Research paper
Pterocarpans induce tumor cell death through persistent mitotic
arrest during prometaphase
Gardenia C.G. Milit
~
ao
a, Marisa P. Prado
b, Cl
audia Pessoa
c, Manoel Odorico de Moraes
c,
Edilberto R. Silveira
d, Mary Anne S. Lima
d, P
ersio Alexandrino Veloso
d,
Letícia V. Costa-Lotufo
c, Gl
aucia M. Machado-Santelli
b,*aDepartamento de Fisiologia e Farmacologia, Universidade Federal de Pernambuco, CEP 50670-901 Recife, Ceara, Brazil
bDepartamento de Biologia Celular e do Desenvolvimento, Instituto de Ci^encias Biomedicas I, Universidade de S~ao Paulo, CP1524, CEP 05508-900 S~ao Paulo,
SP, Brazil
cDepartamento de Fisiologia e Farmacologia, Universidade Federal do Ceara, CEP 6430-270 Fortaleza, Ceara, Brazil
dDepartamento de Química Org^anica e Inorganica, Universidade Federal do Cear^ a, Caixa Postal-12200, 60021-940 Fortaleza, Ceara, Brazil
a r t i c l e
i n f o
Article history:
Received 26 March 2014 Accepted 11 June 2014 Available online 18 June 2014
Keywords:
Pterocarpan Cell cycle Mitotic arrest Breast cancer cell lines Apoptosis
a b s t r a c t
Pterocarpans, a family of isoflavonoids found in the diverseFabaceae, display potent cytotoxic activity over a panel of tumor cell lines, and among those tested, 2,3,9- trimethoxypterocarpan displays the most potent activity. This study evaluates the effects of 2,3,9-trimethoxypterocarpan and its related derivatives on cell cycle progression and microtubule function in select breast cancer cell lines (MCF7, T47d and HS578T). The pterocarpans, with the exception of 3,4-dihydroxy-9-methoxipterocarpan, induced increased frequencies of mitotic cells by inducing arrest in prometaphase. While microtubule organi-zation in interphase cells was not modified during treatment, mitotic cells exhibited high frequencies of monastral spindles surrounded by condensed chromosomes. Immunofluorescence staining with an anti-g-tubulin antibody showed double-dot labeling in the spindle polar region, suggesting that pterocarpan treatment blocked centrosome segregation. We found that this mitotic arrest was reversible when the cells were treated for up to 24 h followed by recovery in drug-free medium, but not after 48-h treatment followed by incubation in drug-free medium. In that case, treated cells typically underwent cell multi-nucleation and apoptosis.
©2014 Elsevier Masson SAS. All rights reserved.
1. Introduction
Given the key role of abnormal cell proliferation in cancer, com-pounds with antimitotic properties represent a major resource for chemotherapeutic discovery. The high dynamics of microtubules are essential for mitosis; therefore, microtubules represent one of the most important targets for cancer chemotherapy. Among the most well known agents that target microtubules are tubulin-binding compounds such as theVinca sp. alkaloids, cryptophycins, halichon-drins, combretastatins, discodermolide, eleutherobins, laulimalide, docetaxel and paclitaxel[1]. More recently, interest has arisen in identifying compounds that target mitotic kinases belonging to the Aurora kinase and polo-like kinase families, as well as motor proteins, which are essential for the assembly and correct function of the mitotic spindle[2]. Although drug discovery is active, there is an
immediate need to identify materials that provide a defined link be-tween mitotic blockage and cell death, and this correlation is para-mount to the translation of a reliable chemotherapeutic.
Pterocarpans are naturally occurring compounds that have a tetracyclic ring system derived from the basic isoflavonoid skeleton and an ether linkage between positions 4 and 20[3]. A previous study on pterocarpans obtained from the Brazilian treePlatymisciumfl o-ribundumdemonstrated that these compounds have cytotoxic ac-tivity against a panel of five tumor cell lines, with 2,3,9-trimethoxypterocarpan being the most potent[4]. Further analyses of these compounds identified antimitotic activity using a sea urchin egg model. In these experiments, 2,3,9-trimethoxypterocarpan pre-sented 1000-times more activity than the antineoplastic agents doxorubicin and etoposide[5].
Pterocarpans induce apoptosis in HL60, which is a leukemia cell line, after 24-h incubation, as evidenced by DNA fragmentation, mitochondrial depolarization and caspase-3 activation, but no membrane destabilization [6]. 2,3,9-trimethoxypterocarpan inhibited the proliferation of a panel of leukemic cell lines in a *Corresponding author. Av. Lineu Prestes, 1524, S~ao Paulo, Brazil. Tel.:þ55 11
30917229; fax:þ55 11 30917402.
E-mail address:glaucia.santelli@gmail.com(G.M. Machado-Santelli).
Contents lists available atScienceDirect
Biochimie
j o u r n a l h o m e p a g e : w w w . e l s e v i e r . c o m / l o c a t e / b i o c h i
http://dx.doi.org/10.1016/j.biochi.2014.06.005
dose-and time-dependent manner: the IC50results after 24 h of
treatment of a panel of four human leukemic cell lines, JURKAT, HL60, K562 and MOLT-4, resulted in IC50values ranging from 8 to
18.8
mM, except for Jurkat cells, which were not affected by the
compound; after 48 h of treatment, the IC50values ranged from 0.3to 2.5
mM; and after 72 h of treatment, the IC
50values ranged from0.3 to 1.6
mM
[7].In the present study, the effects of pterocarpans on human breast cancer cells were further analyzed with the primary goal of determining their mode of action. The drug concentrations were established based on previous IC50 determinations [3] and by
considering 8
mM 3,9-trimethoxypterocarpan as moderate
treat-ment for 24 h. The main focuses were on cytoskeleton organization and cell cycle progression.2. Materials and methods
2.1. Compound isolation
Samples of 2,3,9-trimethoxypterocarpan (1), 3,9-dimethoxypterocarpan (2), 3-hydroxy-9-methoxypterocarpan (3), 3,4-dihydroxy-9-methoxypterocarpan (4) and 3,10-dihydroxy-9-methoxyptrocarpan (5) were isolated (purity above 95%) from the hexane extract of the heartwood ofP.floribundum[2]. All structures were determined by spectroscopy, including one- and two-dimensional NMR methods such as COSY, HMQC, and HMBC, as well as analysis of physical properties and comparison with data from the literature.
Table 1
Phase index of MCF-7 cells treated with1e5over 24 h. The number of cells in
interphase (I), prophase/prometaphase (P), metaphase (M), anaphase (A) and telo-phase (T) was determined by counting 1000. Data are shown as mean of 3 prepa-rations±SD.
Treatment I P M An T
Control 971.5±3.5 14.5±3.5 5.0±0 6.0±0 3.0±0 (1) 8mM 696±21.2 304±21.2* 0 0 0 (2) 90mM 766±19.8 225±26.9* 8±5.6 0 1±1.4 (3) 90mM 959±5.6 32.5±2.1* 3.5±0.7 4.5±3.5 0.5±0.7 (4) 90mM 991.5±3.5 8±2.8 0.5±0.7 0 0 (5) 90mM 883±12.7 105±21.9* 7±7 2±1.4 2.5±0.7 *p<0.05 compared by chi-squared test.
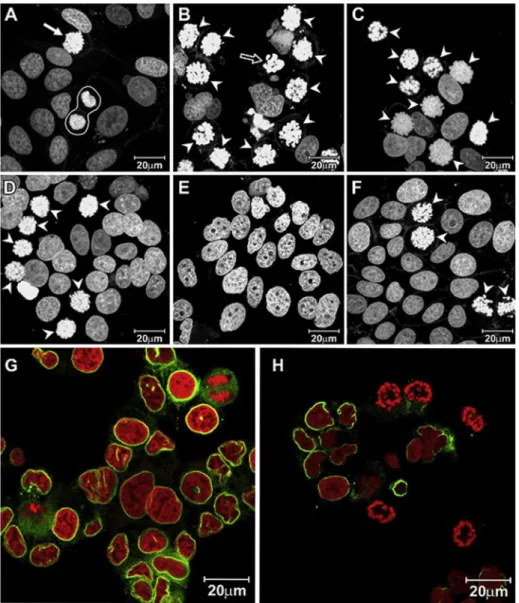
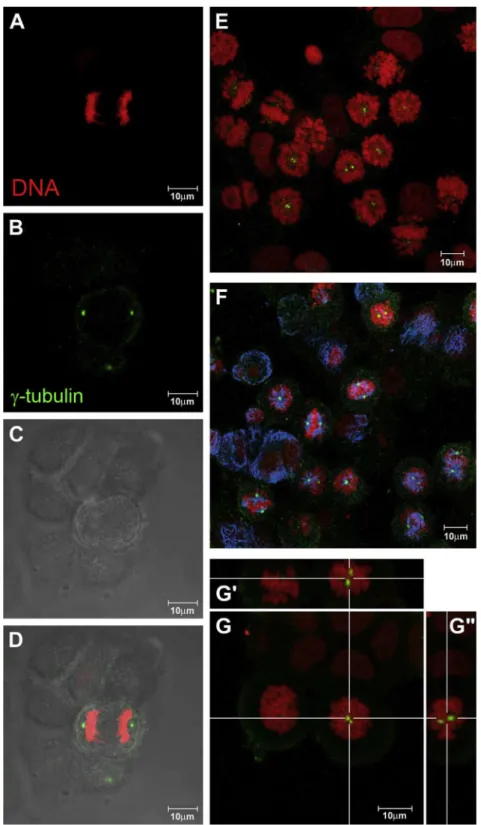
Fig. 1. Laser scanning confocal microscopy images of MCF-7 showingnuclei stained by propidium iodide: (A)control cells showing a normal metaphase (arrow) and telophase (surrounded by white line); (B) cells treated with 8mM1for 24 h; (C) 90mM 2 for 24 h; (D) 90mM3for 24 h; (E) 90mM 4 for 24 h; (F) 90mM5for 24 h. Arrow heads indicate the prometapase/metaphase arrested cells. G and H show images of MCF-7 cells submitted to immunofluorescence with anti-laminB antibody (green), rescpectively, control and treated cells ( 8mM1for 24 h). Nuclei counter stained with propidium iodide (red).
G.C.G. Militao et al. / Biochimie 104 (2014) 147e155
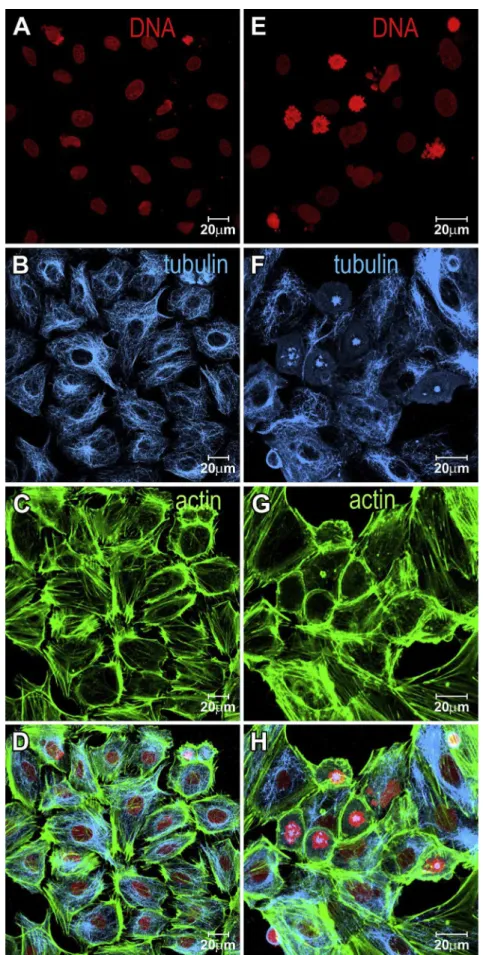
Fig. 2. Laser scanning confocal microscopy images depicting MCF-7 cells. Panels (A, B, C, D) indicate control cells and panels (E, F, G, H) indicate cells treated with 8mM1for 24 h. Splitted channels showing: (A and E) nuclei; (B and F) microtubules; (C and G) actin. Merged channels (D and H). Cells were stained for their nuclei in red using propidium iodide, microtubules in blue using antia-tubulin antibody plus CY5-anti-mouse secondary antibody, and microfilaments in green using FITC-phalloidin.
2.2. Cell lines and culture
MCF-7, T47D and HS578T human breast cancer cells were ob-tained from the American Type Culture Collection (ATCC) and maintained in Dulbecco's Modified Eagle's Medium (DMEM, Sigma Aldrich) supplemented with 10% fetal bovine serum and incubated at 37C in a humidi
fied 5% CO2incubator. The cells were plated in
35-mm dishes at a density of 5104cells/dish in normal culture medium and incubated for 24 h. A previous study on several tumor cell lines indicated that compound1presented cytotoxicity IC50
4a
11b 4
1 3
2
O
6
11a 6a
O 10a
6b
10 9 7
8
OR2
R
1
R
3
OCH3
R4 H
H
R1 R2 R3 R4 1 OCH3 CH3 H H 2 H CH3 H H
3 H H H H
4 H H OH H
5 H H H OH
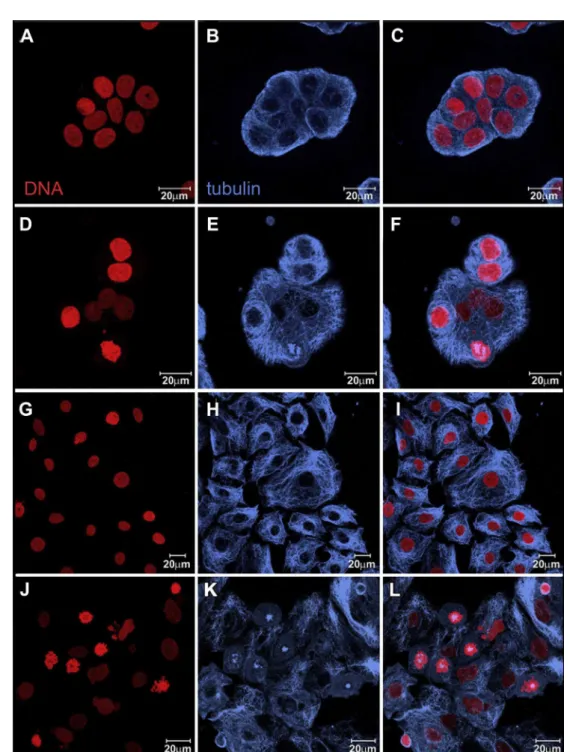
Fig. 3. Laser scanning confocal microscopy imagesshowing T47D control cells (AeC) and T47D cells treated with 8mM1for 24 h(DeF). HS578T control cells (GeI) and treated
with 8mM for 24 h (JeL). Nuclei were stained with propidium iodide (red) and microtubules are in blue, immunoreaction with antia-tubulin antibody plus CY5-anti-mouse
secondary antibody. Each channel is showed separately in the 1st and 2nd columns and merged channels in 3rd column.
G.C.G. Militao et al. / Biochimie 104 (2014) 147e155
values ranging from 8 to 18.8
mM. Thus, in the
first set of experi-ments, the normal medium was replaced by a pterocarpan-containing medium at concentrations of 8mM for
1and 90mM
for2,3,4and5, and the cells were incubated for an additional 24 h.2.3. Cell cycle phase index
Control and treated cells were washed with PBS (phosphate-buffered saline) and fixed with 3.7% formaldehyde in PBS for 25 min. After a second wash with PBS, the cells were permeabilized with 0.5% Triton X-100 for 10 min and then treated with RNase (10 mg/mL) for 15 min. Nuclei were stained by 15
mM propidium
iodide for 15 min, and the coverslips were mounted on slides with Vecta-shield (Vector Laboratories). The number of cells in inter-phase, prointer-phase, metainter-phase, anaphase and telophase was deter-mined by counting 1000 cells on each coverslip using afluorescent microscope. The following characteristics were used to classify the states of mitosis: prophase (condensed chromatids); metaphase(every chromosome has made proper attachments to the mitotic spindle and has congressed to a central position); anaphase (sister chromatid separation and chromosome segregation); telophase (segregated chromosomes begin decondensing)[8].
2.4. Immunofluorescence
MCF-7, T47D and HS578T cells treated with 8
mM
1for 24 h were stained fora-tubulin, lamin B, actin and DNA. The cells were then
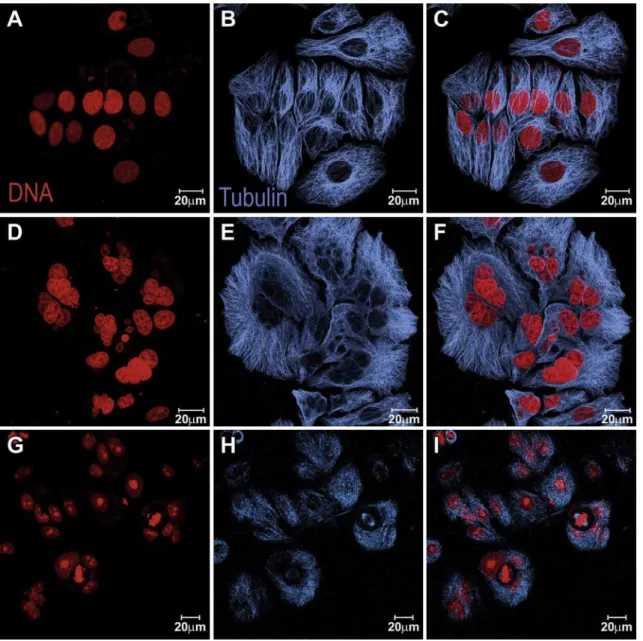
fixed with 3.7% formaldehyde in PBS for 25 min, washed in PBS, treated with 0.5% Triton X-100 for 10 min, and incubated overnight with the primary antibodies anti-a-tubulin or anti-lamin B (Sigma Aldrich). After the incubation, the cells were washed with PBS, the secondary antibody (CY5-anti-mouse or FITC-anti-mouse, Molec-ular Probes, Life Technologies) was added, and the preparations were incubated for 1 h. The actinfilaments were stained with FITC-phalloidin (Sigma Aldrich) for 20 min. For centrosome visualiza-tion, we used primary anti-g-tubulin antibody (Abcam) andFITC-Fig. 4. Laser scanning confocal microscopy images of MCF-7cells recovering after treatment with1. Panels (AeC) control cells; (DeF) cells treated with 8mM1for 24 h plus 24 h recovery in drug-free medium; (GeI) cells treated with 8mM1for 48 h plus 24 h recovery in drug-free medium. Cells were stained for their nuclei in red using propidium iodide (red) and microtubules in blue using antia-tubulin antibody and staining with CY5-anti-mouse secondary antibody. Channels splitted in the 1st and 2nd columns and merged in
3rd column.
labeled anti-rabbit secondary antibody [9]. Nuclei were stained with 15
mM propidium iodide, and after 10 mg/mL RNase treatment
for 10 min, the coverslips were mounted on glass slides with Vecta-shield (Vector Laboratories).To determine if the mitotic arrest was reversible, MCF-7 cells treated with 8
mM
1for 24 h and 48 h were washed with PBS, incubated in a drug-free medium for an additional 24 h and stained for microtubules and nuclei.2.5. Cell cycle analysis
MCF-7 cells were treated with 8
mM
1for 24 h or 48 h. Harvested cells were incubated at 25C for 30 min in a lysis solution con-taining 0.1% sodium citrate, 0.1% Triton X-100 and 75mM propidium
iodide in the dark. The cells were then analyzed byflow cytometry on an EasyCyte System (Guava Technologies Inc.). Five thousand events were evaluated per sample, and cellular debris was omittedFig. 5. Laser scanning confocal microscopy imagesof T47D cells treated with 8mM compound1for 24 h and submitted to immunofluorescence with antia-tubulin (blue) or anti g-tubulin (green) antibodies. Nuclei were stained with propidium iodide (red). Control cells (first column, AeD) showing a normal bipolar mitotic spindle; treated cells (2nd
column, EeG), monastral spindles with twog-tubulin positive dots: (E) chromosomes andg-tubulin; (F) chromosomes,g-tubulin and microtubules; (G) higher magnification of
prometaphase arrested cells with orthogonal sections (G0and G00) evidencing the double dot labeling in the spindle polar region.
G.C.G. Militao et al. / Biochimie 104 (2014) 147e155
from the analysis. To investigate if the G2/M arrest was reversible, MCF-7 cells were treated with 8
mM
1for 24 h or 48 h, washed with PBS, and then incubated in a drug-free medium for an additional 24 h.2.6. Measurement of mitochondrial transmembrane potential
MCF-7 cells were treated with 8
mM or 16
mM
1for 24 h or 48 h in 24-well plates. The mitochondrial transmembrane potential was determined by rhodamine-123 dye retention using aflow cytom-eter and the EasyCyte System (Guava Technologies Inc.) and analyzed using CytoSoft 4.1 (Guava Technologies Inc.). Both adherent and suspension cells were harvested from plates, pelleted and washed with PBS. A solution of rhodamine 123 (13mM) in PBS
was added, and the cells were incubated at 37C for 15 min in the dark. After incubation, the cells were pelleted and washed twice with PBS. The cells were incubated again in PBS for 30 min at 37C in the dark, andfluorescence was measured. Five thousand events were evaluated per sample, and cellular debris was omitted from the analysis.2.7. Statistical analysis
The obtained data are presented as the means±standard de-viation (SD) fromnexperiments. Chi-squared tests were used to compare mitotic phase data, while Student's t test was used to compare DNA content data. To measure the mitochondrial trans-membrane potential, the samples were compared by ANOVA fol-lowed by the Student NewmaneKeuls test. The significance level
was set at 5%.
3. Results
3.1. Pterocarpans interfere with cell cycle progression by arresting cells in prophase/prometaphase
Cell cycle phase index analysis demonstrated that the treat-ment of MCF-7 cells with compounds 1, 2, 3 or 5 for 24 h increased the frequency of prophase/prometaphase cells, and 2,3,9-trimethoxypterocarpan1 displayed the strongest activity. Only 3,4-dihydroxy-9-methoxypterocarpan 4 failed to induce mitotic arrest (Table 1,Fig. 1). Interphasic nuclei predominated in the control cell preparations (97.1%) against a small percentage of mitotic cells, and all mitotic phases were present in these preparations. The interphase and mitotic frequencies changed drastically after 24-h treatment with 8
mM
1, with interphase cells decreasing to 69% and mitotic cells increasing to 31%. The mitotic cells predominantly displayed prophase/prometaphase morphology. T47D cells treated with compound1also presented cell blockage at prophase/prometaphase, which represented 20.0% of treated cells compared to 0.65% of control cells (data not shown).The nuclear morphology corroborated the cell cycle phase index results (Fig. 1). MCF-7 cells treated with compounds1,2,3, or5
presented a high frequency of prophase/prometaphase cells (Fig. 1(A)e(F)). Immunofluorescence with anti-lamin B antibody
showed that treated MCF-7 cells were mitotic and had condensed chromosomes that were organized as rings without a nuclear en-velope, suggesting that the treated cells had passed prophase and were arrested in prometaphase, (Fig. 1(G)e(H)). It is important to
note that the mitotic index analysis was not capable of discrimi-nating between prophase and prometaphase cells.
Interphase cytoskeleton organization did not change after 24-h treatment with compound1because the microtubule network and actin filaments presented similar organization as control cells
(Fig. 2). Similar results were observed for T47-D (Fig. 3(A) and (B)) and HS578T (Fig. 3(C) and (D)) cells because the treated cells pre-sented an increased number of prometaphase cells. The organiza-tion of the microtubules of interphase cells was similar as that of the controls.
To investigate if the mitotic arrest was reversible, MCF-7 cells treated with 8
mM
1for 24 h or 48 h were recovered in drug-free culture medium for an additional 24 h and submitted to immu-nofluorescence (Fig. 4). The effect observed after 24 h of treatment was reversed after a 24-h period in drug-free medium because most treated cells progressed through mitosis and reentered interphase, in spite of the high frequency of bi- or multi-nucleated cells in the culture after recovery. However, longer treatment (48 h) followed by a 24-h drug-free period was not reversible, and most treated cells actually presented delayed, bi-polar mitotic spindles (Fig. 4).During mitosis, the microtubules were organized in a monopolar-like spindle inside the ring of chromosomes. For the control cells in mitosis, immunofluorescence for
g-tubulin showed
one dot at each spindle pole, resulting in two dots per cell. In contrast, double-labeled structures at a single pole of the micro-tubules spindles were observed in treated mitotic cells (Fig. 5), and this location was confirmed using orthogonal sections of the opti-cally sliced Z-stacks that were acquired by confocal microscopy (Fig. 5).3.1.1. Cell cycle analysis and measurement of the mitochondrial transmembrane potential
Treatment of MCF-7 cells with 8
mM
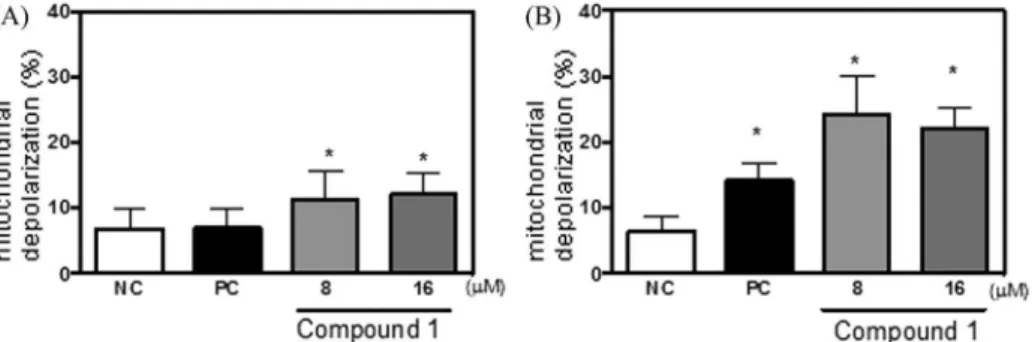
1for 24 h increased the G2/ M frequencies, confirming the morphologyfindings (Table 2). A slight increase in subdiploid DNA was observed, indicating that some treated cells entered apoptosis (Table 2). In addition, after 24-h treatment wit24-h 8mM
1, there was a significant increase in mito-chondrial depolarization: 11.3±1.4% for treated cells compared to 6.8±1% for the negative control (Fig. 6(A)). The increase in incu-bation time to 48 h caused stronger effects, resulting in 38.5% treated cells arrested at G2/M and 14.8% of treated cells showing subdiploid DNA (Table 2). The number of cells displaying mito-chondrial depolarization was also increased after 48 h (Fig. 6(B)).When cells were exposed to drug-free medium after a 24-h treatment with compound 1, these effects were no longer observed, while, after 48-h treatment, 27.6% of treated cells remained arrested at G2/M, which suggested persistent blockage and confirmed the morphological analysis. Moreover, the
Table 2
Cell cycle analysis MCF-7 cells treated with 8mM1byflow cytometry. Data are
shown as mean±SD.
Sub G0 G0/G1 S G2/M Control 24 h 0.9±0.1 53.1±1.9 17.5±1.1 20.51±1.4 Treatment 24 h 1.4±0.2a 40.4±2.0a 15.6±3.2 32±1.7a
Control 24 hþ24 h recovery 0.3±0.1 57.9±2.1 17.7±0.7 19.7±1.5 Treatment 24 hþ24 h
recovery
0.9±0.3b 57.8±1.8 19.5±0.7 16.0±1.2b
Control 48 h 1.1±0.4 60.7±1.7 13.2±0.9 19.8±1.7 Treatment 48 h 14.8±2.2c 31.4±2.7c 11.8±0.9 38.5±4.5c
Control 48 hþ24 h recovery 4.0±1.0 66.9±3.7 9.4±1.3 15.0±1.3 Treatment 48 hþ24 h
recovery
13.4±1.8d 47.9±5.1d 9.8±1.0 27.6±3.6d
Control 48 hþ48 h recovery 2.5±0.8 60.46±5.7 16.10±2.9 14.72±2.0 Treatment 48 hþ48 h
recovery
5.5±1.8e46.28±2.7e16.66±1.3 25.70±2.5e
ap<0.05 compared to 24 h treatment control byttest.
bp<0.05 compared to 24 h treatmentþ24 h recovery control byttest. c p<0.05 compared to 48 h treatment control byttest.
d p<0.05 compared to 48 h treatmentþ24 recovery control byttest. ep<0.05 compared to 48 h treatment
þ48 recovery control byttest.
subdiploid cell number was increased, despite the drug-free me-dium recovery period (Table 2).
4. Discussion
Pterocarpans cause a unique block during prometaphase. Based on this evidence, we now consider this family of compounds as an emerging prospect for further examination as an anticancer agent [4e7]. Among the tested pterocarpans, we found that 2,3,9-trimethoxypterocarpan (1) at 8
mM displayed ef
ficacy in 3 breast cancer cell lines independent of the aggressive features of the cells. The methoxy-group at the C2 position can be considered an important pharmacophoric unit for pterocarpans[4e7]. Moreover, the increasing number of hydroxy groups seems to enhance the nonspecific toxicity of pterocarpans because compounds with this functionality undergo cell death through necrotic processes, while compounds bearing methoxy groups undergo cell cycle arrest fol-lowed by apoptosis[6].Here, we demonstrated that 2,3,9-trimethoxypterocarpan (1) also led to cell cycle arrest in breast cancer cells. We found that the mitotic arrest induced by 1 occurred during prometaphase, as confirmed by the absence of nuclear lamina in lamin B immuno-preparations[8,10]. Persistent prometaphase arrest was followed by apoptosis, as previously observed in leukemia cells[6], while the effects of compound1after shorter exposure (24 h) followed by a 24-h recovery period in drug-free medium were reversible.
Pterocarpan treatment did not interfere with the organization of the cytoskeleton of interphase cells. Both microfilaments and mi-crotubules showed similar distributions in control and treated cells. Although microtubule has been recognized as the classical target for antimitotic drugs, many motor proteins related to the mitotic machinery are emerging as promising targets for anticancer drug development [2,11]. These motor proteins include dynein and different families of kinesin-like proteins. KSP (Kinesin Spindle Protein) is a mitotic spindle motor protein belonging to the kinesin superfamily that plays an essential role in centrosome separation and in organization of the bipolar mitotic spindle[12,13]. Monastrol was thefirst cell-permeable small molecule to target Eg5, a KSP that is required for spindle bipolarity [14]. After structure-relationship studies, a potent and specific inhibitor of KSP, KSP-IA, was developed that recapitulated the biological activities of other KSP-inhibitory agents such as monastrol[15]. Currently, Eg5 in-hibitors, such as ispinesib, AZD-4877 and others, have entered clinical trials[16].
Treatment with compound1 led to an increasing number of prometaphase structures, with chromosomes distributed around the mitotic monopole or asymmetric spindle. Indeed, there was a double-dot-labeled structure localized in the spindle poles of
g-tubulin immunofluorescence preparations, which indicated that the compound blocked the separation of duplicated centrosomes, which is a crucial step in the biogenesis of normal mitotic spindles. Failures in this process could lead to cell death or increasing fre-quencies of multinucleated and aneuploid cells. Inhibitors of KSP, such as monastrol, caused mitotic arrest with a prometaphase-like morphology, thus preventing spindle pole separation and resulting in monopolar spindles[14]. The potential fates of mitotic-arrested cells may include (1) cell death during mitosis by apoptosis or necrosis, (2) exit from mitosis as a 4N cell that terminates in G1 via apoptosis; (3) exit from mitosis as a reproductively dead but viable 4N cell, or (4) exit from mitosis as a 4N cell that can undergo a second passage through the cell cycle[8,17]. Transient exposure to a KSP inhibitor (KSP-IA) caused reversible mitotic arrest, and long treatments or sustained mitotic arrest is required to initiate apoptosis [18]. The present data are in agreement with this behavior because apoptotic cell death was only observed after persistent mitotic arrest induced by1, and the effects observed after 24 h only included early apoptotic features and could be reversed after a drug-free recovery period.
5. Conclusions
In conclusion, the antiproliferative activity of pterocarpans ari-ses with the inhibition of spindle pole separation during mitosis, leading to cell cycle arrest at prometaphase. The long mitotic arrest induced by compound 1 was typically followed by subsequent entry into apoptosis. The presentfindings highlight the anticancer potential of these natural molecules as well as suggest a new mo-lecular tool for further investigations on mitosis.
Conflict of interest
None.
Acknowledgments
We are grateful to Roberto Cabado Modia for his assistance with confocal microscope images. This study was supported by research grants from the S~ao Paulo State Research Foundation (FAPESP) and National Council for Scientific and Technological Development (CNPq).
References
[1] M.A. Jordan, L. Wilson, Microtubules as a target for anticancer drugs, Nat. Rev. Cancer 4 (2004) 253e263.
[2] J.R. Jackson, D.R. Patrick, M.M. Dar, P.S. Huang, Target anti-mitotic therapies: can we improve on tubulin agents? Nat. Rev. Cancer 7 (2007) 107e117. Fig. 6.Effect of compound1on MCF-7 cell mitochondrial transmembrane potential determined byflow cytometer using 13mM rhodamine 123 after incubation for (A) 24 h or (B) 48 h. Negative control (NC) and positive control (PC) are given by DMSO or 0.5mM doxorubicin, respectively. Data are presented as mean values±standard deviation from three independent experiments performed in triplicate. Five thousand events were analyzed in each sample. *,p<0.05 compared to control by ANOVA followed by Student New-maneKeuls test.
G.C.G. Militao et al. / Biochimie 104 (2014) 147e155
[3] T. Maurich, L. Pistelli, G. Turchi, Anti-clastogenic activity of two structurally related pterocarpans purified from Bituminaria bituminosa in cultured human lymphocytes, Mutat. Res. 561 (2004) 75e81.
[4] M.J.C. Falcao, Y.B.M. Pouliquem, M.A.S. Lima, N.V. Gramosa, L.V. Costa-Lotufo,~
G.C.G. Militao, C. Pessoa, M.O. Moraes, E.R. Silveira, Cytotoxic Flavonoids from platymisciumfloribundum, J. Nat. Prod. 68 (2005) 423e426.
[5] G.C.G. Milit~ao, P.C. Jimenez, D.V. Wilke, C. Pessoa, M.J.C. Falc~ao, M.A.S. Lima, E.R. Silveira, M.O. Moraes, L.V. Costa-Lotufo, Antimitotic properties of pter-ocarpans isolated fromPlatymisciumfloribundumon sea urching eggs, Planta Med. 71 (2005) 1e3.
[6] G.C.G. Milit~ao, I.N. Dantas, C. Pessoa, M.J. Falc~ao, E.R. Silveira, M.A. Lima, R. Curi, T. Lima, M.O. Moraes, L.V. Costa-Lotufo, Induction of apoptosis by pterocarpans from Platymisciumfloribundum in HL-60 human leukemia cells, Life Sci. 78 (20) (2006), 2409e2017.
[7] G.C.G. Militao, D.P. Bezerra, C. Pessoa, M.O. de Moraes, F.A.F. da Ponte, M.A.S. Lima, E.R. Silveira, L.V. Costa-Lotufo, Comparative cytotoxicity of 2,3,9-trimethoxypterocarpan in leukemia cell lines (HL-60, Jurkat, Molt-4, and K562) and human peripheral blood mononuclear cells, J. Nat. Med. 61 (2) (2007) 196e199.
[8] B.A.A. Weaver, D.W. Cleveland, Decoding the links between mitosis, cancer, and chemotherapy: the mitotic checkpoint, adaptation, and cell death, Cancer Cell 8 (2005) 7e12.
[9] B.A. Cortez, G.M. Machado-Santelli, Chrysotile effects on human lung cell carcinoma in culture: 3-D reconstruction and DNA quantification by image analysis, BMC Cancer 8 (2008) 181.
[10] R. Manelli-Oliveira, G.M. Machado-Santelli, Cytoskeletal and nuclear alter-ations in human lung tumor cells: a confocal microscope study, Histochem. Cell Biol. 115 (5) (2001) 403e411.
[11] K.W. Wood, W.D. Cornwell, J.R. Jackson, Past and future of the mitotic spindle as an oncology target, Curr. Opin. Pharmacol. 1 (2001) 370e377.
[12] A. Blangy, H.A. Lane, P. d'Herin, M. Harper, M. Kress, E.A. Nigg, Phosphoryla-tion by p34 cdc2 regulates spindle associaPhosphoryla-tion of human Eg5, a kinesin-related motor essencial for bipolar spindle formation in vivo, Cell 83 (1995) 1159e1169.
[13] A.S. Kashina, G.C. Rogers, J.M. Scholey, The bimC family of kinesins: essential bipolar mitotic motors driving centrosome separation, Biochim. Biophys. Acta 1357 (3) (1997) 257e271.
[14] T.U. Mayer, T.M. Kapoor, S.J. Haggarty, R.W. King, S.L. Schreiber, T.J. Mitchison, Small molecular inhibitor of mitotic spindle bipolarity identified in a phenotype-based screen, Science 286 (1999) 972e974.
[15] W. Tao, V.J. South, R.E. Diehl, J.P. Davide, L. Sepp-Lorenzino, M.E. Fraley, K.L. Arrington, R.B. Lobell, An inhibitor of the kinesin spindle protein activates the intrinsic apoptotic pathway independently of p53 and de novo protein synthesis, Mol. Cell. Biol. 27 (2) (2007) 689e698.
[16] K.-S. Chan, C.-G. Kon, H.-Y. Li, Mitosis-targeted anti-cancer therapies: where they stand, Cell. Death Dis. 3 (2012) 411.
[17] C.L. Rieder, H. Maiato, Stuck in division or passing through: what happens when cells cannot satisfy the spindle assembly checkpoint, Dev. Cell 7 (2004) 637e651.
[18] W. Tao, V.J. South, Y. Zhang, J.P. Davide, L. Farrell, N.E. Kohl, L. Sepp-Lorenzino, R.B. Lobell, Induction of apoptosis by an inhibitor of mitotic kinesin KSP re-quires both activation of the spindle assembly checkpoint and mitotic slip-page, Cancer Cell 8 (2005) 49e59.