1
UNIVERSIDADE DE LISBOA
FACULDADE DE CIÊNCIAS
DEPARTAMENTO DE BIOLOGIA ANIMAL
Physiological responses of whitespotted bamboo shark
(Chiloscyllium plagiosum) to high CO
2levels
Eduarda Filipa Campos Pinto
Mestrado Ecologia Marinha
Dissertação orientada por:
Doutor Rui Rosa e
Doutor Tiago Repolho
ii
Agradecimentos
Na nossa vida passamos por várias etapas, grandes desafios pessoais que exigem muito do nosso esforço pessoal. Mas em todas essas etapas, temos pessoas ao nosso redor que nos dão força e nos fazem acreditar em nós quando nós próprios menos acreditamos. São essas pessoas que nos puxam para cima e não nos deixam cair ao chão ajudam-nos a levantar e a continuar de cabeça erguida para atingir o objectivo a que nos propomos. Neste longo e difícil processo que foi a realização desta dissertação, tive muitas pessoas a quem eu agradeço do fundo do meu ser, do fundo do meu coração! Sem elas não sei se seria capaz de continuar a acreditar que isto era possível de ser realizado. Espero conseguir chegar a todos os que ajudaram nesta minha etapa.
Quero começar por agradecer aos meus orientadores Rui e Repolho, com um grande obrigado por me terem aceite como vossa estudante e terem-me dado a oportunidade de realizar esta tese no vosso laboratório, caso contrário não teria sido possível. A toda a equipa do RRlab e do Laboratório Marítimo da Guia, por me terem integrado tão bem neste novo local tão novo para mim. Por me terem dado conhecimento científico e ter oportunidade de ver de perto o que é fazer ciência a sério. Agradecer à Rita, Cátia, Grilo, Borges pelas conversas e momentos de diversão que são possíveis ter entre as horas intensas de trabalho. Agradecer com um especial obrigado às sharky girls, Ana Couto, Catarina e Maria Rita que tanto me aturaram, ao meu choro, às minhas inseguranças e no entanto nunca deixaram de acreditar em mim e que isto era possível acontecer e me deram um suporte enorme na realização desta dissertação.
Agradecer às minhas grandes amigas, Helena, Cátia, Inês, Filipa, Jéssica, Raimundo, Meireles, Catarina por todo o apoio dado nesta fase, por todos os puxa para cima que me fizeram não desistir, a todos os miminhos dados que tão bem sabem quando se está mais em baixo. Ao meu melhor amigo André Alves, que tantas vezes me deu na cabeça, que apesar de estar na Austrália, sempre esteve do meu lado das maneiras possíveis, mesmo quando estava mais difícil de aturar. Obrigado por aturarem esta minha pessoa. Estas são amizades que ficam para a vida toda.
À minha família, os meus pais, a minha irmã, o meu primo que sempre me deram um apoio incondicional em tudo na minha vida, permitindo-me sempre seguir os meus sonhos sem nunca julgarem ou acharem que eu não era capaz. Muito obrigada!
Muito obrigado mesmo, a todos! Pode parecer clichê, nada disto seria possível sem a vossa presença na minha vida.
iii
Abstract
Sharks have been roaming the planet for 400 million years and are vital elements for the health of our oceans. Due to occurring changes in the food-web and anthropogenic pressure from fishing and habitat degradation, sharks populations are now declining sharply. Ocean acidification, caused by continuous release of carbon dioxide (CO2) to the atmosphere, may represent an additional threat. Among other
effects, it may cause physiological disturbances in the organisms and threaten marine ecosystems as we know them, especially the most vulnerable life stages. Hence, the present study focus on the effects that ocean acidification may have on the fitness, metabolism and swimming performance of juvenile whitespotted bamboo sharks (Chiloscyllium plagiosum). After hatching, sharks were placed in either control (pCO2 ~ 400 μatm, pH = 8.0) or high CO2 (pCO2 ~ 900 μatm, pH = 7.7) conditions, according
to the pH levels expected by the end of the century. After an exposure of 45 days, several ecologically important traits were tested, namely their fitness [(i) Fulton condition], metabolic capacity [(i) routine metabolic rate (RMR), (ii) maximum metabolic rate (MMR), (iii) aerobic scope (AS)] and swimming performance [(i) maximum reached velocity, (ii) percentage of time swimming, (iii) number of bursts and (vi) pre and (vii) post-stress ventilation rates]. No changes were observed in their fitness, metabolism and the majority of the swimming performance end-points. Nevertheless, regarding the swimming performance, there was a decrease of the duration of swimming events and a decrease in the post-swimming ventilation rates. Over the past years, these cartilaginous fish have been coping with oscillations in the seawater chemistry and thus appear to be resilient to OA. However, this species’ conservation status is of concern, assessed as Near Threatened, and even the sub-lethal effects observed in this study may potentially reduce the organism’s overall fitness and ultimately impact population dynamics.
iv
Resumo
Desde a Revolução Industrial, o Homem tem continuamente libertado para a atmosfera grandes concentrações de dióxido de carbono (CO2), como resultado da indústria, agricultura e
desflorestação. Paralelamente, uma vez que os gases atmosféricos tendem a equilibrar-se com os do oceano, a concentração de CO2 dissolvido nos oceanos tem também vindo a aumentar. Este aumento a
um ritmo tão acelerado traduz-se inevitavelmente em alterações drásticas, essencialmente provocando uma desregulação do ciclo de carbono. Esta desregulação está intimamente relacionada com a química dos oceanos. O CO2 entra no meio marinho e reage com a água do mar, libertando protões o que torna
este ambiente mais ácido, conduzindo a um processo determinado de acidificação dos oceanos. É expectável que, até ao final do século, as concentrações de CO2 possam ter aumentado de 280 para
1000 µatm, o que se reflete numa diminuição do pH entre 0.13 a 0.4 unidades (uma redução de até 30%). Neste cenário, são expectáveis diversas repercussões nos animais marinhos, nomeadamente a nível fisiológico e comportamental, podendo levar à desregulação do equilíbrio dos ecossistemas marinhos.
O oceano ostenta uma grande diversidade de organismos com diversas estratégias de sobrevivência às várias condições do meio ambiente. A exposição a um nível de pCO2 elevado,
causará um stress maior nestes organismos. O influxo de CO2 por difusão num organismo marinho, dá
lugar a uma tentativa de reequilíbrio entre os vários compartimentos internos, levando ao aumento dos iões H+ e a uma consequente diminuição de pH. Este processo resulta em acidose no organismo, afectando o equilíbrio ácido-base. No entanto, se esta compensação não for atingida há ainda outras espécies que fazem supressão do seu metabolismo como uma estratégia adaptativa de sobrevivência, embora em condições de constante pCO2 elevado, essa estratégia deixa de ser vantajosa. Estes
processos, e as próprias estratégias de compensação implementadas pelo organismo (ex: acumulação de bicarbonatos), requerem uma realocação de energia, que potencialmente poderão comprometer a sua sobrevivência, reprodução e/ou mesmo comportamento, afectando o seu fitness, no geral.
Os tubarões, como predadores de topo, têm um papel fundamental e na estrutura e funcionamento do ecossistema marinho. Trata-se de um grupo ancestral, tendo sobrevivido a diversas extinções em massa. No entanto, e apesar da sua longa história evolutiva, as populações de tubarões têm vindo a decrescer, em grande parte devido ao aumento da pressão antropogénica. O seu ciclo de vida longo, com maturação tardia, e baixa fecundidade, limitam a sua capacidade de adaptação a alterações repentinas no seu ecossistema. Alterações na química do oceano podem representar outro factor de risco para as comunidades de tubarões. Durante bastante tempo foi argumentado que a acidificação dos oceanos não teria efeitos diretos relevantes na fisiologia deste grupo, sendo apenas indirectamente afectados como resultado dum efeito cascata na estrutura do ecossistema, devido à menor quantidade de presas no meio e uma degradação do habitat. Este argumento advém em grande parte da sua longa história evolutiva, ao longo da qual foram expostos a elevados níveis de CO2.
Apenas recentemente começaram a surgir estudos empíricos abordando esta questão, havendo evidências de efeitos directos (ex. alterações fisiológicas e comportamentais) foram reportadas para várias espécies e diferentes estágios do seu ciclo de vida. Alguns tubarões têm ventilação ram, o que faz com que tenham um gasto de energia maior uma vez que têm de estar constantemente a nadar para oxigenar as brânquias, enquanto outros tubarões têm ventilação por bombeamento bucal, o que requer um gasto de energia menor uma vez que podem estar parados no fundo e abrir simplesmente a boca para que haja oxigenação das brânquias. Isto explica porque é que os tubarões com ventilação ram têm taxas metabólicas maiores que os tubarões que fazem ventilação bucal. Através da medição do consumo de oxigénio é possível obter a quantidade de energia que um animal requer para processos essenciais no seu seu ciclo de vida, tais como o seu desenvolvimento, sobrevivência e reprodução. A
v ferramenta mais utilizada para obter as taxas metabólicas de um organismo, quer em repouso, exposto a um stress ou durante uma actividade, é a respirometria (ou calorimetria indirecta).
Este estudo foca-se no efeito que o aumento de CO2 nos oceanos poderá ter no fitness,
fisiologia e performance natatória de uma espécie de tubarão bentónico vulgarmente designada de tubarão bambu (Chiloscyllium plagiosum). Para tal, foram analisados o peso e comprimento, a taxa de consumo de oxigénio para a obtenção das taxas metabólicas e atividade natatória destes organismos. Após eclosão, cada tubarão foi colocado aleatoriamente num tanque individual e exposto a uma de duas condições experimentais: controlo (pH 8.0 | ~390 μatm, n = 5) ou acidificação (pH 7.7 | ~890 μatm, n = 5). Após 45 dias de exposição, foram analisadas diversas características com relevância ecológica, nomeadamente o fitness [(i) condição de Fulton], capacidade metabólica [(i) taxa metabólica de rotina (consumo médio de oxigénio), (ii) taxa metabólica máxima (consumo máximo de oxigénio), (iii) capacidade aeróbica (aerobic scope)] e performance natatória [(i) velocidade máxima nadada, (ii) percentagem de tempo de natação, (iii) número de impulsos caudais e (vi) taxa de ventilação pré e (vii) pós-stress]. Os testes de medição de consumo de oxigénio, os de natação e as ventilações foram realizados numa câmara de natação. Individualmente colocou-se um tubarão na câmara com uma velocidade mínima constante e após período de aclimatação realizavam-se as medições de consumo de oxigénio para determinar a taxa metabólica de rotina tais como a contagem das ventilações em repouso. Posteriormente foi realizado o exercício de exaustão do tubarão para que fosse possível medir o consumo de oxigénio máximo e registar o número de ventilações sobre stress. Quanto aos testes relativos a actividade natatória, o tubarão foi submetido a um aumento gradual da velocidade de circulação até não conseguir manter a posição normal do corpo.
Ao longo desta experiência não se verificou qualquer mortalidade. De facto, são poucos os estudos que relatam uma diminuição da sobrevivência em animais expostos a elevadas concentrações de CO2. De acordo com os resultados deste estudo, esta espécie parece ser resiliente à acidificação dos
oceanos prevista para o futuro. O impacto destas alterações químicas aparenta estar altamente relacionada com a espécie em estudo, dependendo do estádio de desenvolvimento e duração da exposição. Tendo isto em conta, a condição Fulton desta espécie de tubarões, a qual pode ser utilizada como indicador do fitness geral de um organismo, parece não ter sido afectada num cenário de acidificação. Pelo contrário, a taxa de ventilações pós-stress bem como a performance natatória, mais especificamente a percentagem de tempo de natação, diminuíram significativamente. Uma boa performance natatória é essencial para encontrar comida, refúgio e, principalmente enquanto juvenis, para escapar a predadores. Assim, uma diminuição dessa capacidade pode ter graves consequências. Uma vez que animais expostos a elevadas concentrações de CO2 no ambiente podem apresentar
alterações fisiológicas, como consequência de ajustes energéticos que resultam principalmente da reposição do equilíbrio ácido-base.
Em geral, no presente estudo, estes organismos mostraram-se um certo grau de resiliência às condições futuras de acidificação dos oceanos. Nos testes em que os tubarões foram significativamente afectados, uma vez que se trata de um tubarão bentónico e com um estilo de vida lento, esta especie não está particularmente dependente de uma natação intensa durante longos periodos de tempo. Como tal, é possivel que uma realocação de energia esteja a ter lugar e que esta confira ao animal uma maior plasticidade perante as condições futuras. Estudos futuros são necessários para averiguar os efeitos que estas condições podem exercer sobre espécies pelágicas, altamente dependentes nas suas capacidades natatórias. Adicionalmente, estudos que considerem a interação entre a acidificação dos oceanos e outras variáveis ambientais (ex: aquecimento global) devem ser priorizados.
Palavras-chave: acidificação dos oceanos, elasmobrânquios, metabolismo, respirometria,
vii
Index
Agradecimentos ii
Abstract iii
Resumo iv
List of figures and tables viii
List of abbreviations and symbols ix
1. Introduction 1
1.1. Ocean acidification 1
1.2 Impacts of ocean acidification in the marine ecosystem 2
1.3 Impacts of ocean acidification on sharks 2
1.4 Metabolism 3
2. Materials and Methods 7
2.1. Animal collection and acclimation 7
2.2. Fulton condition 8
2.3. Oxygen consumption, aerobic scope and ventilation rates 8
2.4. Swimming performance 9 2.5. Statistical analyses 9 3. Results 10 3.1. Survival 10 3.2. Fulton condition 10 3.3. Physiology 10 3.4. Ventilation Rate 12 3.5. Swimming performance 13 4. Discussion 16
5. Conclusion/ Future directions 17
viii
List of figures and tables
Figure 1.1. The effect of elevated atmospheric carbon dioxide in the ocean’s carbon
system………..……….…1
Figure 1.2. Chiloscyllium plagiosum (Anonymous [Bennett],
1830)...4
Table 1.1. Summary of the available experimentally based studies on the impacts of ocean
acidification (OA) in sharks.……….…………...…………...5
Table 2.1. Seawater carbonate chemistry data of bamboo juvenile
sharks………8
Figure 2.1. Schematic diagram of a swimming
respirometer…………..………...………...8
Figure 3.1. Effects of elevated pCO2 (µatm 900) on Fulton condition on juvenile bamboo sharks
(Chiloscyllium plagiosum). ...……...10
Figure 3.2. Effects of elevated pCO2 on the: a) routine metabolic rate, b) maximum metabolic rate, c)
aerobic scope and d) recovery rate (mg O2 ml-1 g-1 min-1), of juvenile bamboo sharks (Chiloscyllium plagiosum). Values represent mean ± SE. Control n = 5, CO2 n = 5. More statistical details on Table
3.1………..……….12
Figure 3.3. Effects of elevated pCO2 on the ventilation rates, i.e. number of breaths min-1: a)
ventilation rate (while resting), b) ventilation rate (after exhaustion), of juvenile bamboo sharks (Chiloscyllium plagiosum). ……….………….………..13
Figure 3.4. Effects of elevated pCO2 (µatm 900) on the: a) swimming activity; b) maximum velocity,
c) number of bursts of juvenile bamboo sharks (Chiloscyllium plagiosum). Asterisks represent significant differences between treatments. Values represent mean ± SD. Control n = 5, CO2 n = 5.
More statistical details on Table 3.1….………...14
Table 3.1. Results of Generalized Linear Models (GLM) for fulton, routine metabolic rate (RMR),
maximum metabolic rate (MMR), aerobic scope (AS), ventilation rates in rest, ventilation rates in stress, percentage of time spent swimming, maximum velocity reach and number of bursts……….15
ix
List of abbreviations and symbols OA Ocean acidification
CO2 Carbon dioxide
O2 Oxygen
ppm Parts per million
pCO2 Partial pressure of carbon dioxide
H2CO3 Carbonic acid
HCO3- Bicarbonate
H+ Hydrogen ion
pH Power of hydrogen
RMR Routine Metabolic Rate
MMR Maximum Metabolic Rate
AS Aerobic Scope
1
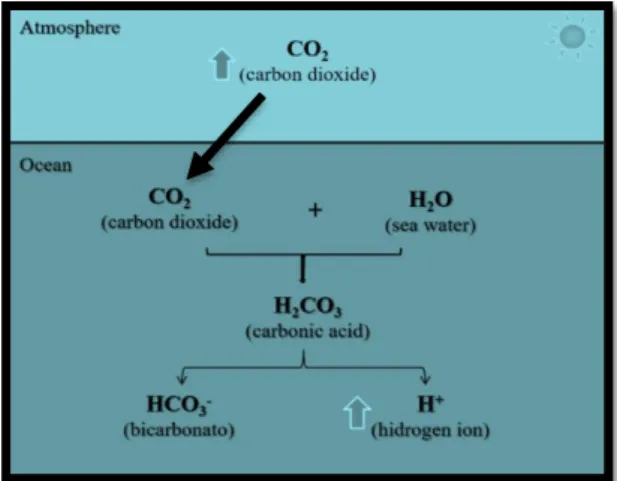
Figure 1.1. Chemical underlying process of ocean
acidification.
1. Introduction
1.1. Ocean acidification
Carbon dioxide (CO2) exists naturally as a constitute of the Earth’s atmosphere,
affecting the heat balance of the planet and the calcium carbonate equilibrium of the ocean (Petit et al., 1999). The carbon cycle has a fundamental regulatory role between climate and ecology (Doney et al., 2007) and the increase of CO2 is expected to change the oceans’
chemistry (Solomon et al., 2010). Since the Industrial Revolution, CO2 has been constantly
emitted as a product of the industry, agriculture and deforestation (Sabine, 2004; Caldeira & Wickett, 2003; Feely et al., 2009), which consequently has increased the amount of CO2
dissolved in the oceans (Pörtner et al., 2004). Chemically, CO2 reacts with water to produce
carbonic acid (H2CO3), which then dissociates to form bicarbonate ions (HCO3-) and protons
(H+); thus, the amount of CO
2 that dissolves in seawater has a strong influence on the resultant
pH of the oceans (Society, 2005). Continuous accumulation of CO2 will increase hydrogen (H+)
and bicarbonate (HCO3-) and decrease the carbonate ions (CO32-) leading to a phenomenon
designated as ocean acidification (OA) (Caldeira, 2005; Zeebe, 2011).
To date, this phenomenon has already caused a decrease of the pH value in 0.1 units and projections suggest that until 2100 it will continue to decrease between 0.13 to 0.4 units (IPCC, 2014). This is extremely concerning, as a lower pH of the seawater may impair animal’s physiology (Rosa, 2008; Rosa et al., 2013; Rosa et al., 2014a) of several marine organisms ( Munday et al., 2010; Domenici et al., 2011; Ferrari et al., 2011; Briffa et al., 2012; Nilsson et al., 2012; Jutfelt et al., 2013; Lai et al., 2015) with potential cascading effects to the ecological system. Hence, defining which species are vulnerable and which are tolerant to higher levels of CO2 is extremely important to assess the impacts that OA might have on the marine ecosystem
2 1.2 Impacts of ocean acidification in the marine ecosystem
The ocean hosts a diversity of marine organisms, from the microscopical phytoplankton to the enormous blue whale, which are adapted with different strategies in order to survive in different conditions (Kroeker et al., 2010). Exposure to higher pCO2 may cause acid–base
disturbances, as chemical reactions take place similarly to the ones in the ocean (Fabry et al., 2008). CO2 enters an organism and reacts with internal body fluids increasing H+ and decreasing
the pH (Pörtner et al., 2004; Fabry et al., 2008). To compensate this chemical change, organisms regulate their acid-base balance with intracellular buffer systems (i.e., HCO3- and Cl-)
(Walsh et al., 1989). This compensatory response may be responsible for several downstream effects, resulting in a variety of physiological changes (Heuer et al., 2014). Nonetheless, these compensatory mechanisms may not be advantageous in a prolonged exposure to high CO2
(Pörtner et al. 2004). If compensation of acid-base imbalance cannot be achieved during a prolonged exposure to high CO2 some species may suppress their metabolism (Pörtner et al.,
1998; Guppy & Withers 1999), which can result from the shutdown of expensive processes (Fabry et al., 2008). Metabolic suppression may result in the reduction of growth and reproductive output, ultimately limiting the survival of the organism (Fabry et al., 2008).
Over the last years, it has become clear that these physicochemical changes may severely impact key biological traits, from calcifying organisms unable to use calcium carbonate (Kleypas et al., 2006), to prey that swims into its predator (Dixson et al., 2010; Munday et al., 2010) and top predators unfit to hunt (Dixson et al., 2015; Pistevos et al., 2015), the negative impacts of OA may reverberate through food webs to the entire ecosystem (Rossoll et al., 2012). High CO2 levels in the seawater leads to a decline of CO32- availability, and as a
consequence calcifying organisms (e.g. corals, molluscs, echinoderms and crustaceans) may be unable to maintain their calcium carbonate structures (Fabry et al. 2008; Gattuso et al., 2015). In contrast, fish appear the most tolerant marine animals, being able to compensate completely both blood plasma pH and intracellular pH (Michaelidis et al., 2007). Tolerant species exhibit greater bicarbonate accumulation and, therefore, compensate more effectively for acidosis (Spicer et al., 2007). Nevertheless, there may be an apex beyond which acid–base regulation starts to compromise ionic balance (Cameron & Iwama 1989) and ultimately leading to physiological impairments (Munday et al., 2009; Rummer et al., 2013).
1.3 Impacts of ocean acidification on sharks
Since most research has focused on teleost fish there is little information available regarding sharks. As apex-predators, sharks occupy upper levels within the marine trophic chain (Compagno 2001), playing a key role (i.e. top down control) in the structure and functioning of marine ecosystems (Dill et al., 2003; Heithaus et al., 2008; Baum & Worm 2009). These animals are one of the most ancient fishes in the marine ecosystem having survived several mass extinctions over the last 400 million years (Grogan & Lund 2004; Kriwet et al., 2008). Unfortunately, populations have been declining sharply (≥ 90 %) mainly due to anthropogenic pressure (Cortés 2000; Chin et al., 2010). The expected changes of the ocean’s chemistry may represent another menace to the shark’s population, and their long life-cycle may limit their potential to adapt to a rapidly changing environment (Briffa et al., 2012).
Under the perspective of their history, it was argued that sharks would not be directly impacted by OA since they have been roaming the oceans through periods of higher CO2 levels
(Rummer & Munday 2017). Instead, they could be indirectly affected by habitat degradation and reduced prey availability, as a result of the cascading effects over the ecosystem structure
3 (Cortés 2000; Chin et al., 2010). As they were expected to be more tolerant to these chemical changes, the direct effects of OA in sharks would not be empirically addressed until recently (review by Rosa et al., 2017). Contrary to what has been argued, recent studies have shown that OA may affect their behavior and physiology (Rosa et al., 2014b; Rosa et al., 2016a; Heinrich et al., 2014; Johnson et al., 2016; Green & Jutfelt 2014; Pistevos et al., 2015; Dixson et al., 2015, summarized in Table 1.1). Sharks exposed to high CO2 showed significant differences in
their swimming behaviour, fulton condition index and their metabolism (Rosa et al., 2014b; Pistevos et al., 2015; Pistevos et al., 2016). In summary, there is cumulative scientific evidence to believe that sharks may be vulnerable to future OA.
1.4 Metabolism
Cartilaginous fishes have a big diversity of forms and life strategies (Compagno, 1990; Compagno, 2001). Some sharks with ram ventilation are more active, with a life strategy more energetic since they must swim continuously to oxygenate their gills (Rosa et al., 2014b; Rummer et al., 2016; Rosa et al.,. 2017). While other sharks can remain stationary by using a buccal pumping respiration, like the catshark, which requires less amount of energy (Clinton E. Brown, 1970). To convert food into energy, organisms use oxygen (O2) through an aerobic
metabolism (Carlson et al., 2004). In this context, measuring the oxygen consumption is a common practice used to measure aerobic metabolism (Carlson et al., 2004). By addressing species metabolism, one can determine energy requirements and associated costs, directly linked to functional needs such as reproduction, development and ultimately survival (Brill, 1996; Whitney et al., 2016).
In this sense, respirometry is a common end tool used to measure oxygen consumption and subsequently estimate an organism’s metabolic rate (Clark et al., 2013; Rummer et al., 2016). Three metabolic measurements can be taken from an organism: the Standard Metabolic Rate (SMR), which is the minimum amount of energy that an animal requires to sustain life and is estimated by measuring the oxygen consumption when the animal is inactive, in a post-absorptive resting state ( Fry, 1947, as cited in Claireaux & Chabot, 2016; Fry, 1957, as cited in Brett, 1964; Carlson et al., 2004); the Routine Metabolic Rate (RMR), that is measured by the metabolic rate of an organism under volitional activity, either in rest or in activity (Carlson et al., 2004); and the Maximum Metabolic Rate (MMR), which is the maximal amount of energy that an organism can metabolize aerobically, measured by the oxygen consumption rate during or after an exhaustive exercise (Norin & Clark, 2016). The total scope of aerobic activity (aerobic scope) can then be estimated from the difference between the MMR and the SMR or RMR and can be defined as the capacity for aerobic metabolism for extra basic maintenance costs, to support biological fitness, such as reproduction, swimming and feeding (Brett, 1964).
The metabolic rate and the division of energy between metabolic processes may overall be affected by CO2 ( Pörtner and Reipschläger, 1996, as cited in Pörtner et al., 2004; Pörtner,
2002). According to Heisler (1986), fish’s main response to high CO2 levels is similar to
elasmobranchs. OA has been reported to affect fish’s metabolism and metabolic rates with the assumption that coping with elevated pCO2 would increase SMR and reduce aerobic scope
(Melzner et al., 2009; Rummer et al., 2013; Heuer & Grosell 2014). To our knowledge, shark’s aerobic scope was maintained under high CO2 treatments, except during embryogenesis (Rosa et al., 2017, review).
Early developed life stages are expected to be the most vulnerable to OA (Dupont, 2014), and these chemical changes may compromise their swimming performance, (Rosa, et al., 2016) and metabolism (Rosa et al., 2014b).
4
Figure 1.2. Chiloscyllium plagiosum
(Anonymous [Bennett], 1830)
Hence, the focus of the present study was to investigate how the increasing levels of CO2 predicted to occur in the future (pCO2 ~ 900 µatm) may influence their fitness, metabolism
and swimming performance of juvenile whitespotted bamboo sharks (Chiloscyllium plagiosum). This species is a tropical benthic shark that inhabits complex reef corals (Compagno, 1990) from the Indo-Pacific region (Chen et al., 2007). They are bottom sharks, with buccal pumping respiration, that use it while resting in reef crevices during the day (Compagno, 1990). Despite their slow life pace, they are able to perform rapid movements, especially during escape response, relying on their swimming abilities (Maia, 2013), which is an energy-demanding ordeal (Lauder, 2016). Considering these key traits, after 45 days of exposure to high CO2
levels predicted to the end of the century (IPCC, 2014), we assessed their overall fitness [(i) Fulton condition], metabolic capacity [(i) routine metabolic rate (RMR), (ii) maximum metabolic rate (MMR), (iii) aerobic scope (AS)] and swimming performance [(i) maximum reached velocity, (ii) percentage of time swimming, (iii) number of bursts and (vi) pre and (vii) post-swimming ventilation rates].
A higher vulnerability of juveniles may eventually become a bottleneck for species survival. In this context, we expected that OA would affect the physiology of this sharks.
5
Table 1.1. Summary of the available experimentally based studies on the impacts of ocean acidification (OA) in sharks. Adapted from Rosa, 2017 review. Abbreviations: R-h, recently hatched;
Hb, haemoglobin; MCHC, mean cell haemoglobin concentration; NA, not applicable.
species Life strategy Life stage pCO2 (μatm) pH Temperature OA effects Reference
Control Treatment Control Treatment Control Treatment
Chiloscyllium punctatum Tropical and benthic Embryos and r-h juveniles 371 - 382 1383 - 1481 8.0 7.5 25.9 – 26.2 29.9 – 30.1 = embryo survival; ↓ juvenile survival; ↓ metabolic rates; ↓ ventilation rates; ↓ Fulton condition (Rosa et al., 2014b) Chiloscyllium punctatum Tropical and benthic R-h juveniles
371 - 382 1383 - 1481 8.0 7.5 25.9 – 26.2 29.9 – 30.1 ↓ brain and muscle aerobic potencial;
↑ brain and muscle anaerobic (LDH) potencial;
↑ peroxidative damage and brain AchE levels;
OA effects heightened with warming (Rosa et al., 2016a) Chiloscyllium punctatum Tropical and benthic R-h juveniles
371 - 382 1383 - 1481 8.0 7.5 25.9 – 26.2 29.9 – 30.1 ↓ pancreatic trypsin levels and alkaline phosphatase activity in shark’s intestine;
↑ both enzyme activities with warming (Rosa et al., 2016b) Hemiscyllium ocellatum Tropical and benthic
Juveniles 397 - 984 608 - 876 8.2 8.0 – 7.9 28.5 NA ↓ plasma [HCO3-], [Hb] and
MHC;
No differences metabolic rates, muscle aerobic and brain, Pcrit, [Hct], plasma [Na+], [K+], [Cl-] and [urea] (Heinrich et al. 2014) Hemiscyllium ocellatum Tropical and benthic
Juveniles 400 615 - 910 8.0 7.9 – 7.8 28.8 NA = foraging and shelter-seeking behaviour (Heinrich et al. 2015) Hemiscyllium ocellatum Tropical and benthic
Embryos 420 945 8.1 7.9 28.3 NA = growth, yolk usage, tail oscillations, gill movements and survival
6
Scyliorhinus canicula
Temperate and benthic
Juveniles 401 993 8.1 7.7 12.7 NA ↑ plasma [HCO3-] and [Na+];
↑ absolute lateralization; ≠ swimming patterns;
No changes in [K+], [Ca2+], [Cl-],
[Hct], MHC, metabolic rates, denticle ultrastructure and growth
(Green & Jutfelt 2014) Heterodontus portusjacksoni Temperate and benthic Embryos and juveniles 400 1000 8.0 7.8 16 19 ↑ energetic demands; ↓ metabolic efficiency;
↓ ability to locate food through olfaction;
↓ growth rates alone or in combination with temperature
(Pistevos, 2015) Heterodontus portusjacksoni Temperate and benthic Embryos and juveniles
400 1000 8.0 7.8 16 19 ↓ chemical and behavioural responses to effective hunting; ↑ energetic demands (Pistevos, 2016) Mustelus canis Temperate and benthopelagic
Adults 405 - 412 734 - 1071 8.1 7.7 – 7.8 19.6 NA Food odour avoidance; ↓ attack behaviour
7
2. Materials and Methods
2.1. Animal collection and acclimation
Ten wild whitespotted bamboo shark (Chiloscyllium plagiosum) eggs were hand collected by fisherman in the Lungsod Ng Cebu (Philippines; around 10º11’N123º580E) and transported by a certified commercial supplier, Tropical Marine Centre (TMC Iberia, Portugal) to the Laboratório Marítimo da Guia (LMG, Cascais, Portugal) aquaculture facilities. Upon arrival, eggs were placed in recirculation aquaculture system (200L total volume), being suspended by strings 5 cm below seawater surface in order to sustain good aeration conditions, at average ambient temperature (26ºC) and pCO2 (400 μatm), until hatching. Embryos were
reared under control conditions (Table 2.1) during at least 30 days.
After hatching, each shark with ~ 10 cm of length, was transferred to an individual 50L opaque tank and randomly assigned to one of the two experimental conditions: control (pH 8.0 | ~400 μatm, n = 5; Table 2.1) and high CO2 (pH 7.7 | ~900 μatm, n = 5; Table 2.1) for 45 days,
according to levels expected for 2100 (Rosa et al. 2014b). Overhead tank illumination was provided, under a 12h light: 12h dark photoperiod with white fluorescent lamps. Each tank was coupled within a recirculation semi-opened system with a 24h drip-system, providing a 50% daily change of UV-irradiated (V2ecton 600, TMC Iberia, Portugal) and 0.35-µm filtered (Harmsco, USA) natural seawater, pumped directly from the sea, with a salinity of 35. Water quality and adequate carbonate speciation were assured with a system of UV-sterilizers (TMC, Chorleywood, UK), protein skimmers (Schuran, Jülich, Germany), wet-dry filters (BioBalls), as well as biological (Ouriço®, Fernando Ribeiro Lda, Portugal), mechanical (Glass wool, Fernando Ribeiro Lda, Portugal) and physical filtration (V2 Skim Pro 450 TMC Iberia, Portugal).
Monitoring of pH values was performed via individual pH probes (GHL, Germany) in each tank and values were automatically adjusted by a Profilux device (GHL, Germany). Downregulation of pH values was achieved by injection of CO2 (certified gas from Air Liquide,
Portugal) or upregulated by aeration with atmospheric filtered air (Sodalime), via a solenoid valve coupled system. Additionally, pH values were daily controlled with a portable pH device (SG8-SevenGoproTM pH/Ion, Mettler Toledo, Switzerland). Seawater carbonate system was
calculated weekly (spectrophotometrically at 595 nm) from total alkalinity and pH measurements (Table 2.1), accordingly (Sarazin et al., 1999). Total dissolved inorganic carbon (CT), pCO2, bicarbonate concentration and aragonite saturation values were calculated using
CO2SYS software (Lewis & Wallace 1998) with dissociation constants from Mehrbach et al. (1973), as refitted by Dickson and Millero (1987). Ammonia, nitrite and nitrate levels (Profi test, Salifert, Holland) were daily checked and kept below detectable levels. Temperature (ºC) and salinity were daily monitored using, respectively, a thermometer (TFX 430, WTW GmbH, Germany) and a refractometer (V2, TMC Iberia, Portugal), and adjusted accordingly. The regulation from temperature was made by electronic heaters. Sharks were fed ad libitum every other day with shrimp, kingfish and squid.
8 Flush-pump Optical oxygen sensor linked to Firesting Optical Oxygen Water flow Water out via flush-pump Outflow from water bath
Figure 2.1. Schematic diagram of a swimming respirometer Table 2.1. Seawater carbonate chemistry data of bamboo juvenile sharks
Temperature (°C) Salinity pH (total scale) AT (μmol kg−1 SW) pCO2 (μatm)
1. 25,7 ± 0,42 36 ± 0,57 8,145 ± 0,09 2251,06 ± 407,16 330,43 ± 72,93
2. 26,0 ± 0,11 35 ± 0,2 8,003 ± 0,11 2269,53 ± 163,6 411,44 ± 35,81
3. 26,1 ± 0,14 35 ± 0,1 7,730 ± 0,01 2323,71 ± 68,81 891,70 ± 17,83 1. Eggs (control); 2. Juveniles (control); 3. Juveniles (high CO2). Carbon dioxide partial pressure (pCO2) was
calculated with CO2SYS using salinity, temperature, pH, and total alkalinity (AT). Values are mean ± SD.
2.2. Fulton condition
Fulton’s condition was calculated based on the weight and length relationship of the animals (Nash et al., 2006). As such, the juvenile bamboo sharks were weighed and measured, both before and after exposure to the experimental conditions, and their Fulton’s condition factor (Froese 2006) was calculated according to the following formula:
K = (W/TL3)/100
W = whole body wet weight (g) TL = total lenght (cm)
To measure the shark, a photo of each one was taken and analyzed on the software ImageJ. To weight, each one was placed inside of a box with the respective water of the system and put in a weight balance.
2.3. Oxygen consumption, aerobic scope and ventilation rates
To measure oxygen consumptions rates (MO2), each shark, now with ~ 16 cm of length,
was placed in a swimming tunnel (swim chamber: 30 x 7.5 x 7.5 cm, 5L; Loligo Systems, Denmark; Fig. 2.1) with water from the corresponding treatment conditions. The swimming tunnel was fitted with 2 mm optodes - fiber-optic oxygen sensors (Firesting, Pyroscience, Aachen, Germany), that were attached to the wall of the chamber, providing information of the MO2 levels inside it (Stokes & Somero, 1999; Frederich & Pörtner, 2000; Clark et al., 2013).
Before each trial, the water was changed and set up to the experimental condition of each shark that will be tested. In order to maintain stable temperature conditions (± 26 ⁰C), the swimming tunnel was immersed in a temperature-controlled bath. Sharks were not fed 24h prior to the swimming tunnel experimental procedure, so residual specific dynamic action can be eliminated.
9 A flux of water at a constant velocity of 0.06 m.s-1 was maintained throughout the trials.
After an acclimation period of ca. 30 minutes, the routine metabolic rate (RMR) was measured in 3 cycles. Each cycle consisted in a period of 10 minutes measuring the MO2 and an interval
with flush to replenish the chamber with new filtered and aerated seawater. In parallel, the pre-stress ventilation rate (number of breaths per minute) was registered 3 times. Then, an exhaustive chase protocol was performed as described in by Clark et al (2013), Rummer et al (2016) and Reidy et al (1994)and consisted in placing the shark in a bucket and chasing it by hand until it no longer responded or tried to escape. After a transfer through air to the swimming tunnel, MO2 was measured to calculate the maximum metabolic rate (MMR) and the post-stress
ventilation rate was registered once more as explained above.
The RMR and MMR values were calculated using the standard formula for intermittent-flow-through respirometry systems (Clark et al., 2013):
MO2 = [(VR – VF) x ΔCwO2] / (Δt x MF))
where VR is the swimming tunnel volume, VF is the shark’s volume, ΔCwO2 is the change in
oxygen concentration in the swimming tunnel, and Δt is the time during which ΔCwO2 is
measured. The aerobic scope was calculated as the difference between MMR and RMR (Killen et al., 2014).
2.4. Swimming performance
Swimming performance was analyzed from video recordings. A single shark was placed in the swimming tunnel holding chamber (swim chamber: 30 x 7.5 x 7.5 cm, 5L; Loligo Systems, Denmark), with water from the corresponding treatment conditions and a constant velocity of 0.06 m.s-1 (ca. 0.5 body length s-1 – from average length). Each trial was filmed for 2h for
posterior video analysis. A single trial consisted in the increase of the water’s velocity by 1 cm min-1 until the shark reached a maximum velocity that it could swim. Whenever a shark stopped swimming, it was encouraged to restart through flux reversal, until exhaustion. Afterwards, the burst swimming performance was measured according to a previously described protocol (Reidy et al., 2000). Hence, the (i) maximum velocity that sharks were able to swim, (ii) the percentage of time swimming, and (iii) number of bursts were registered. A significant water change (> 95%) was made between trials.
2.5. Statistical analyses
To detect significant differences between control and high CO2 treatments a generalized
linear models (GLMs) was used, except for the recovery test. All the data followed a normal distribution, except the number of bursts, percentage of swimming, who followed a poisson and a gamma distribution, respectively. For the recovery test it was used a repeated measures ANOVA test to see the correlation between treatments and time of recovery. Normality and homogeneity of variances were verified by Shapiro Wilks and Levene tests, respectively. All statistical analyses were performed for a significance level of 0.05, using R studio v3.3.2 software (R Core Team, 2016).
10
3. Results
3.1. Survival
Following a 45 days post-hatching exposure to control (pCO2 ~ 390 µatm; n = 5) or high
CO2 (pCO2 ~ 900 µatm; n = 5) conditions, juvenile bamboo sharks had 100% survival rate.
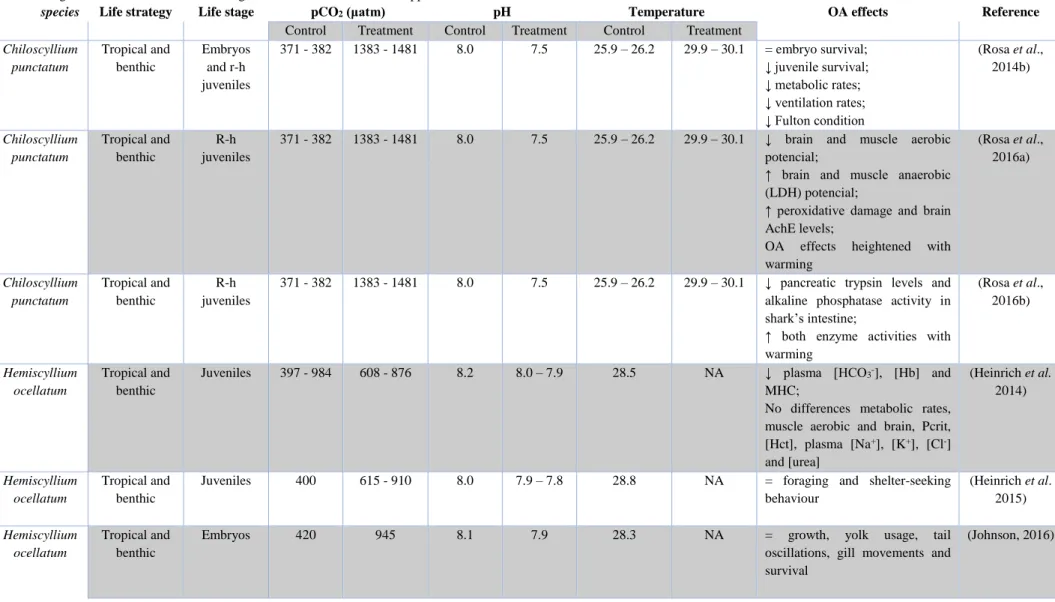
3.2. Fulton condition
Fulton condition (K) of the animals reared under control conditions was of 0.30± 0.01 (mean ± SD) while under high CO2 the mean value was 0.39 ± 0.06. Nonetheless, there were no
significant differences (p > 0.05) between control and high CO2 (Fig. 3.1).
Figure 3.1. Effects of elevated pCO2 (Δ pCO2 ~ 500 µatm) on Fulton condition of juvenile bamboo sharks
(Chiloscyllium plagiosum). Values represent mean ± SD. Control n = 5, High CO2 n = 5. More statistical details on
Table 3.1.
3.3. Physiology
Sharks RMR´s were higher under acidification conditions in comparison to control (Fig.3.2a), with mean values of 6.087 ± 2.245 mg O2 ml-1 g-1 min-1 under higher CO2 levels and
4.056 ± 0.819 mg O2 ml-1 g-1 min-1 under control. Nevertheless, no significant differences
were found between treatments (p > 0.05).
MMR (Fig.3.2b) of sharks exposed to higher CO2 levels were lower than those in the
control treatment, showing a lower oxygen consumption rate, although not statistically significant (p > 0.05). The mean of maximum oxygen consumption under control conditions was 111.22 ± 26.41 mg O2 ml-1 g-1 min-1, while under acidification was 52.04 ± 24.68 mg O2
ml-1 g-1 min-1. Similar to MMR, AS was 107,164 ± 26.088 mg O2 ml-1 g-1 min-1 and 45.96 ±
26.65 mg O2 ml-1 g-1 min-1, under control and acidification conditions, respectively (Fig.3.2c).
Although MO2 was higher in sharks under control conditions, after 30 minutes the recovery
11 ml-1 g-1 min-1 (control) and 0.029 ± 0.020 mg O
2 ml-1 g-1 min-1 (acidification). Between
treatments no significant results were found. However, there was a significant difference (p < 0.05) between times (Fig.3.2d).
a)
b)
12 d)
Figure 3.2. Effects of elevated pCO2 (Δ pCO2 ~ 500 µatm) on the: a) routine metabolic rate, b) maximum metabolic
rate, c) aerobic scope and d) recovery rate (mg O2 ml-1 g-1 min-1) of juvenile bamboo sharks (Chiloscyllium
plagiosum). Values represent mean ± SD. Control n = 5, High CO2 n = 5. More statistical details on Table 3.1.
3.4. Ventilation Rate
Considering pre-stress ventilation rates, no significant differences (p > 0.05) were found between control and high CO2 experimental treatments (Fig.3.3a). On the other hand, post-stress
ventilation rates had a significant difference (p < 0.05) (Fig.3.3b). Mean values ranged from 29.08 ± 1.50 breaths min-1 (pre-stress) to 80.20 ± 4.051 breaths min-1 (post-stress) for control
conditions, with 26.49 ± 1.97 breaths min-1and 66.6 ± 4.09 breaths min-1 for high CO
2 treatment.
13 *
* b)
Figure 3.3. Effects of elevated pCO2 (Δ pCO2 ~ 500 µatm) on the ventilation rates, i.e. number of breaths min-1: a)
ventilation rate (pre-stress), b) ventilation rate (post-stress), of juvenile bamboo sharks (Chiloscyllium plagiosum). Values represent mean ± SD. Asterisks represent significant differences between treatments. Control n = 5, High CO2
n = 5. More statistical details on Table 3.1.
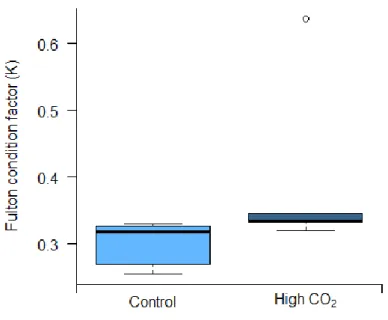
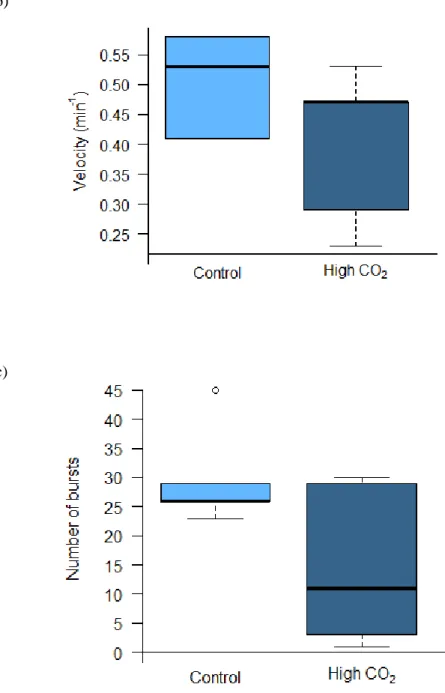
3.5. Swimming performance
Considering the swimming performance [(a) percentage of time swimming (Fig 3.4a), (b) maximum reached velocity (Fig 3.4b), (c) number of bursts (Fig.3.4c)] there were only significant differences (p < 0.05) for the percentage of time spent swimming. The mean value for the maximum reached velocity was 0.492 ± 0.097 in control conditions and 2.176 ± 10.160, the mean percentage of time in control was 11.982 ± 10.161 and 2.176 ± 1.737 in acidification conditions, at least for the number of bursts there is a mean of 29.80 ± 8.76 for control and 14.80 ± 13.93 under acidification.
14 b)
c)
Figure 3.4. Effects of elevated pCO2 (Δ pCO2 ~ 500 µatm) on the: a) percentage of time swimming; b) maximum
velocity, c) number of bursts of juvenile bamboo sharks (Chiloscyllium plagiosum). Asterisks represent significant differences between treatments. Values represent mean ± SD. Control n = 5, High CO2 n = 5. More statistical details
15
Table 3.1. Results of Generalized Linear Models (GLM) for fulton, routine metabolic rate (RMR), maximum
metabolic rate (MMR), aerobic scope (AS), pre-stress ventilation rates, post-stress ventilation rates, percentage of time spent swimming, maximum velocity reach and number of bursts.
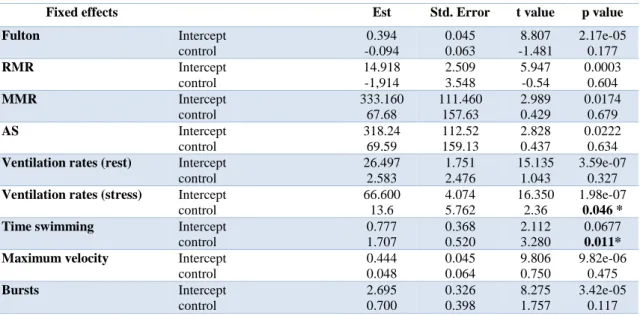
Fixed effects Est Std. Error t value p value
Fulton Intercept control 0.394 -0.094 0.045 0.063 8.807 -1.481 2.17e-05 0.177 RMR Intercept control 14.918 -1,914 2.509 3.548 5.947 -0.54 0.0003 0.604 MMR Intercept control 333.160 67.68 111.460 157.63 2.989 0.429 0.0174 0.679 AS Intercept control 318.24 69.59 112.52 159.13 2.828 0.437 0.0222 0.634
Ventilation rates (rest) Intercept control 26.497 2.583 1.751 2.476 15.135 1.043 3.59e-07 0.327
Ventilation rates (stress) Intercept control 66.600 13.6 4.074 5.762 16.350 2.36 1.98e-07 0.046 *
Time swimming Intercept
control 0.777 1.707 0.368 0.520 2.112 3.280 0.0677 0.011*
Maximum velocity Intercept
control 0.444 0.048 0.045 0.064 9.806 0.750 9.82e-06 0.475 Bursts Intercept control 2.695 0.700 0.326 0.398 8.275 1.757 3.42e-05 0.117
16
4. Discussion
In contrast with previous research with a closely related species (Chiloscyllium punctatum; Rosa et al., 2014b), all animals survived acclimation under both scenarios. A decrease in survival rates under OA-relevant CO2 levels has rarely been documented in fish
(Heuer & Grosell, 2014). Moreover, embryonic exposure to CO2 has been shown to be crucial
to a correct evaluation of the CO2 impacts over teleost fish survival (Baumann et al., 2012). In
this context, the different results obtained for such closely related species may indicate a similar phenomenon in sharks on which we advise further investigation. On the other hand, the pCO2
levels tested here correspond to a more conservative scenario of OA, possibly within the species’ tolerance limits. One should add that the OA-effects on survival have been previously shown to be exacerbated by increased temperatures (Rosa et al., 2014b) and both environmental stressors are expected to act concurrently in the near-future.
Fulton condition, often used as an indicator of the general health status of an organism (Nash et al., 2006), was also not affected by OA-conditions. Indeed, OA impacts over growth-related variables appear to be highly species-specific and dependent on developmental stage and exposure duration (Heuer & Grosell, 2014). Nonetheless, lack of OA-effects over growth-related variables has also been reported in C. punctatum (Rosa et al., 2014b), Scyliorhinus canicula (Green & Jutfelt, 2014) Heterodontus portusjacksoni (Pistevos et al., 2015), Hemiscyllium ocellatum (Johnson et al., 2016). However, despite not being affected by the sole action of high CO2, the Fulton condition of C. punctatum (Rosa et al., 2014b) was greatly
impacted by the combined action of high CO2 and temperature, further highlighting the
importance of multi-stressor studies. Similar results for single and concomitant action of OA and temperature were obtained in H. portusjacksoni fed ad libitum (Pistevos et al., 2015). However, the latter study also showed that the growth of animals reared in mesocosmos, where feeding was dependent on the organisms' hunting skills, was significantly impacted solely by the elevated CO2 levels, mediated by an impairment over their foraging behavior. Indeed, the
impact of environmental stressors is known to be highly context-dependent and the short-cuts often necessary for laboratorial simulations of environmental conditions undermine our ability to produce accurate predictions.
Sharks rely on their swimming performance to search for food, refuge and, particularly as juveniles, to escape predators (Di Santo, 2015). According to our results, sharks exposed to high CO2 conditions showed a reduction on the percentage of time spent swimming. Changes in
swimming behaviour have also been reported in a temperate adult catsharks. Green and Jutfelt, described a decrease in the number of swimming events for Scyliorhinus canicula (Green and Jutfelt, 2014). Notwithstanding, this is the first time an identical result is reported at an early developmental stage and in a tropical species. For this reason, we argue that OA-associated changes in swimming behaviour may extend beyond species, climatic region and life-stage. Elevated pCO2 environments cause pH changes in different body compartments disturbing the
acid-base balance of the organism (Pörtner, 2010). Since swimming is a highly energy-demanding ordeal (Lauder and Di Santo 2015) the energy it demands may be relocated to constant acid–base regulation from exposure to high CO2 (Pörtner et al., 2004), i.e. H+/Na+ and
Cl-/HCO
3- internal exchange (Green and Jutfelt, 2014). Hence, this result could be associated
with a decrease, at a certain point, of their aerobic capacity. The aerobic capacity of animals exposed to high CO2 can decrease (Heuer and Grosell, 2014), as a consequence of these
energetical shifts, since vital processes are prioritized (Heuer and Grosell, 2014) in relation to other energetic needs (Pörtner, 2008). Nonetheless, although sharks exposed to high CO2
17 significantly affected. Similar results have been reported for the epaulette shark (Hemiscyllium ocellatum; Hienrich et al., 2014), a closely related species, with a similar habitat and lifestyle. This is also consistent with the results obtained for the small-spotted catshark (Scyliorhinus canicula; Green & Jutfelt 2014), a temperate and phylogenetically distant species in which no OA-effects over metabolic rates, including aerobic scope, were observed. Moreover, it is noteworthy that the latter study focused on adult animals while we focused on juveniles and both rendered similar responses. One must also keep in mind that acidification is predicted to act along with warming in the future and a study with both stressors may have shown a different result. Indeed, in previous studies tackling both stressors, both embryonic and juvenile stages were affected by OA when in combination with warmer conditions (Rosa et al., 2014b; Pistevos et al., 2015).
Sharks, as osmoconformers, maintain their blood isotonic to their external environment and to compensate the acid-base disturbances caused by exposure to high CO2 they efficiently
transport ions across their gills (Pörtner 2005; Perry & Gilmour 2006; Ishimatsu et al., 2005; Ishimatsu et al., 2008) (Pörtner et al., 2005; Perry & Gilmour, 2006; Ishimatsu et al., 2005, 2008). Ventilation is mostly driven by water oxygenation, which is a limiting factor (Jouve-Duhamel & Truchot, 1983) and there has been growing proof of direct ventilation sensitivity to CO2 in elasmobranchs (Heisler et al., 1988; Burleson & Smatresk, 2000). Correspondingly, the
post-stress ventilation rates of sharks exposed to high CO2 were significantly lower. Ventilation
rates may be influenced by this disruption of the acid-base equilibrium (Heisler et al., 1988). Ultimately, if acid–base imbalance is not compensated properly it can lead to metabolic depression (Pörtner et al., 2004; Fabry et al., 2008). Even though there is no evidence of metabolic depression occurring neither in this species, nor in the closely related bamboo shark (Chiloscyllium punctatum) (Chapman and Renshaw, 2009) or any other species of shark (Renshaw, 2012).
5. Conclusion/ Future directions
Anthropogenic activity in the Indo-Pacific region has been inflicting pressure over this species, from habitat degradation to overfishing (Dulvy et al., 2014). As of now, this shark’s conservation status is of concern, assessed as Near Threatened (Kyne, 2015). OA may further increase the challenges faced by this species. Over the past few years, the number of studies about the direct impacts of OA in sharks has been increasing (see review Rosa et al., 2017; Green & Jutfelt 2014; Rosa et al., 2014b;). In fact, several studies on elasmobranchs have documented an array of physiological changes caused by exposure to OA-relevant levels of CO2
(Rosa et al., 2014b; Green & Jutfelt 20147). Nonetheless, we hypothesize that bamboo sharks may be resilience to OA, which can be related to the fact that they are ancient beings and had successfully already survived several periods of high CO2 (Compagno 1990). Nonetheless,
sharks rely on their swimming performance to search for food, refuge and, particularly as juveniles, to escape predators (Lauder and Di Santo 2015) and thus, even the seemingly negligible effects observed in this study have the potential to reduce the overall fitness of organisms with impacts over population dynamics. However, as relatively slow-living creatures, these animals may have some plasticity regarding small changes in their swimming behaviour. Future studies should also focus on pelagic species that greatly rely on their swimming performance. Moreover, specific dynamic action (SDA) should be measured to effectively evaluate metabolic rates, particularly those associated with vital processes such as digestion. Lastly, we reinforce the significance of multi-stressor studies, as the effects of OA might be increased when combined with other factors, such as the extreme temperatures (Pörtner, 2005) that are expected to occur in the future (IPCC, 2013).
18
6. References
Baum, J.K. & Worm, B., 2009. Cascading top-down effects of changing oceanic predator abundances. Journal of Animal Ecology, 78(4), pp.699–714.
Baumann, H., Talmage, S.C. & Gobler, C.J., 2012. Reduced early life growth and survival in a fish in direct response to increased carbon dioxide. Nature Climate Change, 2(1), pp.38– 41.
Brett, R.J., 1964. The respiratory metabolism and swimming performance of young sockeye salmon. Journal of the Fisheries Research Board of Canada, 21(5), pp.1183–1226.
Briffa, M., de la Haye, K. & Munday, P.L., 2012. High CO 2 and marine animal behaviour: potential mechanisms and ecological consequences. Marine Pollution Bulletin, 64(8), pp.1519–1528.
Brill, R.W., 1996. Selective Advantages Confered by the hight performance physiology of tunas, buillfish and dolphin fish Brill.pdf. Comp Biochem Physiol, 113A(1), pp.3–15. Burleson, M.L. & Smatresk, N.J., 2000. Branchial chemoreceptors mediate ventilatory
responses to hypercapnic acidosis in channel catfish. Comparative Biochemistry and Physiology - A Molecular and Integrative Physiology, 125(3), pp.403–414.
Caldeira, K., 2005. Ocean model predictions of chemistry changes from carbon dioxide emissions to the atmosphere and ocean. Journal of Geophysical Research, 110(C9), p.C09S04. Available at: http://doi.wiley.com/10.1029/2004JC002671.
Caldeira, K. & Wickett, M.E., 2003. Oceanography: anthropogenic carbon and ocean pH. Nature, 425(6956), p.365.
Cameron, J.N. & Iwama, G.K., 1989. Compromises between ionic regulation and acid–base regulation in aquatic animals. Canadian Journal of Zoology, 67(12), pp.3078–3084. Carlson, J., Goldman, K. & Lowe, C., 2004. Metabolism, Energetic Demand, and Endothermy. ,
(March), pp.203–224. Available at:
http://www.crcnetbase.com/doi/abs/10.1201/9780203491317.ch7.
Chen, W.K. et al., 2007. Age and growth estimates of the whitespotted bamboo shark, Chiloscyllium plagiosum, in the northern waters of Taiwan. Zoological Studies, 46(1), pp.92–102.
Chin, A. et al., 2010. An integrated risk assessment for climate change: Analysing the vulnerability of sharks and rays on Australia’s Great Barrier Reef. Global Change Biology, 16(7), pp.1936–1953.
Claireaux, G. & Chabot, D., 2016. Responses by fishes to environmental hypoxia: Integration through Fry’s concept of aerobic metabolic scope. Journal of Fish Biology, 88(1), pp.232– 251.
Clark, T.D., Sandblom, E. & Jutfelt, F., 2013. Aerobic scope measurements of fishes in an era of climate change: respirometry, relevance and recommendations. Journal of Experimental Biology, 216(15), pp.2771–2782. Available at: http://jeb.biologists.org/cgi/doi/10.1242/jeb.084251.
Clinton E. Brown, B.S.M., 1970. of Fish Gills with Analysis of Rarn Yentilation ( Katsuuonus pelarnis ) l Tuna Applicaiion to Skipjack I l , l t t . n r - S . l l u t u. , (198).
Compagno, L.J. V, 1990. Alternative life-history styles of cartilaginous fishes in time and space. Environmental Biology of Fishes, 28(1–4), pp.33–75.
Compagno, L.J. V, 2001. Sharks of the World. An annotated and illustrated catalogue of Shark species known to date - Volume 2. Bullhead, mackerel and carpet sharks (Heterodontformes, Lamniformes and Orectolobiformes). FAO Species Catalogue for Fishery Purposes, 2(1), p.269. Available at: http://www.vliz.be/imis/imis.php?module=ref&.
Cortés, E., 2000. Life History Patterns and Correlations in Sharks. Reviews in Fisheries Science, 8(4), pp.299–344.
Dill, L.M. et al., 2003. Behaviorally Mediated Indirect Interactions in Marine Communities and Their Conservation Implications. Ecology, 84(5), pp.1151–1157.
Dixson, D.L. et al., 2015. Odor tracking in sharks is reduced under future ocean acidification conditions. Global Change Biology, 21(4), pp.1454–1462.
19 Dixson, D.L., Munday, P.L. & Jones, G.P., 2010. Ocean acidification disrupts the innate ability
of fish to detect predator olfactory cues. Ecology Letters, 13(1), pp.68–75.
Domenici, P. et al., 2011. Elevated carbon dioxide affects behavioural lateralization in a coral reef fish. Biology Letters, p.rsbl20110591.
Doney, S.C. & Schimel, D.S., 2007. Carbon and Climate System Coupling on Timescales from the Precambrian to the Anthropocene. Annual Review of Environment and Resources,
32(1), pp.31–66. Available at:
http://www.annualreviews.org/doi/10.1146/annurev.energy.32.041706.124700.
Dulvy, N.K. et al., 2014. Extinction risk and conservation of the world’s sharks and rays. eLife, 2014(3), pp.1–34.
Dupont, S.T. & Thorndyke, M.S., 2014. Direct impacts of near-future OA on Sea Urchins. , (January).
Fabry, V.J. et al., 2008. Impacts of ocean acidification on marine fauna and ecosystem processes. ICES Journal of Marine Science, 65(Dic), pp.414–432.
Feely, R.A., Doney, S.C. & Cooley, S.R., 2009. Ocean Acidification. Oceanography, 22(4), pp.36–47.
Ferrari, M.C.O. et al., 2011. Putting prey and predator into the CO2 equation–qualitative and quantitative effects of ocean acidification on predator–prey interactions. Ecology letters, 14(11), pp.1143–1148.
Frederich, M. & Pörtner, H.O., 2000. Oxygen limitation of thermal tolerance defined by cardiac and ventilatory performance in spider crab, Maja squinado. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology, 279(5), pp.R1531– R1538. Available at: http://ajpregu.physiology.org/content/279/5/R1531.abstract.
Gattuso, J.P. et al., 2015. Contrasting futures for ocean and society from different anthropogenic CO<inf>2</inf> emissions scenarios. Science, 349(6243).
Green, L. & Jutfelt, F., 2014. Elevated carbon dioxide alters the plasma composition and behaviour of a shark. Biology Letters, 10(9), pp.20140538–20140538. Available at: http://rsbl.royalsocietypublishing.org/cgi/doi/10.1098/rsbl.2014.0538.
Grogan, E.D. & Lund, R., 2004. The origin and relationships of early chondrichthyes. Biology of Sharks and their Relatives, (January), pp.3–31.
Guppy, M. & Withers, P., 1999. Metabolic depression in animals: Physiological perspectives and biochemical generalizations. Biological Reviews, 74(1), pp.1–40.
Heinrich, D.D.U. et al., 2014. A product of its environment: The epaulette shark (Hemiscyllium ocellatum) exhibits physiological tolerance to elevated environmental CO2. Conservation Physiology, 2(1), pp.1–12.
Heinrich, D.D.U. et al., 2015. Foraging behaviour of the epaulette shark Hemiscyllium ocellatum is not affected by elevated CO2. ICES Journal of Marine Science, 73(3).
Heisler, N., Toews, D.P. & Holeton, G.F., 1988. Regulation of ventilation and acid-base status in the elasmobranch Scyliorhinus stellaris during hyperoxia-induced hypercapnia. Respiration Physiology, 71(2), pp.227–246.
Heithaus, M.R. et al., 2008. Predicting ecological consequences of marine top predator declines. Trends in Ecology and Evolution, 23(4), pp.202–210.
Heuer, R.M. & Grosell, M., 2014. Physiological impacts of elevated carbon dioxide and ocean acidification on fish. AJP: Regulatory, Integrative and Comparative Physiology, 307(9),
pp.R1061–R1084. Available at:
http://ajpregu.physiology.org/cgi/doi/10.1152/ajpregu.00064.2014. IPCC, 2013. CLIMATE CHANGE 2013 The Physical Science Basis.
IPCC, Climate Change 2014: Synthesis Report, 2014.doi:10.1017/CBO9781107415324
Ishimatsu, A. et al., 2005. Physiological effects on fishes in a high-CO2 world. Journal of Geophysical Research C: Oceans, 110(9), pp.1–8.
Ishimatsu, A., Hayashi, M. & Kikkawa, T., 2008. Fishes in high-CO2, acidified oceans. Marine Ecology Progress Series, 373(1), pp.295–302.
Johnson, M.S. et al., 2016. Will ocean acidification affect the early ontogeny of a tropical oviparous elasmobranch (Hemiscyllium ocellatum)? Conservation Physiology, 4(1), p.cow003.
20 Jouve-Duhamel, A. & Truchot, J.P., 1983. Ventilation in the shore crab Carcinus maenas (L.) as a function of ambient oxygen and carbon dioxide: Field and laboratory studies. Journal of Experimental Marine Biology and Ecology, 70(3), pp.281–296.
Jutfelt, F. et al., 2013. Behavioural disturbances in a temperate fish exposed to sustained high-CO 2 levels. PloS one, 8(6), p.e65825.
Kleypas, J. et al., 2006. Impacts of Ocean Acidification on Coral Reefs and Other Marine Calcifiers : A Guide for Future Research. Report of a workshop held 18-20 April 2005, St. Petersburg FL, sponsored by NSF, NOAA and the U.S. Geological Survey, 18(January), p.88.
Kriwet, J. et al., 2008. First direct evidence of a vertebrate three-level trophic chain in the fossil record. Proceedings. Biological sciences / The Royal Society, 275(1631), pp.181–186. Kroeker, K.J. et al., 2010. Meta-analysis reveals negative yet variable effects of ocean
acidification on marine organisms. Ecology Letters, 13(11), pp.1419–1434. Kyne, A., 2015. Chiloscyllium plagiosum , Whitespotted Bamboo Shark. , 8235.
Lai, F., Jutfelt, F. & Nilsson, G.E., 2015. Altered neurotransmitter function in CO2-exposed stickleback (Gasterosteus aculeatus): a temperate model species for ocean acidification research. Conservation Physiology, 3(1), p.cov018.
Lauder, G. V, 2016. OF ELASMOBRANCH FISHES,
Maia, A. & Wilga, C.D., 2013. Function of dorsal fins in bamboo shark during steady swimming. Zoology (Jena, Germany), 116(4), pp.224–231. Available at: http://www.ncbi.nlm.nih.gov/pubmed/23830781.
Melzner, F. et al., 2009. Physiological basis for high CO 2 tolerance in marine ectothermic animals: pre-adaptation through lifestyle and ontogeny? Biogeosciences, 6(10), pp.2313– 2331.
Michaelidis, B., Spring, A. & Pörtner, H.O., 2007. Effects of long-term acclimation to environmental hypercapnia on extracellular acid-base status and metabolic capacity in Mediterranean fish Sparus aurata. Marine Biology, 150(6), pp.1417–1429.
Munday, P.L. et al., 2010. Replenishment of fish populations is threatened by ocean acidification. Proceedings of the National Academy of Sciences, 107(29), pp.12930– 12934.
Munday, P.L., Crawley, N.E. & Nilsson, G.E., 2009. Interacting effects of elevated temperature and ocean acidification on the aerobic performance of coral reef fishes. Marine Ecology Progress Series, 388, pp.235–242.
Nash, R.D.M. et al., 2006. The Origin of Fulton’s Condition Factor — Setting the Record Straight. Fisheries, 31(5), pp.236–238.
Nilsson, G.E. et al., 2012. Near-future carbon dioxide levels alter fish behaviour by interfering with neurotransmitter function. Nature Climate Change, 2(3), pp.201–204.
Norin, T. & Clark, T.D., 2016. Measurement and relevance of maximum metabolic rate in fishes. Journal of Fish Biology, 88(1), pp.122–151.
Perry, S.F. & Gilmour, K.M., 2006. Acid-base balance and CO2excretion in fish: Unanswered questions and emerging models. Respiratory Physiology and Neurobiology, 154(1–2), pp.199–215.
Petit, J.R. et al., 1999. Climate and atmospheric history of the past 420,000 years from the Vostok ice core, Antarctica. Nature, 399(6735), pp.429–436.
Pimentel, M. et al., 2014. Impact of ocean acidification in the metabolism and swimming behavior of the dolphinfish (Coryphaena hippurus) early larvae. Marine Biology, 161(3), pp.725–729.
Pistevos, J.C.A. et al., 2016. Antagonistic effects of ocean acidification and warming on hunting sharks. Oikos, (7491), pp.0–2.
Pistevos, J.C.A. et al., 2015. Ocean acidification and global warming impair shark hunting behaviour and growth. Scientific Reports, 5, pp.1–10. Available at: http://dx.doi.org/10.1038/srep16293.
Pörtner, H.O., 2002. Climate variations and the physiological basis of temperature dependent biogeography: Systemic to molecular hierarchy of thermal tolerance in animals. Comparative Biochemistry and Physiology - A Molecular and Integrative Physiology,
![Figure 1.2. Chiloscyllium plagiosum (Anonymous [Bennett], 1830)](https://thumb-eu.123doks.com/thumbv2/123dok_br/15258030.1025068/14.892.302.584.129.303/figure-chiloscyllium-plagiosum-anonymous-bennett.webp)