THE IMPACT OF CALCULATION ON THE VALUE OF THERMODYNAMIC PARAMETERS OF COPPER ADSORPTION ON POPLAR SAWDUST
Mirjana M. Brdar*, MКrТЧК B. ŠćТЛКЧ КЧН AХОФsКЧНКr A. TКФКĉТ
University of Novi Sad, Faculty of Technology, Bulevar cara Lazara 1, 21000 Novi Sad, Serbia The adsorption of Cu(II) onto poplar sawdust as an adsorbent is analyzed. The expe-rimental data were fitted by the Langmuir isotherm using four linearized forms at the isotherm along with the original one. The least squares regression method was applied. Using the obtained Langmuir constants by each at methods, the enthalpy, entropy and Gibbs free energy at adsoption were calculated. A comparison of the used linear and non-linear regression methods in view at the goodness of the fit is presented. The coeffi-cient of correlation was adopted as a criterionn to select the best method. The impact of the choice at regression model on the resulting estimates of the thermodynamic para-meters is discussed. The best fit of the experimental data is obtained by the nonlinear regression. Thus, it is recommended to use the Langmuir parameters calculated by the nonlinear regression for estimating the thermodynamic parameters of adsorptin. The differences in the values obtained by different models are not so large to change the basic conclusion that the adsorption of copper ions on poplar sawdust is a spontaneous endo-thermic process i.e. that tested adsorbent has an affinity for copper ions.
KEY WORDS: thermodynamic parameters, Langmuir isotherm, poplar sawdust,
adsorption, copper
INTRODUCTION
The main objective of this study is to evaluate binding characteristics of Cu(II) on Kraft lignin, and to scrutinize feasible application of different adsorption models for description of adsorption on this low-cost and environment-friendly adsorbent. Even though copper is generally considered to be non-toxic for humans, it is known that at a concentration exceeding 5 mg/L it imparts color and undesirable taste to water, and can МКusО КМutО КЧН МСrШЧТМ НТsШrНОrs. TСО АШrХН HОКХtС OrРКЧТгКtТШЧ‟s РuТНОХТЧО ПШr НrТЧФ -ing water (1) recommends maximum permissible concentration for copper of 1 mg/L.
The effectiveness of an adsorbent is estimated on the bases of its capacity, adsorption rate, its mechanical strenght, possibility of regeneration and reuse, and so on. Various mathematical models are used in such evaluations. From experimental data of equilibri-um adsorption at different temperatures, adsorbent capacity, afinity and adsorption
modynamic parameters can be estimated. For the calculations of thermodynamic para-meters the value of coefficient KL of the Langmuir adsorption isotherm, is needed. Thus, the proper setting of KL is essential for a reliable evaluation of the thermodynamic para-meters. If the thermodynamic parameters were properly assessed, they could provide in-depth information regarding the inherent energy and structural changes after adsorption. However, a caution should be paid to any conclusions thus drawn, since the enthalpy and the entropy are not evaluated independently.
Success of Langmuir isotherm undoubtedly reflects its ability to fit a wide variety of adsorption data quite well, but it may also partly reflect the appealing simplicity of the isotherm equation and the ease with which its adjustable parameters can be estimated. Using these two parameters the isotherm can be transformed to a linear form that pro-vides a simple estimation of the parameters by linear regression. This ease of fitting may lead to the Langmuir isotherm enjoying rather more reputation than it deserves since a closer examination often reveals systematic deviations of experimental data from the cal-culated isotherm. Just for these reasons in this paper we examine the impact of the choice among various linearised forms at the isotherm on the reliability of the estimates of the thermodynamic parameters.
The linear method of least squares has been frequently used for finding the parameters of the isotherm (2-5). The transformations of non-linear isotherm equations to linear forms implicitly alter their error structure and may also violate the error distribution and the normality assumptions on which the standard least squares method is based. This implies from a rigorous statistical analysis at calculated parameters.
In this study we analyzed four linear and one non-linear form of Langmuir isotherm model (Table1) and thermodynamic parameters obtained for each model. The method of least squares was used to predict the isotherm by linear regression method. For non-linear regression, the numerical minimization method implemented in Mathematica 6, Wolfram Research software tool, was used.
EXPERIMENTAL
Materials
Water solutions of CuSO4 of different concentrations, were prepared by dilution of 0.25 mol/l stock solutions with distilled water, just before experiments. All chemicals used were of analytical reagent grade.
Kraft lignin was obtained from the sulphate pulping process of poplar and beech wood (70:30) conducted in a pulp mill. Lignin was precipitated from black liquor with sulphuric acid, and washed with distilled water. After filtering and drying at room tem-perature, the lignin was obtained as a fine black-brown powder (particle size <0.25 mm).
Batch adsorption studies
and this pH value is below the on set of copper hydroxid precipitation. The suspension was shaken at 110 rpm, at two temperatures, 20oC and 35oC, up to 3 hours, which was the sufficient contact time (7). The lignin was then filtered through the Gooch G4 crucible, and residual copper ions in the filtrate were determined. The concentration of heavy metal ions in the aqueous solution before ( ) and after adsorption ( ) was determined using a Pye-Unicam SP 191 atomic absorption spectrophotometer. Experiments were carried out in duplicate and the results averaged.
The amount of heavy metal ions adsorbed per specified amount of adsorbent ( ) was calculated as follows:
[1]
Adsorption models
The Langmuir adsorption model
[2]
was used to describe the isothermal equilibrium adsorption, where qm is the maximum amount of heavy metal ions required to form a monolayer on the surface, KL is the LКЧРЦuТr‟s ОquТХТЛrТuЦ МШЧstКЧt, rОХКtОН tШ tСО КППТЧТtв ШП tСО ЛТЧНТЧР sТtОs. TСО LКЧР -muir isotherm is the most commonly used model to study the relation between the con-centration of solute in liquid and solid phases at equilibrium conditions. The Langmuir isotherm equation (8) is derived from simple mass kinetics, assuming monolayer chemi-sorption. This model is based on the assumptions that all sorption sites are equal (ho-mogenous surface), forces of interaction between sorbed molecules are negligible, once a molecule occupies a site no further sorption takes place. In this paper, the experimental data were fitted with four different linearized forms of the Langmuir isotherm, as well as well using the original nonlinear form [2] (Table 1).
Table 1. Models of ishoterm equation
Isotherm Equation Plot
Non-linear model Langmuir q vs.
Linear model
Langmuir 1 vs.
Langmuir 2 vs.
Langmuir 3 vs.
The linearized form of the type Langmuir 1 is the most commonly used linear ex-pression. The expressions of the Langmuir 2 type were also used to explain the equilibria phenomena of dye adsorption process (9). The Langmuir constants qm and KL can be calculated from the plots: C/q versus C, 1/q versus 1/C, q versus q/C, and q/C versus q linearised equations, of the types 1, 2, 3 and 4, respectively.
Adsorption thermodynamics
In the design of the sorption systems, two types of thermodynamic properties, namely the directly measurable properties like temperature, and properties which cannot be mea-sured directly such as equilibrium constant, entropy, S, and free energy, G, are required. Thus, to evaluate the thermodynamic characteristics which cannot be directly measured, the Gibbs free energy, G, was used. The Gibbs free energy and entropy are used to deter-mine the nature of sorption chemical reactions or the nature of the sorption process. The reaction occurs spontaneously at a given temperature if the change of the Gibbs free energy, G has a negative value.
The changes of the thermodynamic parametersG, H and S were computed from the following equations. The standard molar or Gibbs free energy (G) of the adsorption process was calculated as:
[3]
where is the universal gas constant and T the temperature (K).
The standard enthalpy change (H) was calculated from the values of KL parameter at two temperatures T1 andT2 as:
[4]
where KL1 and KL2 are the respective Langmuir parameters at these two temperatures. Finally, the standard entropy change (S) for the process was calculated from the fun-damental thermodynamic relation:
[5]
RESULTS AND DISCUSSION
On the basis of experimental data, it is necessary to determine Langmuir constants, in order to get the estimates of the thermodynamic parameters G, H and S. Various pos-sible ways of determining Langmuir constants result in different values, accompanied by the differences in the estimation of thermodynamic parameters. The coefficient of de-termination was used as an indicator of the isothermal model fiting ability.
transfor-mation of the Langmuir isotherm equation to linear forms shifts the position of minimum of the sum of residual squares. Also, it implicitly alters the error structure, and may also violate the error variance and normality assumptions of the standard regression method. The linear methods using different linearized forms of the Langmuir equation may significantly affect the calculated values of the Langmuir parameters. In this study, the deviations of parameters obtained with various linear models are moderate.
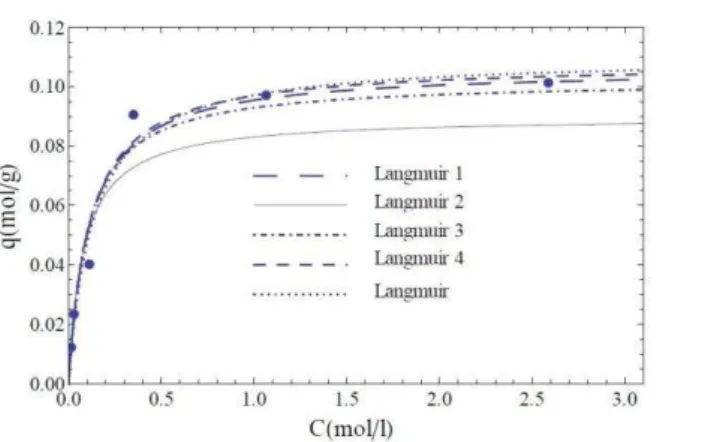
Figure 1. Langmuir isotherms obtained by non-linear method
The entropy, enthalpy and Gibbs free energy changes calculated by equations [3]-[5] with values obtained with various models are presented in Table 2.
Table 2. Adsorption isotherm and thermodynamic parameters obtained by Langmuir model
T
Langmuir 295 20.4918 0.039189 0.979767 -24.3492 58.5240 0.280926
308 7.48479 0.110180 0.968556 -22.8432 0.264179
Langmuir 1 295 14.4417 0.040281 0.960644 -23.4910 27.6258 0.173277
308 8.97732 0.106089 0.961677 -23.3088 0.165372
Langmuir 2 295 22.3207 0.038236 0.973723 -24.5588 35.0254 0.201981
308 12.2161 0.089858 0.856766 -24.0976 0.191958
Langmuir 3 295 21.3509 0.038898 0.978496 -24.4499 44.0462 0.232190
308 10.0051 0.102200 0.949658 -23.5863 0.219586
Langmuir 4 295 20.5096 0.039291 0.979712 -24.3513 49.3451 0.249818
308 8.77322 0.108006 0.963921 -23.2499 0.235698
In this study, the deviation of calculated values for qm, KL, r2 and G could be con-sidered as small to moderate. It is clear that the deviations in KL values are propagated due to the large absolute value of the term:
showed only Langmuir 2 isotherm at atemperature of 308 K (Figure 2). As the non linear least squares method is more accurate than the linear one (2-5, 10-12), it is proposed to calculate the thermodynamic parameters from the Langmuir parameters obtained by non linear methods.
From the estimate of thermodynamic parameters it can be concluded that the process is spontaneous at a given temperatures, because G has a negative value. The positive va-lue of H, indicates that the sorption reaction is endothermic, which means that poplar sawdust has affinity toward copper ions. Generally, the heat evolved during physical ad-sorption is of the same order of magnitude as the heats of condensation, i.e., 2.1–20.9 kJ/mol, while the heats of chemisorption generally fall into a range of 80–200 kJ/mol (13). So, the values of H in Table 1 indicate that Cu(II) adsorption on poplar sawdust should be attributed to a physico-chemical adsorption process rather than to a pure phy-sical or chemical adsorption process.
Since the different low cost adsorbent – heavy metal ions combinations have different values of thermodynamic parameters and it is not reasonable to expect a universal corre-lation between the corresponding enthalpy change and entropy change accompanying the adsorption (14).
Figure 2. Langmuir isotherms obtained on temperature of 308 K
CONCLUSION
Lang-muir linear equations. The process is spontaneous at given temperatures, because G has negative values. The value of H is positive, indicating that the sorption reaction is endo-thermic.
Acknowledgement
The work presented here was supported by the Serbian Ministry of Education and Science (project III44006 and project III43005).
REFERENCES
1. WHO, Guidelines for Drinking Water Quality, Recommendations, vol. I, WHO, Geneva, (1984).
2. Ho Y. S.: Isotherms for the Sorption of Lead onto Peat: Comparison of Linear and Non-Linear Methods, Polish Journal of Environmental Studies 15, 1 (2006) 81-86. 3. Kumar K. V., Porkodi K.: Batch adsorber design for different solution volume/
adsorbent mass ratios using the experimental equilibrium data with fixed solution vo-lume/adsorbent mass ratio of malachite green onto orange peel, Dyes and Pigments 74
(2007) 590-594.
4. Kumar K. V., Porkodi K., Rocha F.: Isotherms and thermodynamics by linear and non-linear regression analysis for the sorption of methylene blue onto activated carbon: Comparison of various error functions, Journal of Hazardous Materials 151
(2008) 794-804.
5. Subramanyam B., Das A.: Linearized and non-linearized isotherm models compara-tive study on adsorption of aqueous phenol solution in soil, Int. J. Environ. Sci. Tech.
6, 4 (2009) 633-640.
6. Dakiky, M., Khamis, M., Manassra, A., Mer'eb, M.: Selective adsorption of chromi-um(VI) in industrial wastewater using low-cost abundantly available adsorbents. Advances Environ. Res. 6 (2002) 533-540.
7. ŠšТЛКЧM., KХКšЧУК M.: ЋtuНв ШП tСО КНsШrptТШЧ ШП МШppОr(II) ТШЧs ПrШЦ аКtОr ШЧtШ wood sawdust, pulp and lignin, Adsorp. Sci. Technol. 22, 3 (2004) 195-206.
8. Langmuir I.: The constitution and fundamental properties of solids and liquids, J. Am. Chem. Soc. 38 (1916) 2221.
9. Gupta V. K., Saini V. K., Jain N.: Adsorption of As(III) from aqueous solutions by iron oxide-coated sand, J. Colloid Interface Sci. 288 (2005) 55-60.
10.Alihosseini A., Taghikhani V., Safekordi A. A., Bastani D.: Equilibrium sorption of crude oil by expanded perlite using different adsorption isotherms at 298.15 k, Int. J. Environ. Sci. Tech. 7, 3 (2010) 591-598.
11.Ho Y. S., Porter J. F., McKay G.: Equilibrium isotherm studies for the sorption of divalent metal ions onto peat: Copper, nickel and lead single component systems, Water, Air and Soil Pollution 141, 14 (2002) 1-33.
12.Kumar K. V., Sivanesan S.: Isotherm parameters for basic dyes onto activated carbon: Comparison of linear and non-linear method, Journal of Hazardous Materials B129
13.Liu Y., Liu Y.J.: Biosorption isotherms, kinetics and thermodynamics, Sep. Purif. Technol. 61 (2008) 229-242.
14.Ramesh A., Lee D.J., Wong J.W.C.: Thermodynamic parameters for adsorption equilibrium of heavy metals and dyes from wastewater with low-cost adsorbents, J. Coll. Interface Sci. 291 (2005) 588-592.
. , . .
, О О , 1, 21000 ,
(II) .
.
LКЧРЦuТr- О О О
,
.
-. ђ
.
. К К
.
Langmuir- ,
ђ
-.
-, Ш К УО
.
О О : О , LКЧРЦuТr- К К, К
, , К