Universidade de Lisboa
Faculdade de Medicina de Lisboa
R
OLE OF
P
LASMODIUM TRANSLATIONALLY REPRESSED
GENE PRODUCTS IN MALARIA TRANSMISSION
Jorge Manuel Santos
Doutoramento em Ciências Biomédicas
Especialidade de Microbiologia e Parasitologia
Universidade de Lisboa
Faculdade de Medicina de Lisboa
R
OLE OF
PLASMODIUM TRANSLATIONALLY REPRESSED
GENE PRODUCTS IN MALARIA TRANSMISSION
Jorge Manuel Santos
Tese orientada pelo Doutor Gunnar R. Mair e co-orientada pela
Professora Doutora Maria M. Mota
Doutoramento em Ciências Biomédicas
Especialidade de Microbiologia e Parasitologia
As opiniões expressas nesta publicação são da exclusiva responsabilidade do seu
autor.
A impressão desta dissertação foi aprovada pelo Conselho
Científico da Faculdade de Medicina de Lisboa em reunião de 28
de Outubro de 2014.
i
T
ABLE OF CONTENTS
A
CKNOWLEDGMENTSiii
A
BBREVIATIONSv
F
IGURE AND TABLEI
NDEXxi
R
ESUMO1
S
UMMARY5
I
NTRODUCTION 9I
–
A
PICOMPLEXA9
II
–
P
LASMODIUM AND MALARIA10
III
–
P
LASMODIUM GENE REGULATION15
IV
–
T
RANSLATIONAL REPRESSION18
IV.1
–
T
RANSLATIONAL REPRESSION IS AN ANCIENT AND CONSERVED MECHANISM18
IV.2
–
T
RANSLATIONAL REPRESSION INPLASMODIUM
19
IV.3
–
T
RANSLATIONAL REPRESSION AND MALARIA TRANSMISSION BLOCKING VACCINES23
V
–
M
ALARIA CRYSTALLOID BODIES26
VI
–
P
ROTEIN PALMITOYLATION INPLASMODIUM AND
RELATEDA
PICOMPLEXA28
VII
–
M
OTILITY AND INVASION OF MALARIA PARASITES30
ii
EPSF:
A NOVEL CRYSTALLOID BODY PROTEIN WITH A CRITICALROLE FOR
P
LASMODIUM SPOROZOITE DEVELOPMENT39
A
BSTRACT40
M
ETHODS41
R
ESULTS44
D
ISCUSSION48
S
UPPLEMENTARYF
IGURES ANDT
ABLES51
P
ALMITOYLATION DEFINES KEY EVENTS DURING MALARIA LIFE CYCLE PROGRESSION IN THE MOSQUITO VECTOR 61A
BSTRACT62
M
ETHODS63
R
ESULTS69
D
ISCUSSION81
S
UPPLEMENTARYF
IGURES ANDT
ABLES87
LIMP:
A SURFACE PROTEIN ESSENTIAL FOR MALARIA PARASITE MOTILITY AND INVASION THROUGH REGULATION OF ADHESION SITE TURNOVER105
A
BSTRACT106
M
ETHODS107
R
ESULTS114
D
ISCUSSION123
S
UPPLEMENTARYF
IGURES ANDT
ABLES131
G
ENERALD
ISCUSSION 141iii
A
CKNOWLEDGMENTS
I would like to start by thanking Gunnar Mair, my supervisor, for receiving me in his lab, for accepting me as his student and for giving me freedom to try things my way. Also for teaching me all the basic and advanced tricks of working on Plasmodium molecular biology, and for setting the pace for my PhD while insisting on perfection and always expecting the best from me. I thank Maria Mota, my co-supervisor, for her determined and pragmatic character that many times helped me to see with clarity through the fog. Thank you also for your encouragement words and honest opinions throughout these years.
Thank you Céline Carret, for the close supervision when I most needed, Miguel Prudêncio, for the attentive eye to my work and my person, and Rita Zilhão, for being a true tutor since college days. After all, it is thanks to you that I have embraced the decision of doing a PhD.
I have to thank many people at Instituto de Medicina Molecular, colleagues that became friends, for their help on the daily life in the lab but not less important for the lovely crazy environment we all lived together, especially in the end of the working days: Afonso Almeida, Alice Melão, Ana Guerreiro, Ana Parreira, Ana Rita Gomes, António Mendes, Bethania Cassani, Fabien Guegan, Fernanda Baptista, Filipa Ferreira, Filipa Teixeira, Francisco Branco, Hélder Ribeiro, Inês Albuquerque, Iset Vera, Joana Pissarra, Leonor Pinho, Mafalda Pimentel, Margarida Vaz, Marija Markovic, Marta Machado, Miguel Duarte, Patrícia Inácio, Patrícia Meireles, Patrícia Silva, Rita Domingues, Sandra Trindade, Sílvia Madeira, Telma Lança. My apologies to those who I might have missed here, namely my partners in the IMM/CAML PhD Students Commission. We all have lived unforgettable moments together. My thoughtful gratitude goes to Vanessa Luís, for the special guidance in the last phase of my work, and for the sincere friendship, and to Andreia Pinto, for some of the most exciting moments of my journey at IMM; I simply loved working with both of you.
I am thankful to Chris Janse and Blandine Franke-Fayard for kindly receiving me in their lab in Leiden, The Netherlands, where I have generated with their help most of the parasite mutants used in the present work. Our collaboration was certainly fruitful and I am sure essential for the accomplishment of my PhD goals. Thank you Hans, Jai and Onny, it would not be possible without your support as well. A special word for Blandine, one of the kindest and wisest persons I ever met in Science. I wish all the very best for your career as I know you also aspire for mine.
I thank Fundação para a Ciência e a Tecnologia (FCT) for awarding me a PhD fellowship during the four years of this work, as well as for financially supporting my stay in Chris Janse’s lab in late 2011.
My final words go to my closest friends and family. Amado, Antas, Gaspar, Guilherme, Inês, Joel, Lima, Maria, Marialva, Nelson, Palha, Pena (also an IMM fellow) and Sara: when we are together, problems just do not seem so overwhelming and everything feels like the old days;
iv
thank you for that. I am sincerely grateful for my mother’s support, not just during this last four years but also throughout my life. She was and is unconditionally proud of me and that pride has always kept me going. She deserves my absolute admiration and is undoubtedly my hero in life. Thank you for staying by my side in the good but mostly in the difficult moments.
Finally, I deeply thank my dear Diogo, who has been by my side since the beginning of this journey and before, who has constantly kept me on track, brought my feet back to ground when I felt lost and above all, who never let me forget what should be the priorities in life. May I be able to absorb your strength for many more years to come.
v
A
BBREVIATIONS
2-BMP 2-bromopalmitate
ABE Acyl-Biotin Exchange
Alba acetylation lowers binding affinity
ALD aldolase
AMA1 apical membrane antigen 1
AP2 Apetala2
AP2-O Apetala2 in ookinetes
AP2-Sp Apetala2 in sporozoites
ApiAP2 Apicomplexan Apetala2
ATP adenosine triphosphate
BRE Bruno response element
BSA Bovine Serum Albumin
CAP cyclase-associated protein
CB crystalloid body
cDNA complementary DNA
CDPK calcium-dependent protein kinase
CelTOS cell-traversal protein for ookinetes and sporozoites
CHT1 chitinase
CITH C. elegans CAR-1 and fly Trailer hitch Homologue
CM cerebral malaria
CPEB cytoplasmic polyadenylation element binding protein
CRD cysteine-rich domain
CSP circumsporozoite protein
CTRP circumsporozoite- and TRAP-related protein
DARF DOZI-associated repressor factors
DEAD Asp-Glu-Ala-Asp = Aspartate-Glutamate-Alanine-Aspartate
dhfr/ts dihydrofolate reductase/thymidylate synthase
DHHC Asp-His-His-Cys = Aspartate-Histidine-Histidine-Cysteine
vi
DMSO dimethyl sulfoxide
DNA deoxyribonucleic acid
DOZI Development Of Zygote Inhibited
DTT dithiothreitol
EEF exoerythrocytic from
eef1a eukaryotic translation elongation factor 1α
eIF2α eukaryotic translation initiation factor 2α eIF4E eukaryotic translation initiation factor 4E
eIF4G eukaryotic translation initiation factor 4G
EM electron microscopy
EMP1 Erythrocyte Membrane Protein 1
EPSF Essential Protein for Sporozoite Formation
EST Expressed Sequence Tag
EuPathDB Eukaryotic Pathogen Database Resources
FACS fluorescence-activated cell sorting
F-actin filamentous actin
FBS Fetal Bovine Serum
FIGE Field-Inversion Gel Electrophoresis
FLP-FRT flippase-flippase recognition target
FPKM Fragments Per Kilobase of transcript per Million mapped reads
GAK cyclin G-associated kinase
GAP glideosome-associated protein
GAPM glideosome associated protein with multiple membrane spans
gDNA genomic DNA
GEST gamete egress and sporozoite traversal
GFP green fluorescent protein
GMP cyclic guanosine monophosphate
HA haemagglutinin
Hda2 histone deacetylase 2
vii
HoMu Homologue of Musashi1
HP1 heterochromatin protein 1
HPRT hypoxanthine-guanine phosphoribosyltransferase
HRP horseradish peroxidase
HSP20 heat shock protein 20
HSP70 heat shock protein 70
HSPG heparan sulphate proteoglycan
i.p. intraperitoneally
i.v. intravenously
ICP inhibitor of cysteine proteases
IFA immunofluorescence assay
IgG immunoglobulin G
IK2 eukaryotic translation initiation factor 2α kinase IMC inner membrane complex
IP immunoprecipitation
iRBC infected red blood cell
ISP IMC sub-compartment protein
KH K-homology
KO knock-out
lncRNA long non-coding RNA
Lsm like-Sm
MAEBL merozoite adhesive erythrocytic binding-like protein
MAOP membrane-attack ookinete protein
MAP-2 mitogen-activated protein kinase 2
MBOAT membrane-bound O-acyl-transferase
mRNA messenger ribonucleic acid
mRNP messenger ribonucleoprotein
MSP1 merozoite surface protein 1
MTBV malaria transmission blocking vaccine
viii
MTOC microtubule organizing centre
MyoA Myosin A
ncRNA non-coding RNA
NIMA Never in Mitosis Gene A
NRE Nanos response element
ORF open reading frame
p.i. post-infection
PABP poly(A)-binding protein
PAM pregnancy-associated malaria
PAT palmitoyl-S-acyl-transferase
Pb Plasmodium berghei
PBE Pumilio binding element
P-body processing-body
PBS Phosphate Buffered Saline
PCR polymerase chain reaction
PCRMP Plasmodium cysteine repeat modular protein
Pf Plasmodium falciparum
PFA paraformaldehyde
Pg Plasmodium gallinaceum
PGAM phosphoglycerate mutase family member
PK7 protein kinase 7
PlasmoDB Plasmodium Genomics Resource
PlasmoGEM Plasmodium Genetic Modification Project
PM plasma membrane
PP protein phosphatase
PPKL kelch-like motifs protein phosphatase
PPLP Plasmodium perforin-like protein
PPM metallo-dependent protein phosphatase
PRE Prx Regulatory Element
ix
PSII photosystem II
PSOP putative secreted ookinete protein
PTM post-translational modification
PTPLA protein tyrosine phosphatase-like A homolog
Puf Pumilio
PUM-HD Pumilio homology domain
PV parasitophorous vacuole
PVM parasitophorous vacuole membrane
Pvs Plasmodium vivax
Py Plasmodium yoelii
qPCR quantitative real-time PCR
RBC red blood cell
RBS ribosome binding site
RFP red fluorescent protein
RMgmDB Rodent Malaria genetically modified Parasites Database
RNA ribonucleic acid
RNAi RNA interference
RNA-IP RNA-immunoprecipitation
RNAseq RNA sequencing
RON4 rhoptry neck protein 4
RPMI Roswell Park Memorial Institute medium
RT reverse transcriptase or room temperature
RT-PCR reverse transcriptase-polymerase chain reaction
S6/TREP sporozoite-specific gene 6/TRAP-related protein
SA severe anaemia
SDS-PAGE sodium dodecyl sulfate-polyacrylamide gel electrophoresis
SEM standard error of the mean
SHLP1 Shewanella-like protein phosphatase 1
SIP2 SPE2-interacting protein
x
SOAP secreted ookinete adhesive protein
SP signal peptide
SPECT sporozoite microneme protein essential for cell traversal
SPM subpellicular microtubule protein
SR serine/arginine
SRPK serine/arginine-rich protein kinase
Sub2 subtilisin-like serine protease (subtilase)
TB transmission blocking
TEM transmission electron microscopy
TF transcription factor
Tg Toxoplasma gondii
TJ tight junction
TLP TRAP-like protein
TM transmembrane
ToxoDB Toxoplasma Genomics Resource
TR translational repression
TRAP thrombospondin-related anonymous protein
TRSP thrombospondin-related sporozoite protein
UIS upregulated in infectious sporozoites
UTR untranslated region
VSP variant-specific surface protein
WARP von Willebrand factor A domain-related protein
WT wild-type
xi
F
IGURE AND
T
ABLE
I
NDEX
I
NTRODUCTIONFigure 1
– Life cycle of Plasmodium falciparum 12
Figure 2
– Dynamics of Plasmodium development in the mosquito vector 13
Figure 3
– Putative mRNP responsible for translational repression in
Plasmodium berghei female gametocytes 20
Figure 4
– Model for the Plasmodium gliding motility apparatus 30
EPSF:
A NOVEL CRYSTALLOID BODY PROTEIN WITH A CRITICAL ROLE FORP
LASMODIUM SPOROZOITE DEVELOPMENTFigure 1
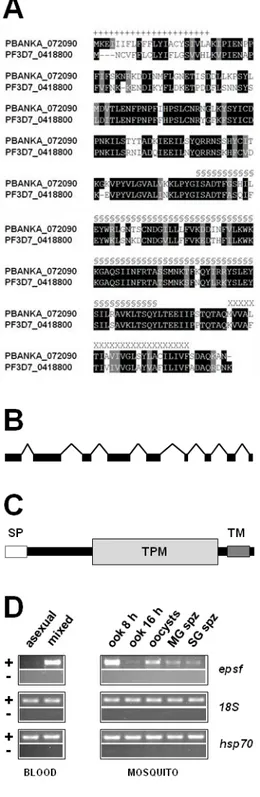
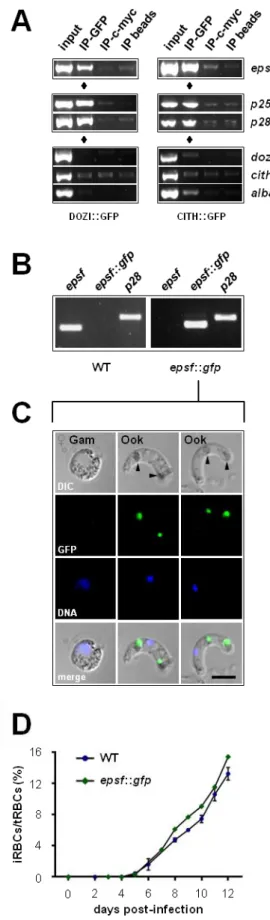
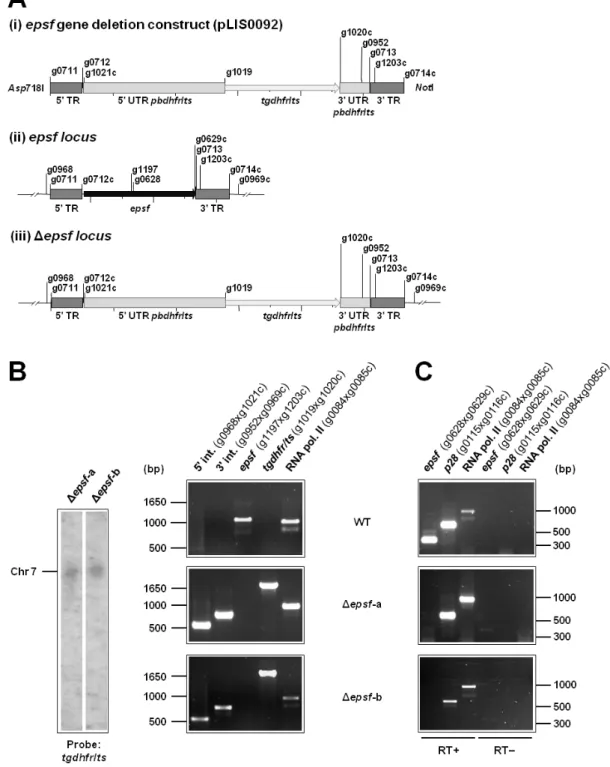
– Organisation of epsf gene and protein and its mRNA expression profile 45
Figure 2
– epsf is translationally repressed in gametocytes and expressed
in ookinetes 46
Figure 3
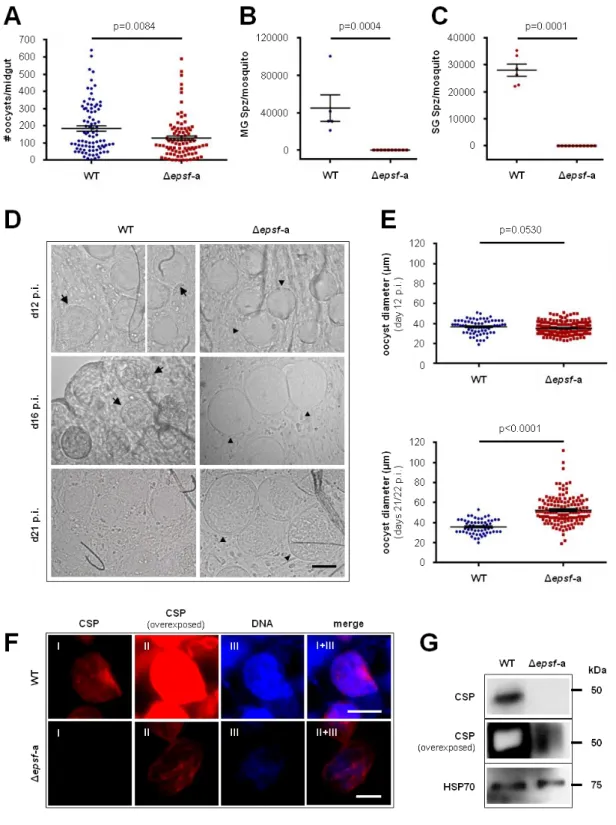
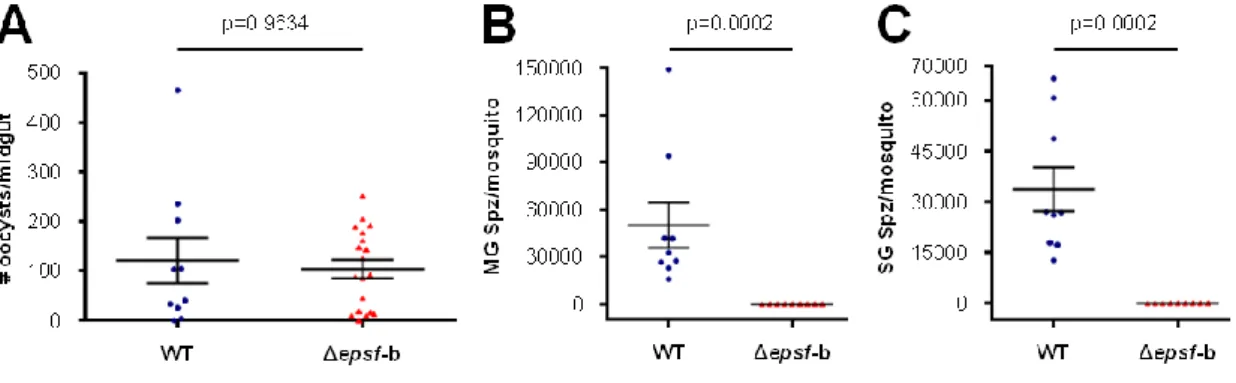
– Δepsf-a oocysts do not sporulate and lack circumsporozoite protein 47
Figure S1
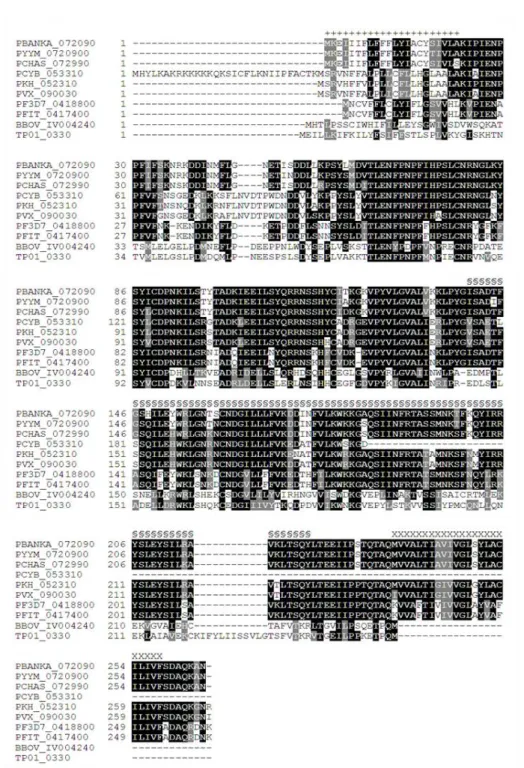
– EPSF is conserved in apicomplexan parasites 51
Figure S2
– epsf is not transcribed in liver stages 51
Figure S3
– Generation and genotyping of epsf::gfp parasite line 52
Figure S4
– Generation and genotyping of Δepsf parasite lines 53
Figure S5
– Δepsf-b oocysts do not sporulate 54
Figure S6
– Δepsf mutants do not transmit to naïve mice 54
Table S1
– Primers used in the present work 55
Table S2
– Parasite transfection experiments 59
Table S3
– Summary of phenotypes of the P. berghei mutants generated
in the present study 59
P
ALMITOYLATION DEFINES KEY EVENTS DURING MALARIA LIFE CYCLE PROGRESSION IN THE MOSQUITO VECTORFigure 1
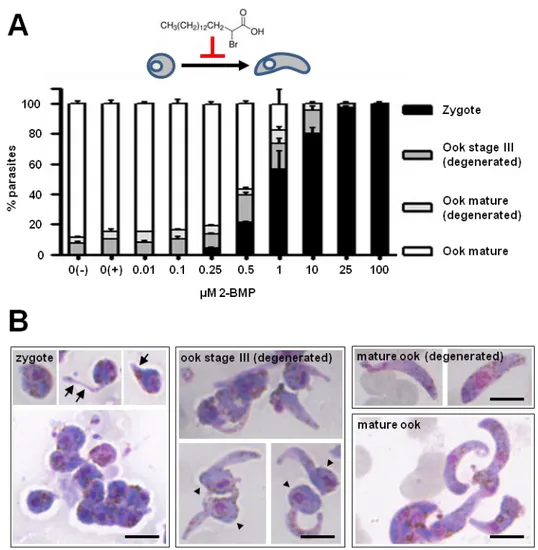
– Inhibition of protein palmitoylation arrests ookinete development 69
xii
Figure 2
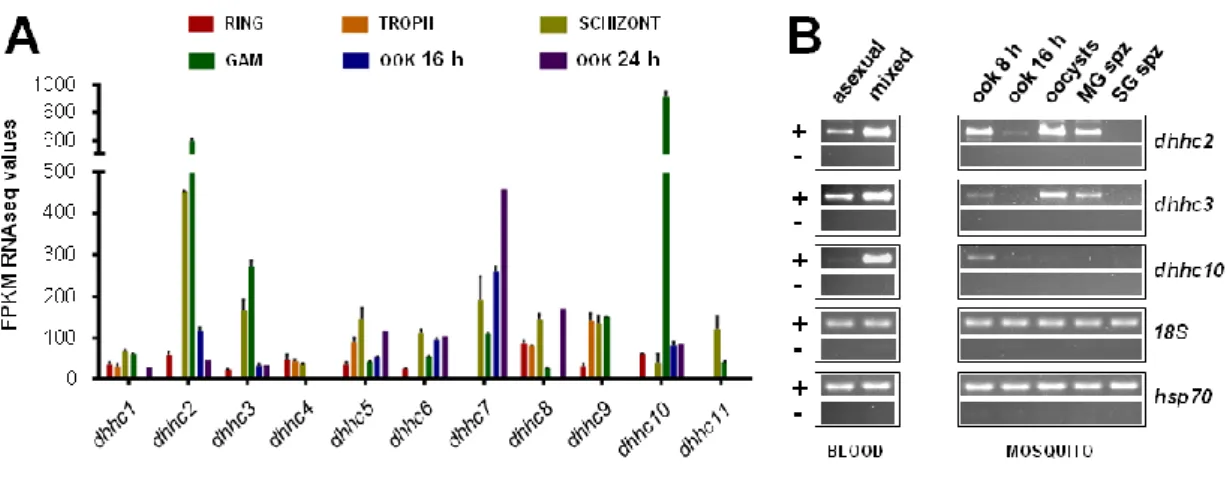
– mRNA expression of P. berghei dhhc genes 70
Figure 3
– Δdhhc10 oocysts do not sporulate and lack circumsporozoite protein 72
Figure 4
– Δdhhc10 mutants are impaired in sporozoite development and
do not transmit to naïve mice 74
Figure 5
– dhhc10 is translationally repressed in gametocytes and expressed
in ookinetes 75
Figure 6
– Δdhhc10 ookinetes lack crystalloid bodies and associated
haemozoin clusters 76
Figure 7
– Absence of crystalloid bodies in Δdhhc10 parasites leads to
mislocalisation of LAP2 77
Figure 8
– DHHC2 and DHHC3 are expressed in blood and mosquito stages 78
Figure 9
– Sporozoite invasion but not motility is dependent on protein
palmitoylation 80
Figure S1
– dhhc2, dhhc3 and dhhc10 are not transcribed in liver stages 87
Figure S2
– Generation and genotyping of Δdhhc10 parasite lines 88
Figure S3
– Generation and genotyping of dhhc10::gfp parasite line 89
Figure S4
– DHHC10 localisation in ookinetes 90
Figure S5
– Generation and genotyping of dhhc2::gfp parasite line 91
Figure S6
– Generation and genotyping of dhhc3::gfp parasite line 92
Figure S7
– Generation and genotyping of dhhc2::gfp-3'UTR parasite line 93
Figure S8
– Generation and genotyping of dhhc3::gfp-3'UTR parasite line 94
Figure S9
– DHHC2 and DHHC3 are expressed in blood and mosquito stages 95
Figure S10
– dhhc2 and dhhc3 transcripts are bound by DOZI/CITH-defined
mRNPs 95
Table S1
– Primers used in the present work 96
Table S2
– Parasite transfection experiments 100
Table S3
– Summary of Plasmodium DHHC-PAT expression data 101
Table S4
– Summary of phenotypes of the P. berghei mutants generated in
the present study 103
xiii
Table S5
– Identification of subpellicular network and IMC-associated proteins
shown to be palmitoylated 104
LIMP:
A SURFACE PROTEIN ESSENTIAL FOR MALARIA PARASITE MOTILITY AND INVASION THROUGH REGULATION OF ADHESION SITE TURNOVERFigure 1
– Gene and protein structure of limp 114
Figure 2
– limp is translationally repressed in gametocytes and expressed in
ookinetes 115
Figure 3
– Δlimp parasites suffer cumulative population loss during mosquito
passage 116
Figure 4
– limp mRNA and protein expression profiles 117
Figure 5
– Δlimp parasites do not transmit to naïve mice 118
Figure 6
– Δlimp parasites are severely impaired in establishing liver infection
in vitro 119
Figure 7
– Δlimp parasites are greatly impaired in gliding motility but do not
show altered cellular ultrastructure 120
Figure 8
– LIMP::GFP is localised to the parasite plasma membrane 122
Figure 9
– limp::gfp parasites present productive gliding and invasion 124
Figure 10
– limp::gfp salivary gland sporozoites glide with a limp 126
Figure S1
– Generation and genotyping of limp::gfp parasite line 131
Figure S2
– Generation and genotyping of Δlimp parasite lines 132
Figure S3
– Δlimp parasites develop normal blood stage parasitaemia
and gametocytaemias over the course of infection 133
Figure S4
– Δlimp ookinetes show WT morphology 133
Figure S5
– limp is not transcribed in liver stages 133
Figure S6
– limp::gfp parasites establish normal liver infection 134
Table S1
– Primers used in the present work 135
Table S2
– Parasite transfection experiments 138
Table S3
– Summary of phenotypes of the P. berghei mutants generated
in the present study 139
xiv
Table S4
– Quantification of gold particles in the immuno-EM specimens
1
R
ESUMO
A Malária é uma doença causada por espécies parasitas do género Plasmodium pertencentes ao filo Apicomplexa. Estes parasitas causam doença no seu hospedeiro quando invadem e se desenvolvem dentro de eritrócitos e apresentam um ciclo de vida deveras complexo, incluindo uma fase de transmissão por um mosquito fêmea do género Anopheles. A infecção deste vector depende de células precursoras sexuais designadas gametócitos que se desenvolvem na corrente sanguínea do hospedeiro vertebrado. Nesta fase, os parasitas apresentam dimorfismo sexual, evidente quer a nível morfológico, quer molecular. Por forma a colonizar com sucesso o intestino médio do mosquito, os gametócitos femininos armazenam de forma quiescente ácidos ribonucleicos mensageiros (mRNAs) específicos em ribonucleoproteínas mensageiras (mRNPs) através de um mecanismo molecular designado repressão da tradução. Após uma refeição sanguínea, estes transcriptos são traduzidos no zigoto em desenvolvimento, iniciando desta forma as alterações morfológicas e funcionais que permitem aos parasitas formar o oocineto dotado de motilidade e estabelecer um oocisto replicativo por debaixo do epitélio do intestino médio.
Previamente à transição entre zigoto e oocineto, a repressão da tradução de determinados mRNAs depende dum complexo multiproteico onde as proteínas DOZI e CITH são componentes centrais. Quando estes estão ausentes, os mRNAs outrora silenciados são sobexpressos, prevenindo assim a expressão não oportuna de proteínas na corrente sanguínea do hospedeiro que poderia gerar anticorpos de bloqueio da transmissão da Malária. Parasitas depletados em DOZI ou CITH realizam fertilização, mas ficam impossibilitados de prosseguir mais além no seu desenvolvimento. Os primeiros genes traducionalmente reprimidos a serem identificados foram p25 e p28; estes codificam para as duas mais abundantes proteínas de superfície do oocineto envolvidas na sobrevivência do oocineto, na penetração do epitélio do intestino médio e no desenvolvimento dos oocistos. A repressão da tradução destes mRNAs depende de uma região rica em uridinas nas suas regiões não traduzidas (UTRs), nomeadamente na UTR 5’ de p25 e na UTR 3’ de p28. Para além disto, estes genes são também dos alvos líder no desenvolvimento de vacinas de bloqueio da transmissão da Malária. Tais vacinas baseiam-se na presença de anticorpos contra antigénios de Plasmodium que, quando ingeridos por um mosquito durante uma refeição sanguínea, se ligam ao parasita em desenvolvimento, prevenindo assim a infecção do vector. É portanto evidente que a repressão da tradução é crucial para a produção cronometrada de moléculas do parasita que de outra forma poderiam gerar anticorpos de bloqueio da transmissão com consequências deletérias para a transmissão de Plasmodium.
Existem evidências de que a repressão da tradução também ocorre em esporozoítos nas glândulas salivares do mosquito. Os esporozoítos são a forma infecciosa do parasita responsável pela transmissão da doença do mosquito para um hospedeiro não infectado. A deleção do gene puf2 em P. berghei, a espécie causadora de Malária em roedores, resulta em alterações transcripcionais e traducionais que normalmente têm lugar em estádios hepáticos do
2
parasita. Estas modificações moleculares precedem alterações morfológicas reminiscentes da transição esporozoíto-forma exoeritrocítica quando o parasita ainda se encontra no interior das glândulas salivares do mosquito, sugerindo que a quiescência do esporozoíto depende do controlo pós-transcripcional de certos mRNAs e da função da proteína Pumilio 2 ou Puf2.
O presente trabalho centra-se nos factores que controlam a progressão do ciclo de vida do parasita no mosquito, desde o despontar do desenvolvimento sexual ainda no interior do eritrócito, à formação do esporozoíto e à invasão do fígado. O mesmo caracteriza a função de novos produtos génicos traducionalmente reprimidos de P. berghei e determina a sua contribuição para o desenvolvimento do parasita no interior do mosquito e, consequentemente, para a sua transmissão. Este estudo debruça-se essencialmente em três genes principais –
epsf, dhhc10 e limp – e suas correspondentes proteínas. Todos estes genes são sobexpressos
em gametócitos de parasitas Δdozi. É aqui demonstrado que os mesmos são traducionalmente reprimidos em gametócitos femininos, que os seus transcritos interagem com DOZI e CITH e, uma vez traduzidos, as respectivas proteínas se localizam nos corpos cristalóides do oocineto, organelos efémeros e enigmáticos cuja função não é ainda totalmente conhecida.
EPSF (Essential Protein for Sporozoite Formation) é uma proteína conservada entre os membros do filo Apicomplexa mas a sua função era até à data desconhecida. Esta proteína apresenta, embora com baixa probabilidade bioinformática, um domínio semelhante a TPM, que em plantas é conhecido por participar no ciclo de síntese/degradação de proteínas do fotossistema II no cloroplasto. DHHC10 é um membro de uma família evolutivamente conservada de palmitoil-S-acil-transferases (PATs) caracterizada pela presença de um domínio rico em cisteínas compreendendo o motivo Asp-His-His-Cys (DHHC), ou por vezes DHYC. Estas proteínas são responsáveis pela adição de palmitato (ácido gordo de cadeia C-16) a resíduos de cisteína de outras proteínas, uma modificação pós-traducional reversível que modifica a afinidade dos seus alvos para membranas lipídicas. Por sua vez, LIMP é uma pequena proteína (110 aminoácidos de comprimento) exclusiva do género Plasmodium e não exibe qualquer domínio funcional identificável.
Os mutantes Δepsf estabelecem números normais de oocistos que permanecem vacuolizados e não desenvolvem esporozoítos. Os mesmos atingem dimensões superiores aos oocistos wild-type (WT) sem concomitante replicação de ácido desoxirribonucleico (DNA) ou expressão de circumsporozoite protein. Consequentemente, os parasitas Δepsf não apresentam qualquer capacidade de transmissão.
Os mutantes Δdhhc10 apresentam um fenótipo muito semelhante ao nível dos oocistos, não obstante neste caso a replicação do seu DNA não ser afectada. Apesar da palmitoilação de proteínas e a relevância de DHHC-PATs para a biologia das fases sanguíneas de Plasmodium terem sido foco de muita atenção recentemente, nada é sabido actualmente acerca da importância desta modificação pós-traducional no desenvolvimento do parasita no seu vector. Através do uso de inibidores e de modificação genética este estudo demonstra pela primeira vez que a palmitoilação é crucial para processos biológicos que têm lugar em diversas
3
fases da infecção do mosquito: é indispensável para a transformação do zigoto em oocineto e define a eficiência de invasão de hepatócitos por esporozoítos, sendo no entanto redundante para a motilidade do tipo gliding e para o atravessamento de células hospedeiras. Ao remover
dhhc10 é demonstrado neste trabalho que esta DHHC-PAT é basilar para a correcta
localização de outros componentes dos corpos cristalóides e para a própria formação destes organelos.
Tal como para outras proteínas localizadas nos corpos cristalóides (membros da família proteica de adesinas LCCL), EPSF e DHHC10 têm um papel essencial na esporogonia de P.
berghei (formação de esporozoítos dentro dos oocistos) e portanto na progressão do ciclo de
vida do parasita no mosquito. Os dados aqui apresentados reforçam a noção de que proteínas residentes dos corpos cristalóides são vitais para a esporulação dos oocistos. LIMP também se localiza nos corpos cristalóides do oocineto mas, contrariamente a EPSF e DHHC10, a sua função nada tem que ver com a formação de esporozoítos. De facto, os oocistos Δlimp são igualmente eficientes na produção de esporozoítos quanto parasitas WT.
Este ensaio estabelece LIMP como um novo factor de motilidade do tipo gliding específico de parasitas da Malária. O mesmo é importante para uma eficiente infectividade do oocineto e essencial para a motilidade do esporozoíto, assim com para a sua capacidade de aderir, atravessar e invadir células do fígado. A ausência desta proteína reduz a transmissão para o mosquito em metade, a invasão das glândulas salivares em dez vezes e torna os parasitas incapazes de infectar ratinhos naïve, quer por picada de mosquito, quer por injecção intravenosa de esporozoítos. Por um lado, os parasitas depletados em LIMP não dispõem de qualquer motilidade. Por outro, a fusão deste factor com a proteína fluorescente GFP resulta num movimento imperfeito caracterizado por velocidade reduzida e frequente alongamento do esporozoíto, particularidades relacionadas com uma redução da reciclagem de locais de adesão entre o parasita e o substrato. Conjuntamente com a localização de LIMP na membrana plasmática do esporozoíto, os actuais resultados sugerem que LIMP desempenha um papel crítico no estabelecimento/dissolução de locais de adesão durante o movimento do parasita e invasão de células hospedeiras.
De forma geral, a presente dissertação sublinha a relevância da repressão da tradução e dos seus mRNAs alvo para a biologia de Plasmodium e demonstra que os genes traducionalmente reprimidos são cruciais para a transmissão do parasita e sua sobrevivência em múltiplas fases do seu desenvolvimento no interior do mosquito. Este trabalho indicia novas abordagens conceptuais no combate à Malária, nomeadamente a inibição da formação de corpos cristalóides e a interferência com a maquinaria de motilidade do parasita.
5
S
UMMARY
Malaria is caused by parasite species of the genus Plasmodium belonging to the phylum Apicomplexa. Plasmodium parasites cause disease when they infect red blood cells of the mammalian host and present a very complex life cycle that includes transmission by a female anopheline mosquito vector. The infection of this vector depends on sexual precursor cells called gametocytes that develop in the blood stream of the vertebrate host. At this stage, parasites display sexual dimorphism that is evident at both morphological and molecular levels. In order to successfully colonise the mosquito midgut, female gametocytes quiescently store specific messenger ribonucleic acids (mRNAs) in messenger ribonucleoproteins (mRNPs) by a molecular mechanism designated translational repression (TR). Following a blood meal, these transcripts are translated in the developing zygote, thereby initiating the morphological and functional changes that allow the parasites to form the motile ookinete and establish a replicating oocyst underneath the midgut epithelium cell layer.
Prior to the zygote-to-ookinete transformation (ZOT), TR of selected mRNAs depends on a multiprotein complex where DOZI and CITH are core components. When absent, the otherwise silenced mRNAs are downregulated, preventing mistimed protein expression in the mammalian host’s blood stream that could allow for the generation of malaria transmission blocking antibodies. Parasites lacking DOZI or CITH take part in fertilisation but cannot develop further. The first Plasmodium translationally repressed genes to be identified were p25 and p28; they encode for the two major ookinete surface proteins involved in ookinete survival, penetration of midgut epithelium and development into oocysts. TR of these mRNAs depends on uridine-rich regions in their untranslated regions (UTRs), namely in the 5’ UTR of p25 and in the 3’ UTR of p28. Additionally, these have also been the gold standard and leading target candidates for malaria transmission blocking vaccines (MTBVs). These vaccines rely on the presence of antibodies against Plasmodium antigens that, when taken up by a mosquito during a blood meal, bind to the developing parasite preventing infection of the vector. It is then evident that TR is paramount for the timely production of parasite molecules that otherwise could raise transmission blocking antibodies with deleterious consequences for Plasmodium transmission.
TR is also believed to take place in salivary gland sporozoites, the mammalian-infective parasite form that is responsible for disease transmission from the mosquito to the human or rodent host. Deletion of puf2 in the rodent malaria parasite species P. berghei results in transcriptional and translational alterations that normally take place in hepatic stages. These molecular modifications precede morphological changes reminiscent of sporozoite-to-liver stage transformation while still in the mosquito salivary glands, suggesting that sporozoite quiescence relies on post-transcriptional control of certain mRNAs and on the function of the protein Pumilio 2 or Puf2.
We are interested in the factors that drive parasite life cycle progression in the mosquito vector, from the onset of sexual development in the erythrocyte to sporozoite formation and liver
6
cell invasion. In the present study we characterised the function of novel putative translationally repressed gene products of P. berghei and determine their contribution to parasite development within the mosquito and consequently to malaria transmission. Three main genes (and corresponding proteins) are addressed herein – epsf, dhhc10 and limp – all of which were found downregulated in the Δdozi parasite line. We show here that they are translationally repressed in female gametocytes, are bound by DOZI and CITH and are targeted to the ookinete crystalloid bodies (CBs) upon translation, a short-lived and enigmatic organelle with unclear function.
EPSF (Essential Protein for Sporozoite Formation) is conserved among apicomplexans but its function was so far unknown. This protein contains, although with low probability, a bioinformatically annotated TPM-similar domain that in plants has been implicated in the synthesis/degradation cycle of photosystem II proteins in the chloroplast. DHHC10 is a member of an evolutionarily conserved family of palmitoyl-S-acyl-transferases (PATs) characterised by the presence of an Asp-His-His-Cys (DHHC) motif (sometimes DHYC) within a cysteine-rich domain. These proteins are responsible for the addition of palmitate (C-16-long chain fatty acids) to cysteine residues of other proteins, a reversible post-translational modification (PTM) that tethers its targets to lipid membranes. On the other hand, LIMP is a small (110 amino acids-long) protein unique to Plasmodium spp. and exhibits no identifiable functional domains.
Δepsf mutants establish normal oocyst numbers but fail to develop sporozoites, remain vacuolated and continue to increase in size without concomitant deoxyribonucleic acid (DNA) replication or circumsporozoite protein expression. Consequently, Δepsf parasites are completely blocked in transmission.
Δdhhc10 mutants show a very similar oocyst phenotype despite the fact that DNA replication does not seem to be affected in this case. Although protein palmitoylation and the relevance of DHHC-PATs in Plasmodium biology have recently been the focus of much interest in blood stage parasites, nothing is know about the importance of such PTM in parasite development within its mosquito vector. Using drug inhibitor and gene deletion studies we show for the first time that palmitoylation is crucial for the execution of developmental and cell biological processes during several phases of mosquito stage infection: it is indispensable for ZOT and defines the efficiency of sporozoite invasion of the host hepatocyte while being redundant for rapid protein turn-over during gliding motility and cell traversal. By deleting
dhhc10 we demonstrate that this DHHC-PAT is key to the correct targeting of other CB
components and to the formation of the ookinete CB itself.
Similar to other CB-associated proteins (the members of the LCCL protein family of adhesins), both EPSF and DHHC10 have an essential role for P. berghei sporogony (formation of sporozoites within oocysts) and therefore for progression of the parasite life cycle in the mosquito. Our data thus further establishes the vital function of CB-resident proteins in oocyst sporulation. LIMP is also localised to the ookinete CBs but, on the contrary to EPSF and
7
DHHC10, is not involved in the formation of sporozoites. In fact, Δlimp oocysts are equally efficient as wild-type (WT) parasites in producing midgut sporozoites.
We establish LIMP as a novel malaria-specific gliding motility factor that is important for efficient ookinete infectivity and essential for sporozoite motility and capability to adhere, traverse and invade host liver cells. The absence of this protein reduces transmission to the mosquito vector by half, salivary gland invasion 10-fold and renders parasites unable to infect naïve mice by mosquito bite or when injected intravenously. While LIMP-depleted parasites display no gliding motility, in situ green fluorescent protein (GFP) tagging of this protein resulted in a limping movement characterised by reduced speed and frequent stretching, which has been correlated with reduced turnover of parasite-substrate adhesion sites. Together with the plasma membrane localisation of LIMP in sporozoites, our results suggest that LIMP plays a critical role in regulating the attachment/detachment of adhesion sites during gliding motility and invasion of host target cells.
Overall, this dissertation highlights the relevance of TR and its target mRNAs for
Plasmodium biology and demonstrates that translationally repressed gene products are crucial
for parasite transmission and survival at multiple steps of its development within the mosquito. This work raises new conceptual approaches to combat malaria, namely by inhibiting the formation of CBs and interfering with the parasite gliding motility machinery.
9
I
NTRODUCTION
I
–
A
PICOMPLEXAThe phylum Apicomplexa is composed of a large group of unicellular, eukaryotic protozoa and contains obligate intracellular parasites causing disease to humans, livestock, wild animals and invertebrates, such as those belonging to the genera Plasmodium, Toxoplasma,
Cryptosporidium, Babesia and Theileria [1]. The hallmark of the phylum from which the name
Apicomplexa derives is the presence of an apical complex involved in motility and host cell adhesion and penetration and composed of secretory organelles such as rhoptries and micronemes and one or more polar rings [2]. Polar rings are circular structures from which subpellicular microtubules emanate and were thus postulated to be a microtubule organizing centre (MTOC) [3]. Some members such as Toxoplasma possess a set of spirally arranged tubulin fibers forming a truncated cone structure called conoid [4]. This organelle is thought to play a mechanical role in invasion of host cells [2]. Another characteristic feature of most apicomplexans is the apicoplast, a non-photosynthetic plastid thought to be derived from the engulfment of a red alga in a secondary endosymbiotic event [5, 6]. As a result, the apicoplast presents not two, but four membranes, with the outermost and second outermost membranes deriving from the parasite and algal plasma membranes, respectively [5, 6]. The overall function of this organelle is still elusive but evidence exists that it is involved in type II fatty acid, isoprenoid, iron-sulphur cluster and haem synthesis [6].
10
II
–
P
LASMODIUM AND MALARIAAlthough the global malaria burden has decreased in last few years, it still exerts a severe negative impact on the social and economic development of most African and South-East Asian countries. According to World Health Organization estimates, there were 219 million malaria cases worldwide in 2010, including 660 thousand deaths. Most of the cases (80%) and deaths (91%) occur in sub-Saharan Africa. Strikingly, 86% of the malaria deaths took place in children under 5 years of age. Plasmodium falciparum, the deadliest human parasite species, was responsible for 90% of the total malaria cases in that year [7].
Malaria is caused by parasites species of the genus Plasmodium belonging to the phylum Apicomplexa and is a complex disease including several different symptoms and clinical manifestations. The invasion of the liver and development of the parasite within hepatocytes are disease-free phases of the parasitic infection. The pathogenic process starts when parasites infect the red blood cells (RBCs) of its host. The first and most common signs of malaria infection are periodic fever peaks and chills. These symptoms occur at the time of erythrocyte rupture, a necessary step for the subsequent infection of new RBC and continuation of parasite asexual multiplication, and are believed to be caused by the release of malarial toxins. Consecutive RBC burst typically leads to anaemia [8]. Severe forms of disease exist and include cerebral malaria (CM), acute lung injury, acute respiratory distress syndrome, pregnancy-associated malaria (PAM) and severe anaemia (SA) [9]. In addition to SA, which affects mostly children under the age of 3, CM, accompanied by characteristic seizures and sometimes coma, is one of the leading causes of death in non-immune patients and African children [8]. The onset of this severe condition depends on high blood parasitaemias [8] but also on the concomitant sequestration of CD8-expressing T cells and infected RBCs (iRBCs) in the brain, as determined in animal models [10]. In P. falciparum, the endothelial cytoadherence feature of iRBCs depends on the expression of PfEMP1 (Erythrocyte Membrane Protein 1) at the surface of the iRBC, a protein encoded by the var family of genes [11, 12]. Non-immune women are particularly susceptible to PAM, which may lead to abortions and stillbirths. Other frequent consequences of PAM for the newborns are low birth weight, caused by intrauterine growth retardation and/or premature delivery, neonatal infection and mortality. These pregnancy outcomes are likely to be associated with the accumulation of iRBCs in the placental vasculature [8]. In agreement with this idea, VAR2CSA, a PfEMP1 variant, is implicated in iRBC sequestration in the placenta [13].
Malaria parasites are transmitted from and to mammalian hosts (human, simian and rodent) during the blood meal of female anopheline mosquitoes (Anopheles spp.), which feed on blood in order to produce and lay eggs. On the other hand, culicine mosquitoes such as
Aedes spp. and Culex spp. are, similarly to Anopheles spp., capable of transmitting P. gallinaceum and P. relictum to avian vertebrate hosts but have never been reported transmitting
mammalian parasites [14]. Out of the numerous Plasmodium species known to date, only four routinely cause disease in humans: P. falciparum, P. vivax, P. malariae and P. ovale [15].
11
Traditionally viewed as a zoonotic infectious agent, P. knowlesi has recently been reported in humans [16, 17]. Five major anopheline species act as human malaria vectors in Africa: A.
gambiae, A. arabiensis, A. funestus, A. nili and A. moucheti. They all belong to species
complexes that are very difficult to distinguish based on morphological characters. The predominance and overlap of these species largely depends on the geographic region and ecosystems but in certain areas as much as all five species co-habit and transmit parasites to humans [18].
The malaria parasite was first discovered in Algiers by Alphonse Laveran, a French army surgeon who observed flagellated cells moving in a blood sample from a malaria patient. He was observing what today is known as the exflagellation of male gametocytes [19]. The erythrocytic cycle and the name Plasmodium were established a few years later by Ettiore Marchiafava and Angelo Celli [20]. The vectorial capacity of Anophelles mosquitoes for
Plasmodium parasites was only discovered in 1902 by Ronal Ross, a British medical doctor
working at the Presidency General Hospital in Calcutta at the time. By carefully examining histological sections of stomachs from mosquitoes fed on malaria patients four days before he found perfectly round parasite forms: the oocyst [21]. One year later he describes the complete parasite ‘metamorphosis’ in the mosquito, including the ookinete to oocyst development, the release of sporozoites into the haemocoel and their journey to the salivary glands, using P.
relictum (then called Proteosoma) infecting sparrows and larks [22]. This major scientific
breakthrough earned Sir Ross the second Nobel Prize for Physiology or Medicine in 1902. However, it was only in the second half of the 20th century that the whole malaria life cycle became complete, when the English protozoologist Cyril Garnham (in collaboration with is colleague Hugh Shortt) unravelled the exoerythrocytic stage of species infecting non-human primate hosts [23-25] and later on of the human malaria parasites P. vivax [26, 27], P.
falciparum [28], P. ovale [29] and P. malariae [30].
The Plasmodium life cycle is complex. Within the RBCs, the majority of parasites follow an asexual multiplication cycle termed schizogony [31], while some develop into non-dividing sexual precursor cells called gametocytes [32, 33]. Following a mosquito blood meal, the ingested male (micro) and female (macro) gametocytes form male and female gametes, respectively; these mate inside the mosquito midgut and the resulting zygote develops into an ookinete. This motile parasite form escapes from the midgut peritrophic matrix, a chitin-containing sheath secreted by midgut cells to contain the blood meal [34], traverses the midgut epithelium and establishes a sessile oocyst that produces thousands of sporozoites. Oocyst rupture results in the release of motile sporozoites that migrate through the haemocoel to the mosquito salivary glands where they await another blood meal [35, 36]. After being injected into the skin of the vertebrate host (e.g., human or mouse model), the parasites migrate to the blood stream and reach the liver where they traverse a few cells before definitely establishing themselves within a hepatocyte [37]. There, a single sporozoite develops into an exoerythrocytic from (EEF), which releases thousands of newly formed, RBC-infective merozoites into the
12
Figure 1 – Life cycle of Plasmodium falciparum.
The complete life cycle including the liver and blood stages of infection in the human host, as well as the gut and salivary gland phases in the mosquito vector are depicted. [Adapted from Bannister and Mitchell,
Trends Parasitol 2003, 19(5): 209-13]
bloodstream [38]. Some species such as the non-human primate parasite P. cynomolgi and the human parasites P. vivax and P. ovale are capable of producing liver dormant cells called hypnozoites, responsible for malaria relapses (with concomitant blood parasitaemias) months or even years after the infectious bite took place. The discovery of this deviation to the standard life cycle is also due to the work of Garnham as well as other British and American scientists [39, 40]. Please see Figure 1 for a diagram of the complete life cycle of P. falciparum.
13
Figure 2 – Dynamics of Plasmodium development in the mosquito vector.
The sequence of developmental events (top) is accompanied by drastic fluctuations in parasite density (bottom). The plot should is merely an indication of changes in density over time and should not be regarded as an indication of absolute parasite numbers. [Adapted from Baton and Ranford-Cartwright,
Trends Parasitol 2005, 21(12): 573-80]
The developmental time frame of each one of Plasmodium life cycle steps is highly variable and species-dependent. For example, in P. berghei, a rodent malaria species, the exoerythrocytic phase takes about 48 hours, but can last up to 16 days depending on the parasite species [38]. The completion of the asexual blood cycle is achieved in 24 hours in P.
berghei [41], P. chabaudi (another species infecting rodents) [42] and P. knowlesi [43]. In P. falciparum this cycle takes approximately 48 hours [44-46] but is isolate-dependent.
Gametocyte maturation takes 7-8 days in the case of P. falciparum, passing through five morphological stages [32, 36], and 26-30 hours in P. berghei, only slightly longer than the duration of the asexual cycle [32]. Fertilisation occurs within approximately one hour after mosquito blood feeding and zygote-to-ookinete transformation (ZOT) in different Plasmodium species varies from 10 to 30 hours. The entire duration of parasite development within the mosquito, from the ingestion of infected blood to the presence of infective sporozoites in the salivary glands, is complete in approximately 14 days at 26 ○C in the case of P. falciparum [36] and takes at least 18 days at 20 ○C in the case of P. berghei.
Two major bottlenecks exist in the malaria life cycle, each one of them followed by extensive population expansion: they occur during transmission to and from the mosquito vector. While it is estimated that less than 5% of the 10–10,000 female gametocytes ingested during a blood meal successfully develops into oocysts [35, 36] (Figure 2), not more than a few hundred sporozoites are injected in the skin of the host during mosquito probing prior to a subsequent blood feeding [35, 38, 47]. In between, a less pronounced population constraint takes place during development within the mosquito: only a quarter of the sporozoites that are released from the oocysts to the mosquito hemolymph successfully reach the salivary glands, which can harbour up to 100,000 parasites [35]. Therefore, intervention strategies targeting such density limiting stages are likely to be successful, as substantially less parasitic cells need to be eliminated at those specific moments. Recently, a studied performed in
laboratory-14
controlled vector populations has shown that the administration of the transmission blocking (TB) drug atovaquone to infected mice reduces parasite burden and prevalence of infection in mosquitoes by 57 and 32%, respectively. Even so, this mild effect was capable of eliminating malaria from both the mosquito and mouse populations in only three cycles of transmission at low mosquito biting rates, i.e., one or two potentially infectious mosquito bites per mouse in each generation. At higher transmission rates, parasites were however not eliminated from either population, indicating that more efficacious TB strategies would be needed to achieve malaria elimination in this scenario [48].
15
III
–
P
LASMODIUM GENE REGULATIONThe Plasmodium proteome is tightly regulated throughout its intricate life cycle, with high percentages of stage-specific proteins [49-51]. How exactly malaria parasites control gene expression to achieve such specificity is still being unravelled but involves molecular mechanisms at the transcriptional, post-transcriptional and translational level. One very relevant observation with great impact on how these organisms regulate gene expression is that they do not possess the core RNA interference (RNAi) machinery components, such as Argonaute and Dicer [52].
The mutually exclusive expression of var genes has been extensively studied and depends to a great extent on epigenetic regulators such as chromatin-remodelling proteins, histone modification marks, histone variants and long non-coding RNAs (lncRNAs) [53]. Conserved eukaryotic epigenetic factors have been correlated with gene expression on a P.
falciparum genome-wide scale. The dynamic occupancy pattern of eight euchromatic and
heterochromatic histone marks in either histone H3 (H3K4me3, H3K9ac, H3K14ac and H3K56ac) or H4 (H4K8ac, H4K16ac, H4K20me1 and H4K4,8,12,16ac) displayed a strong positive correlation with transcript levels across the parasite genome and intraerythrocytic development cycle. Interestingly, and similarly to what is observed in plants, these histone modifications were mainly associated with the 5’ end of coding sequences and not with intergenic regions [54]. Non-coding RNAs (ncRNAs) may also contribute to epigenetic regulation of transcription by possibly binding to general histone-modifying enzymes and providing them with target specificity [55]. Recently, an unprecedented type of post-transcriptional gene regulation mechanism involving RNase II-mediated degradation of nascent RNAs was shown to contribute to the silencing of upsA-type var genes in P. falciparum, known to be associated with obstruction of brain blood vessels in cerebral malaria human patients [56].
Evidence suggests that nuclear positioning of specific loci participates in the activation/silencing of genes. For instance, silent var genes are observed in perinuclear clusters while they move towards the nucleus centre upon activation. Nonetheless, general active transcription sites are also observed at the nuclear periphery [57]. Overall P. falciparum genome architecture has also been recently implicated in gene regulation, with a strong correlation between the transcription of specific genes and their spatial localisation within the three-dimensional genome structure [58].
Unlike most eukaryotes, not many transcription factors (TFs) are known in the genomes of Plasmodium parasites. Only one family of TFs is known in malaria parasites, the Apicomplexan Apetala2 (ApiAP2) family [59], with 27 predicted members in P. falciparum and 26 in P. berghei (PlasmoDB version 11.1). Toxoplasma gondii strains on the other hand encode up to 68 of such proteins in their genomes ([60, 61] and ToxoDB version 11.0). This is a family of plant TFs characterised by the presence of at least one AP2 domain, a DNA binding domain of approximately 60 amino acids [62].
16
Given the scarcity of TFs, locus-specific, promoter-based transcriptional control of gene expression was long thought not to be the dominant mode of gene regulation in Plasmodium. A significant delay between the transcript and protein maximum abundances for a vast percentage of the malarial genes pointed towards this view [63]. Instead, post-transcriptional mechanisms would play a major role in achieving a tight regulation of gene expression [64]. But recent and current work done on the ApiAP2 gene family is challenging this theory.
Remarkably, the AP2 domains of different ApiAP2 proteins show specific affinities for distinct DNA motifs enriched in the upstream region of functionally-related genes, underscoring the importance of both cis- and trans-acting factors in parasite gene regulation [65]. In recent years, specific ApiAp2 TFs have been ascribed to defined biological processes and life cycle stage transitions. The ApiAP2 member PfSIP2 (SPE2-interacting protein) was shown to co-localise with PfHP1 (heterochromatin protein 1) and to bind almost exclusively to heterochromatic domains upstream of subtelomeric var genes containing the SPE2 DNA motif. Interestingly, this work described for the first time the participation of an ApiAP2 TF in the formation of heterochromatin (tightly condensed form of DNA) and thus in gene silencing in P.
falciparum blood stages [66]. Also in P. falciparum, it was recently demonstrated that sexual
commitment is dependent on the DNA-binding protein PfAP2-G [67], which in turn is negatively controlled by PfHP1 [68] and PfHda2 (histone deacetylase 2) [69]. Both these epigenetic regulators contribute to the maintenance of heterochromatin at the pfap2-g locus. Similarly, the
P. berghei ortholog PbAP2-G was also shown to be a master regulator of sexual differentiation
in this rodent malaria parasite species, while a second transcription factor, PbAP2-G2, modulates the numbers of gametocytes, as its absence does not result in the complete abolishment of gametocytogenesis [70]. AP2-O (Apetala2 in ookinetes) on the other hand was shown to regulate the expression of P. berghei ookinete-specific genes, particularly invasion-related factors, by binding to a DNA motif in their 5’ upstream regions [71]. The AP2 domain of another ApiAP2 TF in P. berghei, AP2-Sp (Apetala2 in sporozoites), was found to bind to a conserved cis element in the promoter region of known sporozoite-specific genes. In agreement with this finding, ablation of AP2-Sp led to the development of abortive oocysts in mosquito midguts, demonstrating its essentiality for sporozoite formation, most likely through the transcriptional activation of genes necessary for sporozoite morphogenesis [72]. On the other hand, AP2-L is essential for the development of malaria liver stages and responsible for the expression of uis3, uis4, exp1 and lisp1, genes encoding for proteins of the parasitophorous vacuole membrane (PVM) surrounding intrahepatic parasites. Although AP2-L-depleted parasites invade hepatocytes normally, they arrest development during liver schizogony [73]. Lastly, a non-ApiAP2 intraerythrocytic stage TF of P. falciparum but well conserved within the
Plasmodium genus was shown to activate the expression of pf1-cys-prx [74], a
trophozoite/schizont-specific peroxiredoxin family member [75, 76]. This TF was named PREBP (PRE Binding Protein) based on its ability to bind to DNA stretches including the 102 base pair-long Prx Regulatory Element (PRE) and contains four K-homology (KH) domains [74] characteristic of single-stranded DNA and RNA binding proteins [77, 78].
17
Regardless of the continuously growing evidence that TFs are master regulators of parasite virulence and development, co-transcriptional and post-transcriptional mechanisms of gene regulation are critical players in controlling gene expression in Plasmodium. One example of co-transcriptional mechanisms is pre-mRNA alternative splicing. Approximately 53% of P.
falciparum genes are predicted to contain introns ([79] and PlasmoDB version 11.1) and many
of these give rise to alternative mRNA splice variants, which arise through use of alternative 5’ and/or 3’ splice sites, intron retention/creation or exon skipping. Splicing can also be observed in the 5’ and 3’ untranslated regions (UTRs) of certain genes [80, 81]. In some cases, these alternative splicing events generate mature mRNAs containing premature stop codons [81], which might encode for non-functional proteins or instead, protein variants with distinct functions and subcellular localisations [82]. One important post-transcriptional mechanism of malaria gene expression control is translational repression (TR), by which specific mRNAs are kept translationally quiescent in the parasite cytoplasm in association with protein repressor complexes until the right environmental cues trigger their release from such complexes and initiate protein synthesis. TR in Plasmodium and as a general evolutionarily conserved mechanism will be described in greater detail in the next section of this Introduction.
Finally, a polysome profiling study along the parasite erythrocytic cell cycle identified a delay between the peak of mRNA abundance in steady-state versus that associated with polysomes for over 30% of P. falciparum genes, implying a strong translational control of gene expression. Abundant and widespread transcription of ncRNAs which could influence translation efficiency was also highlighted in the same work [83]. Over 300 antisense, ncRNAs were also detected in another study on P. falciparum blood stages and believed to act as silencers of the cognate genes in the transition from asexual to sexual development [84].
18
IV
–
T
RANSLATIONAL REPRESSIONIV.1–TRANSLATIONAL REPRESSION IS AN ANCIENT AND CONSERVED MECHANISM
TR is a broad molecular concept that consists in inhibiting the translation of certain genes/transcripts. It is an evolutionarily conserved post-transcriptional gene regulatory process exerted by both prokaryotic and eukaryotic organisms which general principle is to interfere with the formation of translational initiation complexes.
In bacteria this can be achieved by three mechanisms: a temperature-induced mRNA conformational change that generates a cis-acting mRNA sequence responsible for preventing ribosomal 30S subunit access to the ribosome binding site (RBS), binding of a protein to its own mRNA in a negative loop feedback effect and binding of a trans-acting allosteric repressor to the mRNA. The first two mechanisms are called competitive mechanisms as they compete with the ribosome for binding to the RBS. The latter one is an entrapment mechanism, as the repressor does not impair interaction of ribosomal subunits with the RBS, but instead induces entrapped ternary complexes that inhibit the formation of stable translational initiation complexes [85, 86].
In eukaryotes, TR is accomplished through the binding of microRNAs or RNA binding proteins to target mRNAs [87-89]. Although not fully dissected, a model for the involvement of microRNAs in TR suggests they interact with GW182 and Argonaute proteins leading to: (i) competition with the translation initiation factor eIF4G for association with poly(A)-binding protein (PABP), (ii) prevention of 60S-40S ribosomal subunit interaction to form 80S ribosomes, (iii) ribosome stalling along the mRNA, (iv) premature translation termination and/or (v) co-translational degradation of synthesised proteins [89]. One of the best studied families of translational repressors is the Puf family of RNA binding proteins. This is an evolutionarily conserved family present in highly divergent eukaryotic organisms, from yeast to zebrafish,
Xenopus, mouse, humans and plants, but also in the invertebrates Drosophila, Anopheles and Caenorhabditis elegans. Puf proteins are related to the Pumilio protein of Drosophila and the
fem-3 mRNA binding factor of C. elegans (founder members of the family) and typically contain eight tandem Pumilio homology domains (PUM-HDs). They control mRNA translation by promoting its repression or degradation and generally interact with transcripts by binding to conserved motifs [UGUA/G(N)1-2AUA] on their UTRs, often the 3’ UTR, the so called Pumilio binding elements (PBEs) [90-93].
TR allows a bi-dimensional regulation of gene expression (i.e., both in time and in space). For example, in the case of Drosophila, hunchback mRNA (encoding for a transcription factor critical for embryonic patterning) is specifically repressed in posterior regions of the fly embryo. This is mediated by the binding of a protein complex containing Pumilio and Nanos proteins to the Nanos response elements (NREs) on hunchback 3’ UTR and ensures that Hunchback protein is exclusively expressed at the anterior pole of the embryo [94, 95]. The