RevistaBrasileiradeFarmacognosia25(2015)588–591
w w w. s b f g n o s i a . o r g . b r / r e v i s t a
Original
Article
Is
phototridachiahydropyrone
a
true
natural
product?
夽
Margherita
Gavagnin
∗,
Ernesto
Mollo,
Guido
Cimino
ConsiglioNazionaledelleRicerche,IstitutodiChimicaBiomolecolare,Pozzuoli,Italy
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received29April2015 Accepted9July2015
Availableonline1October2015
Keywords:
Marinenaturalproducts Polypropionates Sacoglossans Tridachiahydropyrones
a
b
s
t
r
a
c
t
Theoccurrenceof(−)-phototridachiahydropyrone(5)innaturehasbeenproven.Thiscompoundhas beennowidentifiedasminorcomponentoftheextractofmarinesacoglossanmolluskElysiacrispata fromwhichthemain(−)-tridachiahydropyrone(4)waspreviouslydescribed.Synthetic(±)-5was for-merlyobtainedbyMoses’groupbybiomimeticphotochemicalconversionof(±)-tridachiahydropyrone (4).Thesameauthorssuggestedthatcompound5hadtobeanaturalproductderivedfromprecursor 4“yettobediscovered”.ComparisonofCDprofilesofnatural(−)-4and(−)-5indicatedthesame abso-luteconfigurationforbothcompounds.Thisevidenceisinagreementwiththeconcertedmechanism proposedforthephotochemicalconversion.
©2015SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Allrightsreserved.
Introduction
The solar radiation by penetrating the sea surface strongly influencesthephysical,biologicalandchemicalprocessesofsea flora and fauna, forcing marine organisms to adopt strategies for defendingthemselvesto harmfulUV radiation (Ireland and Scheuer,1979).Thisoccursparticularlyinshallowwaterswhere theexpositiontosunlightis intense.Amongtheorganisms liv-inginhighlyphotophilichabitats,agroupofherbivorousmarine opisthobranchgastropodsbelongingtothefamilyPlakobranchidae (Mollusca:Gastropoda:Sacoglossa)areknownas“solar-powered mollusks”(Rudman,1998;Rumphoetal.,2000).Actually,these animalsassimilatechloroplastsfromsiphonaceousmarinealgae and maintainthe active organelles for several monthsin their own tissues where they carry out the photosynthesis (Jensen, 1997; Rumphoet al., 2000,2008; Evertsenet al., 2007). Natu-ralproductsfromplakobranchidsincludephoto-active␥-pyrone
polypropionate-derivedcompoundsthathavebeensuggestedto serveassunscreens toprotectthemollusks fromdamagingUV radiation (Ireland and Scheuer, 1979). The molecular network of these polypropionatesdisplays complex cyclic structures all includinga distinctive ␥-pyronemoiety bearing an ␣-methoxy
group. Starting from the first report of tridachione in the late 1970s from the Pacific Elysia (=Tridachiella) diomedea (Ireland etal.,1978;IrelandandFaulkner,1981),acertainnumberofsuch
夽 DedicatedtoProf.RosângelaDeAlmeidaEpifanio’smemory.Shespentone
yearinourinstitutegiving,withherenthusiasm,culture,will,andrigorouswork, relevantcontributionstomarinechemistry.
∗ Correspondingauthor.
E-mail:mgavagnin@icb.cnr.it(M.Gavagnin).
␥-pyronepolypropionateshavebeen describedsofar from
dif-ferentplakobranchideanspeciescollectedindistinctgeographical areas(reviewedbyCiminoetal.,1999;CiminoandGhiselin,2009; recentreportsbyDíaz-Marreroetal.,2008;Carboneetal.,2013). Thisisinagreementwiththesuggestionthatthesemetabolites aresynthesizeddenovoratherthansimplyderivingfromdietary sources(IrelandandScheuer,1979;IrelandandFaulkner,1981; Gavagninetal.,1994b;Díaz-Marreroetal.,2008).The biosynthe-sisofpolypropionatesinplakobranchideansacoglossanshasbeen rigorouslyproveninsomespeciesbyinvivofeedingexperiments (IrelandandScheuer,1979;Gavagninetal.,1994a;Cutignanoetal., 2009).
Fourdistinctstructuralarchitecturescanberecognizedin plako-branchideanpolypropionates:1,3-cyclohexadienederivatives,e.g. 9,10-deoxytridachione (1) (Ireland and Faulkner, 1981); bicy-clo[3.1.0]hexanes, e.g. photodeoxytridachione (2) (Ireland and Scheuer, 1979); bicyclo[4.2.0] hexanes, e.g. ocellapyrone A (3) (Manzoetal.,2005;MillerandTrauner,2005);andfused pyrone-containingbicyclicringderivatives,e.g.tridachiahydropyrone(4) (Gavagninetal.,1996;Jefferyetal.,2005;Sharmaetal.,2008).
The photochemical relationship between cyclohexadiene-containingandbicyclohexene-containingsacoglossan polypropi-onateswasdemonstratedbyphotoconversionof1into2inboth
invitro(IrelandandFaulkner,1981;Zuidemaetal.,2005)andinvivo
(IrelandandScheuer,1979).Theinvitroexperimentsdemonstrated thattheconversionof1into2occurswithretentionofoptical activ-ityaccordingtothe[2a+2a]rearrangementmechanismproposed
byIrelandandFaulkner(1981).Thealternativebiradicalpathway
viaatripletexcitedstateprocesshasbeenalsosuggested(Zuidema etal.,2005).Theinvivoexperimentsledtotheobservationthat thenaturallight-dependentprocessmaynotbeenzymaticandis promptedwhentheUVradiationpenetratingthedorsalsurfaceof
http://dx.doi.org/10.1016/j.bjp.2015.07.028
M.Gavagninetal./RevistaBrasileiradeFarmacognosia25(2015)588–591 589
themolluskexceedstheabsorptionlimitsofthe␥-pyronemoiety,
consistentwiththesunscreenprotectiverolesuggestedforthese polypropionates(IrelandandScheuer,1979).Thephotochemistry ofplakobranchideanpolypropionateshasbeenextensivelystudied inthelasttenyearsandseveralsynthesesincludingbiomimetic synthesis have been appeared in the literature (reviewed by:
Beaudry et al., 2005; Miller and Trauner, 2006; Sharma et al., 2011).Based onthesestudies, ithasbeenproposedthat many complexpolypropionatemetabolitesmaybederived biosynthet-icallyfromlinearpolyeneswithallE-configuration(Mosesetal., 2003;Rodriguezetal.,2007).Allthecorecyclicstructuresshould be formedthough mechanisms involving theE–Z double bond isomerizationfollowedbythermaland/orphotochemical electro-cyclizationwith[4+2]cycloadditionreactionsor[2+2]concerted rearrangements.Supportingthishypothesis,anumberofdiverse polypropionatesfromplakobranchidshavebeensynthesized start-ing froma linear tetraene-pyroneprecursor (Eade etal., 2008; Sharmaetal.,2009).
Inthisgroupofcomplexmolecules,tridachiahydropyronesare uniquemembersexhibitingthemostinterestingandunusual struc-turalmotifswiththe␥-pyroneformingpartofthecoreframework
andtherearrangementofC-12methylgroupshiftedtotheC-13 position.Theprototype(−)-tridachiahydropyrone(4)wasisolated severalyears ago by us froma Venezuelancollection of Elysia crispata(Gavagninetal.,1996)andtheoriginallyproposed struc-ture(4a)waslaterreassignedas4bysynthesis(Jefferyetal.,2005; Sharmaetal.,2008,2009).Relatedoxidizedderivativeswerealso described fromPlacobranchus ocellatus (Fu etal., 2000; Sharma etal.,2009).
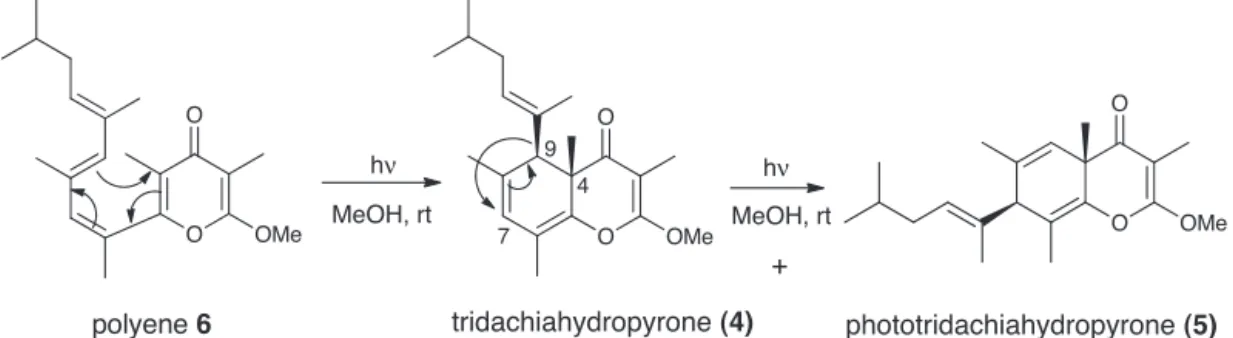
Surprisingly, biomimetic photochemical synthesis of (± )-tridachiahydropyrone (4)performedby Moses’s group(Sharma etal.,2009,2011)ledtotheadditionalunprecedented polypro-pionate(±)-5,whichwasobtainedbyphotochemicalconversion of4andnamedphototridachiahydropyrone.Theauthorssuggested thatcompound5,thestructureofwhichwassecuredbyX-ray anal-ysis,couldbeanaturalproduct“yettobediscovered”(Sharmaetal., 2009;SharmaandMoses,2010).
Now,wehavere-examinedtheextractofE.crispata,thesame as previously investigated (Gavagnin et al., 1996, 1997, 2000), withtheaim toverifythis hypothesis. A minormetabolite co-occurringwith(−)-tridachiahydropyrone(4)hadbeen detected at that time but thestructure was not determined. We report herethecharacterizationofthiscompound,justidentifiedas(− )-phototridachiahydropyrone(5).
O
OCH3 O
O
OCH3
OCH3
OCH3 OCH3
OCH3 O
1 2
3 O
O H
O O
4
O O
4a
O O
5 3
1 5 4 17
20 9
7 18
10
16 19 12
15
14
Materialsandmethods
Generalprocedures
Si-gel chromatography was performed by using precoated MerckF254platesandMerckKieselgel60powder.Optical rota-tionsweremeasuredonaJascoDIP370digital polarimeter.The UVspectraandCDcurveswererecordedonaAgilent8453 spec-trophotometer and JASCO 710spectropolarimeter, respectively. TheIRspectraweretakenonaBio-RadFTS7spectrophotometer.
1Hand13CNMRspectrawererecordedonaBrukerWM500MHz
andaBrukerAM400MHzspectrometersinCDCl3;chemicalshifts
arereportedinpartspermillionreferencedtoCHCl3 asinternal
standard(ı 7.26forprotonandı 77.00forcarbon).EI-MSspectra weremeasuredonaTRIO2000VGCarloErbaspectrometer.
Biologicalmaterial
Elysiacrispataindividuals(25animals,averagesize8cm)were collectedbySCUBAdiversoffMochima(Venezuela)atadepthof 3–10m,in November1993,asithasbeenpreviouslydescribed (Gavagninetal.,1996,1997).ThemolluskswereidentifiedbyProf. J.Ortea(UniversidaddeOviedo),immediatelyfrozenand subse-quentlytransferredtoIstitutodiChimicaBiomolecolarelaboratory, inItaly.
Purificationofphototridachiahydropyrone(5)andacquisitionof spectroscopicdata
Asithasbeenalreadyreportedinthepreviouspapers(Gavagnin etal.,1996,1997),thefrozenmaterialwasexhaustivelyextracted withacetone.Thediethylether-solubleportion(1.16g)ofthe ace-toneextractwasanalyzedbyTLCandthenfractionatedbySi-gel columnchromatography(lightpetroleumether/diethylether gra-dient)togiveaseriesofpolypropionates(Gavagninetal.,1996, 1997),including(−)-tridachiahydropyrone(4),whichwasthemain component (15.8mg)ofthefractionseluted bylightpetroleum ether/diethylether,9:1.Additionallesspolarfractionsthatwere atthattimecollectedhavebeennowcombined(10.7mg)and sub-mittedtoSi-gelchromatography(lightpetroleumether/CHCl3,6:4)
togivepurecompound5(3.5mg).
(−)-Phototridachiahydropyrone (5): oil; [␣]D −46.0◦ (CHCl3,
c=0.35);CD(n-hexane,c=3.9×10−5)
max[]:307(−7353),270
590 M.Gavagninetal./RevistaBrasileiradeFarmacognosia25(2015)588–591
O O
OMe
phototridachiahydropyrone
(5)
O O
OMe
polyene
6
O O
OMe
tridachiahydropyrone
(4)
hν hν
MeOH, rt MeOH, rt
+
94
7
Scheme1.Biomimeticconversionofpolyene6into4and5.
FromSharmaetal.(2009).
Table1
NMRdataaofphototridachiahydropyrone(5).
Carbon ıH(mult,J) ıC m HMBC(C→H)
1 – 165.7 s H3-20; OMe
2 – 87.9 s H3-20
3 – 194.8 s H-9;H3-17;H3-20
4 – 44.6 s H-7;H-9;H3-17
5 – 145.0 s H-7;H-9;H3-17;H3-19
6 – 117.4 s H-7;H3-19
7 3.08(s) 57.5 d H-9;H-11;H3-18;H3-19
8 – 131.8 s H-7;H3-18
9 5.95(s) 12.8 d H-7,H3-17;H3-18
10 – 133.4 s H-7;H2-12;H3-16
11 5.41(bt,7.1) 129.2 d H-7;H2-12;H3-16
12 1.96(dd,7.1,6.9) 37.4 t H-11;H3-14;H3-15
13 1.65(m) 28.9 d H2-12;H3-14;H3-15
14 0.91(d,6.6) 22.4 q H2-12
15 0.91(d,6.6) 22.4 q H2-12
16 1.38(bs) 11.9 q H-7;H-11 17 1.34(s) 27.6 q H-7 18 1.60(s) 21.0 q H-7;H-9 19 1.64(s) 13.1 q H-7
20 1.64(s) 6.7 q –
OMe 3.99(s) 55.0 q –
aBrukerWM500andAM400MHzspectrometers;CDCl
3;assignmentsmadeby 1H–1HCOSY,HSQCandHMBCexperiments(J=6and10Hz).
1613(broad)cm−1;UV(MeOH)
max271(ε =6400)nm;EIMS,m/z (%):330(M+,6),315(36),243(50),233(59),173(100);HREIMS,
m/z330.2210(C21H30O3requires330.2195).1Hand13CNMRin Table1.
Resultsanddiscussion
TheextractofthesacoglossanE.crispatahasbeenre-considered withtheaimtoidentifycompound5amongtheminormetabolites whichwerenotpreviouslydescribed(Gavagninetal.,1996,1997). Inparticular,thelesspolarfractionobtainedbythefirst chromato-graphicfractionationofcrudediethyletherextractofE.crispata
(Gavagninetal.,1996,1997)hasbeenre-analyzed.Further purifi-cationstepsofthisfractionhadledtotheisolationofthemain component,(−)-tridachiahydropyrone(4),aspreviouslydescribed (Gavagninetal.,1996).Aminormorepolarrelatedcompoundhad alsobeendetectedinthesamefractionatthattime(unpublished data)butitwasnotpurifiedandcharacterizedbyspectroscopic analysis.However,apreliminary1HNMRanalysisofan
unpuri-fiedsamplehadshowedastructuralrelationshipwithcompound
4.Thefractionscontainingthisunreportedcompoundhavebeen nowcombined,checkedby1HNMRandthensubmittedtoSi-gel
purificationtogive3.5mgofpure(−)-phototridachiahydropyrone (5).
Compound 5 had the molecular formula C21H3003 and the
EIMS fragmentation pattern the same as that observed for
tridachiahydropyrone(4).AnalysisofNMRspectraof5confirmed theclosestructural relationshipwith4, inparticular indicating thepresenceof thebicycliccoreincludingthe␥-pyroneringas
wellasofthelateralalkylchain.Acheckofpublisheddata (Sup-portinginformationinSharmaetal.,2009)confirmedthatnatural polypropionate5wasphototridachiahydropyrone.Fullassignment ofprotonandcarbonvaluesthatwasnotpreviouslyreportedis listedinTable1.
Asitwasmentionedbefore,(±)-phototridachiahydropyrone(5) wasanunexpectedproductformedinthecourseof photochem-icalelectrocyclicconversionof␥-pyronepolyeneprecursor6to
(±)-tridachiahydropyrone(4)underirradiation witha UVlamp (Scheme1accordingtoSharmaetal.,2009).Aselectivetandem sequenceof photochemicaltransformationswasobserved:first, theformationof4bycyclizationof6and,subsequently,the con-versionof4into5,underthesamereactionconditions.Prolonged irradiationof4resultedintothecompleteandirreversible con-versionto5suggestingthatphototridachiahydropyrone(5)isthe preferredphotochemicalproduct(Sharmaetal.,2009).
Thisconversionwassuggestedtooccurthrougha photochemi-cal1,3-sigmatropicmigrationoflateralalkylchainfromC-9toC-7 accordingtoretentionoftherelativeconfigurationofmethylatC-4 andthesidechainin5withrespectto4.
Natural(−)-phototridachiahydropyrone(5)isopticallyactive and displays the CD profile identical with that of natural (− )-tridachiahydropyrone (4) (Fig. 1) implying the same absolute configuration.However,theabsolutestereochemistryof tridachi-ahydropyronesremainstobedeterminedandthusenantiomers drawninstructures4and5havebeenchosenarbitrarily.
Theisolationofpropionate5fromE.crispatasupportstheideas thatledtopredictionsofitsexistenceinnature.Natural5,infact, couldbegenerated from4 through a concertedphotochemical mechanismaccordingtosyntheticprocess(Sharmaetal.,2009; SharmaandMoses,2010).Inthenaturalhabitat,this transforma-tionappearstobeonlypartialasindicatedbytheapproximate ratiooftridachiahydropyrones(4:5,5:1)detectedintheE.crispata
extract.ThisismostlikelyduetotheattenuationofUVlightinthe seawaterbythemaskingeffectinfluencedbyseveralfactorssuch asdissolvedorganicmaterials,depthofwater,temperature,etc.
(SharmaandMoses,2010).
M.Gavagninetal./RevistaBrasileiradeFarmacognosia25(2015)588–591 591
3.000E+04
–3.000E+04 [θ]
200.0 WL [nm] 400.0
a
3.000E+04
–4.000E+04 [θ]
200.0 WL [nm] 400.0
b
Fig. 1.CD curves []of (a) (−)-phototridachiahydropyrone (5) and (b) (− )-tridachiahydropyrone(4).
Authorscontributions
EMcontributedinrunningthepurificationwork.MGsupervised thelaboratorywork,analyzedspectroscopicdata,anddraftedthe paper.GCcontributedtoanalysisofthedataandtocriticalreading ofthemanuscript.Alltheauthorshavereadthefinalmanuscript andapprovedthesubmission.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgements
Thisresearchworkwaspartiallyfinanced byPORCampania FESR2007–2013–Project“FARMABIONET:Reteintegrataperle BiotecnologieApplicateamolecoleadattivitàfarmacologica”.
References
Beaudry,C.M.,Malerich,J.P.,Trauner,D.,2005.Biosyntheticandbiomimetic elec-trocyclizations.Chem.Rev.105,4757–4778.
Carbone,M.,Muniain,C.,Castelluccio,F.,Iannicelli,O.,Gavagnin,M.,2013.First chemicalstudyofthesacoglossanElysiapatagonica:isolationofa␥-pyrone propionatehydroperoxide.Biochem.Syst.Ecol.49,172–175.
Cimino,G.,Fontana,A.,Gavagnin,M.,1999.Marineopisthobranchmolluscs: chem-istryandecologyinsacoglossansanddorids.Curr.Org.Chem.3,327–372. Cimino,G.,Ghiselin,M.T.,2009.Chemicaldefenseandtheevolutionof
opistho-branchgastropods.Proc.Calif.Acad.Sci.60,175–422.
Cutignano,A.,Cimino,G.,Villani,G.,Fontana,A.,2009.Shapingthe polypropi-onatebiosynthesisinthesolar-poweredmolluscElysiaviridis.ChemBioChem 10,315–322.
Díaz-Marrero,A.R.,Cueto,M.,D’Croz,L.,Darias,J.,2008.Validatingand endoper-oxideasakeyintermediateinthebiosynthesisofelysiapyrones.Org.Lett.10, 3057–30060.
Evertsen,J.,Burghardt,I.,Johnsen,G.,Wägele,H.,2007.Retentionoffunctional chloroplastsinsomesacoglossansfromtheIndo-PacificandMediterranean. Mar.Biol.151,2159–2166.
Eade,S.J.,Walter,M.W.,Byrne,C.,Odell,B.,Rodriguez,R.,Baldwin,J.E.,Adlington, R.M.,Moses,J.E.,2008.Biomimeticsynthesisofpyrone-derivednatural prod-ucts:exploringchemicalpathwaysfromauniquepolyketideprecursor.J.Org. Chem.73,4830–4839.
Fu,X.,Hong,E.P.,Schmitz,F.J.,2000.Newpolypropionatepyronesfromthe Philip-pinesacoglossanmolluscPlacobranchusocellatus.Tetrahedron56,8989–8993. Gavagnin,M.,Marin,A.,Mollo,E.,Crispino,A.,Villani,G.,Cimino,G.,1994a. Sec-ondarymetabolitesfromMediterraneanElysioidea:originandbiologicalrole. Comp.Biochem.Physiol.108B,107–115.
Gavagnin,M.,Spinella,A.,Castelluccio,F.,Arnaldo,M.,Cimino,G.,1994b. Polypropi-onatesfromtheMediterraneanmolluskElysiatimida.J.Nat.Prod.57,298–304. Gavagnin, M., Mollo,E., Cimino, G.,Ortea,J., 1996. Anew␥
-dihydropyrone-propionatefromtheCaribbeansacoglossanTridachiacrispata.TetrahedronLett. 37,4259–4261.
Gavagnin,M.,Mollo,E.,Montanaro,D.,Castelluccio,F.,Ortea,J.,Cimino,G.,1997. AnoveldietarysesquiterpenefromthemarinesacoglossanTridachiacrispata. Nat.Prod.Lett.10,151–156.
Gavagnin,M.,Mollo,E.,Montanaro,D.,Ortea,J.,Cimino,G.,2000.Chemicalstudies ofCaribbeansacoglossans:dietaryrelationshipswithgreenalgaeandecological implications.J.Chem.Ecol.26,1563–1578.
Ireland,C.,Faulkner,D.J.,Solheim,B.A.,Clardy,J.,1978.Tridachione,a propionate-derivedmetaboliteoftheopisthobranchmolluscTridachielladiomedea.J.Am. Chem.Soc.100,1002–1003.
Ireland,C.,Scheuer,P.J.,1979.Photosyntheticmarinemollusks:invivo14C incorpo-rationintometabolitesofthesacoglossanPlacobranchusocellatus.Science205, 922–923.
Ireland,C.,Faulkner,D.J.,1981.ThemetabolitesofmarinemolluscsTridachiella diomedeaandTridachiacrispata.Tetrahedron37(Suppl.1),233–240. Jeffery,D.W.,Perkins,M.V.,White,J.M.,2005.Synthesisoftheputativestructureof
tridachiahydropyrone.Org.Lett.7,1581–1584.
Jensen,K.,(PhDthesis)1997.Systematics,PhylogenyandEvolutionoftheSacoglossa (Mollusca,Opisthobranchia).ZoologiskMuseum,University ofCopenhagen, Denmark.
Manzo,E.,Ciavatta,M.L.,Gavagnin,M.,Mollo,E.,Wahidulla,S.,Cimino,G.,2005. New␥-pyronepropionatesfromtheIndianOceansacoglossanPlacobranchus ocellatus.TetrahedronLett.46,465–468.
Miller,A.K.,Trauner,D.,2005.Miningthetetraenemanifold:totalsynthesisof com-plexpyronesfromPlacobranchusocellatus.Angew.Chem.Int.Ed.44,4602–4606. Miller,A.K.,Trauner,D.,2006.Mappingthechemistryofhighlyunsaturatedpyrone
polyketides.Synlett14,2295–2316.
Moses,J.E.,Baldwin,J.E.,Brückner,S.,Eade,S.J.,Adlington,R.M.,2003.Biomimetic studiesonpolyenes.Org.Biomol.Chem.1,3670–3684.
Powell,K.J.,Sharma,P., Richens,J.L.,Davis, B.M.,Moses,J.E.,O’Shea, P.,2012. Interactionsofmarine-derived␥-pyronenaturalproductswithphospholipid
membranes.Phys.Chem.Chem.Phys.14,14489–14491.
Rodriguez,R.,Adlington,R.M.,Eade,S.J.,Walter,M.W.,Baldwin,J.E.,Moses,J.E., 2007.TotalsynthesisofcyerceneAandthebiomimeticsynthesisof(± )-9,10-deoxytridachioneand(±)-ocellapyroneA.Tetrahedron63,4500–4509. Rudman,W.B.,1998.Solar-PoweredSeaSlugs.SeaSlugForum.AustralianMuseum,
Sydney,http://www.seaslugforum.net/find/solarpow.
Rumpho,M.E.,Summer,E.J.,Manhart,J.R.,2000.Solar-poweredseaslugs, Mol-lusc/algalchloroplastsymbiosis.PlantPhysiol.123,29–38.
Rumpho,M.E.,Worful,J.M.,Lee,J.,Kannan,K.,Tyler,M.S.,Bhattacharya,D.,Moustafa, A.,Manhart,J.R.,2008.HorizontalgenetransferofthealgalnucleargenepsbO tothephotosyntheticseaslugElysiachlorotica.Proc.Natl.Acad.Sci.U.S.A.105, 17867–17871.
Sharma,P.,Griffiths,N.,Moses,J.E.,2008.Biomimeticsynthesisandstructural reass-ignmentrevisionof(±)tridachiahydropyrones.Opt.Lett.10,4025–4027. Sharma,P.,Lygo,B.,Lewis,W.,Moses,J.E.,2009.Biomimeticsynthesisandstructural
reassignmentofthetridachiahydropyrones.J.Am.Chem.Soc.131,5966–5972. Sharma,P.,Moses,J.E.,2010.Photochemicalstudiesofthetridachiahydropyrones
inseawater.Synlett,525–528.
Sharma,P.,Powell,K.J.,Burnley,J.,Awaad,A.S.,Moses,J.E.,2011.Totalsynthesisof polypropionate-derived␥-pyronenaturalproducts.Synthesis18,2865–2892.
![Fig. 1. CD curves [ ] of (a) (−)-phototridachiahydropyrone (5) and (b) (−)- (−)-tridachiahydropyrone (4).](https://thumb-eu.123doks.com/thumbv2/123dok_br/14929875.501269/4.918.74.439.79.688/fig-cd-curves-phototridachiahydropyrone-b-tridachiahydropyrone.webp)