AGUIDA MARIA ALVES PEREIRA MORALES
CARACTERIZAÇÃO MOLECULAR DA RESISTÊNCIA À FERRUGEM ASIÁTICA DA SOJA MEDIADA PELO GENE Rpp4
Tese apresentada à Universidade Federal de Viçosa, como parte das exigências do Programa de Pós-Graduação em Genética e Melhoramento, para obtenção do título de Doctor Scientiae.
VIÇOSA
“A fé em Deus nos faz crer no incrível, ver o invisível e realizar o impossível”
À minha grande amiga Selma Pereira dos Santos (in memoriam), que sempre será meu exemplo de coragem e determinação. Sua dedicação pela ciência ficará sempre em minha memória. Saudades...
Dedico.
Ao meu esposo Alan Alves Pereira. Obrigada por estar sempre presente na minha vida. Te amo!
Agradecimentos
A Deus por ter me dado tudo que eu tenho, e por estar sempre presente na minha vida.
Aos meus pais Vivaldo e Elizabeth por sempre me apoiarem. Vocês foram fundamentais.
Aos meus irmãos Kiko, Vivaldo e Ana Paula muitas saudades da convivência com vocês. Amo vocês demais.
Aos meus sobrinhos que enchem minha vida de alegria, Gabriel, Isabela e Julinha.
Às minhas amigas Amaralina e Dani, obrigada por sempre estarem presentes, pelos bons momentos no Skype durante minha estadia nos EUA.
Ao meu avô Manoel pelo exemplo a ser seguido. Te amo muito.
Aos meus avós Anália e Francisco, aqueles que deram o passo inicial, meus sinceros agradecimentos. Nada disso teria acontecido sem a ajuda de vocês! Meu muito obrigada!
Ao Paulo e Théa, obrigada por tudo. Vocês tornaram a nossa estadia em Viçosa mais “bela”.
À Eva muito obrigada pela amizade.
Ao Dr. Aluízio Borém, muito obrigada pela amizade e confiança. Você será sempre meu exemplo a ser seguido. Quero ser igual à você quando eu crescer!!!
Ao Dr. Ricardo Vilela Abdelnoor meu muito obrigado por ter me dado a oportunidade de trabalhar em um renomado laboratório de Biotecnologia Vegetal, e muito obrigada pela amizade.
À Dra. Michelle Graham meus sinceros agradecidos. Muito obrigada por me fazer acreditar na pesquisa cada dia mais. Sua paixão pelo seu trabalho me contagia. I miss you so much!!!
Ao Dr. Marcelo Ehlers Loureiro por ter aberto as portas de seu laboratório onde eu pude conviver com pessoas que levarei para a minha vida toda.
À Edna e Rita, secretárias da pós graduação, por sempre estarem abertas a me ajudar.
Aos meus amigos do laboratório de Fisiologia Molecular de Plantas – UFV, Sabrina (minha Nurse querida), Rose e Mercês, meu muito obrigada.
Aos meus amigos Aleson e Gustavo, saudades infinitas de nossas conversas na escada do lab. Com vocês por perto tudo é muito mais fashion!!!
À minha grande amiga ermã Viviane e ao amigo Everton, meu muito obrigada por tudo. Obrigada pelo ombro amigo. Tenho certeza que Deus colocou vocês na minha vida.
Às minhas grandes amigas e companheiras de estatística, Daniele, Jaque, Juliana e Lorêta. Foi muito bom nosso tempo de estudo. Vocês são um orgulho para mim. Sucesso sempre e saudades meninas.
Aos meus amigos do laboratório de Genética de Soja do USDA em Ames, Lori, Brian e Christie. It was really funny work with you guys!!! I hope to see you soon. Thanks.
Aos meus amigos de Ames que tornaram nossa estadia nos EUA muito mais fácil, Juliana, Nick, Victor, Carine, Dani, Silvia, Jeff, Camila, Bruna, Monica, Aldane, Ângela, Bruno, Jana, Karla, Laura, Lucas, Marianna, Mônica, Paulo, Alissa e Fran. Obrigado pelos momentos de descontração, amizade, festas e churrascos. Aos analistas e funcionários do laboratório de Biotecnologia Vegetal da Embrapa Soja, Silvana, Danielle, Márcia, César, Renan e Verinha, meu muito obrigada pela ajuda e amizade.
Aos meus amigos do laboratório de Biotecnologia Vegetal da Embrapa Soja, Adriana, Mayra, Renata, Fabiana, Lizandra, Noelle, Valéria (feia), Tati (monster), Michelle, Idenize, Cynara, Euziane, Gislaine, Larissa, Ciça, Patrícia, Juliane, Juliana , Ju, André, João Vitor, Lucas, Rodrigo, Mayla, Kleber e Cibele, é muito bom trabalhar com vocês.
Aos amigos Salvador, Lívia, Elton, Renata por fazerem parte do trio fantástico, obrigada pelos momentos de descontração, jantinhas e pela grande amizade...
Biografia
Sumário
Resumo ... ix
Abstract ... xi
Review: Advances on molecular studies of the interaction soybean - Asian rust .... 1
Abstract ... 1
Asian Soybean Rust ... 3
Molecular interaction between soybean and ASR ... 5
References ... 9
Combining Transcriptome Analyses and Virus Induced Gene Silencing to Identify Genes in the Rpp4-mediated Asian Soybean Rust Resistance Pathway. ... 14
Abstract ... 14
1. Introduction ... 16
2. Materials and methods ... 19
2.1. Silencing of Rpp4 via virus induced gene silencing ... 19
2.2. RNA extraction and isolation ... 19
2.3. Microarray analyses ... 20
2.4. Statistical analysis and array processing ... 20
2.5. Annotation of differentially expressed probes ... 20
2.6. Bioinformatics analysis of cis-elements ... 21
3. Results ... 22
3.1. Comparisons of gene expression in PI459025B in Rpp4 silenced plants and empty vec tor treated plants, each inoculated with P. pachyrhizi. ... 22
3.2. Gene Expression from Rpp4 silenced plants ... 22
3.3. Bioinformatics analysis of cis-elements ... 29
3.4. MEME and MAST analysis ... 32
3.5. Unique genes identified in microarray Rpp4 silenced plants. ... 34
4. Discussion ... 36
5. Conclusion ... 40
6. Acknowledgments ... 40
Expression Analyses of Candidate Resistance Genes in the Rpp4 Asian Soybean
Rust Resistance Locus ... 49
Abstract ... 49
1. Introduction ... 50
2. Materials and methods ... 51
2.1. Pathogen isolation and plant inoculation ... 51
2.2. RNA extraction, isolation and DNase-treatment. ... 52
2.3. Efficiency curve ... 52
2.4. Relative quantification of candidate R-genes in the Rpp4 locus ... 53
2.5. Analysis of Alternative Splicing ... 56
3. Results ... 56
4. Discussion ... 63
5. Conclusions ... 66
6. Acknowledgments ... 66
Resumo
MORALES, Aguida Maria Alves Pereira, D.Sc., Universidade Federal de Viçosa, Julho de 2011. Caracterização molecular da resistência à ferrugem asiática da soja mediada pelo gene Rpp4. Orientador: Aluízio Borém de Oliveira. Co-orientadores: Ricardo Vilela Abdelnoor e Ney Sussumu Sakiyama.
Abstract
MORALES, Aguida Maria Alves Pereira, D.Sc., Universidade Federal de Viçosa, July, 2011. Molecular characterization of resistance to Asian soybean rust mediated by Rpp4. Adviser: Aluízio Borém de Oliveira. Co-advisers: Ricardo Vilela Abdelnoor and Ney Sussumu Sakiyama.
significantly over represented in our differentially expressed genes when compared to all genes in the soybean genome, several with known roles in defense. Finally, to elucidate which genes are exclusively related to Rpp4-mediated signaling, we have compared the results of our experiment with microarray results from Rpp2, Rpp3 and Rpp4 resistant and susceptible reactions. We have identified 101 genes unique to the Rpp4-signaling pathway. In addition, in order to obtain more information about Rpp4 function, we used real time quantitative PCR (RT-qPCR) to analyze the expression of all Rpp4 genes in different plant tissues, in different stages of development and after inoculation with P. pachyrhizi. We have developed a single pair of primers from the NBD domain that allow us to monitor the expression of all ten genes. Direct sequencing of the RT-qPCR product differentiates between the ten genes. In addition we examined the occurrence of alternative splice Rpp4 gene under inoculation effect.
Chapter I
Review: Advances on molecular studies of the interaction soybean - Asian rust
Revisão: Avanços dos estudos moleculares da interação da soja - ferrugem asiática
Abstract
Effective management practices are essential for controlling rust outbreaks. The main control method used is the application of fungicides, which substantially increase the cost of production and are harmful to the environment. Prevention is still the best way to avoid more significant losses in soybean production. Alternatives, such as planting resistant varieties to the fungus, are also very important. The use of resistant or tolerant varieties is the most promising method for control of Asian soybean rust. Recently, five single dominant genes to specific soybean rust isolates were described; Rpp1, Rpp2, Rpp3, Rpp4 and Rpp5. However, little is known about the molecular interaction between soybean and soybean rust and on the molecular pathway triggered by pathogen recognition. Understanding the molecular mechanisms involved in defense responses is of primary importance in planning strategies for controlling stress and consequently increasing plant adaptation to limiting conditions.
Resumo
Práticas efetivas são necessárias para o controle da ferrugem. O principal método de controle utilizado é a aplicação de fungicidas, o que aumentará substancialmente o custo de produção e são prejudiciais ao meio ambiente. A prevenção ainda é a melhor maneira de evitar mais perdas significativas na produção de soja. Alternativas, como o plantio de variedades resistentes ao fungo, também são importantes. O uso de variedades resistentes ou tolerantes é o método mais promissor para o controle da ferrugem asiática da soja. Recentemente, cinco genes de resistência a ferrugem da soja foram descritos Rpp1, Rpp2, Rpp3, Rpp4 e Rpp5. No entanto, pouco se sabe sobre a interação molecular entre a planta de soja e o fungo da ferrugem asiática e as rotas desencadeadas na planta pelo reconhecimento do patógeno. Compreender os mecanismos moleculares envolvidos nas respostas de defesa é de primordial importância no planejamento de estratégias para controle do estresse e, consequentemente, para aumentar a adaptação das plantas a condições limitantes.
Asian Soybean Rust
Asian Soybean Rust (ASR) is caused by Phakopsora pachyrhizi Syd. & Syd; uredial anamorph; Malupa sojae (syn. Uredo sojae); Domain Eukaryota; Kingdom Fungi; Phylum Basidiomycota; Order Uredinales; Class Urediniomycetes; Family Phakopsoraceae; Genus Phakopsora (Index Fungorum 2010). Rust is considered a polycyclic disease. The fungus is able to complete several generations in a single cycle of the host. Temperatures and humidity that favor the growth and development of soybean plants also favor the development of rust (Zambolim 2006). According to Freire et al. (2008) the South and North American continents were free of P. pachyrhizi until 2001. Then P. pachyrhizi was first reported in Paraguay (Morel and Yorinori 2002), and became established in Bolivia, Argentina (Rossi 2003) and Brazil (Yorinori et al. 2005) in 2002/2003. In 2004, ASR was reported for the first time in the USA (Schneider et al. 2005). These authors estimated that the disease caused yield losses varying from 10 to 80%.
According to Ono et al. (1992) P. pachyrhizi and Phakopsora meibomiae, the American rust, have wide host ranges and are able to sporulate on 31 species in 17 genera of leguminous plants. Rust samples taken from wild host plants are able to infect a broad range of plant species in greenhouse environments (Jarvie 2009). Recently, new host species from 25 genera were identified in greenhouse evaluations, including 12 genera that had not been reported previously (Slaminko et al. 2008). The presence of a susceptible host, viable pathogen spores and suitable environmental conditions are prerequisites for the development of a soybean rust epidemic. The optimum temperature for urediniospore germination ranges between 12 and 27°C. Urediniospore germination is greater in darkness and requires a period of leaf moisture. Germination takes about 6 hours in optimum temperature and moisture conditions (Kochman 1977).
increases with lesion age and groups of spores (urediniospores) are expelled from each pustule (uredinia) through a central pore (Sinclair 1989).
The disease destroys leaf tissue resulting in reduced photosynthetic activity, premature defoliation and reduced life cycle. In addition, the premature leaf abscission prevents grain maturation (Sinclair 1989) and rust infection during pod formation or seed fill can cause embryo abortion and pod abscission (Yorinori et al. 2005). The cumulative effect of rust on production translates into lower seed weight and reduces the number of pods and seeds (Sinclair 1989).
P. pachyrhizi forms asexual uredospores on short stalks within a uredium 5-8 days after inoculation on colonized leaves. Uredospores are released from uredia through an ostiole and dispersed by wind. Under appropriate conditions, uredospores germinate a single germ tube and the penetration occurs directly thought epidermis, but can also occur through stomatal openings (Zambolim 2006). Penetration by P. pachyrhizi starts with the formation of a funnel-shaped structure, termed the appressorial cone, within the appressorium. This cone is contiguous with the cell wall of the penetration hypha, which is also referred to as the transepidermal vesicle. On penetration, the epidermal cells collapse, become disorganized and show signs of cell death (Panstruga 2003, Mendgen et al. 2006). After penetration, the hypha grows through the epidermal cell and reaches the intercellular space. The primary hypha may branch to form secondary hypha and finally, haustorium mother cells differentiate in close contact with mesophyll cells. The haustorium provides a wide contact surface within the host cell for acquisition of sugars and amino acids through a symport proton gradient (Mendgen et al. 2006).
Prevention is still the best way to avoid more significant losses in soybean production. One method is to offset the timing of soybean production and pods reach maturity in condition that do not favor P. pachyrhizi. In addition, lowering inoculum levels by implementation of a soybean-free period is important. Alternatives such as using resistant varieties to the fungus also are important. However, resistance does not mean that the disease does not occur, but it allows greater stability and efficiency of chemical control (Anuário Brasileiro de Soja 2009). Recently soybean cultivars resistant to the fungus were released in Brazil. These varieties boast characteristics that curb fungal growth and ensure higher production stability, reducing the losses induced by the disease, and the environmental impacts caused by repeated fungicide applications.
Molecular interaction between soybean and ASR
Immunity to P. pachyrhizi occurs when no visual lesions are produced by the soybean plant. A resistant response leads to the formation of RB lesions indicating a hypersensitive reaction. A susceptible response occurs when TAN lesions develop indicating fungal growth and development. The genetics of resistance of five single dominant genes to specific soybean rust isolates has been described: Rpp1, Rpp2, Rpp3, Rpp4 and Rpp5 (Bromfield and Hartwig 1980, Mclean and Bith 1980, Hartwig and Bromfield 1983, Hartwig 1986, Garcia et al. 2008).
In order to identify new sources of resistance in soybean, Miles et al. (2006) evaluated the entire United States Department of Agriculture (USDA) germplasm collection (16,000 accessions) against a mixture of five P. pachyrhizi isolates. After two rounds of evaluation, only 850 accessions were identified with even partial tolerance or resistance reactions to P. pachyrhizi, which correlates to less than 5% of USDA germplasm collection.
pachyrhizi broke the resistance conferred by genes Rpp1, and Rpp3, while Rpp2, Rpp4 and Rpp5 remain resistant (Arias et al. 2004).
Although Rpp2- and Rpp4-mediated resistances have been stable in Brazil (Hartman et al. 2005), single, dominantly inherited R gene-mediated resistance against P. pachyrhizi has been overcome in nature several times because of the great capacity of the fungus to develop new races. Generally, this scenario of the breakdown of R gene-mediated resistance is known as the ‘boom and bust’ syndrome. In addition to pyramiding known Rpp resistance genes into modern cultivars to create a more durable and broad-spectrum disease resistance, the recruitment of novel sources of resistance to P. pachyrhizi is desirable (Goellner et al. 2010).
Along with single gene resistance, partial resistance to soybean rust has been described (Hartman et al. 2005). This kind of resistance may be controlled by minor genes and may be expressed as reduced uredinial number and size, a longer latent period, and other components related to fungal reproduction. Recently, the average number of uredinia per lesion and average uredinial diameter were reported to be components of partial resistance in soybean rust and were a reflection of fungal growth in the host tissue (Bonde et al. 2006).
All described Rpp genes have been already mapped on soybean chromosomes (Chr), Rpp1 was mapped on chromosome 18, Rpp2 on Chr 16, Rpp3 on Chr 6, Rpp4 on Chr 18 and Rpp5 on Chr 3, (Garcia et al. 2008, Hyten et al. 2007, Hyten et al. 2009, Silva et al. 2008b) Additionally, some alleles have been mapped to the same chromosomes, Rpp1b was mapped on Chr 18, Rpp? Hyuuga on Chr 6 (Chakraborty et al. 2009, Monteros et al. 2007)
(Virus-induced gene silencing) demonstrated that silencing of the Rpp4 candidate genes diminished resistance in PI459025B (that carries Rpp4 resistance allele), confirming that one of the genes in the cluster is responsible for resistance.
There is clear evidence of the evolutionary forces acting on the Rpp4 locus. Differences in gene number between Wm82 and PI459025B are likely due to duplication or unequal recombination. In addition, given the similarity of all Rpp4 candidate genes between genotypes, it is possible that small amino acids differences may play a key role in resistance (Meyer et al. 2009).
Little is known about the molecular interaction between soybean and P. pachyrhizi and the defense pathways triggered by pathogen recognition. Understanding the molecular mechanisms involved in defense responses is of primary importance in planning strategies for controlling stress and consequently to increase plant adaptation to limiting conditions. The development of sequencing techniques and gene expression analysis on a large scale, combined with novel bioinformatics tools for data analysis have facilitated the structuring of extremely valuable databases for developing strategies for genetic engineering.
the second response likely relates to R-gene detection of P. pachyrhizi (Posada-Buitrago and Frederick 2005, Tremblay et al. 2009).
In a similar approach, Panthee et al. (2007) identified genes that might be involved in a defense response against P. pachyrhizi by susceptible soybean cv.5601 plants 72h after infection (hai) using microarray. Most of the induced genes had defense and stress related functions such as genes encoding an SA-related protein, heat shock protein (HSP), leaf senescence-associated receptor like kinase, and chalcone synthase. Silva et al. (2008a) identified genes activated during resistant and susceptible interactions with the P. pachyrhizi in soybean (PI230970- Rpp2 resistance). By analysis of cDNA microarrays, they identified 65 transcripts differentially expressed. These genes were involved in the production of reactive oxygen species, phytoalexins and antimicrobial proteins, cell death and senescence, modification, stabilization and protein degradation, control of gene expression and reinforcement of cell wall.
Recently, Pandey et al. (2011) combined the work of Van de Mortel et al. (2007) with VIGS, to screen 140 candidate genes that might play a role in Rpp2-mediated resistance toward P. pachyrhizi. This study identified 11 genes that compromised Rpp2-mediated resistance when silenced, including GmEDS1, GmNPR1, GmPAD4, GmPAL1, five predicted transcription factors, an O-methyl transferase, and a cytochrome P450 monooxygenase. Additionally, a large scale transcript profiling approach conducted with soybean plants (accession PI200492) has revealed an up regulation in gene expression for lipoxygenases and peroxidases in an incompatible interaction, suggesting an important function for these genes in Rpp1-mediated resistance (Choi et al. 2008).
similarly (E-value < 10-5) to sequences deposited in the NCBI non-redundant protein database. These genes were assigned putative roles in primary metabolism, gene and protein expression, cell structure and growth, cell division, cell signaling and cell communication (Posada-Buitrago and Frederick 2005). Recently a cDNA library was constructed from uredinia separated from host tissue by laser-captured microdissection (Tremblay et al. 2009). About 80% of identified genes in this study shared no homology to previously described Phakopsora genes. This result demonstrates stage-specific gene expression in the development of uredinia.
While the techniques have proven effective at looking at genes downstream of Rpp genes, more research is needed to identify potential candidate genes that could be used to engineer sustainable resistance into soybean against P. pachyrhizi.
References
Anuário Brasileiro de Soja (2009) Editora Gazeta, Santa Cruz do Sul, 127p.
Arias CA, Ribeiro AS, Yorinori JT, Brogin RL, Oliveira MF, Toledo JFF (2004) Inheritance of resistance of soybean to rust (Phakospora pachyrhizi sidow). In: World soybean research conference. Foz do Iguaçu, p.100.
Bonde MR, Nester SE, Austin CN, Stone CL, Frederick RD, Hartman GL, Miles MR (2006) Evaluation of virulence of Phakopsora pachyrhizi and P. meibomiae isolates. Plant Disease 90: 708-716.
Bromfield KR and Hartwig EE (1980) Resistance to soybean rust and mode of inheritance. Crop Science 20: 254-255.
Chakraborty N, Curley J, Frederick RD, Hyten, DL, Nelson RL, Hartman GL, Diers, B (2009) Mapping and Confirmation of a New Allele at Rpp1 from Soybean PI 594538A Conferring RB Lesion–Type Resistance to Soybean Rust. Crop Science 49: 783-790.
Freire MCM, de Oliveira LO, de Almeida AMR, Schuster I, Moreira MA, Liebenberg MM, Mienie CMS (2008) Evolutionary history of Phakopsora pachyrhizi (the Asian soybean rust) in Brazil based on nucleotide sequences of the internal transcribed spacer region of the nuclear ribosomal DNA. Genetics and Molecular Biology 31: 920-931.
Garcia A, Calvo É, Souza Kiihl R, Harada A, Hiromoto D, Vieira L (2008) Molecular mapping of soybean rust (Phakopsora pachyrhizi) resistance genes: discovery of a novel locus and alleles. Theoretical and Applied Genetics 117: 545-553. Goellner K, Loehrer Langenback C, Conrath U.; Akoch EC, Schaffrat HU (2010)
Phakopsora pachyrhizi, the causal agent of Asian soybean rust. Molecular Plant Pathology 2:169–177.
Hartman GL, Miles MR and Frederick RD (2005) Breeding for resistance to soybean rust. Plant Disease 89: 664-666.
Hartwig EE (1986) Identification of a fourth major gene conferring resistance to soybean rust. Crop Science 26: 1135-1136.
Hartwig EE and Bromfield KR (1983) Relationships among 3 genes conferring specific resistance to rust in soybeans. Crop. Science 23: 237-239.
Hyten DL, Hartman GL, Nelson RL, Frederick RD, Concibido VC, Narvel JM, Cregan PB (2007) Map location of the Rpp1 locus that confers resistance to soybean rust in soybean. Crop Science 47: 837-838.
Hyten DL, Smith JR, Frederick RD, Tuchekr ML, Song Q, Cregan PB (2009) Bulked segregant analysis using the GoldenGate assay to locate the Rpp3 locus that confers resistance to soybean rust in soybean. Crop Science 49: 265-271.
Index Fungorum (2010) Available at http://data.gbif.org/datasets/resource/1752. Accessed on September 11, 2010.
Jarvie JA (2009) A review of soybean rust from a South African perspective. South African Journal of Science 105: 103-108.
Kochman JK (1977) Soybean rust in Australia. In: Ford RE, Sinclair JB (eds.) Rust of soybean-The problem and research needs. International Agricultural Publications, Manila, p. 44-48.
Laperuta LDC, Arias CAA, Ribeiro AS, Rachid BF, Pierozzi PHB, Toledo JFF, Pípolo AE, Carneiro GED (2008) New Genes Conferring Resistance to Asian Soybean Rust: Allelic Testing for the Rpp2 and Rpp4 Loci. Pesquisa Agropecuária Brasileira 43: 1741-1747.
Mackey D and McFall AJ (2006) MAMPs and MIMPs: proposed classifications for inducers of innate immunity. Molecular Microbiology 61: 1365-1371.
McLean RJ and Byth D (1980) Inheritance of resistance to rust (Phakopsora pachyrhizi) in soybean. Australian Journal of Agricultural Research 31: 951-956.
Mendgen K, Krieg PA, Rebagliati MR, Maniatis T, Zinn K, Green MR (2006) Volatiles modulate the development of plant pathogenic rust fungi. Planta 224: 1353-1361.
Meyer JDF, Silva DCG, Yang C, Pedley KF, Zhang C, Van de Mortel M, Hill JH, Shoemaker RC, Abdelnoor RV, Whitham SA, Graham MA (2009) Identification and analyses of candidate genes for Rpp4-mediated resistance to Asian soybean rust in Soybean. Plant Physiology 150: 295-307.
Miles MR, Frederick RD and Hartman GL (2006) Evaluation of soybean germplasm for resistance to Phakopsora pachyrhizi. Online. Plant Health Progress doi:10.1094 / PHP- 2006-0104-01-RS.
Miles MR, Frederick RD and Hartman GL (2003) Soybean rust: is the U.S. soybean crop at risk? Available at http://www.apsnet.org/online/feature/rust/. Accessed on July 13, 2003.
Monteros MJ, Missaoui AM, Phillips DV, Walker DR, Boerma HR (2007) Mapping and confirmation of the ‘Hyuuga’ red-brown lesion resistance gene for Asian soybean rust. Crop Science 47: 829-834.
Morel WM and Yorinori JT (2002) Situacion de la roja de la soja en el Paraguay. Bol. de Diulgacion N° 44. Ministerio de Agricultura y Granaderia, Centro Regional de Investigacion Agricola, Capitan Miranda, Paraguay.
Ono Y, Buritica P and Hennen JF (1992) Delimitation of Phakopsora, Physopella and Cerotelium and their species on Leguminosae. Mycological Research 96: 825-850.
Soybean Rust Resistance Pathway Mediated by Rpp2. Molecular Plant-Microbe Interactions 24: 194-206.
Panthee DR, Yuan JS, Wright DL, Marois JJ, Mailhot D, Stewart JRCN (2007) Gene expression analysis in soybean in response to the causal agent of Asian soybean rust (Phakopsora pachyrhizi Sydow) in an early growth stage. Functional and Integrative Genomics 7: 291-301.
Panstruga R (2003) Establishing compatibility between plants and obligate biotrophic pathogens. Current Opinion in Plant Biology 6: 320-326.
Pierozzi PHB, Ribeiro AS, Moreira JUV, Laperuta LC, Rachid BF, Lima WF, Arias CAA, Olivera MF, Toledo JFF (2008) New soybean (Glycine max, Fabales, Fabaceae) sources of qualitative genetic resistance to Asian soybean rust caused by Phakopsora pachyrhizi (Uredinales, Phakopsoraceae). Genetics and Molecular Biology 31: 505-511.
Posada-Buitrago ML and Frederick RD (2005) Expressed sequence tag analysis of the soybean rust pathogen Phakopsora pachyrhizi. Fungal Genetics and Biology 42: 949-962.
Rossi RL (2003) First report of Phakopsora pachyrhizi, the causal organism of soybean rust in the province of Misiones, Argentina. Plant Disease 87: 102. Schmutz J, Cannon SB, Schlueter J, Ma J, Mitros T, Nelson W, Hyten DL, Song Q,
Thelen JJ, Cheng J (2010) Genome sequence of the palaeopolyploid soybean. Nature 463: 178-183
Schneider RW, Hollier CA, Whitam HK, Palm ME, McKemy JM, Hernández JR, Levy L, DeVries-Paterson R (2005) First report of soybean rust caused by Phakopsora pachyrhizi in the continental United States. Plant Disease 89: 774-774
Silva DCG, Stolf R, Van de Mortel M, Lemos NG, Santos JVM, Pereira RM, Bineck E, Almeida AMR, Nepomuceno AL, Yamanaka N, Marcelino FC, Baum TJ, Whitham S, Lemos EGM, Abdelnoor RV (2008a) Transcritos da soja induzidos durante interação com a ferrugem asiática. In: 54o Congresso Brasileiro de Genética. Salvador, p. 319.
mapping of two loci that confer resistance to Asian rust in soybean. Theoretical and Applied Genetics 117: 57-63.
Sinclair JB (1989) Threats to soybean production in the tropics: red leaf blotch and leaf rust. Plant Disease 73: 604-606.
Slaminko TL, Miles MR, Frederick RD, Bonde MR, Hartman GL (2008) New legume hosts of Phakopsora pachyrhizi Based on Greenhouse Evaluations. Plant Disease 92: 767-771.
Tremblay A, Hosseini P, Alkharouf N, Li S, Matthews BF (2010) Transcriptome analysis of a compatible response by Glycine max to Phakopsora pachyrhizi infection. Plant Science 179: 183-193.
Tremblay A, Li S, Scheffler BE, Matthews BF (2009) Laser capture microdissection and expressed sequence tag analysis of uredinia formed by Phakopsora pachyrhizi, the causal agent of Asian soybean rust. Physiological and Molecular Plant Pathology 73: 163-174.
Van de Mortel M, Recknor JC, Graham MA, Nettleton D, Dittman JD, Nelson RT, Godoy CV, Abdelnoor RV, Almeida AMR, Baum TJ, Whitham SA (2007) Distinct biphasic mRNA changes in response to Asian soybean rust infection. Molecular Plant-Microbe Interactions 20: 887-899.
Yamaoka Y, Fujiwara Y, Kakishima M, Katsuya K, Yamada K, Hagiwara H (2002) Pathogenic races of Phakopsora pachyrhizi on soybean and wild host plants collected in Japan. Journal of General Plant Pathology 68: 52-56.
Yorinori JT, Paiva WM, Frederick RD, Costamilan LM, Bertagnoli PF, Hartman GL, Godoy CV, Nunes JJ (2005) Epidemics of soybean rust (Phakopsora pachyrhizi) in Brazil and Paraguay from 2001 to 2003. Plant Disease 89: 675-677.
Chapter II
Combining Transcriptome Analyses and Virus Induced Gene Silencing to Identify Genes in the Rpp4-mediated Asian Soybean Rust Resistance Pathway.
Abstract
Five Asian Soybean Rust (ASR) resistance loci have been identified and mapped in soybean genome: Rpp1, Rpp2, Rpp3, Rpp4 and Rpp5. Of particular interest is Rpp4, mapped on chromosome 18, which has remained stable and confers resistance against Phakopsora pachyrhizi isolates from around the world.
resistance and metabolism. Statistical analyses of overrepresented biological process and molecular function gene ontology functional categories highlighted the importance of genes involved in lignin biosynthesis, flavonoid biosynthesis, response to oxidative stress and phenylpropanoid biosynthesis for defense. To identify transcription factor active in the Rpp4 signaling pathway, we used Clover (cis-element over representation) software and the TRANSFAC (transcription factor database) to identify transcription factor binding sites over-represented in the promoters of our differentially expressed genes. This study allow us also the identification of 33 transcription factor-binding sites significantly over represented in our differentially expressed genes when compared to all genes in the soybean genome, several with known roles in defense. Finally, to elucidate which genes are exclusively related to Rpp4-mediated signaling, we have compared the results of our experiment with microarray results from Rpp2, Rpp3 and Rpp4 resistant and susceptible reactions. We have identified 101 genes unique to the Rpp4-signaling pathway.
1. Introduction
Asian Soybean rust (ASR) is caused by the obligate fungus Phakopsora pachyrhizi Sydow and was first reported in Brazil in 2001 (Yorinori et al., 2005). The disease is considered polycyclic, since the fungus is able to complete several generations in a single life-cycle of the host. Temperatures that favor the growth and development of soybean also favor the development of rust (Zambolin, 2006). The disease destroys leaf tissue, resulting in reduced photosynthetic activity, premature defoliation and reduced life cycle. The cumulative effect of rust on soybean production translates into lower seed weight and reduces the number of pods and seeds (Sinclair, 1989). Currently, P. pachyrhizi is one of the most important economical threats for soybean growers in South America. In Brazil, a recent study documented a two-year field trial that showed that rust was responsible for 37-67% of soybean seed yield losses (Kumudini et al., 2008).
mediated resistance in the resistant parent PI459025B, indicating Rpp4 is a member of the same gene cluster.
Recently transcriptomic techniques have been successful in characterizing soybean pathogen interactions to identify changes in host gene expression following inoculation. In soybean, transcriptomic approaches have identified genes involved in susceptibility and resistance against soybean cyst nematode (Heterodera glycines), Phytophthora stem and root rot (Phytopthora sojae), soybean mosaic virus (Pseudomonas syringae), soybean aphid (Aphis glycines) and Asian soybean rust (P. pachyrhizi) (Alkharouf et al., 2006; Ithal et al., 2007; Moy et al., 2004; Zabala et al., 2006; Zou et al., 2005; van de Mortel et al., 2007). Standardized microarray platforms provide inexpensive, genotype independent means to associate gene expression with gene function. Further, microarray analyses accelerate the understanding of host pathogen interactions, because a large fraction of the genome can be analyzed simultaneously and different bioinformatics methods can be used to identify related groups of genes that are activated or repressed in various regulatory pathways (Kato-Maeda et al., 2001).
Perhaps the most comprehensive transcriptomic studies thus have been on Rpp2 mediated defense. Van de Mortel et al. (2007) examined a seven day time course of ASR infection in resistant (mediated by Rpp2) and susceptible genotypes. A biphasic response to P. pachyrhizi was seen in both genotypes. At 12 hours post inoculation with ASR, both genotypes had induction of basal defense. However, 24 hours after infection, defense gene expression returned to mock-inoculated levels. At 72 hours post infection, a second round of defense gene expression occurred in the resistant genotype, likely due to Rpp2-mediated signaling. While this secondary defense response was also detected in the susceptible interaction, it did not occur until 96 hours post infection and never at the same magnitude observed in the resistant parent. Surprisingly, while greater levels of defense-related gene induction were observed in the resistant parent, greater numbers of differentially expressed genes were observed in the susceptible parent.
This work was followed by Pandey et al. (2011) who used virus induced gene silencing to try and disrupt the Rpp2-mediated signaling pathway in an Rpp2 resistant genotype. The authors identified 140 candidate genes that could potentially be involved in Rpp2-mediated defense signaling using the work of van de Mortel et al. (2007) and soybean orthologs of known defense signaling genes and transcription factors. Eleven genes were identified in the Rpp2-mediated signaling pathway, required for Rpp2-mediated resistance. These included four soybean orthologs of known defense genes (GmEDS1, GmNRP1, GmPAD4 and GmPal1), five predicted transcription factors (GmWRKY36, GmWRKY40, GmWRKY45, GmDBTF and GmMYB84), an O-methyl transferase (GmO-MT) and a cytochrome P450 (GmCYP83E12). Combining their results with data from other plant-pathogen systems allowed the characterization of Rpp2 signaling cascade, even though Rpp2 has yet to be cloned.
2. Materials and methods
2.1. Silencing of Rpp4 via virus induced gene silencing
The VIGS plants used in our analyses are the same plants described by Meyer et al. (2009). In brief, a portion of the LRR domain of the Rpp4 candidate genes from Williams 82 was cloned into RNA2 of the BPMV VIGS vector (Zhang et al., 2009). Co-bombardment of BPMV RNAs 1 and 2 on Wm82 leaves was used to generate inoculum for further experiments. After three weeks, BMPV infected tissue was collected, lyophilized and shipped to the Foreign Disease-Weed Science Research Unit at Fort Detrick, Maryland. At Fort Detrick, the resistant soybean genotype PI459025B was grown in a growth chamber, and two weeks after germination, plants were rub inoculated with test VIGS constructs. Each construct was tested on six plants. Three weeks later, plants were inoculated with P. pachyrhizi isolate LA04-1. Two weeks later plants were evaluated for resistance. Controls included no treatment, mock inoculation and empty BPMV constructs. Three independent replicates of the experiment were performed. After the completion of each replicate experiment, leaves were collected from three LRR-BPMV VIGS plants and three empty-vector LRR-BPMV plants, all infected with P. pachyrhizi. This provided three biological replicates and three technical replicates to use for microarray analyses. Leaves were flash frozen in liquid nitrogen and stored at -80 C.
2.2. RNA extraction and isolation
2.3. Microarray analyses
Labeling, hybridization, and scanning were performed at the Iowa State University GeneChip Facility. Labeled target cRNA was synthesized from 5 µg of total RNA using the GeneChip® One-Cycle Target Labeling and Control Reagents kit (Affymetrix, Santa Clara, CA) according to manufacturer’s instructions. Fragment cRNA (10 µg) were hybridized to GeneChip® Soybean Genome Array (Affymetrix®, Santa Clara, CA) according to manufacturer’s instructions. cRNA quality was verified on an Agilent 2100 BioAnalyzer equipped with an RNA Nano LabChip®. Microarrays were scanned with a GCS3000 7G scanner (Affymetrix, Santa Clara, CA).
2.4. Statistical analysis and array processing
Raw expression values from the .CEL files generated during array processing were read into R (R development core team 2006). The data was analyzed in the 'affy' background and corrected using the RMA function, normalized using the invariant set and summarized using the median polish command. Only perfect match probes were considered. Expression values were fit to a linear model using the limma package and a contrast matrix comparing treatments was applied. Expression values were corrected for multiple testing by an empirical Bayesian correction (eBayes) and fdr (false discovery rate). Genes differentially expressed between the Rpp4 silenced plants and the empty vector treated plants were identified by a fold change equal to or greater than 1 or -1 and a P-value equal to or less than 0.05. Since the data is in log2 form, a fold change of 1 equals a 2-fold difference in expression between samples.
2.5. Annotation of differentially expressed probes
compared to the predicted cDNAs from the soybean whole genome assembly (version 1.0,Schmutz et al., 2010) using BLASTN (Altschul et al., 1997). If matching soybean cDNAs could not be identified or multiple cDNAs from multiple genes could not be distinguished, the Affymetrix consensus sequence was used in place of the soybean cDNA for further analyses. The soybean predicted cDNAs, and when necessary the Affymetrix consensus sequences, were compared to the UniProt protein database (version June 2008, Apweiler et al., 2004) and predicted cDNAs from the A. thaliana genome (version 8, The Arabidopsis Information Resource, www.arabidopsis.org) using BLASTX (E<10-6, Altschul et al., 1997). TAIR Gene Ontology (GO) terms (Berardini et al., 2004) were assigned based on the top A. thaliana sequence identified. Fisher’s exact test (Fisher, 1966) with a Bonferroni correction (Bonferroni, 1935) was used to identify overrepresented Gene ontology categories or transcription factor classes.
2.6. Bioinformatics analysis of cis-elements
Each of the differentially expressed genes identified was assigned to a cDNA from the whole soybean genome assembly (Schmutz et al., 2010) using BLASTN (Altshul et al., 1997, E<10-30, percent identity >95). Using the coordinates of the corresponding soybean cDNA, custom perl scripts were used to extract 1000 bases of promoter sequence from whole soybean genome assembly. Clover (Frith et al., 2004), in combination with the TransFAC transcription factor matrix (Wingender et al., 1996), was used to identify overrepresented transcription factor binding sites in the promoters of the differentially expressed genes. As a background control, the results were compared to 1000 bases of promoter sequence from all predicted soybean genes excluding transposable elements.
predicted soybean genes excluding transposable elements. This would identity genes not present on the array that could also be differentially expressed in response to Rpp4.
3. Results
3.1. Comparisons of gene expression in PI459025B in Rpp4 silenced plants and empty vec tor treated plants, each inoculated with P. pachyrhizi.
We previously developed VIGS constructs from the Rpp4 locus in the susceptible parent, Williams 82 (Meyer et al., 2009). Based on mapping and sequencing data, we hypothesized that Rpp4 in PI459025B was a member of NBS-LRR cluster characterized in Williams82. To test this hypothesis, we developed VIGS constructs from the LRR domain of the Williams82 R-genes. PI459025B plants, carrying Rpp4, were treated with the VIGS constructs and tested for changes in resistance to P. pachyrhizi. The LRR VIGS constructs silenced Rpp4, leading to susceptibility to P. pachyrhizi (Meyer et al., 2009), Mock treated plants and empty VIGS vector treated PI459025B plants maintained resistance to P. pachyrhizi. In this study, leaf tissue from these same experiments was collected and frozen to allow future studies of the Rpp4-signaling pathway.
3.2. Gene Expression from Rpp4 silenced plants
from Rpp4 silenced (LRR VIGS construct) and non-silenced plants (treated with empty vector), we should be able to identify genes downstream of Rpp4 in the signaling pathway controlling resistance to P. pachyrhizi.
A total of 383 genes were significantly differentially expressed (P-value < 0.05) between Rpp4-silenced and control silenced plants, each inoculated with P. pachyrhizi, being 22 regulated, and 361 down regulated. Most of the up-regulated genes show similarity to genes encoding known proteins such as Pectin acetylesterase, Aspartyl protease, GDP mannose pyrophosphorylase, phosphatidylinositol transfer protein PDR16, among others, while several of the down-regulated genes identified share sequence similarity with genes encoding known proteins were related to defense, disease resistance and metabolism (Table 1).
Table 1. List of the most greatly induced and suppressed annotated genes in
Rpp4 silenced plants 14 days after inoculation (dai) by ASR (p-value< 0.05).
Probe set Gene annotation Fold change P-value
Up-Regulated
Gma.5599.1.A1_at hypothetical protein 2.00196 7.50E-07
GmaAffx.78729.1.S1_at SUGAR-1-PHOSPHATE GUANYL
TRANSFERASE
2.06924 1.14E-06
GmaAffx.68386.1.S1_at unknown protein 2.07344 2.50E-07
Gma.14098.1.A1_at PENTATRICOPEPTIDE
REPEAT-CONTAINING PROTEIN
2.13734 5.30E-07
Gma.13925.1.A1_at ALDOSE-1-EPIMERASE 2.20873 5.35E-06
Gma.5963.1.S1_at SUGAR TRANSPORTER 2.22862 1.96E-06
GmaAffx.86638.1.S1_at unknown protein 2.24613 9.20E-07
GmaAffx.85211.1.S1_at SERINE/THREONINE-PROTEIN
KINASE WNK (WITH NO LYSINE)-RELATED
2.24767 1.08E-05
GmaAffx.88028.1.A1_at unknown protein 2.28299 4.20E-07
Gma.18082.1.S1_at hypothetical protein 2.30806 1.10E-07
GmaAffx.79275.1.S1_s_at CYTOCHROME P450 2.30924 5.87E-06
GmaAffx.35140.1.S1_at Phosphatidylinositol transfer protein
PDR16 and related proteins
2.31658 8.89E-06
GmaAffx.61395.1.A1_at unknown protein 2.35599 1.69E-05
Gma.2961.1.S1_at GLUCOSYL/GLUCURONOSYL
TRANSFERASES
Gma.6498.1.A1_at Aspartyl protease 2.54087 2.15E-05
GmaAffx.53274.1.S1_at unknown protein 2.57811 6.10E-07
Gma.7454.1.S1_a_at hypothetical protein 2.65087 2.27E-06
Gma.1007.2.S1_at unknown protein 2.78284 3.00E-07
GmaAffx.4935.2.S1_at unknown protein 2.78974 1.20E-07
GmaAffx.48606.1.S1_at unknown protein 3.24535 7.10E-07
GmaAffx.4935.1.S1_at Pectin acetylesterase and similar
proteins
4.09302 3.85E-06
Gma.4755.1.S1_at unknown protein 4.38654 6.95E-05
Down-regulated
GmaAffx.93635.1.S1_s_at Cystein-rich secretory protein
(CRISP/SCP/TPX1)-related
-11.26476 3.71E-05
GmaAffx.77637.1.S1_at chalcone and stilbene synthases -9.83177 7.64E-05
Gma.10150.1.A1_at Iron/ascorbate family oxidoreductases -8.99014 7.17E-05
GmaAffx.92564.1.S1_at hypothetical protein -8.59062 6.15E-05
GmaAffx.92558.1.S1_s_at Iron/ascorbate family oxidoreductases -7.50755 3.90E-06
Gma.14338.1.A1_at hypothetical protein -7.28335 0.00013166
Gma.3604.4.S1_s_at caffeoyl-CoA_O-methyltransferase -6.74917 6.30E-07
GmaAffx.57966.1.S1_at PAR1 protein -6.54803 3.68E-06
GmaAffx.18868.1.S1_s_at NADH:flavin
oxidoreductase/12-oxophytodienoate reductase
-6.41068 1.27E-06
Gma.2586.1.S1_at unkown protein -6.20006 3.46E-05
Gma.17873.1.S1_s_at hypothetical protein -5.91209 0.0002849
GmaAffx.92410.1.S1_s_at Flavonol reductase/cinnamoyl-CoA
reductase
-5.56145 7.20E-07
Gma.15958.1.S1_at hypothetical protein -5.43274 6.70E-05
Gma.79.4.S1_s_at hypothetical protein -5.40399 5.79E-05
Gma.9072.1.S1_at chalcone and stilbene synthases -5.21972 2.69E-06
Gma.1269.1.S1_at alcohol dehydrogenase -5.10924 5.40E-07
Gma.10820.1.S1_at Hydroxyindole-O-methyltransferase and
related SAM-dependent methyltransferases
-5.09748 5.14E-06
Gma.3988.1.S1_at Glyoxalase -5.07278 5.26E-05
GmaAffx.92479.1.S1_s_at alcohol dehydrogenase -5.05661 5.42E-06
Gma.16913.1.S1_s_at hypothetical protein -5.01768 8.10E-05
GmaAffx.74923.1.S1_at Iron/ascorbate family oxidoreductases -4.90309 4.60E-07
GmaAffx.42893.1.A1_at Reductases with broad range of
substrate specificities
-4.89001 3.90E-07
GmaAffx.91071.1.S1_at Reductases with broad range of substrate specificities
-4.86401 1.20E-07
GmaAffx.90009.1.S1_s_at hypothetical protein -4.82904 1.41E-05
GmaAffx.82647.1.S1_at Peroxidase/oxygenase -4.78257 6.57E-05
GmaAffx.7258.1.S1_s_at unkown protein -4.72723 1.47E-05
GmaAffx.88105.1.S1_at hypothetical protein -4.68377 5.92E-06
GmaAffx.83910.1.S1_at NADH:flavin
oxidoreductase/12-oxophytodienoate reductase
-4.57422 1.07E-05
GmaAffx.92070.1.S1_at caffeoyl-CoA_O-methyltransferase -4.49304 1.20E-07
GmaAffx.50446.1.S1_at unkown protein -4.45622 0.00023923
GmaAffx.50670.1.A1_at Kynurenine aminotransferase, glutamine
transaminase K
-4.44107 4.35E-06
GmaAffx.84342.1.S1_x_at unkown protein -4.41364 0.00021469
GmaAffx.21548.1.S1_at UDP-glucuronosyl and UDP-glucosyl
transferase
-4.36898 3.44E-06
GmaAffx.4716.1.S1_at Flavonol reductase/cinnamoyl-CoA
reductase
-4.33782 3.10E-07
Gma.6211.1.S1_at unkown protein -4.33512 2.65E-06
Gma.4716.2.S1_at unkown protein -4.31655 9.07E-05
Gma.16709.2.S1_s_at Cytochrome P450 CYP2 subfamily -4.24676 1.21E-06
GmaAffx.92894.1.S1_s_at Peroxidase/oxygenase -4.20954 1.45E-05
Gma.169.1.S1_at hypothetical protein -4.20737 2.77E-05
Gma.3473.1.S1_at Molecular chaperone (small heat-shock
protein Hsp26/Hsp42)
-4.15882 8.75E-05
Gma.17851.1.S1_at unkown protein -4.14922 5.21E-05
GmaAffx.86629.1.S1_at Glycosyl hydrolases -4.14421 1.50E-07
Gma.6549.1.S1_at Defense-related protein containing SCP
domain
-4.13849 8.02E-06
GmaAffx.83919.1.S1_at Hydroxyindole-O-methyltransferase and
related SAM-dependent methyltransferases
-4.06296 6.27E-05
Gma.144.1.S1_at unkown protein -4.06067 1.41E-06
Gma.169.1.S1_x_at Glycosyl hydrolases -4.04885 1.01E-05
GmaAffx.215.1.S1_at ATPase -4.03547 3.75E-06
GmaAffx.18940.1.S1_at carbonate dehydratase -4.02311 3.36E-06
significantly overrepresented in our differentially expressed gene list when compared to all genes represented on the soybean array. We identified sixteen GO biological process categories significantly overrepresented in our data set (Table 2). Of these, three were associated with defense or stress responses. Similarly, we identified 13 overrepresented molecular function GO categories including methyl transferases, peroxidases, and enzymes involved in redox reactions, lignin sythesis and flavonoid biosynthesis.
Table 2. Significantly (P<.05) overrepresented Gene Ontology (GO) biological process and molecular function terms found in P. pachyrhizi regulated probe sets as determined by Fisher’s exact test and Bonferroni correction.
GO Term GO Description Corresponding probe sets present on array
Number of P. pachyrhizi -responsive probe sets P-value following Bonferroni correction
GO Biological Process
GO:0009809 Lignin biosynthetic
process
109 17 0.00E+00
GO:0009813 Flavonoid biosynthetic
process
102 19 0.00E+00
GO:0006979 Response to oxidative
stress
402 29 1.40E-07
GO:0010422 Regulation of
brassinosteroid biosynthetic process
8 6 1.87E-07
GO:0001561 Fatty acid
alpha-oxidation
6 5 2.15E-06
GO:0016131 Brassinosteroid
metabolic process
11 6 2.90E-06
GO:0009807 Lignan biosynthetic
process
23 7 2.40E-05
GO:0009699 Phenylpropanoid
biosynthetic process
49 9 4.62E-05
GO:0010224 Response to UV-B 86 11 1.03E-04
GO:0009411 Response to UV 62 9 3.67E-04
GO:0051347 Positive regulation of
transferase activity
4 3 4.10E-03
GO:0051555 Flavonol biosynthetic
process
50 7 6.30E-03
GO:0009435 NAD biosynthetic
process
GO:0009827 Plant-type cell wall modification
15 4 2.26E-02
GO:0006334 Nucleosome assembly 89 8 4.39E-02
GO:0010260 Organ senescence 18 4 4.84E-02
GO Molecular Function
GO:0016614 Oxidoreductase activity,
acting on CH-OH group of donors
8 6 3.69E-08
GO:0042409 Caffeoyl-CoA
O-methyltransferase activity
18 6 2.24E-05
GO:0004601 Peroxidase activity 118 12 3.00E-05
GO:0005199 Structural constituent of
cell wall
61 9 4.01E-05
GO:0047763 Caffeate
O-methyltransferase activity
34 7 7.95E-05
GO:0045430 Chalcone isomerase
activity
14 5 1.69E-04
GO:0045548 Phenylalanine
ammonia-lyase activity
10 4 1.23E-03
GO:0008987 Quinolinate synthetase A
activity
4 3 1.67E-03
GO:0010283 Pinoresinol reductase
activity
4 3 1.67E-03
GO:0008171 O-methyltransferase
activity
60 7 4.07E-03
GO:0004553 Hydrolase activity,
hydrolyzing O-glycosyl compounds
228 13 5.95E-03
GO:0016682 Oxidoreductase activity 19 4 2.04E-02
GO:0004338 Glucan
1,3-beta-glucosidase activity
2 2 2.81E-02
glutathione-s-transferases. Among the transcription factors, we found a heat shock protein, four protein with Myb domain and three bZip transcription factors (Figure 1 A). Analyzing the secondary metabolism overview we identified 71 genes related to phenylpropanoid, lignin, lignan, and flavonoid pathways (Figure 1B). Twenty-genes were related to the phenylpropanoid synthesis and included genes such as phenylalanine ammonia lyase (PAL-1), phenylalanine ammonia lyase (PAL-2), 4-coumarate: CoA ligase-3 (4Cl3), caffeoyl-CoA 3-O-methyltransferase, ferulate-5-hydroxylase, and cinnamyl-alcohol dehydrogenase. Twenty genes were identified belonging to the ligin and lignan pathways and include the O-methyltransferase family 1, O-methyltransferase family 2 protein, phenylalanine ammonia lyase and ferulate-5-hydroxylase. Analysis of the flavonoid pathway identified eight genes including chalcone flavonone isomerase, chalcone synthase 7 and chalcone synthase 4.
B)
Figure 1. MapMan visualization of the Rpp4 regulated probes identified in the Rpp4 VIGS microarray experiment. A) Biotic stress overview, 137 genes identified, B) Secondary metabolism overview, 71 genes identified. The expression level of each probe is associated with a specific color, red- down regulated genes, blue- up regulated genes.
Comparison of the overrepresented gene ontology terms to the results of Mapman revealed the two approaches were complimentary. The gene ontology approach was much more stringent and identified individual pathways important in resistance. The Mapman approach tied these pathways together to form a general picture of defense.
3.3. Bioinformatics analysis of cis-elements
binding sites over represented (P<0.05) in the promoters of differentially expressed genes when compared to promoters of all genes (minus transposable elements) in the soybean genome. From this analysis, we identified 33 transcription factor binding sites (TFBD) significantly over represented in the differentially expressed. Many of the transcription factors binding sites were related to defense including MYB80, MYBBAS1, MYB.PH3, and CRF-2 (Table 3).
Table 3. List of Transcription Factor Binding Sites identified in promoters of
Rpp4- regulated genes.
Nomenclature Raw Score
p-value Reference Function
CPRF-3 47.2 0 Weisshaar et al.,
1991
Involved in light-induced gene expression
TGA1b 34.6 0 Niggewe et al.,
2000
auxin, salicylic acid, light (disease resistance)
LIM1 212 0 Wang et al.,
2009
involved in lignin biosynthesis
OCSBF-1 7.18 0 Singh et al., 1990 expression during plant development
HBP-1b 38 0 Tabata et al.,
1991
phosphorylation is required for DNA-binding (histone genes)
ROM 74.8 0 unknown
HBP-1a 53.9 0 Tabata et al.,
1991
involved in the cell cycle-dependent expression of Wheat core histone genes
TAF-1 65.9 0 Oeda et al., 1991 transcriptional activator
CPRF-3 45.3 0 Weisshaar et al.,
1991
involved in light-induced gene expression
TGA1b 38.8 0 Niggewe et al.,
2000
auxin, salicylic acid, light (disease resistance)
AtMYB-84 22.8 0 Martin and
Paz-Ares, 1997
Protein REGULATOR OF AXILLARY MERISTEMS 3 (disease response -PAL)
P 35.1 0.001 unknown
EmBP-1b 22.4 0.001 Carlini et al.,
1999
may be involved in mediating ABA-response
C1 76.9 0.002 Piazza et al.,
2001
anthocyanin biosynthesis
RITA-1 14.4 0.002 Izawa et al.,
1994
seed development
PCF2 57.9 0.003 Kosugi and
Ohashi, 1997
bind to site in the promoter proliferating cell nuclear antigen (PCNA) gene.
CG1 72.5 0.004 Staiger et al.,
1990
light-inducible expression (chalcone Synthase promoter)
1999 phenylpropanoid biosynthetic gene and in early plant defense response
TGA1a 21.5 0.006 Niggewe et al.,
2000
auxin, salicylic acid, light (disease resistance)
CPRF-1 46.9 0.007 Weisshaar et al.,
1991
involved in light-induced gene expression
CPRF-2 32.8 0.007 Kircher et al.,
1999
CPRF-2 is transported from the cytosol into the nucleus upon irradiation due to action of hytochrome photoreceptors PhyA and PhyB
Alfin1 218 0.008 Bastola et al.,
1998
may play a role in the regulated expression of PRP2 in alfafa roots and contribute to salt tolerance in these plants
TAF-1 40 0.009 Oeda et al., 1991 transcriptional activator
OSBZ8 35.9 0.01 Mukherjee et al.,
2006
induced by Abscisic acid, increase after dehydration
HBP-1a 35.9 0.015 Tabata et al.,
1991
Involved in the cell cycle-dependent expression of wheat core histone genes
Opaque-2 19.2 0.02 Schmidt et al.,
1990
involved in the regulation of seed storage protein synthesis
MYBAS1 30.6 0.025 Yang et al., 2001 induced by water deficit stress
GBP 39.8 0.026 unknown
TRAB1 34.3 0.03 Hobo et al., 1999 involved in ABA-regulated transcription
TGA1a 9.38 0.032 Niggewe et al.,
2000
auxin, salicylic acid, light (disease resistance)
ATHB-9 -3.29 0.033 Prigge et al.,
2005
Probable transcription factor involved in the determination of adaxial-abaxial polarity in ovule primordium (UniProt)
MYB80 -3.45 0.046 Li et al., 1999 disease response
ZAP1 -0.691 0.047 Pater et al., 1996 transcriptional activator
Figure 2. Number of transcripiton factor binding sites found in the promoters of Rpp4-regulated genes. Each of the differentially expressed genes identified was assigned to a cDNA from the whole soybean genome assembly using BLASTN. Custom Perl scripts were used to extract 1000 bases of promoter sequence for all differentially expressed genes. Clover and Transfac were used to identify overrepresented transcription factor binding sites (TFBD) in the promoters. Thirty-three TFBD were identified, with varied frequency.
3.4. MEME and MAST analysis
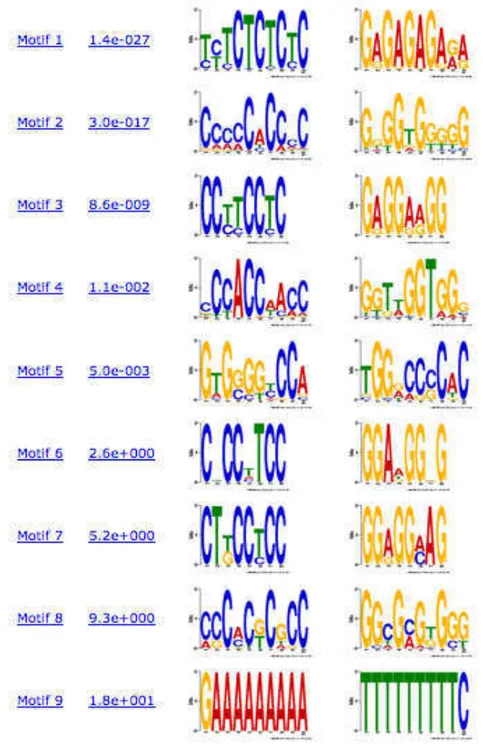
Figure 3. Motif Overview. We used MEME (Bailey and Elkan, 1994) to find probable transcription factor binding sites in soybean genes. The Figure shows 9 motifs identified with the respective motif number, p-value and logo of protein motif. Logos are use to visualize conserved nucleotides in the motif. Only motifs with P-values greater than 0.05 were used for further analysis.
would identify additional genes not present on the soybean genome array that could be downstream of Rpp4. Using MAST to analyze our five significant motifs, we identified 667 promoters in the soybean genome containing one or more motifs (Table 4).
Table 4. Identification of the frequency of motif by MAST.
Motif ID Number of times a motif was found with a single promoter
Total number of time a motif was found
Motif 1 551 1280
Motif 2 489 775
Motif 3 290 409
Motif 4 275 349
Motif 5 361 185
The frequency of the motifs varied greatly, for example, Motif 1 was found in 551 different promoters while Motif 5 was found only in 167 promoters. The number of times a motif was found with a single promoter also varied, Motif 1 was found anywhere from 0 to 18 times while Motif 5 was found from 0 to 2 times. The next step in the analysis will be to annotate the gene corresponding to the identified promoters to see if they have roles in defense.
3.5. Unique genes identified in microarray Rpp4 silenced plants.
(unpublished) measure gene express across twelve days using two soybean genotypes (PI450925B (Rpp4) and Williams 82) and a single P. pachyrhizi isolate. These experiments identified 894, 8447, and 5,806 differentially expressed genes associated with the resistance response governed by Rpp2R, Rpp3R and Rpp4R, respectively. Similarly, 1516, 1827 and 5,365 differentially expressed genes were associated with the susceptible response (Rpp2S, Rpp3S and Rpp4S). To try to elucidate which genes are exclusively related to Rpp4 VIGS experiment, we have overlapped the differentially expressed genes identified by microarray Rpp4 silenced plants with other microarray data from different genotypes carrying genes for resistance as Rpp2, Rpp3 and Rpp4 and susceptible genotypes reactions (Table 5).
Table 5. List of genotypes and timepoints used to overlap the differentially expressed genes identified by microarray Rpp4 silenced plants.
R- gene Resistant interaction Susceptible interaction
Timepoints (hours after inoculation)
Rpp2 PI230970/Brazil P.
pachyrhizi isolate
Embrapa48/Brazil P.
pachyrhizi isolate
6, 12, 18, 24, 36, 48, 72, 96, 120, 168
Rpp3 PI462312, P.
pachyrhizi HW94-1
PI462312, P. pachyrhizi
TW80-2
12, 24, 72, 144, 216, 288
Rpp4 PI459025B, P.
pachyrhizi HW94-1
Williams, P. pachyrhizi
isolate HW94-1
12, 24, 72, 144, 216, 288
Rpp4 VIGS PI459025B, empty
vector silenced, LA04-1
PI459025B, Rpp4
silencing vector silenced, LA04-1
336
4. Discussion
In this study, we combine the power of transcriptomics and virus induced gene silencing to characterize genes involved in the Rpp4-mediated Asian Soybean Rust resistance pathway. Our experiment uses a single soybean genotype (PI459025B) with two different VIGS vectors previously described by Meyer et al. (2009). The first vector is a BPMV silencing vector lacking a silencing target. This construct does not alter the expression of Rpp4, so PI459025B remains resistant to P. pachyrhizi. The second construct, developed from the LRR of the Rpp4 candidate genes in Williams 82, is able to silence the expression of Rpp4 in PI459025B, leading to susceptibility to P. pachyrhizi. Following silencing and P. pachyrhizi inoculation, these plants differ only in the expression of Rpp4 and genes downstream of Rpp4 in the resistance pathway. By isolating and comparing RNA from both plants, we have identified 383 genes downstream of Rpp4 important in Rpp4-mediated defense. Of these, 101 were unique and had not been identified in previous microarray experiments (van de Mortel et al. 2007, Schneider et al. (unpublished), Freeman et al. (unpublished). These genes corresponded to several different biological pathways including transcription factors related to biotic stress (AtbZIP9 and bZIP61), genes involved in cell wall structure (UDP-D- galactose 3 epimerase) and secondary metabolites (phenylpropanoids (OMT1), flavonoids (chalcone synthase) and dihydroflavonoids (cinnamyl-alcohol- dehydrogenase).
indicates that they regulate seed storage protein production by interacting with the PBF protein (Vicente-Carbajosa et al., 1997). Few publications have focused on the biological function of group C bZIPs. ATbZIP 10 also belongs to group C and was shown to be involved in oxidadtive stress response, particularly during defense against the biotrophic pathogen Hyaloperonospora parasitica (Kamida et al., 2006). Recently a microarray analysis revealed 231 genes differentially expressed between two genotypes (WT and an ATbZIP-9 mutant) leading to some possible connections between ATbZIP9 and energy metabolism, abiotic stresses, jasmonic acid, ethylene and salicylic acid signaling (Vilela et al., 2009). A promoter fusion with GUS revealed that ATbZIP9 expression is restricted to the phloem of all organs analyzed. ATbZIP9 mRNA accumulation was also shown to be repressed by glucose and induced by abscissic acid and cytokinin (Silveira et al., 2007). In addition, in vitro phosphorylation experiments show that ATbZIP9 is phosphorylated, suggesting a signaling role in the cell (Kircheler et al., 2010).
abundance of the gene was stimulated by 2.5-fold within 24 h of wounding treatment. Promoter analysis confirmed that the gene promoter was capable of directing expression of the GUS reporter in both wounded and unwounded leaves of transgenic plants, indicating that the gene promoter is systemically responsive to wounding stimuli (Sun et al., 2011).
In plants, the phenylpropanoid pathway has a role in defense (Subramanian et al., 2005). In this category we identified genes such as cinnamyl-alcohol dehydrogenase (CAD). CAD catalyses the conversion of the cinnamyl aldehydes to cynnamyl alcohols, this is the last step in the synthesis of monolignols before their polimerization in cell walls (Ma, 2010). High levels of 1bCAD1 mRNA were found in the roots of sweet potato. The 1bCAD1 gene transcripts were highly induced by cold, wounding and reactive oxygen species (Kim et al., 2010). Interestingly, analyses of transcriptional regulation of the 1bCAD1 promoter-GUS revealed that 1bCAD1 promoter expression was strong in the roots, but barely detectable in the cotyledons. The identification of CAD in our microarray analysis implies that successful defense against P. pachyrhizi involves modification and fortification of cell walls.
Many of the probes we identified were related to proline-rich extensins. Extensins (HRGPs) play an essential role in biotic and abiotic stress responses due to their abilities to cross-link and strengthen the cell wall. The plant cell wall has been established as one of the most important structures of plants as it harbors many vital functions for the plant. Besides providing stability to the plant and counterbalancing internal turgor pressure, it offers protection from injury and pathogen attack. In addition, cell wall-mediated resistance in plants forms the first line of defense against pathogens (Deepak et al., 2010).