temperature [9].
To address these issues, the element Bi, which is non-toxic and not bio-accumulative [12], was considered, as
puriied Bi metal is often used in the preparation of a number of pharmaceutical products [13]. More recently, it has been shown that low iron Mg-5Bi-1(Si, Ca) alloys are suitable for use as small bone implants, stents, intravascular coils or wires [14]. From these reports, it is believed that Mg-Bi alloys have great potential for application as orthopedic implant materials.
ρ × ×
∆ × =
t A
m CR 8.76
1 Experimental procedures
1.1 Sample preparation
The nominal compositions of the alloys studied were Mg-3wt.%Bi (B3), Mg-6wt.% Bi (B6), and Mg-9wt.% Bi (B9). Ingots of each alloy were prepared by melting 99.9% pure Mg and 99.9% pure Bi in a steel crucible under the protective gas mixture of the N2 + CH2FCF3 (4%) using an electrical resistance furnace. The melts were then poured into steel molds that were preheated to 200 ºC.
1.2 Microstructure characterization
The microstructural characteristics of the as-cast billets before and after immersion tests were analyzed using X-ray diffraction (XRD), optical microscopy (OM), and a scanning electron microscope (SEM) equipped with an energy dispersive X-ray spectrometer (EDS). The secondary dendrite arm spacing (SDAS) and volume fractions of second phases were averaged using a computer aided method from values measured from 5 optical and SEM micrographs.
1.3 Electrochemical and immersion tests
The corrosion behavior of the as-cast alloys was investigated using immersion tests, electrochemical impedance spectroscopy (EIS) measurements, and potentio-dynamic polarization tests in Hank’s solution at 37 ºC. Prior to the immersion test, the alloy samples with dimension of 15 mm ×15 mm ×10 mm were mechanically ground using SiC papers up to 3000 grit. The corrosion rate CR (mm•a
-1
) was calculated according to the following equation [17]:
where △m is weight loss in g, A is exposed surface area in m2, t is total immersion time in hours, and ρ is the density in g•cm-3. For the electrochemical tests, a conventional three-electrode cell was used with platinum as counter electrode, a saturated calomel electrode as the reference electrode and the specimens mounted in epoxy resin with an exposed area of 1 cm2 as working electrodes at a constant scan rate of 0.5 mV•s-1. The electrochemical impedance spectroscopy (EIS) measurements were in the frequency range of 104 to 10-2 Hz at open circuit
potential with AC amplitude of 10 mV after the specimens had been immersed for 1 h. All electrochemical tests were conducted at least three times to conirm reproducibility.
2 Results and discussion
2.1 Microstructure
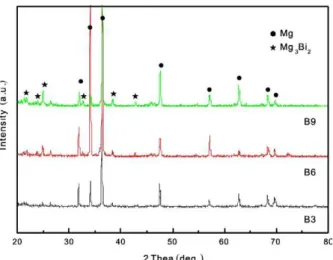
The XRD patterns of the studied alloys are shown in Fig. 1. As indicated, the as-cast alloys are mainly composed of α-Mg and Mg3Bi2 phase. It should be noted that the intensities of Mg3Bi2 peaks increased as the Bi content increased from 3wt.% to 9wt.% Bi, suggesting that the fraction of Mg3Bi2 phase increased.
Figure 2 shows the OM and SEM micrographs of as-cast Mg-Bi alloys. All the alloys exhibited typical dendritic structure. When there was an increase in Bi content, the SDAS of the studied alloys signiicantly decreased from 41.2 μm (B3) to 25.4 μm (B9). Similar to previously reported results [18]
, the formation of constitutional undercooling in a diffusion layer ahead of the solid/liquid interface results in the segregation of the alloying elements at the front of the grain growth, which limits the grain growth, hence refining the grains. Furthermore, the relatively small grain size in the B9 compared to the B6 and B3 alloys is related to the larger volume fraction of the continuous net-like Mg3Bi2 phase (Fig. 2d) in the B9 alloy, which acts as a barrier to grain growth.
The SEM micrographs of the studied alloys are presented in Fig. 3. As indicated, the distribution and morphology of the second phase are greatly dependent on the Bi content. In addition to a few eutectic phases, a rod-shaped Mg3Bi2 phase presenting at the grain boundaries and a partial divorced spherical-shaped Mg3Bi2 phase around the vicinity of the eutectic phase can be observed in the B3 alloy. Generally, during the last stage of solidiication, the reduced temperature of the residual liquid [19] results in breaking of the secondary dendrites, which can promote the formation of divorced Mg3Bi2 phase particles. With a further increase in Bi element, the morphology of the second phase changed from rod-shaped and discontinuous to massive-like and continuous net-like in the B9 alloy. Furthermore, some globular net-like phases were distributed in the α-Mg matrix as denoted by the red arrow in Fig. 3(d). Subsequent EDS analysis revealed that the globular net-like phases contain Mg and Bi at an atomic ratio of ~2, which is very close to the atomic ratio of Mg3Bi2.
2.2 Corrosion behavior in Hank’s solution
2.2.1 Polarization tests
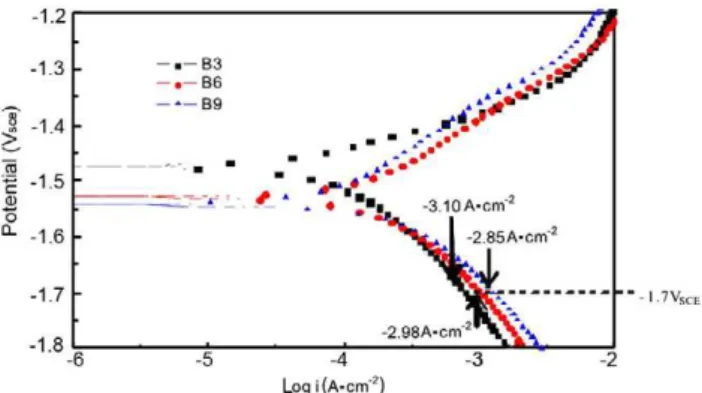
The polarization curves (Fig. 4) were notably changed by an increase in Bi content. The cathodic branch of the curve showed that the B3 alloy has a lower cathodic current density (icathodic) than the B6 and B9 alloys. The icathodic values measured at -1.7 VSCE
Fig. 1: XRD patterns showing constituents of as-cast Mg-Bi alloys
Fig. 2: OM and SEM micrographs of as-cast Mg-Bi alloys: (a) B3, (b) B6, (c, d) B9
Fig. 3: SEM micrographs of as-cast Mg-Bi alloys: (a) B3, (b) B9, (c, d) enlarged images of the area marked by dotted rectangle in B3 and B9 alloys
were-3.10 A·cm-2 (B3), - 2.98 A·cm-2 (B6) and -2.85 A· cm-2 (B9), respectively. What is more, the cathode polarization current of hydrogen evolution reaction on the B9 alloy was much higher than that on the B3 and B6 alloys, which was ascribed to the relatively low over potential of the cathodic hydrogen evolution reaction on the B9 alloy in comparison with the other two alloys.
T h e m e a s u r e d c o r r o s i o n potential (Ecorr), current density (Icorr) and polarization resistance (Rp) are summarized in Table 1. The Rp was calculated according to the following equation [20]:
As given in Table 1, the Rp value of the B3 alloy is much higher than that of the B6 and B9 alloys, with Rp value being
3530.76 Ω·cm2. In other words, high Rp values denote a low corrosion rate, which implies that the corrosion resistance of as-cast Mg-Bi alloys is reduced when there is an increase in Bi content.
2.2.2 EIS tests
Nyquist plots of the studied alloys are shown in Fig. 5(a). All the Nyquist plots show two depressed semicircles: a large capacitive l o o p a t h i g h e r f r e q u e n c i e s (HF), followed by a secondary c a p a c i t i v e l o o p a t m e d i u m frequencies (MF), indicating similar corrosion behavior of the studied alloys during the (2) Rp
immersion in Hank’s solution after 1 h. The high capacitive loop corresponds to hydrogen evolution and is controlled by charge transfer [21]. The medium capacitive loop is related to the presence of the corrosion product ilm on the alloy surface [22]. Based on the Nyquist plots, the equivalent circuit was shown in Fig. 5(b). In this model, Rs was the solution resistance. Charge transfer resistance (Rct) and a double layer capacitance (CPE1) described the high frequency capacitive loop (HF). The film resistance (Rf) and CPE2 described the middle frequency capacitive loop (MF).
The parameter values (Rs, CPE1, Rct, CPE2 and Rf ) are listed in Table 2. For the studied alloys, with an increase in Bi content, the Rct decreased from 430.9 Ω·cm
2 to 278.7 Ω
·cm2. Clearly, the Rct values are normally used as the factor evaluating the corrosion resistance of the alloys [7]. The above results indicate that the increase in Bi content degenerates the corrosion resistance of as-cast Mg-Bi alloys.
2.2.3 Immersion tests
The 72 h immersion test results of the Mg-xBi alloys in Hank’s solution at 37 ºC are presented in Fig. 6. The corrosion rates of the three kinds of as-cast Mg-Bi alloys calculated from the hydrogen evolution measurement were 1.32 (B3), 3.60 (B6) and 8.07 mm•a-1 (B9). Based on the weight loss rate results, it was found that the corrosion resistance was mainly associated with the volume fraction of Mg3Bi2 phase and grain size. The volume fractions of the Mg3Bi2 phase were in the order of B3< B6<B9; meanwhile, the corrosion rate of alloys increased in the same order. It demonstrated that an increased fraction of Mg3Bi2 phase led to poor corrosion resistance of the as-cast binary Mg-Bi alloys. Similarly, a recent report [23] showed that the coarse particles act as more active sites for the initiation of pitting corrosion than do the fine particles in extruded Mg-Sn based alloys. Furthermore, recent research [24, 25] also indicated that the grain boundary can act as a physical corrosion barrier. The alloys with small grain size create more grain boundaries favorable for the prevention of the corrosion process. As mentioned above, increasing the Bi content leads to the reinement of grain size. Thus we can assume that the reduced SDAS brings positive effects on the corrosion resistance of as-cast Mg-Bi alloys.
Fig. 4: Polarization curves of as-cast Mg-Bi alloys
Alloy Ecorr
(VSCE)
Icorr
(mA·cm-2)
Rp
(Ω·cm2)
Corrosion rate (mm·a-1)
B3 -1.475 0.092 3530.76 1.32
B6 -1.530 0.188 2658.60 3.60
B9 -1.544 0.196 2117.11 8.07
Table. 1: Results obtained from polarization curves and weight loss tests of studied alloys
Fig. 5: (a) Nyquist plots and (b) equivalent circuit of as-cast Mg-Bi alloys
Alloy Rs (ohm)
CPE1
(F·cm-2)
Rct
(ohm·cm2)
CPE2
(F·cm-2)
Rf
(ohm·cm2)
B3 29.80 2.261×10-5 430.9 2.419×10-3 152.4
B6 29.23 2.384×10-5 358.7 1.771×10-3 133.7
B9 24.45 2.956×10-5 278.7 3.124×10-3 104.8 Table 2: Results obtained from impedance diagrams of as-cast Mg-Bi alloys
Fig. 6: Corrosion rate, volume fraction of Mg3Bi2
phase and SDAS of three as-cast Mg-Bi alloys
2.2.4 Corroded surface morphologies of as-cast Mg-Bi alloys
Corroded surface morphologies under SEM of the studied alloys after immersion in Hank’s solution for 72 h with removal of the corrosion products are shown in Fig. 7. In general, relatively severe corrosion was found to occur at positions where Mg3Bi2 phase existed in the studied alloys. Corrosion
ongoing corrosion process, partial α-Mg disappeared and left many corrosion pits (on and/or around grain boundaries) on the surfaces. Note that the corrosion pits presented around both the particle-like (marked as A) and massive-shaped Mg3Bi2 (marked as B) phases at grain boundaries. This indicates that the corrosion process was mainly governed by the fraction and distribution site of the Mg3Bi2 phase. This is the reason why severe corrosion can be observed at and/or around the grain boundaries. In addition, due to the aggressive Cl- in the Hank’s solution, a few corrosion pits can also be observed in the center of the α-Mg matrix (marked as C). It is worth mentioning that pitting corrosion occurred on the surfaces of all the alloys immersed in Hank’s solution for 72 h shown in Fig. 7. It has been reported that pit formation corresponds to the low-frequency inductive loops of Nyquist plots [22]
. However, low-frequency inductive loops were not observed in Nyquist plots as shown in Fig. 5. The above mentioned disagreement was presumably due to the localized corrosion which rarely occurred in the Mg-Bi alloys in the short immersion time of 1 h during the EIS test.
Generally speaking, the standard electrode potentials were +0.216 and -2.363 V for Bi and Mg, respectively. During the corrosion process, the α-Mg matrix acted as an anode and Mg3Bi2 phase as a cathode, forming the micro-galvanic corrosion driven by the micro-galvanic effect. Thus, when Mg-Bi alloys were immersed in Hank’s solution, the dissolution reaction initiated from the grain boundaries as a result of dissolution of Mg (reaction 3 below). Meanwhile, the cathodic reaction occurred at the same time due to the occurrence of galvanic corrosion accompanied by hydrogen evolution (reaction 4). The secondary corrosion products Mg(OH)2 piled up on the surface
Fig. 7:
SEM micrographs showing corroded surface morphologies of as-cast Mg-Bi alloys after immersion in Hank's solution for 72 h: (a) B3, (b) B6, (c) B9 alloys
(Corrosion pits around the particle-like Mg3Bi2 phase, massive-shaped Mg3Bi2 phase and inside the α-Mg matrix are marked A, B and C, respectively).
Fig. 8: Schematic diagrams of corrosion process of as-cast Mg-Bi alloys
of the studied alloys. Accordingly, the corrosion of the studied alloys can be expressed according to the following three equations:
Anodic reaction (3)
Cathodic reaction (4)
The second corrosion production (5) Mg Mg2+ + 2e
-2H2O + 2e
- H
2 +2OH
-
Mg2+ + 2OH- Mg(OH)
2
Figure 8 shows the sketch diagram of the corrosion process of as-cast binary Mg-Bi alloys. At the early stage of the corrosion process, the corrosion preferentially occurred at the interface between the α-Mg matrix and the Mg3Bi2 phase due to the micro-galvanic effect. With the prolonged time, corrosion propagated and extended to the center of the α-Mg matrix. As a result, severely localized corrosion pits were visible around the Mg3Bi2 phase, suggesting that the Mg3Bi2 phase had a positive effect on development of pitting, as shown in Fig. 7 and Fig. 8.
rates of the B9 alloys were much faster than those of the other alloys. This disagreement is mainly attributed to the continuous network structure of the Mg3Bi2 phase, which acts as a micro-cathode, resulting in an increase in the cathode-to-anode area and accelerating the corrosion process. It is thus clear that the corrosion behavior of the as-cast binary Mg-Bi alloys was mainly controlled by the fraction of the second phase particles rather than the grain size.
3 Conclusions
The change of corrosion behavior of binary Mg-xBi (x=3, 6 and 9wt.%) alloys with respect to their microstructures has been investigated and the following conclusions can be drawn: (1) For the modiied alloys with Bi, the addition of Bi can reine the secondary dendrite arm spacing (SDAS) from 41.2 μm of the as-cast Mg-3wt.%Bi to 25.4 μm of the as-cast Mg-9wt.%Bi and lead to high volume fraction of Mg3Bi2.
(2) The polarization and EIS tests are characterized by decreasing the polarization resistance (Rp) values from 3530.76
to 2117.11 Ω·cm2; the charge transfer resistance (R
ct) decreased
from 430.9 to 278.7 Ω·cm2 as the Bi content increased from 3wt.% to 9wt.%. The electrochemical tests results indicated that the addition of Bi content can reduce the corrosion resistance of the Mg-xBi alloys.
(3) The reduced corrosion resistance was mainly ascribed to the increasing fraction of the second phase particles, which brings positive effects on the development of pitting.
References
[1] Liu Zhaoming and Gao Hong. Microstructure and tensile properties of thixo-diecast AZ91D magnesium alloy. China Foundry, 2013, 10: 288-293.
[2] Yang L, Huang Y, Feyerabend F, et al. Microstructure, mechanical and corrosion properties of Mg-Dy-Gd-Zr alloys for medical applications. Acta Biomaterialia, 2013, 9: 8499-8508.
[3] Liu Chen, Yang Huazhe, Wan Peng, et al. Study on biodegradation
of the second phase Mg17Al12 in Mg-Al-Zn Alloys: In vitro
experiment and thermodynamic calculation. Materials Science and Engineering C, 2014, 35: 1-7.
[4] Wu Guosong, Xu Ruizhen, Feng Kai, et al. Retardation of surface corrosion of biodegradable magnesium-based materials by aluminum ion implantation. Applied Surface Science, 2012, 258(19): 7651-7657.
[5] Gu X N, Zheng W, Cheng Y F. A study on alkaline heat treated Mg-Ca alloy for the control of the bio-corrosion rate. Acta Biomaterialia, 2009, 5(7): 2790-2799.
[6] Qin Chunling, Xiao Tongna, Li Yongyan, et al. Corrosion behavior of Mg-Zn-Ca amorphous alloys with Nd addition in simulated body luids. China Foundry, 2014, 11: 503-509.
[7] Anik M and Celikten G. Analysis of the electrochemical reaction
behavior of alloy AZ91 by EIS technique in H3PO4/KOH buffered
K2SO4 solutions. Corrosion Science, 2007, 49(4): 1878-1894.
[8] Liu Qiang, Cheng Weili, Zhang Hui, et al. The role of Ca on the
microstructure and corrosion behavior of Mg-8Sn-1Al-1Zn-Ca alloys. Journal of Alloys and Compounds, 2014, 590: 162-167. [9] Li Zijian, Gu Xunan, Lou Siquan, et al. The development of binary
Mg-Ca alloys for use as biodegradable materials within bone.
Biomaterials, 2008, 29(10): 1329-1344.
[10] Yumiko N, Yukari T, Yasuhide T, et al. Differences in behavior among the chlorides of seven rare earth elements administered intravenously to rats. Fundamental and Applied Toxicology, 1997, 37: 106-116.
[11] El-Rahman S S. Neuropathology of aluminum toxicity in rats (glutamate and GABA impairment). Pharmacological Research, 2003, 47: 189-194.
[12] Fowler B A and Sexton M J. Bismuth (Chapter 22). In: G. F. Nordberg, B. A. Fowler, M. Nordberg, et al. Handbook on the Toxicology of Metals. Third Ed., Academic Press, Burlington, 2007: 433-443.
[13] Wa n Yi z a o , X i o n g G u a n g y a o , e t a l . P r e p a r a t i o n a n d characterization of a new biomedical magnesium-calcium alloy. Material & Design, 2008, 29(10): 2034-2037.
[14] Remennik S, Bartsch I, Willbold E, et al. New, fast corroding high ductility Mg-Bi-Ca and Mg-Bi-Si alloys, with no clinically observable gas formation in bone implants. Materials Science and Engineering C, 2011, 176(20): 1653-1659.
[15] Zhao Yuhua and Wang Meng. Microstructure and mechanical properties of Mg-Bi alloys. Foundry, 2012, 61(7): 758-763. (In Chinese)
[16] Zhang Hui, Han Bao, Xu Chunxiang, et al. Effect of Sn content on microstructure and mechanical properties of binary Mg-Bi magnesium alloy. Foundry, 2014, 63(11):1138-1141. (In Chinese) [17] Dhanapal A, Rajendra Boopathy S, and Balasubramanian, V.
Inluence of pH value, chloride ion concentration and immersion time on corrosion rate of friction-stir-welded AZ61A magnesium alloy weldments. Journal of Alloys and Compounds, 2012, 523: 49-60.
[18] Fu J W and Yang Y S. Formation of the solidiied microstructure in Mg-Sn binary alloy. Journal of Crystal Growth, 2011, 322(1): 84 -90.
[19] Chen Jihua, Chen Zhenhua, Yan Hongge, et al. Effects of Sn addition on microstructure and mechanical properties of Mg-Zn-Al alloys. Journal of Mg-Zn-Alloys and Compounds, 2008, 461(1-2): 209-215.
[20] Ma Ying, Guo Yang, Zhang Zhongming, et al. Effect of Ca on the microstructure and corrosion resistance of as-cast Mg-4Zn alloy. Transactions of Materials and Heat Treatment, 2015, (36): 62-69. (In Chinese)
[21] Cai Shuhua, Lei Ting, Li Nianfeng, et al. Effects of Zn on microstructure, mechanical properties and corrosion behavior of Mg-Zn alloys. Materials Science and Engineering C, 2012, 32: 2570-2577.
[22] Bonora P L, Andrei M, Eliezer A, et al. Corrosion behaviour of stressed magnesium alloys. Corrosion Science, 2002, 44(4): 729-749.
[23] Ha H Y, Kang J Y, Yim C D, et al. Role of hydrogen evolution rate in determining the corrosion rate of extruded Mg-5Sn-(1-4 wt%)Zn alloys. Corrosion Science, 2014, 89: 275-285.
[24] Sun Yu, Zhang Baoping, Wang Yin, et al. Preparation and characterization of a new biomedical Mg-Zn-Ca alloy. Materials & Design, 2012, 34: 58-64.
[25] Aung Naing Naing and Zhou Wei. Effect of grain size and twins on corrosion behaviour of AZ31B magnesium alloy. Corrosion
Science, 2010, 52(2): 589-594.
This study was supported by the National Natural Science Foundation of China (Grant no.51404166), Shanxi Province Science Foundation for Youths (2013021013-4), the Research Project Supported by Shanxi Scholarship Council of China (2014-023), the Technological Innovation Programs of Higher Education Institutions in Shanxi (Grant no. 2014120), and the Advanced Programs of Department of Human