w w w . j c o l . o r g . b r
Journal
of
Coloproctology
Original
Article
Effect
of
lecithin
on
oxidative
stress
in
an
experimental
model
of
rats
colitis
induced
by
acetic
acid
Josieli
Raskopf
Colares
a,b,c,
Elizângela
Gonc¸alves
Schemitt
b,c,d,
Renata
Minuzzo
Hartmann
b,c,d,
Rosa
Maria
Moura
e,
Maria
Isabel
Morgan-Martins
b,c,
Henrique
Sarubbi
Fillmann
c,f,∗,
Lúcio
Fillmann
f,
Norma
Possa
Marroni
a,b,c,d,e,f,gaBioHealth,UniversidadeLuteranadoBrasil(ULBRA),Canoas,RS,Brazil
bLaboratoryofOxidativeStressandAntioxidants,UniversidadeLuteranadoBrasil(ULBRA),Canoas,RS,Brazil
cLaboratoryofHepatologyandExperimentalGastroenterology,HospitaldeClínicasdePortoAlegre(HCPA),UniversidadeFederaldoRio
GrandedoSul(UFRGS),PortoAlegre,RS,Brazil
dMedicalSciences,UniversidadeFederaldoRioGrandedoSul(UFRGS),PortoAlegre,RS,Brazil
eAppliedToxicology,UniversidadeLuteranadoBrasil(ULBRA),Canoas,RS,Brazil
fPontifíciaUniversidadeCatólicadoRioGrandedoSul(PUCRS),PortoAlegre,RS,Brazil
gPhysiology,UniversidadeFederaldoRioGrandedoSul(UFRGS),PortoAlegre,RS,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received12November2015 Accepted26March2016 Availableonline13April2016
Keywords: Ulcerativecolitis
Inflammatoryboweldisease Lecithin
Oxidativestress
a
b
s
t
r
a
c
t
Ulcerativecolitis(UC)isaninflammatorydiseasethataffectsthebowels.Reactiveoxygen species(ROS)areinvolvedintheprogressofUC.
Objective:EvaluatetheantioxidanteffectoflecithininanexperimentalmodelofacuteUC
inducedbyadministrationofaceticacid(AA)inrats.
Methods:Lecithin(0.5mL/kg/day)administeredorally2daysbeforeandafterinductionof
colitiswith4%AAinavolumeof4mL.Twenty-fivemaleWistarratsweredividedin5groups: control(CO);control+lecithin(CO+LE);colitis(CL);colitis+lecithin(CL+LE);lecithin+colitis (LE+CL).Analsphincterpressure,LPO(TBARS),andantioxidantactivityofenzymes super-oxidedismutase(SOD)andcatalase(CAT)weremeasured,andahistologicalanalysiswith H&Ewasperformed.
Resultsanddiscussion:AnalsphincterpressurewassignificantlysmallerintheCOgroup,
lecithintreatmentincreaseditinpre-andpost-treatedgroups.LPOandSODactivitywere increasedintheCOgroupanddecreasedinthelecithin-treatedgroups.CATactivitywas increasedinCOgroupanddecreasedinlecithingroups.Thehistologicalanalysisshowed damagetothebowelswithdestructionofcrypts,edema,andinflammatoryinfiltrate.Use oflecithinpreservedthecryptsanddecreasedtheedema.
∗ Correspondingauthor.
E-mail:henrique@fillmann.com.br(H.S.Fillmann). http://dx.doi.org/10.1016/j.jcol.2016.03.002
Conclusion: Ulcerativecolitisincreasedlipidperoxidation,andtheuseoflecithinwas effec-tivereducingdamagetothebowelsinthemodelofexperimentalcolitis.
©2016SociedadeBrasileiradeColoproctologia.PublishedbyElsevierEditoraLtda.Thisis anopenaccessarticleundertheCCBY-NC-NDlicense (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Efeito
da
lecitina
sobre
o
estresse
oxidativo
no
modelo
experimental
de
colite
induzida
por
ácido
acético
em
ratos
Palavras-chave: Retocoliteulcerativa
Doenc¸ainflamatóriaintestinal Lecitina
Estresseoxidativo
r
e
s
u
m
o
Aretocoliteulcerativa(RCUI)éumadoenc¸aintestinalinflamatória.Espéciesreativasde oxigênio(ERO)estãoenvolvidasnoprogressodaRCUI.
Objetivo:AvaliaroefeitoantioxidantedelecitinaemmodeloexperimentaldeRCUIinduzida
pelaadministrac¸ãodeácidoacético(AA)emratos.
Métodos:ALecitina(0,5mL/kg/dia)foiadministradaporviaoral2diasanteseapósainduc¸ão
decolitecomAA.VinteecincoratosWistarmachosforamdivididosem5grupos: con-trole(CO);controle+lecitina(CO+LE);colite(CL);colite+lecitina(CL+LE);lecitina+colite (LE+CL).Foramavaliadas:pressãodoesfíncteranal,lipoperoxidac¸ão(LPO),atividade antiox-idantedasenzimassuperóxidodismutase(SOD)ecatalase(CAT),efoirealizadaumaanálise histológicacomH&E.
Resultadosediscussão:ApressãodoesfíncteranalfoisignificativamentemenornogrupoCL,
otratamentocomlecitinaaumentouapressãonosgrupospréepóstratados.ALPOe ativi-dadedaSODaumentaramnogrupoCLediminuiramnosgrupostratadoscomlecitina. A atividadeda CATfoiaumentadanogrupoCLediminuiunosgruposcomlecitina.A análisehistológicamostroudanosaointestinocomdestruic¸ãodascriptas,edemae infil-tradoinflamatório.Ousodelecitinaproporcionouumapreservac¸ãodascriptasediminuic¸ão doedema.
Conclusão: ARCUIaumentaaLPO,autilizac¸ãodelecitinafoieficaznareduc¸ãodosdanos
aointestinoinduzidoporAAnomodelodecoliteexperimental.
©2016SociedadeBrasileiradeColoproctologia.PublicadoporElsevierEditoraLtda.Este éumartigoOpenAccesssobalicençadeCCBY-NC-ND (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Inflammatoryboweldisease(IBD)ischaracterizedbychronic inflammation of the gastrointestinal tract, and numerous physiopathogenic mechanisms may be associated with its etiology, such as those of genetic, dietary, immunological, infectious,parasitical, post-radioactive, ischemic and envi-ronmentalorder.1Idiopathiculcerativerectocolitis(IURC)and Crohn’sdiseasearethemostcommonformsofincidenceof IBDanditsetiologyisnotfullyclarified.2,3
IBDposes a serious global healthproblem, as it affects primarilyyoungpeople andhassevereandchronicclinical presentations,occurringallovertheworld.3
Research suggests that oxidative stress may be impor-tant inthe activity and developmentofIBD. Other studies showedthatreactiveoxygenspecies(ROS)aregeneratedin excess in individuals with colitis as compared to normal individuals.2,4
TheexperimentalmodelofcolitisperformedbyFillmann etal.1suggeststhatbesidesROS,nitricoxideisinvolvedinthis situation,triggeringinhibitoryactiononsmoothmuscles, pro-motingrelaxationoftheanalsphincterandtherebyadecrease inanalsphincterpressurelevels.
Theincreaseinthe generationofROSinulcerative coli-tistriggersanimbalanceinthecellredoxstatusandthereby anincreaseinfreeradicals(FR).Suchincreaseoverwhelms the antioxidant defense capabilitiesof the cell, thus char-acterizing oxidativestress(OS),whichinturn triggerslipid peroxidation(LPO),leadingtodisruptionofdisulfidebridgesof lipidsbybreakingthemandlossofcellintegrity,destabilizing itandleadingtocelldeath.5
Theorganismhasadefensesystemagainstoxidantagents composed of enzymatic activity, i.e., enzymes superoxide dismutase(SOD),catalase(CAT)andglutathioneperoxidase (GPx);andnon-enzymaticagents,suchasglutathione(GSH), vitamins(A,C,E),flavonoids,andothercompoundspresentin food,suchaslecithin.Thefunctionofantioxidants(AOX)isto maintaintheredoxbalancebykeepingROSlevelslow,thereby preventingtheformationoffreeradicalssuchassuperoxide anion,hydrogenperoxide,andthemostharmfulone,hydroxyl radical.1,2,5
apolarportion,therebyinsertingitselfinthecellmembrane, maintainingits integrity, asit can react withfreeradicals, sweepingthemandpreventingLPO.7
Thelecithinmoleculeiscomposedofcholine,phosphate and fatty acids, and its importance lies in the fact that it isthelargestlipid componentofthebody.Cholinepresent inlecithinisthedietary component requiredforthe func-tioning of all cells. Lecithin or its metabolites, including phospholipids,ensurethe structuralintegrityandsignaling functions of cell membranes.8 Choline acts as a precur-sorforthe biosynthesisofphosphatidylcholine (PC),which in turn plays an important role in the intestinal absorp-tionoflipids,asit increasesthemicellarsolubilityforming chylomicrons.9
Inthisstudyweevaluatedtheabilityoflecithintoreduce tissueoxidativestressintheexperimentalmodelofcolitisby aceticacidinWistarrats.
Materials
and
methods
Twenty-fivemaleWistarratsweighing300gwereused.They were divided infive groupsas follows:control(CO), colitis (CL),control+lecithin(CO+LE),colitis+lecithin(CL+LE)and lecithin+colitis(LE+CL).
Theanimalswere keptinthe vivariumofthe Universi-dadeLuteranadoBrasil(ULBRA)throughouttheexperiment, in12hlight/darkcycleandtemperaturebetween20and25◦C.
Waterandfoodweregivenadlibitum.Themodelchosenfor colitisinductionwasadaptedfromYamada10andTannahill etal.11 Theanimalsreceivedintracolonicadministrationof 4%acetic acid ina volume of4mL byenema. Thegroups receivedlecithin0.5mL/animal48hbeforeandimmediately after induction of colitis once a day until the end of the experiment.Thedrugusedinthisexperimentwasfromthe SunflowerIndustryandlaboratoryFitotherapicLtdaand con-tained0.5mgofeggoil.
After pressure measurements, animals were anaes-thetizedwithxylazinehydrochloride50mg/kgandketamine hydrochloride 100mg/kg body weight ip for removal of the distal colon (8cm). Subsequently, euthanasia was per-formed by exsanguination under anesthesia. Experiments followed a protocol approved by the Animal Ethics Com-mittee of the Lutheran University of Brazil (ULBRA) with therecommendationsoftheEuropeanUnionregarding ani-mal experimentation: Directive of the European Counsel 86/609/EEC.12
Analsphincterpressuremeasurements
Before euthanasia the animals were lightly anaesthetized withIsoflurane®tomeasureanalsphincterpressure. Anorec-talmanometrywasperformedincmofH2O(Proctossystem, Viotti,SP)withaballooncatheter.13
Histologicalanalysis
Forhistologicalexamination,aportionoftheintestinewas placed in buffered formalin and subsequently included in paraffin blocks to obtain 3-m thick cuts using a rotary
microtome. We performed standard histological examina-tion stainingwithhematoxylin–eosin(HE). Theslideswere analyzedwithabinocularmicroscopeLABOPHOTNIKONat magnificationof200×.
Intestinehomogenates
The intestines were weighed and homogenized for 40s in an Ultra-Turrax (IKA-WERK) centrifuge at4◦C in the
pres-enceof1.15%KCl(5mLpergramoftissue)andmethylphenyl sulfonylfluoride(MPSF)ataconcentrationof100mMin iso-propanol(10LpermLofKCladded).Thenthehomogenates
werecentrifugedfor10minat3000rpminarefrigerated cen-trifuge(SORVALLSuperT21 –CondensedOperatingKendro Laboratory Products –USA). Thesupernatant was pipetted into Eppendorf flasks and the precipitate was discarded. The samples were stored again at −80◦C for posterior
analyses.14
Protein
ProteinswerequantifiedaccordingtoLowryandcolleagues, usingastandardsolutionofbovinealbuminataconcentration of1mg/mL.Samplesweremeasuredspectrophotometrically at 625nm, and values expressed in mg/mL. The values wereusedtocalculatethiobarbituricacidreactivesubstance (TBARS)andantioxidantenzymelevels.15
Lipidperoxidation
The amount of aldehydes generated by lipid peroxidation is measured by the TBARS method, which determines the amountofsubstancesreactingwiththiobarbituricacid. Sam-ples were incubated at 100◦C for 15min after addition of
500L of 0.37% thiobarbituric acid in 15% trichloroacetic
acid and centrifuged at3000rpm (1612.8×g) for10minat 4◦C.Absorbancewasdetermined spectrophotometricallyat
535nm.16
Antioxidantsenzymeanalyses
The analysis of superoxide dismutase (SOD) is based on theinhibitionofthereactionofthesuperoxideradicalwith adrenaline, detected spectrophotometricallyat 480nm and valuesexpressedinU/mgprot.17Theanalysisofcatalase(CAT) activityisbasedonmeasuringthedecreaseinhydrogen per-oxide,detectedspectrophotometricallyat240nmandvalues expressedinpmol/mgprot.18
Statisticanalysis
70
A
B
Pressure
TBARS
a
a
b
b b
b
60
50
40
30
cm/H
2
O
nmoles/mgprot
20
10
0
5
4
3
2
1
0
CO CO+LE CL LE+CL CL+LE
CO CO+LE CL LE+CL CL+LE
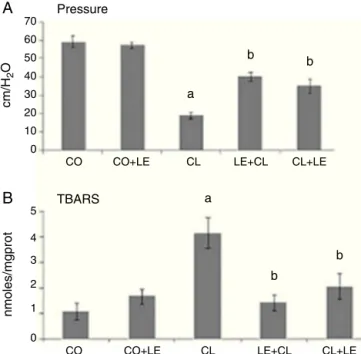
Fig.1–(A)Meanvaluesofanalsphincterpressureinthe differentgroupsstudied(cm/H2O):*significantincreaseof analsphincterpressureintheCLgroupascomparedtothe othergroups(p<0.05),#significantdecreaseofanal
sphincterpressureintheLE+CLandCL+LEgroupsas comparedtotheCLgroup(p<0.05).
(B)Evaluationoflipoperoxidationthroughthetechniqueof thiobarbituricacidreactivesubstances(TBARS)(nmol/mg prot):*significantincreaseoflipoperoxidationintheCL groupascomparedtotheothergroups(p<0.05),
#significantincreaseoflipoperoxidationintheCLgroupas
comparedtotheothergroups(p<0.05),#significant
decreaseoflipoperoxidationintheLE+CLandCL+LECL groupsascomparedtotheCLgroup(p<0.05).
Results
Analsphincterpressureandlipidperoxidation
Theadministrationoflecithinincreasedsphincterpressure by114%intheLE+CLgroupandby86%intheCL+LEgroup (Fig.1A).LPOevaluationbyTBARSshowedthatgroups receiv-inglecithintreatment(CL+LEandLE+CL)had significantly decreasedLPOascomparedtothegroupwithcolitis(p<0.05) (Fig.1B),whileintheCLgrouptherewasanincreaseof119% inrelationtoCOandCO+LEgroups.
Superoxidedismutase(SOD)andcatalase(CAT)activity
Fig.2showsthevaluesofSODandCATactivitiesacrossthe differentgroups.NotethatbothSODandCATdecreased sig-nificantlyinthegroupstreatedwithLE(CL+LEandLE+CL)as comparedtothecolitisgroup(p<0.05)(Fig.2AandB).
Histopathologicalanalysis
Theslideswerestainedwithhematoxylin–eosin(HE)and ana-lyzedat200×magnification.Fig.3Ashowsaphotomicrograph
400
USOD/mgprot
nmoles/mgprot
SOD
A
B
CATa
b b
a
b b
300
200
100
0
1
0.8
0.6
0.4
0.2
0
CO CO+LE CL LE+CL CL+LE
CO CO+LE CL LE+CL CL+LE
Fig.2–(A)MeanvaluesofSODactivityinthebowelacross thedifferentgroupsstudied(USOD/mg/prot):*significant increaseofSODactivityintheCLgroupascomparedtothe othergroups(p<0.05),#significantdecreaseofSODactivity
inLE+CLandCL+LEgroupsascomparedtotheCLgroup (p<0.05).
(B)MeanvaluesofCATactivityinthebowelsofthe differentgroupsstudied:*significantincreaseinCAT activityintheCLgroupsascomparedtotheothergroups (p<0.05),#significantdecreaseofCATactivityintheLE+CL
andCL+LEascomparedtotheCLgroup(p<0.05).
ofananimalinthecontrolgroup(CO).Notetheintegrityof crypts(CP)withsimpleglandularepitheliumandnormal sub-mucosa(SM).Fig.3Bshowsaphotomicrographofananimalin thelecithincontrolgroup(CO+LE)withsimilararchitectureto thecontrolgroup.Fig.3Cshowsaphotomicrographfromthe colitisgroup(CL).Notethechangesinthearchitectureofthe colon,destructionofCP,extensivesubmucosaledema(E)and inflammatoryinfiltrate(IF).Fig.3Dshowsprophylactic treat-mentwithlecithin(LE+CL),withlesspreservationofCPand nodecreaseinE.Fig.3Eisfromananimalinthecolitisgroup treatedwithlecithin(CL+LE).NotethepreservationofCPwith glandularepithelium,andlessinflammatoryinfiltrate.
Discussion
Theetiologyofulcerativecolitisisnotwellunderstand,and there are several experimental models with similar phys-iopathogeny that are used toinvestigate its toxic or acute presentation. Acetic acid causes intestinal injury, and the developmentofinflammationisconsidered oneofthe fea-turesofcolitis.19
A
CP
CP
SM
CP
CP
CP
SM
SM
CO
CL
CO+LE 200x
200x
200x
LE+CL
CL+LE
200x 200x
B
C
D
E
Fig.3–Photomicrographofthedistalportionoftheintestineinthedifferentgroups.(A)Control(CO);(B)control+lecithin (CO+LE);(C)colitis(CL);(D)lecithin+colitis(LE+CL);(E)colitis+lecithin(CL+LE).Magnification200×.
animalsinthecolitis(CL)andinthelecithin-treated(LE+CL and CL+LE) groups, where the inflammatory process was foundtobereduced.
Amicroscopicanalysiswithhematoxylin-eosin(HE) con-firmsinjuryresultingfrominflammatoryprocessinthecolitis group(Fig.3C),wherethereisdisruptionofcryptsandedema inthesubmucosa.Inanimalsofthecontrolgroups,onenotes thatthe architectureofthe intestinaltissueshows normal cryptsandsubmucosa.
Preservationandrestorationofthecryptsand reduction ofedemawere observedintheanimalsreceiving LEeither beforeoraftercolitisinduction,whichdemonstratesthatLEis abletoreduceinflammation,corroboratingotherstudiesthat usedotherantioxidantsandwhichdemonstratedreduction ofinjuriesbroughtaboutbycolitis.4,20,21
Oxidativedamageisassociatedwithcolitisbytheincrease inROSandNOS,withgenerationoffreeradicalswhichcause celldestructionleadingtoleukocyteinfiltrationandreleaseof inflammatorymediatorsaswellascytokines,triggering oxida-tivestress.1,19,22
By evaluatingtheLPOtriggeredbytheoxidativeprocess (Fig.1B),weseethatLEwaseffectiveinreducingLPOinthe CLgroups,bothinanimalspre-treatedwithLEandinthose post-treatedwiththedrug.Thisisevidenceofasignificant reductionintheoxidativedamageinducedbycolitis.
LEisamoleculewithamphotericcharacteristic,thereby adheringtotheplasmamembrane,whichismadeupof phos-pholipids. Therebyit subtracts the anions offree radicals, sweepingthemandpreventingthemfromtriggeringLPO,as seenintheTBARSanalysis.
Tahan et al.23 administered melatonin astreatment for experimentalcolitisandshowedsignificantreductioninthe treatedversusuntreatedgroupwithcolitis.Al-rejaieetal.22 used naringenin (a flavonoid present in citrus fruits) as treatment and observedreduced LPOin thetreated group, suggestingROSreduction.
importantroleinprovisionfortheprocessesofmotility regu-lationandcytoprotectionofthelargeintestine.6,24
Inthisstudy,themeasurementofanalsphincterpressure byanorectalmanometryshowedthatthe colitisgrouphad significantdecreaseinsphincterpressureascomparedtothe othergroups.Inapreviouswork,Hartmannetal.2foundthat animalswithcolitisinducedbyaceticacidshowedsignificant increaseinNOintheintestinewithconsequentdecreaseof sphincterpressure.
ResearchbyFillmannetal.25;Kretzmannetal.20;Hartmann etal.,2showedthatadministrationofsuchantioxidant sub-stances as glutamineand Boswellia serrata were associated withincreaseofanalsphincterpressureintreatedanimals. Thesefindingsareinagreementwithourdata,asthe admin-istrationofLEinthedifferenttreatedgroupswaseffectivein increasinganalsphincterpressure,possiblybydecreasingNO, inflammationandLPO,aspreviouslyreported.
Theenzymesystem,suchassuperoxidedismutase(SOD) and catalase (CAT), prevents accumulation of superoxide anion(O2•)andhydrogenperoxide(H2O2),preventingthe for-mationofthemostreactivefreeradical,thehydroxylradical (HO•), thus being considered the maindefenseline. These
enzymesarefoundnotonlyinmitochondriabutalsointhe cytosol(CAT),wheremostfreeradicalsaregenerated.5 How-ever,SODincreasemayresultinincreaseintheformationof H2O2,whichmaycauseitsaccumulationintissuesand con-sequentincreaseinHO•throughtheFenton&Haber–Weiss
reaction,leadingtooxidativestress.24
Wefounda significant increasein SODand CAT activi-tiesinthe colitisgroup.Wesuggest thatthis increasewas duetotheactivationofamechanismtocompensateforthe damagecausedbythe actionofacetic acidintheintestine oftheanimals.SODiscrucialfortheredoxbalance, dismu-tatingthe superoxideanioninto hydrogen peroxide(H2O2), andCATdegradesintowaterandoxygen,therebyavoiding the formationofHO• radical26 protectingagainst oxidative damage.Lecithin-treated animals (LE+CLand CL+LE) pre-sented reducedSOD and CATactivities, becausetreatment significantlydecreaseslipoperoxidationandrestoresthe oxi-dant/antioxidant balance in the organism, thus protecting againsttheoxidativedamageinducedbyaceticacid.
Wecanconcludethataceticacidiseffectiveasan exper-imentalmodelin inducingcolitis,leading toinflammation andoxidativedamageinthelargebowel.Lecithin administra-tionwaseffectiveinreducingtheinflammatoryprocessinthe bowels,decreasingoxidativestressbysignificantlydecreasing LPOandrestoringantioxidant defenses.Thesefindings are supportive of the use of antioxidants in the treatment of inflammatoryboweldisease,butfurtherstudiesarerequired toclarifytheprotectiveeffectinhumans.
Funding
CNPq,CAPES,FAPERGS.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
r
e
f
e
r
e
n
c
e
s
1.FillmannHS,KretzmannN,LlesuyS,FillmannLS,MarroniNP. OPapeldoÓxidoNítriconaPressãoAnalEsfincterianade RatosSubmetidosàColiteExperimental.RevBras Coloproctol.2006;26:437–42.
2.HartmannRM,MorganMartinsMI,TieppoJ,FillmannHS, MarroniNP.EffectofBoswelliaserrataonantioxidantstatusin anexperimentalmodelofcolitisratsinducedbyaceticacid. DigDisSci.2012;57:2038–44.
3.SouzaMH,TronconLE,RodriguesCM,VianaCF,OnofrePH, MonteiroRA,etal.Trendsintheoccurrence(1980–1999)and clinicalfeaturesofCrohn’sdiseaseandulcerativecolitisina universityhospitalinsoutheasternBrazil.ArqGastroenterol. 2002;39:98–105.
4.BeheraJP,MohantyB,RamaniYR,RathB,PradhanS.Effectof aqueousextractofAeglemarmelosunripefruiton
inflammatoryboweldisease.IndianJPharmacol. 2012;44:614–8.
5.HalliwellB,GutteridgeJ.Freeradicalsinbiologyandmedicine. 4thed.NewYork:OxfordUniversityPressInc.;2007.
6.JaldinRG,FalcãoFilhoHA,SequeiraJL,YoshidaWB.O processoateroscleróticoemartériasdecoelhossubmetidosà
dietasuplementadacomgemadeovo:modeloexperimental
debaixocusto.JVascBras.2006;5:247–56.
7.AraújoJ.Químicadealimentos:teoriaeprática.2ed.Vic¸osa: editoraUFV;1995.p.335.
8.DonavanSM,MarM-H,ZeiselSH.Cholineandcholineester concentrationsinporcinemilkthroughoutlactation.JNutr Biochem.1997;8:603–7.
9.JiangY,NohSK,KooSI.Eggphosphatidylcholinedecreases thelymphaticabsorptionofcholesterolinrats.JNutr. 2001;131:2358–63.
10.YamadaY,PostSR,WangK,TagerHS,BellGI,SeinoS.Cloning andfunctionalcharacterisationofafamilyofhumanand mousesomatostatinreceptorsexpressedinbrain, gastrointestinaltractandkidney.ProcNatlAcadSci. 1992;89:251–5.
11.TannahillL,KleinR,SchachnerM.Theneurotrophin receptorstrkAandtrkBareinhibitoryforneuriteoutgrowth. EurJNeurosci.1995;7:1424–8.
12.E.E.C.CouncilDirective86/609/EECof24November1986on theapproximationoflaws,regulationsandadministrative provisionsoftheMemberStatesregardingtheprotectionof animalsusedforexperimentalandotherscientificpurposes. OffJEurCommun.1986;L358:1–29.
13.ReadNW,SunWM.Anorectalmanometry.In:HenryMM,
SwashM,editors.Coloproctologyandthepelvicfloor.2nded. London:Butterworth-HeinemannLtd.;1992.p.119–45. 14.LlesuySF,MileiJ,MolinaH,BoverisA,MileiS.Comparisonof
lipidperoxidationandmyocardiadamageinducedby adriamycinand4′-epiadrimicininmice.Tumori.
1985;71:241–9.
15.LowryB,RosenbroughNJ,FarrAL,RandallRJ.Protein measurementwiththefolinphenolreagent.JBiolChem. 1951;193:265–75.
16.BuegeJA,AustSD.Microsomallipidperoxidation.Methods Enzymol.1978;52:302–10.
17.MisraHP,FridovichI.Theroleofsuperoxideanioninthe autooxidationofepinephrineandasimpleassayfor superoxidedismutase.JBiolChem.1972;24: 3170–5.
18.BoverisA,ChanceB.Themitochondrialgenerationof hydrogenperoxide.Generalpropertiesandeffectof hyperbaricoxygen.Biochemistry.1973;134:707–16. 19.TahanG,AytacE,AytekinH,GundunF,DogusoyG,AydinS,
antioxidantactivitiesinaceticacid-inducedulcerativecolitis inrats.CanJSurg.2011;54:333–8.
20.KretzmannNA,FillmannH,MaurizJL,MarroniCA,MarroniN, González-GallegoJ,etal.Effectsofglutamineon
proinflammatorygeneexpressionandactivationofnuclear factorkappaBandsignaltransducersandactivatorsof transcriptioninTNBS-inducedcolitis.InflammBowelDis. 2008;14:1504–13.
21.WadieW,Abdel-AzizH,ZakiHF,KelberO,WeiserD,Khayyal MT.STW5iseffectiveindextransulfatesodium-induced colitisinrats.IntJColorectalDis.2012;24:1445–53. 22.Al-RejaieSS,AbuohashishHM,Al-EnaziMM,Al-AssafAH,
ParmarMY,AhmedMM.Protectiveeffectofnaringeninon aceticacid-inducedulcerativecolitisinrats.WorldJ Gastroenterol.2013;19:5633–44.
23.TahanG,GramignoliR,MarongiuF,AktolgaS,CetinkayaA, TahanV.Melatoninexpressespowerfulanti-inflammatory andantioxidantactivitiesresultingincompleteimprovement
ofacetic-acid-inducedcolitisinrats.DigDisSci. 2011;56:715–20.
24.SathyasaikumarKV,SwapnaI,ReddyPVB,MurthyCRK,Gupta AD,SenthilkumaranB,etal.Fulminanthepaticfailureinrats inducesoxidativestressdifferentiallyincerebralcortex,
cerebellumandponsmedulla.NeurochemRes.
2007;32:517–24.
25.FillmannH,KretzmannNA,San-MiguelB,LlesuyS,Marroni N,González-GallegoJ,etal.Glutamineinhibits
over-expressionofpro-inflammmatorygenesand down-regulatesthenuclearfactorkappaBpathwayinan experimentalmodelofcolitisintherat.Toxicology. 2007;236:217–26.