www.bjorl.org
Brazilian
Journal
of
OTORHINOLARYNGOLOGY
ORIGINAL
ARTICLE
Association
between
desloratadine
and
prednisolone
in
the
treatment
of
children
with
acute
symptoms
of
allergic
rhinitis:
a
double-blind,
randomized
and
controlled
clinical
trial
夽
Gustavo
F.
Wandalsen
a,
Carolina
Miranda
b,c,
Luis
Felipe
Ensina
a,d,
Flavio
Sano
e,
Roberto
Bleul
Amazonas
f,g,h,
Joyce
Macedo
da
Silva
i,
Dirceu
Solé
a,∗aUniversidadeFederaldeSãoPaulo(Unifesp),EscolaPaulistadeMedicina,(EPM)DepartamentodePediatria,SãoPaulo,
SP,Brazil
bUniversidadeFederaldeSãoPaulo(Unifesp),EscolaPaulistadeMedicina,(EPM)DepartamentodeGinecologia,SãoPaulo,
SP,Brazil
cFundac¸ãodeApoioàEscolaPaulistadeMedicina(FAP),SãoPaulo,SP,Brazil dUniversidadedeSantoAmaro(Unisa),ClínicaMédica,SãoPaulo,SP,Brazil eHospitalNipo-Brasileiro,Pediatria,SãoPaulo,SP,Brazil
fUniversidadeEstadualdeCampinas(Unicamp),Campinas,SP,Brazil gFundac¸ãoGetúlioVargas,MBAemMarketing,RiodeJaneiro,RJ,Brazil hGrupoNCFarma,SãoPaulo,SP,Brazil
iGrupoNCFarma,PesquisaClínicaeFarmacovigilância,SãoPaulo,SP,Brazil
Received6June2016;accepted14August2016 Availableonline13September2016
KEYWORDS
Allergicrhinitis; Desloratadine; Dexchlorpheniramine; Prednisolone;
Betamethasone
Abstract
Introduction:A combinationofantihistaminesandoralcorticosteroidsisoftenusedtotreat acutesymptomsofallergicrhinitis.
Objective: Toevaluate safetyand efficacy ofdesloratadine plusprednisolone inthe treat-mentofacutesymptomsofchildren (2---12years)withallergicrhinitis,andtocompareitto dexchlorpheniramineplusbetamethasone.
Methods:Childrenwith moderate/severepersistentallergicrhinitisandsymptomatic (nasal symptomsscore[0---12]≥6) wereallocatedinadouble-blind, randomizedfashiontoreceive dexchlorpheniramine plusbetamethasone (n=105;three daily doses) or desloratadineplus prednisolone (n=105;singledose followedby two ofplacebo)for 7days.Atthe beginning andendoftheevaluation, thefollowingwere obtained:nasalsymptomsscore,extranasal
夽 Pleasecitethisarticleas:WandalsenGF,MirandaC,EnsinaLF,SanoF,AmazonasRB,SilvaJM,etal.Associationbetweendesloratadine
andprednisoloneinthetreatmentofchildrenwithacutesymptomsofallergicrhinitis:adouble-blind,randomizedandcontrolledclinical trial.BrazJOtorhinolaryngol.2017;83:633---9.
∗Correspondingauthor.
E-mail:dirceu.sole@unifesp.br(D.Solé).
PeerReviewundertheresponsibilityofAssociac¸ãoBrasileiradeOtorrinolaringologiaeCirurgiaCérvico-Facial.
http://dx.doi.org/10.1016/j.bjorl.2016.08.009
symptoms score, peak nasal inspiratory flow, blood biochemistry, and electrocardiogram. Ninety-sixchildrenofthedexchlorpheniramineplusbetamethasonegroupand98ofthe deslo-ratadineplusprednisolonegroupcompletedtheprotocol.
Results:Thetwogroupsweresimilarregardinginitialandfinalnasalsymptomsscores,extra nasalsymptomsscoresandpeaknasalinspiratoryflow. Adropof76.4%and79.1%for nasal symptomsscore,86.0%and79.2%for extranasalsymptomsscore,aswellasanincreaseof 25.2%and24.3%forpeaknasalinspiratoryflowoccurredforthosetreatedwithdesloratadine plusprednisoloneanddexchlorpheniramineplusbetamethasone,respectively.Therewereno significantchangesinbloodchemistry.Sinustachycardiawasthemostfrequent electrocardio-gramchange,butwithnoclinicalsignificance.Drowsinesswasreportedsignificantlymoreoften amongthoseofdexchlorpheniramineplusbetamethasonegroup(17.14%×8.57%,respectively).
Conclusion:Thedesloratadineplusprednisolonecombinationwasabletoeffectivelycontrol acutesymptomsofrhinitisinchildren,improvingsymptomsandnasalfunction.Comparedto thedexchlorpheniramineplusbetamethasonecombination,itshowedsimilarclinicalaction, butwithalowerincidenceofadverseeventsandhigherdosingconvenience.
© 2016 Publishedby Elsevier EditoraLtda. on behalf ofAssociac¸˜ao Brasileira de Otorrino-laringologiaeCirurgiaC´ervico-Facial.ThisisanopenaccessarticleundertheCCBYlicense (http://creativecommons.org/licenses/by/4.0/).
PALAVRAS-CHAVE
Rinitealérgica; Desloratadina; Dexclorfeniramina; Prednisolona; Betametasona
Associac¸ãoentredesloratadinaeprednisolonanotratamentodecrianc¸as comsintomasagudosderinitealérgica:ensaioclínicoduplo-cego,randomizado econtrolado
Resumo
Introduc¸ão:A associac¸ãoentre anti-histamínicos e corticosteroides orais éfrequentemente empregadanotratamentodesintomasagudosdeRiniteAlérgica.
Objetivo:Avaliaraseguranc¸aeeficáciadaassociac¸ãodesloratadina+prednisolonano trata-mento de sintomas agudos de crianc¸as (2---12 anos) com rinite alérgica e compará-la à de dexclorfeniramina+betametasona.
Método: Crianc¸ascomrinitealérgica persistentemoderada/graveesintomáticas(escorede sintomasnasais[0---12]≥6)foramalocadasdemododuplo-cegoerandômicoparareceberem dexclorfeniramina+betametasona(n=105;trêsdosesdiárias)oudesloratadina+prednisolona (n=105;doseúnicaseguidaporduasdeplacebo)por7dias.Aoinícioefinaldaavaliac¸ãoforam obtidos:escoredesintomasnasais,escoredesintomasextra-nasais,picodefluxoinspiratório nasal,bioquímicasanguíneaeeletrocardiograma.Dototal,96crianc¸asdogrupo dexclorfeni-ramina+betametasonae98dogrupodesloratadina+prednisolonaconcluíramoprotocolo.
Resultados: Osdoisgruposforamiguaiscomrelac¸ãoaoescoredesintomasnasais,escorede sin-tomasnasaisextra-nasaisepicodefluxoinspiratórionasaliniciaisefinais.Observou-sequedade 76,4%e79,1%nosescoresparaescoredesintomasnasais,de86,0%e79,2%paraescorede sin-tomasextra-nasais,assimcomoincrementode25,2%ede24,3%paraopicodefluxoinspiratório nasalparaosgruposdesloratadina+prednisolonaedexclorfeniramina+betametasona, respec-tivamente.Nãohouvealterac¸õessignificativasdabioquímicasanguínea.Taquicardiasinusalfoi aalterac¸ãodoeletrocardiogramamaisencontrada,massemsignificânciaclínica.Sonolência foisignificantementemaisreferidaentreostratadoscomdexclorfeniramina+betametasonado queentreosdesloratadina+prednisolona(8,57%×17,14%,respectivamente).
Conclusão:Aassociac¸ãodesloratadina+prednisolonafoicapazdecontrolar efetivamenteos sintomasagudosderiniteemcrianc¸as,melhorandosintomaseafunc¸ãonasal.Nacomparac¸ão comaassociac¸ãodexclorfeniramina+betametasona,demonstrouac¸ãoclínicasemelhante,mas commenorincidênciadeeventosadversosemaiorcomodidadeposológica.
©2016Publicado porElsevier EditoraLtda.em nome deAssociac¸˜ao Brasileirade Otorrino-laringologia eCirurgiaC´ervico-Facial.Este ´eum artigo OpenAccess sobuma licenc¸aCC BY (http://creativecommons.org/licenses/by/4.0/).
Introduction
ARIAinitiative(AllergicRhinitisanditsImpact onAsthma) recommendsthattreatmentofallergicrhinitis(AR)isscaled accordingtotheseverityandpersistenceofthedisease.1
recommendedsincetheycausefeweradverseeffects (seda-tion, anticholinergic action, among others) and have a convenient dosage (single daily dose).1 Among the many
available in our country, the following standout: fexofe-nadine,desloratadine,ebastine,levocetirizine,rupatadine and,morerecently,bilastine.
Desloratadine (descarboethoxyloratadine), the primary metaboliteofloratadine,isaselectiveantagonistof second-generation H1 receptors. It has a half-life of 27h, its absorptionisnotaffectedbyfood,itsmetabolismand elimi-nationarenotalteredbyage,raceandgender,2anditisnot
affected by the simultaneousadministration of macrolide antibiotics, ketoconazole and cyclosporine.3 In studies in
patientswithAR,desloratadinewaseffectiveincontrolling nasal,extranasalsymptoms,evenafterasingledose.4---6
Systemic corticosteroids (CS) are generally used when symptomsarenotcontrolledwithenvironmentalortopical measures,orinmoreseverecaseswithairwaycompromise ormajorassociatedmorbidity.1 Comparedtotopicalnasal
CS,thesystemicadministrationhastheadvantageof reach-ing all parts of the nose and paranasal sinuses, even in participantswithseverenasalcongestionandnasalpolyps.7
Although the simultaneous use of anti-H1 and oral CS is not recommended by ARIA in the treatment of AR, it has been widely used. An auditing of sales (units) of pharmaceuticals in 2015 showed that the association of dextrochloropheniramine andbetamethasoneaccountsfor 34.79% of sales of products available in this segment in Brazil.8On theotherhand,theuseofanti-H1 andoralCS
associationinthetreatmentofARisrare.
Theobjectivesofthisstudyweretoevaluatetheefficacy andsafetyofdesloratadine+prednisoloneassociation(oral solution;DP)inthecontrolof acutenasalandextra-nasal symptomsin children withmoderate-severe persistent AR (PAR)andtocompareitsactionwiththetradeassociation dexchlorpheniramine+betamethasone(syrup;DB).
Methods
This prospective, multicenter, double-blind, randomized, controlledstudyofparallelgroupshadtheparticipationof 210 children (2---12 years) withmoderate/severe PAR (1). Thesubjectsexhibitedclinicalfeaturesconsistentwiththe diagnosis of AR(recurrent nasal symptoms,and sensitiza-tiontoairborneallergensbythepresenceofspecificIgE).In addition,theyhadnasalsymptomsscores(NSS)---≥6during thepreviousweek.NSSassessnasalobstruction,rhinorrhea, sneezingandnasalitchingfrom0(absent)to3(severe) giv-ing a possible maximum score of 12 points. All subjects’ parents/guardianssignedtheinformedconsentdocument.
Patientsonspecificallergenimmunotherapy,thosewith previoustreatmentinthelast15dayswithoral corticoste-roids or topicalnasalor oral antihistamines,those witha chronicdisease(hematopoietic,cardiovascular,renal, neu-rological, psychiatric and autoimmune disorders) as well as those with uncontrolled asthma, chronic rhinosinusitis and/oranatomicalabnormalitiesoftheupperairways,were notadmittedtothestudy.
Duringselection(Visit0---V0),patientswereevaluated clinicallyandscored according tothe NSS,and the extra-nasalsymptoms score--- ExNSS. This evaluates itchyeyes,
itchypalate, ocular hyperemia andtearing,and is scored from0(absent)to3(severe),foramaximumpossiblescore of12points.Furthermore,a peripheralblood sample was collected for CBC, transaminase, urea, creatinine, biliru-bindosage,andanelectrocardiogram(ECG)wasperformed. When subjects returned at day 5 (±2), if there were no laboratory abnormalities, patients were allocated in ran-domorder,indouble-blindfashion,tothetreatmentgroups, accordingtotheactiveprinciple:DPorDB.Atthisvisit,they wereclinicallyreevaluated,andthoseoversixyearsofage underwentthemeasurementofpeaknasalinspiratoryflow (PNIF)(V1).Afterreceivingtheguidelinesonthedailyfilling ofnasalsymptomsandtheregistrationofpossibleadverse events,patientswerereleasedandtoldtoreturnin7(±2) days(V2)whentheyunderwentallclinical andlaboratory testsagain.
Treatmentregimens
Patientswererandomizedby electronicCRF (CaseReport Form) at the time of enrollment in the study using the criterionof blocksofsix treatments,withthreebeingDP and three DB, and received the drugs as follows: (a) DP --- desloratadine (0.5mg/mL) and prednisolone (4mg/mL) combinedin oralsolution,or DB---commerciallyavailable (Celestamine®, Mantecorp, Brazil) a combination of
dex-chlorpheniraminemaleate(0.4mg/mL)andbetamethasone (0.05mg/mL) syrup. Children under 6 years who started treatmentwiththeformulationofDPreceived2.5mLorally (vialA)complementedby twootheroral dosesofplacebo (vialsBandC),atintervalsof8h.PatientstreatedwithDB receivedthreeoraldosesof 2.5mL(vialsA,BandC)also at8-hintervals.Patientsolderthan6 yearsreceived dou-blethedose(5mL)employingthesamedistributionofvials. Vialsofbothtreatmentregimensandthoseofplacebowere identical,andthesamevehiclewasused,soastohavethe sameflavor.Thevialswerelabeledaccordingtothe recom-mendationsbyANVISA.9Randomizationcodeswerebroken
onlyafteranalyzingthedata.
Dailynasalsymptoms,self-assessment
questionnaireandreportofadverseevents
Those responsible for the patients were instructed tofill outthe diary of symptoms(sneezing, itching, runny nose and nasal obstruction) with respect to its interference in daily activities (0=no symptoms, 1=mild symptoms, 2=symptomsthatinterferewithdailyroutine,butnotsleep; and 3=symptoms that interfere with sleep). The sum of scoreswasthescoreofeachdayoftreatment.
Inaddition,thoseresponsibleforthepatientswerealso askedtoanswertheself-assessmentquestionnaireregarding theuseofprescribedmedication (didnottake,took25%, took50%,took75%,tookeverything---100%)oneachdayof treatment,aswellasonthepresenceofanyadverseevents suchassomnolence,headache,tremors,amongothers.
Peaknasalinspiratoryflow(PNIF)
Clement-Clarke, England) were conducted after patients blewtheirnoses,in triplicaterecording thehighest value accordingtoexistingrecommendations.10
Samplecalculation
Becauseitis anon-inferioritystudyof parallelgroups,we employedastheprimaryvariablethechange ofNSS, hav-ing admission as basis. For this purpose, we estimated a 50%reductionofNSSaftertreatment,andconsideringthe maximum differenceof 0.5point between the two treat-mentgroups,andstandarddeviationof0.5point,withan alphaerror of5%,and95% testpowerwouldbenecessary toinclude86patientspergroup.Estimatinglossesofupto 20%ofpatientsincluded,thetotalnumberofpatientstobe includedis210;105ineachtreatmentgroup.
Statisticalanalysis
According to the nature of the variables analyzed, para-metricor non-parametrictestswereused,fixing thelevel ofrejection ofthe nullhypothesis at 5%.Fortheanalysis tobecarriedout,weusedSASsystem (StatisticalAnalysis System),version9.1.3.
Theprotocol wasapprovedbytheEthicsCommitteeof theUniversidadeFederaldeSãoPaulo---EscolaPaulistade MedicinaandHospitalSãoPaulo,aswellasthatofallthe centersinvolved, andit wasrecorded in ClinicalTrials.org PRSundernumberNCT01529229.Allguardianssignedthe informedconsent,andchildrenolderthan6yearstheassent form.
Results
One-hundredandninety-five patientscompletedthestudy (DPn=98andDBn=97).TwopatientsfromtheDBgroup, and one of the DP group were excluded due to adverse events, threeof DBand threeof the DPwere eliminated duetopoorcompliance,oneoftheDBgroupandoneofthe DPgroupwereexcludedduetoprotocolviolation,oneofDB andtwoofDPgroupabandonedthestudy,andonepatient oftheDBgroupwithdrewtheinformedconsent.
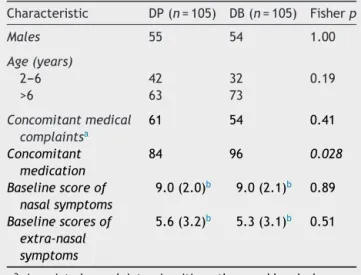
Table1 summarizesthe main clinicalcharacteristics of thepatientsaccordingtothereceivedtreatmentregimen. Wefound thatexceptfor theuseof additionaldrugsthat weresignificantlyhigheramongthoseintheDBgroup,the twogroups weresimilar, especiallyregarding NSS, ExNSS, andlaboratoryabnormalities.
Patients treated withDP showed a reduction of 76.4% ofNSS; 86.0% of ExNSS, andan increase of 25.2% ofPNIF compared to baseline (Table 2). Those treated with DB showeda reductionof 79.1%of NSS; 79.2% ofExNSS, and 24.3%increaseinPNIFcomparedtobaseline(Table2).The comparativeanalysisoftheseparametersbetweenthetwo treatmentregimens either at thebeginning or at theend didnotshowsignificantdifferences.
In Fig. 1, we observed average values of reduction in NSSand ExNSS regarding the firstday of evaluation, dur-ingthe7daysofmonitoring,accordingtothetwotreatment groups.Wefoundthatthetworegimensprovidedsignificant reduction,butnosignificantdifferencesbetweenthem.
Table 1 Characteristics of children with
moder-ate/severe persistent allergic rhinitis at admission, according to the treatment regimen received: DP (desloratadine+prednisolone) or DB (dexchlorpheni-ramine+betamethasone)---treatmentintention.
Characteristic DP(n=105) DB(n=105) Fisherp
Males 55 54 1.00
Age(years)
2---6 42 32 0.19
>6 63 73
Concomitantmedical complaintsa
61 54 0.41
Concomitant medication
84 96 0.028
Baselinescoreof nasalsymptoms
9.0(2.0)b 9.0(2.1)b 0.89
Baselinescoresof extra-nasal symptoms
5.6(3.2)b 5.3(3.1)b 0.51
a Associatedcomplaints,sinusitis,asthmaandheadache. b Standarddeviation.
2 3 4 5 6 7 8 9
1 2 3 4 5 6 7
Reduction in score of symptoms
DP
Days
DB DPEx DBEx
Figure 1 Progression of score (average) of nasal and extranasal (Ex) symptomsaccording to thetreatment group: desloratadine+prednisolone (DP and DPEx, respectively) or dexchlorpheniramine+betamethasone (DB and DBEx, respec-tively)accordingtodifferentdays.
Thetreatment regimen wasfollowedby almostall the patients in both study groups. When asked about the responseobtainedafterthetreatmentreceived,therewas nosignificantdifferencebetweenthefrequencyofpatients whoreportedbeingmuchbetter/betterafterthetreatment received(Table3).
Table2 Average score(standard deviation)ofnasal symptoms(NSS), ofextra-nasal symptoms(ExNSS)andofpeak nasal inspiratoryflow(PNIF)ofchildrenwithmoderate/severepersistentallergicrhinitisatadmission(V1)andaftertreatment(V2; 7±2days)withDP(desloratadine+prednisolone)orDB(dexchlorpheniramine+betamethasone).
DP(n=98) DB(n=97)
V1 V2 V1−V2 V1 V2 V1−V2
NSS 8.9(2.0) 2.1(2.3) 6.8 9.1(2.1) 1.9(2.3) 7.2
ExNSS 5.7(3.1) 0.8(1.2) 4.9 5.3(3.2) 1.1(2.2) 4.2
DP(n=61) DB(n=70)
V1 V2 V1−V2 V1 V2 V1−V2
Peaknasalinspiratoryflow(L/min)
PNIF 70.3(23.9) 83.6(26.3) 13.3 64.5(21.9) 80.3(25.0) 15.8
Mann---Whitney.
NSS,ExNSSandPNIF-V1:DP=DB;V2:DP=DB;V1−V2:DP=DB. DPandDB---NSS,ExNSSandPNIF:V1>V2---p<0.05.
Table 3 Global assessment ofthe participant regarding receivedtreatment:DP(desloratadine+prednisolone)orDB (dexchlorpheniramine+betamethasone).
Assessmentofparticipant DP DB
n(%) n(%)
Muchbetter/better 96(98.0) 94(96.9) Unchanged/worse 2(2.0) 3(3.1) Total 98(100.0) 97(100.0)
Fisher’sexacttest---p=0.682.
allpatients,thesechangeswerenotfollowedbysignificant clinicalsymptoms,andwereconsidered irrelevant:mainly sinustachycardia,physiologicalsinusarrhythmia, intraven-tricularconductiondelay.SixpatientsintheDPgroupand11 intheDBgrouppresentedchangesattheendofthestudy, buthadnormalECG tracingatthebeginning,adifference thatwasnotsignificant(p=0.52).
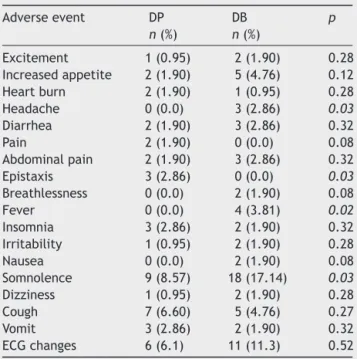
Asforreportedadverseevents,somnolencewasthemost reported, being significantly higher among those treated withDB(Table 4).Headache andfever had similar occur-rences.However,epistaxiswasreportedsignificantlymore often amongDPpatients (Table4). Allthese events were classifiedasnotsevere(Table4).
Discussion
Both treatment regimens were effective in controlling acute symptomsof children andadolescents with moder-ate/severePAR,revealedbythereductionofNSSandExNSS, aswellastheincreaseofPNIF(Table2).Itisworthnoting thatthepatientsanalyzedhadapictureofARofmoderate tosevereintensity,whichoftenmakesthetreatmentmore challenging.1
AlthoughthereductionofNSScomparedtobaseline val-ues was 76.4% for those treated with DP, and 79.1% for those treated with DB, there were still patients who did notachievefullcontrolofnasalandextra-nasalsymptoms. AILAstudy(AllergiesinLatinAmerica)conductedto deter-minetheprevalenceofARinthepopulationofsomeLatin
Table 4 Adverse events reported by at least 1%
of patients, according to the treatment group: desloratadine+prednisolone (DP) or dexchlorpheni-ramine+betamethasone(DB).
Adverseevent DP DB p
n(%) n(%)
Excitement 1(0.95) 2(1.90) 0.28 Increasedappetite 2(1.90) 5(4.76) 0.12 Heartburn 2(1.90) 1(0.95) 0.28 Headache 0(0.0) 3(2.86) 0.03
Diarrhea 2(1.90) 3(2.86) 0.32 Pain 2(1.90) 0(0.0) 0.08 Abdominalpain 2(1.90) 3(2.86) 0.32 Epistaxis 3(2.86) 0(0.0) 0.03
Breathlessness 0(0.0) 2(1.90) 0.08 Fever 0(0.0) 4(3.81) 0.02
Insomnia 3(2.86) 2(1.90) 0.32 Irritability 1(0.95) 2(1.90) 0.28 Nausea 0(0.0) 2(1.90) 0.08 Somnolence 9(8.57) 18(17.14) 0.03
Dizziness 1(0.95) 2(1.90) 0.28 Cough 7(6.60) 5(4.76) 0.27 Vomit 3(2.86) 2(1.90) 0.32 ECGchanges 6(6.1) 11(11.3) 0.52
Fisher’sexacttest
Italictype---significantlydifferent.
Americancountriesdocumentedthatmanypatients identi-fiedashaving ARfrequently changed treatment regimens because they considered them ineffective, and the use of combination of drugs of different classes wascommon among them.11 A similar finding was observed by other
researchers.12,13
allergicrhinitis.10Bothtreatmentregimensprovided
signif-icant increase in nasal patencywith an increase in mean valuesofPNIFcloseto25%ofbaseline.Thisfindingcanbe consideredclinicallyrelevant.Asacomparison,nasal provo-cationstudiesconsidervariationinPNIFvaluesoftheorder of20%fordefiningrelevantnasalobstruction and comple-tionofthetest.14,15
Regardingsafetyevaluationoftreatmentregimensused, we found that the frequency of drowsiness among those treatedwithDB wastwotimes higherthan thaton those treatedwithDP(Table4).Inaddition,althoughthenumber ofpatientswithECGchangesatbaselinewashigheramong thosein theDPgroup, thisdifferencedisappeared at the endofthestudywhenonlysixpatientsofDPgroupand11 oftheDBgroupstillhadsuchchanges.
Although first-generation, or classic, anti-H1 has been usedinthetreatmentofallergicdiseases sincethe1940s, safety studies are scarce and more recent. These drugs havebeen developed fromthesamebase molecule, simi-lartothemuscariniccholinergicantagonists,tranquilizers, antipsychotics and anti-hypertensive agents, and due to their low selectivity for H1 receptors, theyinteract with receptors of other active amines causing antimuscarinic, anti-␣-adrenergic and anti-serotonin effects. Since they
crosstheblood-brainbarrier easily,theybindtobrain H1-receptorsand interfere withneurotransmitterfunction of histaminecausingdrowsiness,sedation,fatigue,decreased readiness, worsening of cognitive function, memory and psychomotorperformance.16Thisexplainsthehigher
preva-lenceof drowsiness among the subjectsin the DPgroup, sincedexchlorpheniramineis representativeof theclassic orfirstgenerationanti-H1.17
It was from 1980 on that the second generation of anti-H1 emerged and started being used in large scale, with no side effects previously associated with the first-generationagents.However,theoccurrenceofcardiotoxic effectswasassociatedwithsomeofthem:terfenadineand astemizole.17Thisfactwasdocumentedtobedueto
compe-titionforhepaticmetabolicpathway,thecytochromeP450 system, by those drugs, ketoconazole, macrolide antibi-otics, and other agents, which would result in a high circulatinglevelsofthatanti-H1agentthatwerepotentially cardiotoxic.Thisfactleadtothereplacementofthese anti-H1agentsbyneweragentsofsimilarchemicalstructurethat hadnoneoftheseadverseeffects,buthadthesamepower ofaction.17
Anotherimportantfactistheindiscriminateuseoffirst generationanti-H1agentsamonginfantsandchildrenthat came to be twice greater than drugs of the second gen-eration. This widespread use for many years created the false impression that they were as safe as the second generation.16 Inaddition,manydoctorsprescribethemfor
their sedative effect, believing that patients will have a better sleep. This idea proved to be wrong since these first-generation anti-H1 agents prevent the patient from reachingtheREMstageofsleep,makingitineffective.18,19
Thecurrentconsensusdoesnotrecommendtheuseof first-generationanti-H1forthetreatmentofallergicrhinitis,and recommends the use of second-generation anti-H1 agents for their greater safety and lower incidence of adverse events.19
Conclusion
Inconclusion,althoughbothtreatmentregimenshave pro-videdeffectivecontrolofthesymptomsofPAR,DPshowed to be more advantageous due to its convenient dosage schedule (once a day) and lower frequency of adverse effects.
Funding
Funding authorityfor StudiesandProjects (FINEP)--- Inno-vationandResearch,Brazil,andEMS/AS---SãoPaulo,Brazil (Process:01.12.0094.00;referenceatFINEP-1375/10).
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
References
1.BousquetJ,KhaltaevN,CruzA,DenburgJ,FokkensW,Togias A,etal.AllergicRhinitisanditsImpactonAsthma(ARIA)2008 update.Allergy.2008;63Suppl.:S8---160.
2.AffrimeM,BanfieldC,GuptaS,CohenA,Boutros T,Thonoor M, et al. A pharmacokinetic profile of desloratadine in healthyadultsincludingelderlysubjects.ClinPharmacokinet. 2002;41:21---8.
3.BanfieldC,HerronJ,KeungA,PadhiD,AffrimeM.Desloratadine hasnoelectrocardiographicorpharmacodynamicinteractions withketoconazole.ClinPharmacokinet.2002;41:37---44. 4.Kreutner W, Hey JA, Anthes J, Barnett A, Young S, Tozzi
S.Preclinicalpharmacologyofdesloratadine,a selectiveand nonsedating histamine H1 receptor antagonist: 1st commu-nication: receptor selectivity, antihistamine activity, and antiallergeniceffects.Arzneimittelforschung.2000;50:345---52. 5.LippertU,Kruger-KrasagakesS,MollerA,KiesslingU,Czametzki BM.PharmacologicalmodulationofIL-6andIL-8secretionby theH1-antagonistdescarboethoxy-loratadineand dexametha-sonebyhumanmastandbasophiliccelllines.ExpDermatol. 1995;4:272---6.
6.BousquetJ,Bachert C,CanonicaGW,MullolJ,Van Cauwen-berge P, Bindslev Jensen C,et al. Efficacy of desloratadine in intermittent allergic rhinitis: a GA(2)LEN study. Allergy. 2009;64:1516---23.
7.BrooksCD,TitusCR,HeisslerCT.Vasoconstrictorand corticos-teroidresponsivecomponentofallergicnasalmucosalswelling. AnnAllergy.1988;6:151---6.
8.Global Pharmaceuticals Marketing Channel Reference report. Available at http://www.imshealth.com/en/solution-areas/market-insights[accessedJune2015].
9.Rotulagemdemedicamentos.Availableathttp://www.anvisa. gov.br/medicamentos/pesquisa/rotulagempesquisa%20clinica. pdf[accessed09.09.13].
10.NathanR,EcclesR,HowarthP,SteinsvagS,TogiasA.Objective monitoringofnasalpatencyandnasalphysiologyinrhinitis.J AllergyClinImmunol.2005;115Suppl.1:S442---59.
11.NeffenH,MelloJFJr,SoleD,NaspitzCK,DoderoAE,GarzaHL, etal.NasalallergiesintheLatinAmericanpopulation:results fromtheAllergiesinLatinAmericasurvey.AllergyAsthmaProc. 2010;31Suppl.1:S9---27.
themanagementofallergicrhinitisbyphysiciansandpatients (ISMAR).WorldAllergyOrganJ.2015;8:10.
13.PriceD,ScaddingG,RyanD,BachertC,CanonicaGW,MullolJ, etal.Thehiddenburdenofadultallergicrhinitis:UKhealthcare resourceutilizationsurvey.ClinTranslAllergy.2015;5:39. 14.VanGervenL,BoeckxstaensG,JorissenM,FokkensW,Hellings
P. Short-time cold dry air exposure: a useful diagnostic tool for nasal hyperresponsiveness.Laryngoscope. 2012;122: 2615---20.
15.ChusakulS,PhannasoC,SangsarsriS,AeumjaturapatS, Snid-vongsK.House-dustmitenasalprovocation:adiagnostictool inperennialrhinitis.AmJRhinolAllergy.2010;24:133---6. 16.Church MK, Maurer M, Simons FER, Bindslev-Jensen C, van
Cauwenberge P, Bousquet J, et al. Risk of first-generation
H1-antihistamine:a GA2LEN position paper.Allergy. 2010;65: 459---66.
17.HolgateS,CanonicaGW,SimonsFE,TaglialatelaM,TharpM, TimmermanH,etal.ConsensusGrouponNew-Generation Anti-histamines(CONGA):presentstatusandrecommendations.Clin ExpAllergy.2003;33:1305---24.
18.MerensteinD,Diener-WestM,HalbowerAC,KristA,RubinHR. The trial ofinfant response to diphenhydranime: the TIRED study---arandomized,controlled,patient-orientedtrial.Arch PediatrAdolescMed.2006;160:707---12.