BJRS
RADIATION SCIENCES
07-2A (2019) 01-14ISSN: 2319-0612 Accepted: 2018-12-18
Influence of adsorption parameters on uranium adsorption
capability by biochar derived from macauba coconut
residue
S. N. Guilhen
a; J. Coleti
b; J. A. S. Tenório
b; D. A. Fungaro
a aComissão Nacional de Energia Nuclear, Instituto de Pesquisas Energéticas e Nucleares, Centro de Química e Meio Ambiente, 05508-000, São Paulo, SP, Brasil.
snguilhen@ipen.br
b
Universidade de São Paulo, Escola Politécnica, Departamento de Engenharia Metalúrgica e de Materiais, 05508-030, São Paulo, SP, Brasil.
ABSTRACT
Biochar (BC) is a carbon-rich product obtained when biomass is thermally decomposed at relatively low temperatures (under 700ºC) and limited supply of oxygen in a process called pyrolysis. Because of its porous structure, charged sur-face and sursur-face functional groups, BC exhibits a great potential as an adsorbent. Its characteristics strongly depend on the feedstock and the pyrolysis conditions. The aim of this study was to evaluate the adsorption potential for the remov-al of uranium, U(VI) from aqueous solutions using BC obtained through slow pyrolysis of the macauba coconut endo-carp. The influence of parameters such as pH, sorbent dose and initial concentration on the adsorption of U(VI) was investigated. The BC obtained at 350 °C (BC350) presented a removal percentage of approx. 80 %, demonstrating its applicability for the treatment of uranium contaminated aqueous solutions.
1. INTRODUCTION
Radioactive uranium aqueous wastes emerge as a result of many distinct nuclear activities such as mining, research, fuel cycle and nuclear medicine. Environmental issues have become increasingly important in the last few decades especially due to the large number of uranium production facilities which have been taken out of operation recently. Decommissioning and rehabilitation have become a concern, since the remediation of formerly utilized sites involves a broad investigation of the envi-ronment and the extent to which it has been affected, before applying the necessary administrative and technical actions to restore it under the auspicious control of regulatory agencies [1].
Monitoring of the water from uranium mills is required to ensure that all constituents are within regulatory compliance. Equally, the treatment of uranium contaminated wastewater is essential be-fore its discharge into the environment. Combined techniques and operations such as chemical pre-cipitation, reverse osmosis, solvent extraction, micellar ultrafiltration and adsorption are normally required for the treatment of uranium contaminated wastewaters and liquid radioactive wastes [2, 3]. Among the possible techniques, adsorption stands out for its efficiency and specificity, requiring a simple design and low investment [4].
Several adsorbents for uranium have been reported in literature comprising biological adsorbents such as the fungus Aspergillus niger [5], the green algae Scenedesmus obliquus [6], the aquatic plant E. Canadensis [7] and the fungus Rhizopus arrhizus [8], inorganic adsorbents such as the modified bentonite [9] and organic adsorbents such as carbon microspheres [10] and IPN poly-mer [11].
Agricultural by-products have been studied as a sustainable solution for wastewater treatment [12], providing a wide range of renewable sources for the production of biochar, a porous stable material. Several studies have demonstrated that biochar can be applied for wastewater treatment because it effectively removes heavy metals from aqueous solutions [13, 14]. Biochars derived from pine nee-dles, switchgrass, rice husk and eucalyptus [15–18] have been applied for uranium sorptive removal from aqueous solutions.
Biochar may be produced using slow pyrolysis technique, in which the biomass is heated to rela-tively low temperatures (< 700 °C) in the absence of oxygen [19]. Heating rate, residence time and
temperature have a direct impact in the distribution and yield of each of the biochar [20, 21]. Of these parameters, the maximum temperature to which the biomass is subjected in the pyrolysis reac-tor, called highest treatment temperature (HTT) has the greatest overall influence on the biochar properties [22, 23, 24].
Macauba (Acrocomia aculeata) is a palm tree prevalent in the Brazilian savannah, which coconut is used to produce oil. As a residue of the nut-oil extraction, the endocarp has a potential to be used as feedstock for biochar production, because of its elevated lignin content [25]. By doing so, value is imparted to the residue.
In this study, the influence of parameters such as pH, adsorbent dose and initial concentration on the adsorption of U(VI) was investigated for the macauba biochar obtained at 350 °C (BC350) in order to optimize the adsorption conditions for the removal of U (VI) from aqueous solutions. The selec-tion of the optimal parameters is part of the preliminary studies which allow to maximize the BC’s performance, being very important, not only for the application itself, but for the realization of fur-ther experiments and characterizations.
2. MATERIALS AND METHODS
2.1. Materials
The macaúba coconut endocarp was supplied by Soleá (João Pinheiro, MG, Brazil). After skin and pulp removal from the coconut, the chestnut, constituted by the endocarp and almond, was broken using a jaw crusher, allowing the separation of the endocarp.
A standard solution of 1000 mg L-1 of uranium was prepared by dissolution of a U3O8 certified ref-erence material (CRM 129-A, natural 238U) supplied by New Brunswick Laboratory (New Bruns-wick, NJ, USA). This solution was used to prepare diluted solutions used for adsorption and cali-bration experiments. All solutions were prepared using ultrapure water (18.2 MΩ cm resistivity) and Merck’s analytical grade nitric acid, HNO3 65 % (Darmstadt, Germany).
After sampling for removal of dirt and unbroken coconuts, the endocarp were shredded in a cutting mill passing over a 3/8 Mesh screen and subsequently oven-dried at 100 ºC for 3 hours.
2.3. Biochar production
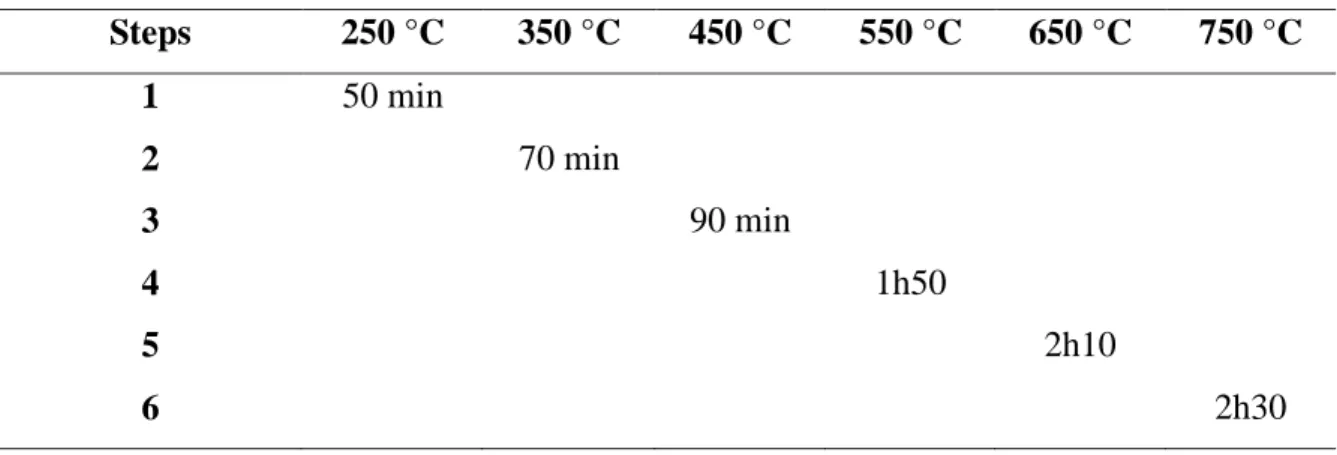
Pyrolysis of the endocarp was conducted using a Thermo Fisher (Asheville, NC, USA), Lindberg Blue M, horizontal tubular steel reactor heated by an electric furnace at the Laboratory of Recy-cling, Waste Treatment and Extraction (Polytechnic School, University of Sao Paulo) under inert argon atmosphere. Six different temperatures were independently adjusted and ranged from 250ºC to 750ºC. The carbonized samples were identified as “BCT”, in which “T” corresponds to the HTT; for instance: BC250 when the HTT is 250ºC. The dried endocarp was subjected to six different HTTs, as shown in Table 1.
For each HTT, approximately 30 g of endocarp was processed applying an argon (Ar) gas flow of 40 mL min-1. The sample was kept at each HTT for 1 h (residence time). By the end of the process, the furnace was switched off and the reactor was allowed to cool.
Table 1: Pyrolysis heating steps at a heating rate of 5 °C min-1.
Steps 250 °C 350 °C 450 °C 550 °C 650 °C 750 °C 1 50 min 2 70 min 3 90 min 4 1h50 5 2h10 6 2h30
The argon flow proceeded during the cooling process in order to purge the reactor of any remaining pyrolysis gases and to prevent any oxygen exposure of the biochar while still above ignition tem-perature.
After that, the alumina crucible containing the biochar was removed from the furnace and allowed to cool at room temperature in a desiccator. Finally, the biochar was ground in a cutting mill pass-ing over a 120 Mesh screen and stored in hermetic polypropylene bottles.
2.4. Sorption experiments
Equilibrium adsorption experiments were conducted in triplicates using batch technique and the experiments were performed in a rotary shaker using 100 mL glass beakers, 120 rpm stirring rate at room temperature (25 °C) during 24 hours contact time, to ensure the attainment of the system’s equilibrium. The adsorbent was separated by filtration using a SCP Science (Baie-D’Urfé, QC, Canada) 0.45 micron Teflon membrane filter (DigiFILTER).
The U(VI) concentration in the reminiscent filtrate solution was subjected to a determination by inductively coupled plasma optical emission spectrometry using a Spectro ARCOS ICP OES (Kleve, NRW, Germany). The adsorption of U(VI) was evaluated as a function of pH, adsorbent dose, initial concentration and contact time. The initial pH was adjusted using 0.1 mol L-1 HNO3 and 0.1 mol L-1 NaOH solutions.
The adsorption capacity, qt (mg g-1), of the adsorbent was calculated using the Eq. (1):
(1)
where qt is the adsorbed amount of adsorbate per gram of adsorbent at any time t, C0 and Ct the concentrations of the adsorbate in the initial solution and at any time t, respectively (mg L-1); V the volume of the adsorbate solution added (L) and M the amount of the adsorbent used (g).
The extraction efficiency, R (%), was determined through the following equation:
where R is the efficiency of extraction or percentage removal, C0 (mg L-1) is the initial concentra-tion of each adsorbate and Ct (mg L-1) represents the concentration of the adsorbate at time t.
3. RESULTS AND DISCUSSION
3.1 Effect of pH
The pH of the aqueous solution affects the surface charge of the adsorbents as well as the degree of ionization and speciation of the solute. The pH values were evaluated in a range between 2.0 and 7.0, while the other parameters (adsorbent dosage of 10 g L-1 and U initial concentration of 5 mg L -1
) were held constant.
BC350 was selected for the preliminary experiments based on a previous study [26], in which the gravimetric yield factor was used to identify the temperature at which to obtain a biochar with the maximum fixed carbon content and gravimetric yield. In this case, the gravimetric yield obtained for BC350 was of 46.09 % and the fixed carbon content was of 60.22 %, rendering a gravimetric yield factor of 0.28 %, which, by comparison, qualified 350 °C as the operational HTT.
The influence of pH on the removal of U(VI) ions onto macauba biochar is presented in Figure 2.
Figure 1: Effect of initial pH on the adsorption capacity of U(VI) onto BC350; C0(U) = 5 mg L-1,
As can be seen in Figure 2, the adsorption ability for uranium onto BC350 strongly depends of the pH value. The uptake amount of U(VI) reached the maximum adsorption capacity at pH 3.0. At lower pH values (pH < 3.5), over 95 % of the uranium is present as free uranyl ions (UO22+). How-ever, when the solution pH is too low, the amount of protons (H+) can compete with the uranyl cati-ons for adsorption sites in the biochar. Thus, the adsorption decreases when pH < 3. However, as the solution pH is gradually increased, the presence of different mononuclear and polynuclear ura-nium (VI) hydrolysis products in the form of [(UO2)p(OH)q](2p-q)+ responds for the decrease of the adsorption, since these species have lower affinity with the adsorbent [7].
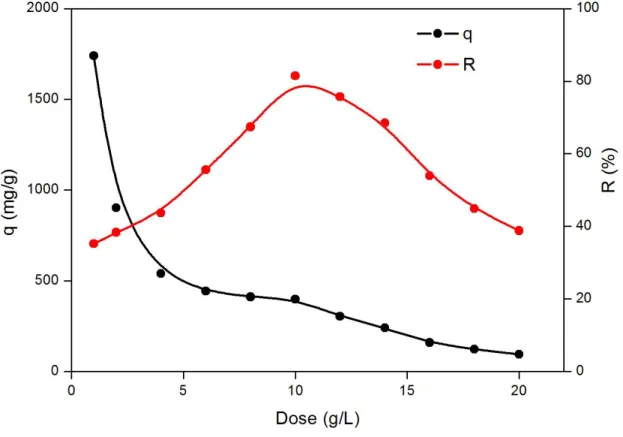
The adsorbent dosage is an important parameter because it is associated to the determination of the adsorption capacity of an adsorbent for a given concentration of adsorbate. The adsorption studies of U(VI) onto biochar materials were conducted by varying the adsorbent dosage from 1 to 20 g L-1. The influence of adsorbent dosage on adsorption of U(VI) ion is shown in Fig. 3.
Figure 2: Effect of the adsorbent dose on the adsorption of U(VI) onto BC350; C0(U) = 5 mg L-1,
pH = 3, contact time = 24 h; T= 25 ± 2 °C.
As seen in Figure 3, increasing the adsorbent dose up to 20 g L-1 substantially decreases the adsorp-tion capacity (the amount adsorbed per unit mass of adsorbent) for U(VI), which is mainly due to the sites remaining unsaturated during the adsorption process and can be mathematically explained [27]. Another reason may be attributed to the particle interactions, such as aggregation, caused by high adsorbent concentration [28]. Such aggregation would lead to a decrease in the total surface
area of the adsorbent [29]. Thus, further increase of adsorbent dosage does not afford exhaustive adsorption of U(VI). However, in terms of removal, the maximum effect is observed when a dose of 10 g L-1 is used. Therefore, this dose was adopted for adsorption experiments.
3.3. Effect of initial concentration
The initial concentration (C0) of the U(VI) aqueous solution was evaluated for a range of concentra-tions from 1 to 50 mg L-1. The experiments were performed using a dose of sorbent of 10 g L-1 and adjusting the solutions to pH 3.
The influence of initial concentration (C0) on adsorption of U(VI) ion is shown in Figure 4. An in-crease of the adsorption capacity (qt) is observed when C0 was increased from 1 to 5 mg L-1, reach-ing a qt maximum at 5 mg L-1. As the concentration was increased to higher concentrations, a steady decrease of qt was observed until 25 mg L-1 after which it appears to assume a constant be-havior.
Figure 3: Effect of the initial concentration on the adsorption of U(VI) onto BC350; pH = 3;
This is in accordance to the selected dose, which was optimized for the given initial concentration (C0 = 5 mg L-1). In the event of working with more concentrated solutions, new correlations to other doses should be evaluated.
3.4. Adsorption on BC350
The adsorption of U (VI) onto BC350 was investigated using an initial U concentration of 5 mg L-1, pH 3 and 10 g L-1 biochar dose. The adsorption capacity obtained for BC350 was of 408 mg g-1 and the removal efficiency was of 80.1 %. These results point to the promising potential of the macauba biochar as an adsorbent for the treatment of uranium containing aqueous solutions and were enabled through the optimization of the adsorption conditions, at which the BC350 can be applied to its
maximum efficiency. Additionally, it must be emphasized that the obtained biochar didn’t undergo activation steps.
4. CONCLUSION
Batch experiments were employed to evaluate the adsorption capacity of the biochar derived from macauba coconut residue for removing uranium ions from aqueous solutions aiming its application for the treatment of liquid wastes and contaminated wastewaters. The biochar was obtained using slow pyrolysis under inert argon atmosphere. The results revealed that the optimal conditions were achieved at pH 3, adsorbent amount of 10 g L-1 and U (VI) initial concentration of 5 mg L-1. A re-moval efficiency of 80 % was achieved for BC350 using a heating rate of 5 °C min-1 and a resi-dence time of 1 h. The results demonstrated the potential of the macauba biochar as a value-added material for uranium removal from aqueous radioactive wastes.
5. ACKNOWLEDGMENT
Special thanks to Soleá (João Pinheiro, MG, Brazil) for supplying the macauba endocarp.
REFERENCES
1. NEA/OECD - Nuclear Energy Agency Organization for Economic Co-Operation and Develop-ment, Environmental Activities in Uranium Mining and Milling, Paris: OECD, 1999, 177p. 2. SAKR, K.; SAYED, M.S; HAFEZ, M. B. Immobilization of radioactive waste in mixture of ce-ment, clay and polymer. Journal of Radioanalytical Nuclear Chemistry, v. 256, n. 2, p. 179-184, 2003.
3. OZDEMIR, T.; USANMAZ, A. Use of poly(methyl methacrylate) in radioactive waste manage-ment: I, radiation stability and degradation. Progress in Nuclear Energy, v. 51, n. 2, p. 240-245, 2009.
4. IAEA – International Atomic Energy Agency, Combined methods for liquid radioactive waste
treatment, IAEA-TECDOC-1336, Vienna: IAEA, 2003, 250p.
5. TSURUTA, T. Removal and recovery of uranyl ion using various microorganisms. Journal of
Bioscience and Bioengineering, v. 94, p. 23–28, 2002.
6. ZHANG, X. Z.; LUO, S. G.; YANG, Q.; ZHANG, H. L.; LI, J. Y. Accumulation of uranium at low concentration by green alga Scnenedesmus obliquus. Journal of Applied Phycology, v. 9, p. 64–71, 1997.
7. YI, Z.; YAO, J. ZHU, M.; CHEN, H.; WANG, F.; YUAN, Z.; LIU, X. Batch study of uranium biosorption by Elodea Canadensis biomass. Journal of Radioanalytical Nuclear Chemistry, v. 310, p. 505–513, 2016.
8. WANG, S.; PENG, Y. Natural zeolites as effective adsorbents in water and wastewater treatment.
Chemical Engineering Journal, v. 156, p. 11-24, 2010.
9. ZAREH, M. M.; ALDAHER, A.; HUSSEIN, A. E. M.; MAHFOUZ, M. G.; SOLIMAN, M. Uranium adsorption from a liquid waste using thermally and chemically modified bentonite.
Jour-nal of RadioaJour-nalytical Nuclear Chemistry, v. 295, p. 1153–1159, 2013.
10. ZHANG, X. F.; WANG, J.; LI, R. M.; LIU, Q.; LI, L.; YU, J.; ZHANG, M. L.; LIU, L. H. Effi-cient removal of uranium (VI) from aqueous systems by heat-treated carbon microspheres.
Envionmnetal Science Pollution Research, v. 20, p. 8202–8209, 2013e.
11. AYCIK, G. A.; GURELLIER, R. Comparative studies for the determinations of uranyl ion ad-sorption onto interpenetrating polymer networks (IPNs). Journal of Radioanalytical Nuclear
Chemistry, v. 273, n. 3, p. 713–718, 2007.
12. DE GISI, S.; LOFRANO, G.; GRASSI, M.; NOTARNICOLA, M. Characteristics and adsorp-tion capacities of low-cost sorbents for wastewater treatment: A review. Sustainable Materials
and Technologies, v. 9, p. 10-40, 2016.
13. INYANG; M. I.; GAO, B; YAO, Y.; XUE, Y; ZIMMERMAN, A.; MOSA, A; PULLAMMANAPPALLIL, P.; OK, Y. S.; CAO, X.A review of biochar as a low-cost adsorbent for aqueous heavy metal removal. Critical Reviews in Environmental Science and Technology, v. 46, n. 4, 2016.
14. PATRA, J. M.; PANDA, S. S.; DHAL, N. K. Biochar as a low-cost adsorbent for heavy metal removal: A review. Internat J of Res in Biosci, v. 6, n. 1, p. 1-7, 2017.
15. ZHANG, Z. B.; CAO, X. H.; LIANG, P.; LIU, Y. H. Adsorption of uranium from aqueous solu-tion using biochar produced by hydrothermal carbonizasolu-tion. Journal of Radioanalytical Nuclear
16. KUMAR, S.; LOGANATHAN, V. A.; GUPTA, R. B.; BARNETT, M. O. An assessment of U (VI) removal from groundwater using biochar produced from hydrothermal carbonization. Journal
of Environmental Management, v. 92, p. 2504–2512, 2011.
17. WANG, S.; GUO, W.; GAO, F.; WANG, Y.; GAO, Y. Lead and uranium sorptive removal from aqueous solution using magnetic and nonmagnetic fast pyrolysis rice husk biochars. RSC
Ad-vances, v. 24, 2018.
18. MISHRA, V.; SURESHKUMAR, M. K.; GUPTA, N.; KAUSHK, C. P. Study on sorption char-acteristics of uranium onto biochar derived from eucalyptus wood. Water, Air, & Soil Pollution, v. 228, n. 8, 2017.
19. LEHMANN, S. J. Biochar for Environmental Management, 1st ed. Oxford: Earthscan, 2009. 20. BRIDGWATER, A. V.; PEACOCKE, G. V. C. Fast pyrolysis processes for biomass.
Renewa-ble SustainaRenewa-ble Energy Reviews, v. 4, p. 1-73, 2000.
21. LU, Q.; LI, W-Z.; ZHU, X-F. Overview of fuel properties of biomass fast pyrolysis oils.
Ener-gy Conversion and Management, v. 50, p. 1376-1383, 2009.
22. ANTAL, M. J. J.; GRONLI, M. The art, science, and technology of charcoal production.
Indus-trial and Engineering Chemistry Research, v. 42, p. 1619-1640, 2003.
23. LUA, A. C.; YANG, T.; GUO, J. Effects of pyrolysis conditions on the properties of activated carbons prepared from pistachio-nut shells. Journal of Analytical and Applied Pyrolysis, v. 72, p. 279-287, 2004.
24. ÖZÇIMEN, D.; ERSOY-MERIÇBOYU, A. A study on the carbonisation of grapeseed and chestnut shell. Fuel Processing Technology, v. 89, p. 1041-1046, 2008.
25. RIOS, R. D. F.; FONSECA, R. M.; CREN, E. C.; ANDRADE, M. H. C. Adsorção de fenol no carvão ativado produzido a partir do endocarpo do fruto da macaúba. In: XX Congresso Brasileiro
de Engenharia Química, 2014, Florianópolis. Annals... Florianópolis: COBEQ 2014.
26. GUILHEN, S. N.; MASEK, O.; ORTIZ, N.; FUNGARO, D. A. Pyrolytic temperature evalua-tion of macauba biochar for uranium adsorpevalua-tion from aqueous soluevalua-tions. In: Biochar: Producevalua-tion,
Characterization and Applications Conference, 2017, Alba, Annals… Alba: ECI Conference,
2017.
27. ROYER, B.; CARDOSO, N. F.; LIMA, E. C.; RUIZ, V. S. O.; MACEDO, T. R.; AIROLDI, C. Organofunctionalized kenyaite for dye removal from aqueous solution. Journal of Colloid and
Interface Science, v. 336, p. 398-405, 2009.
28. ŐZACAR, M.; SENGIL, I. A.Adsorption of metal complex dyes from aqueous solutions by pine sawdust. Bioresource Technology, v. 96, p. 791-795, 2005.
29. SHUKLA, A.; ZHANG, Y. H.; DUBEY, P.; MARGRAVE, J. L.; SHUKLA, S. S. The role of sawdust in the removal of unwanted materials from water. J Hazard Mat, v. 95, p. 137-152, 2002.