NADPH-diaphorase activity in area 17
of the squirrel monkey visual cortex:
neuropil pattern, cell morphology and
laminar distribution
1Departamento de Fisiologia, Centro de Ciências Biológicas,
Universidade Federal do Pará, Belém, PA, Brasil
2Programa de Neurobiologia, Instituto de Biofísica Carlos Chagas Filho,
Universidade Federal do Rio de Janeiro, Rio de Janeiro, RJ, Brasil J.G. Franca1,2,
J.L.M. do-Nascimento1,
C.W. Picanço-Diniz1,
J.A.S. Quaresma1
and A.L.C. Silva1
Abstract
We studied the distribution of NADPH-diaphorase activity in the visual cortex of normal adult New World monkeys (Saimiri sciureus) using the
malic enzyme “indirect” method. NADPH-diaphorase neuropil activity had a heterogeneous distribution. In coronal sections, it had a clear laminar pattern that was coincident with Nissl-stained layers. In tangen-tial sections, we observed blobs in supragranular layers of V1 and stripes throughout the entire V2. We quantified and compared the tangential distribution of NADPH-diaphorase and cytochrome oxidase blobs in adjacent sections of the supragranular layers of V1. Although their spatial distributions were rather similar, the two enzymes did not always overlap. The histochemical reaction also revealed two different types of stained cells: a slightly stained subpopulation and a subgroup of deeply stained neurons resembling a Golgi impregnation. These neurons were sparsely spined non-pyramidal cells. Their dendritic arbors were very well stained but their axons were not always evident. In the gray matter, heavily stained neurons showed different dendritic arbor morphologies. However, most of the strongly reactive cells lay in the subjacent white matter, where they presented a more homogenous morphology. Our results demonstrate that the pattern of NADPH-diaphorase activity is similar to that previously described in Old World monkeys.
Correspondence
J.G. Franca
Laboratório de Neurobiologia 2 Instituto de Biofísica, UFRJ CCS, Bloco G, 2º andar 21949-900 Rio de Janeiro, RJ Brasil
Fax: 55 (021) 280-8193 E-mail: jgfranca@ibccf.biof.ufrj.br Research supported by FINEP/FADESP,
UFPA/PROPESP and CNPq.
Received October 25, 1995 Accepted July 10, 1997
Key words
·Nitric oxide synthase · Neocortex
·NADPH-diaphorase
·Cytochrome oxidase ·Primates
Introduction
NADPH-diaphorase (NADPHd) activity in the central nervous system has been stud-ied since Thomas and Pearse (1) described the “solitary cells” that survive various neu-rodegenerative and ischemic insults (2,3). NADPHd histochemistry has revealed the distribution of nitric oxide synthase in fixed tissue (4). Brain nitric oxide synthase (bNOS) is a calcium/calmodulin-dependent enzyme
(5) that synthesizes nitric oxide (NO) through NMDA receptor activation (6). NO is a gas that might act as a diffusible retrograde mes-senger that enhances the activation of the presynaptic terminal (6).
(10). Thus, a simple histochemical proce-dure allows detailed localization of NOS in fixed material. NOS utilizes NADPH as a cofactor to reduce the chromogen nitroblue tetrazolium, a yellowish salt, to diformazan that has a bluish appearance. This approach has been widely used to study the central nervous system of many non-primate spe-cies including rats (10,11), guinea pigs (12), opossums (13) and cats (14). In a previous report, Sandell (15) described the pattern of NADPHd activity in the visual cortex of the Rhesus monkey. Until recently there were no data on the distribution of this enzyme in the visual cortex of New World monkeys.
In the present study we used the “indi-rect” histochemical method to describe the morphology and laminar distribution of re-active neurons of the squirrel monkey. This animal presents some remarkable differences in the organization of neocortical architec-ture (16). We wondered if any difference in the NADPHd pattern could be detected, re-garding neuropil distribution and cell distri-bution and morphology. We also quantified the distribution of NADPHd and cytochrome oxidase activities in the neuropil of supra-granular layers in order to detect any differ-ence in the blob pattern revealed by these two different enzymes. No quantitative data on the distribution of NADPHd in New World monkeys have been published.
Material and Methods
Five adult Saimiri sciureus monkeys (three males and two females) were deeply anesthetized with an overdose of barbiturate (200-400 mg/kg Thionembutal). The ani-mals were subsequently perfused through the left ventricle with 0.9% saline followed by 4% paraformaldehyde in 0.1 M sodium phosphate buffer (PB), pH 7.2-7.4, followed by cryoprotective sucrose solutions. About 1 liter of each solution was used for perfusion. Cryoprotectant solutions were essential to keep the tissue well preserved during and
after freezing microtomy. Thick microtome sections (100-200 µm) were washed 3 times in 0.1 M PB for 10 min each and then incu-bated with the histochemical solutions. For NADPHd histochemistry, free-floating sec-tions were incubated in a solution containing 0.6% malic acid, 0.03% nitroblue tetrazo-lium (NBT), 1% dimethylsulfoxide, 0.03% manganese chloride, 0.1% ß-nicotinamide adenine dinucleotide phosphate (Sigma Chemical Co., St. Louis, MO) and 3% Triton X-100 in 0.1 M Tris, pH 8.2. The cytochrome oxidase solution consisted of 0.06% diaminobenzidine (Sigma), 0.03% cytochrome c (Sigma) and 4.5% saccharose in 0.1 M PB. The sections were incubated under continuous shaking at 37oC for about 6 h.
After the histochemical procedures, the sections were rinsed in 0.1 M Tris buffer, pH 7.4, mounted on gelatinized slides, dehy-drated and coverslipped using DPX or Entellan as embedding medium. Five hemi-spheres, one from each animal, were used to obtain complete series of the occipital lobe in four section planes (parasagittal, coronal, horizontal and tangential). Sections from these hemispheres were treated to detect NADPHd activity and some sections were selected for Nissl counterstaining.
Results
NADPH-diaphorase neuropil activity compared to cytochrome oxidase pattern
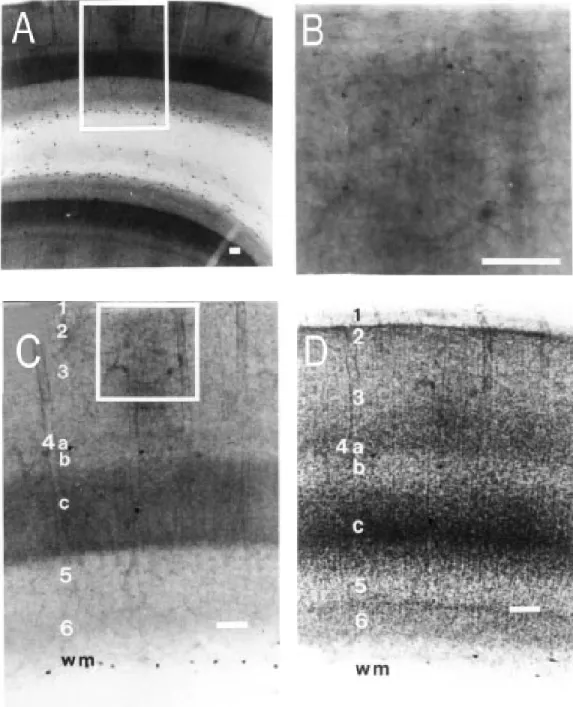
The bluish appearance of formazan was dispersed as a background activity that re-vealed NADPHd-reactive neuropil. Strong reactive regions were dark blue. Intense and light stained neuropil regions were orga-nized in a fashion related to cortical layers. A clear laminar pattern was observed from pial surface to white matter. This was equiva-lent to cortical layers 1, 2/3, 4A, 4B, 4C, 5 and 6 (Figure 1A,C,D). Layer 1 presented a number of NADPHd-positive fibers running parallel to the pial surface. Just below this layer and bordering layer 2 we found a thin layer virtually devoid of NADPHd activity. The limit between layers 2 and 3 was not well defined. NADPHd-rich zones in the neuropil formed rows of blobs (Figure 1A) which could be better observed in tangential sections through layers 2 and 3 (see below). NADPHd blobs could not be found in infragranular layers. Layer 4A was thin and more densely stained than the adjacent lay-ers 3 and 4B (Figure 1A,C,D). All laylay-ers presented faint longitudinal striations (17) better recognized in well-stained layers us-ing high power microscopy (layer 4C is the best example). In tangential sections, layer 4A did not present the honeycomb pattern described in Rhesus monkeys (15,17). Layer 4C was clearly labeled. This is a distinctive architectural feature of V1 that can be used to limit this cortical area. The border of this layer with the underlying layer 5 showed an even more intense NADPHd activity. This darker region comprised about 1/5 of layer 4C as a whole. Layers 5 and 6 were much less intensely stained than the other laminae. Layer 6 showed slightly higher NADPHd activity than layer 5 (Figure 1A,C).
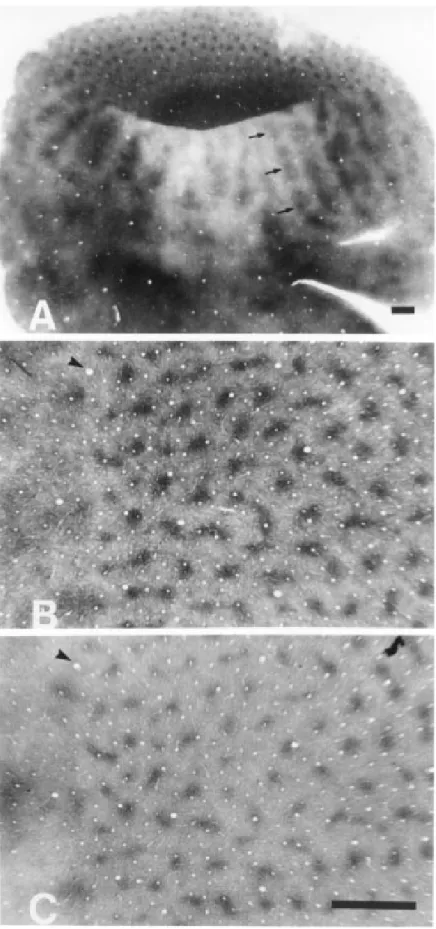
In tangential sections, the distinction be-tween V1 and V2 was very clear because of the blob and stripe patterns, respectively
(Figure 2A). In V1, NADPHd- (Figure 2B) and cytochrome oxidase-rich zones (Figure 2C) appeared as rows of blobs separated by interblob regions where enzymatic activity was poor. Most of the blobs were isolated and rounded. In some instances, blobs were linked by bridges of higher neuropil activity. The t-test applied to samples from adjacent
sections treated to detect NADPHd or cyto-chrome oxidase activity (Figure 3A,B) pointed to significant differences between blob areas revealed by cytochrome oxidase (36,280 µm2, N = 126, SD = 11,884) and NADPHd (42,582 µm2, N = 121, SD = 14,272, 2-tailed t-test, P<0.001) (Figure
3A,B). Nearest neighbor blob distances were similar when NADPHd and cytochrome oxi-dase were compared (375 x 364 µm, respec-tively, 2-tailed t-test, P = 0.546, N1 = 126,
N2 = 130, SD1 = 65.8, SD2 = 65.3) (Figure 3C,D). The spatial distribution of the two blob systems was not random. Nearest neigh-bor distances of NADPHd or cytochrome oxidase blobs were significantly different from nearest neighbor distances measured in a random dot pattern of the same area and density (P<0.05) (Figure 3E). Additionally, in the operculum the number of NADPHd blobs per mm2 of striate cortex was, on average, quite similar to that reported by Horton (17) in Rhesus monkey (4.6/mm2 x 4.8/mm2, respectively).
The spatial relationship between the two enzymes was further investigated by align-ing adjacent cytochrome oxidase and NADPHd sections using blood vessels as landmarks. Figure 4 shows the result of this procedure for the equivalent fields depicted in Figure 2B and C. Although the two enzy-matic systems have a similar spatial distribu-tion they are not identical.
ter thin stripe and thick stripe distances were 2 mm (SD = 0.5 mm) and 1.5 mm (SD = 0.4 mm), respectively. Thin stripes extended from the anterior border of V1 to the anterior margin of V2. Different from thin stripes, thick stripes did not touch the V1/V2 border. Both types of stripes seemed to present ir-regular enzymatic activity, suggesting the occurrence of separate compartments along the stripe. These compartments were charac-terized by zones rich in enzymatic activity isolated by thin paler transverse regions (Fig-ure 2A). The anterior limit of V2 could be identified by the disappearance of the stripe pattern.
Morphology and distribution of NADPH-diaphorase-positive neurons
In addition to neuropil activity, NADPH-diaphorase revealed two different subpopu-lations of labeled cells that were not ob-served by means of cytochrome oxidase his-tochemistry. The first group of cells was composed of a large number of small lightly stained NADPHd-positive somata. Labeling of these cells was so faint that only their cell bodies could be observed by light micros-copy (Figure 1B). Primary processes could be distinguished in a few cases. These cells were found to be scattered throughout all layers but they were much more numerous in laminae 2 and 3. Modifications in fixation procedures could intensify their labeling. Lightly labeled cells were much more
merous in tissue processed by light parafor-maldehyde fixation (i.e., tissue exposed to fixative for less than 20 min and/or using lower concentrations of paraformaldehyde) or using glutaraldehyde as fixative (data not shown).
The second subpopulation of labeled cells presented fine anatomical details both in dendrites and axons (Figure 5). Similar to what is observed with Golgi staining, the
neuron processes and soma were so strongly labeled that we were not able to identify organelles or the cell nucleus. However, NADPHd-positive cells were much more frequent than Golgi-impregnated neurons in all cortical layers. In area 17 gray matter, the distribution and morphology of these NADPHd-positive neurons were heteroge-neous. The axons of NADPHd-positive cells were usually very thin and faintly stained. They could be followed for more than 200 µm from the cell body in one typical neuron (Figure 5D). In some cases, they could be followed for more than 1 mm (e.g., layer 5 or white matter cells projecting up to layer 1, data not shown). Axons could arise from any point in somata or primary dendrites and followed an ascending or descending initial course that could be changed in a recurrent trajectory giving rise to a number of short collaterals (e.g., Figure 5B). In most cases we observed boutons “en passant” (18) and, in a few cases, “club like” boutons (18) that were suggestive of synaptic sites.
All stained neurons were non-pyramidal sparsely spined cells which resembled the neurons described by Peters and Regidor (19) except for the fact that NADPHd neu-rons presented many varicosities along the dendrites. Gray matter neurons had cell bod-ies of different shapes. Dendritic arboriza-tion also varied from one neuron to another. NADPHd-positive cells showed dendritic arborization in the immediate vicinity of the cell body. Some of the NADPHd-positive neurons had dendritic trees that were elon-gated in opposite directions (Figure 5B). These neurons were similar to the bi-tufted cells reported by Fairén et al. (20). We did not detect any strict correlation between the pattern of dendritic arborization and laminar position. However, there were some excep-tions. At the bottom of layer 6, most neurons had horizontally oriented dendrites which resembled the neurons impregnated by the Golgi staining method described by Tömböl (21). Another exception was layer 4C
neu-Number Number Number Number Number 40 30 20 10 0 40 30 20 10 0
0.2 0.4 0.6 0.8 1.0
0.2 0.4 0.6 0.8 1.0 Area (µm2 x 106)
B - Cytox
A - Diaphorase
Blob areas 100 80 60 40 20 0
0 200 400 600
100 80 60 40 20 0
0 200 400 600
100 80 60 40 20 0
0 200 400 600
Nearest neighbor distance (µm) C D E Diaphorase Fitted normal distribution Cytox Fitted normal distribution Randon points Fitted normal distribution
Figure 3 - Quantitative analysis of blob distribution in a tangen-tial section. A and B, Histograms of blob areas as measured in the section reacted for diaphorase (A) and cytochrome oxidase (Cytox) (B). C, D and E, Histo-grams of nearest neighbor dis-tances between blobs in the section processed for diapho-rase (C), and cytochrome oxi-dase (D). These distributions are different from that obtained for a random dot pattern of the same density (E).
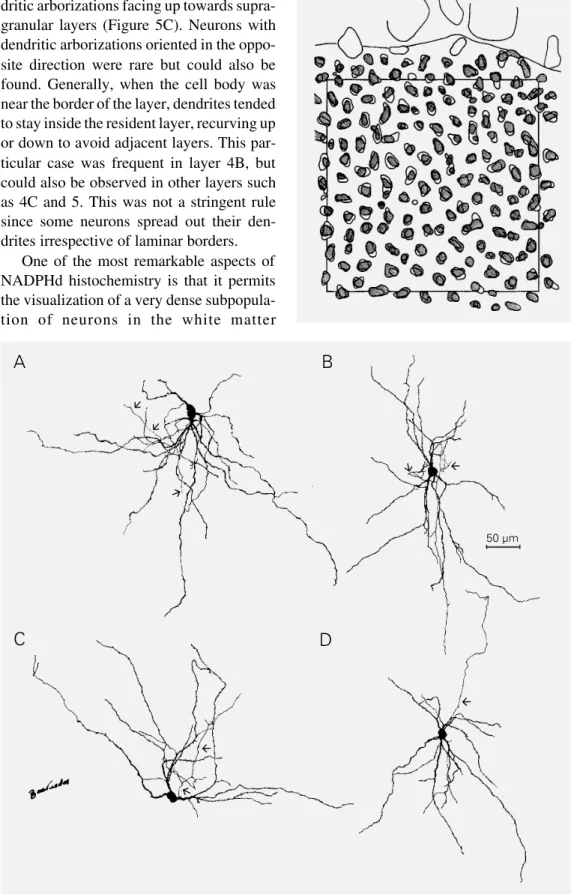
rons. In some cases, these neurons had den-dritic arborizations facing up towards supra-granular layers (Figure 5C). Neurons with dendritic arborizations oriented in the oppo-site direction were rare but could also be found. Generally, when the cell body was near the border of the layer, dendrites tended to stay inside the resident layer, recurving up or down to avoid adjacent layers. This par-ticular case was frequent in layer 4B, but could also be observed in other layers such as 4C and 5. This was not a stringent rule since some neurons spread out their den-drites irrespective of laminar borders.
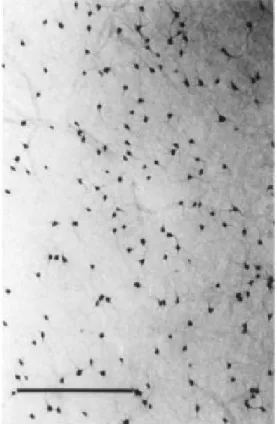
One of the most remarkable aspects of NADPHd histochemistry is that it permits the visualization of a very dense subpopula-tion of neurons in the white matter
Figure 4 - Camera lucida draw-ing representdraw-ing the superim-posed blob pattern obtained by using the blood vessels as land-marks. The same sections as il-lustrated in Figure 2B and 2C were used. Blobs revealed by cytochrome oxidase histochem-istry are hatched. Area of the square is 25 mm2.
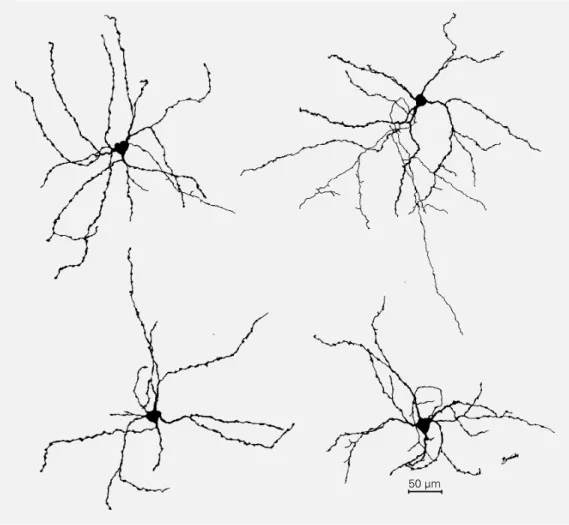
Figure 5 - Camera lucida draw-ings of NADPH-diaphorase-posi-tive neurons from different cor-tical layers of V1 gray matter as seen in a parasagittal section. Although their dendritic arboriza-tion seems quite variable, none of the cells are pyramidal. Supra-granular layer neurons (A,B), layer 4C neurons (C), and a neu-ron from layer 5 (D) are illus-trated. The arrows point to axons.
A
B
C
D
50 µm
à
à
à
à
à
à
à
à
(Figure 6). These neurons have not been identified previously by means of any other technique in the adult brain (15). In V1, most NADPHd neurons were in the white matter (considering just the first 150 µm below the inferior border of layer 6). These neurons comprised about 80% of the total population of heavily labeled cells (laminar distribution in V1 gray matter was the following: supra-granular layers = 11.9%, layer 4C = 3.3%, infragranular layers = 4.1%; N = 2256). White matter cell distribution and morphology were more homogeneous than those of gray mat-ter neurons. In non-tangential sections, white matter cells exhibited dendritic arborization oriented along the tangential plane. Some dendrites extended to the gray matter. In tangential sections, white matter cell den-drites displayed a radial distribution (Fig-ures 6 and 7), a pattern suggesting that these neurons might form a neural network with significant dendritic overlap. Axons of these neurons extended towards the white and/or gray matter. Additionally, some of these
axons bifurcated after a few hundred mi-crometers giving off many short collaterals. In a few cases, we followed axons from white matter cells up to layer 1. These axons had many thin collaterals and often pre-sented boutons “en passant”. Terminal boutons were less frequent.
In the white matter we also identified many thick NADPHd-positive axons that could not be attributed to any of the reactive cells in the section. These axons could be observed in a course parallel to the pial surface and were 2 to 3 times thicker than axons of neocortical NADPHd neurons. These thick axons could be followed for more than 1 mm before being sectioned on the surface of the slice. We do not know the origin or the target of these axons, but we think they may arise from subcortical projec-tions since their morphology was different from that of axons of cortical NADPHd-positive cells.
Since no remarkable qualitative morpho-logical differences could be assigned to the neurons from different cortical areas or from gray vs white matter, we decided to compare
soma areas of well-impregnated NADPHd neurons in V1, V2 and white matter. V1 soma areas ranged from 98 to 240 µm2 (mean = 154 µm2, N = 182, SD = 32.9 µm2). No remarkable difference could be found in V2, that had similar soma areas (mean = 144.5 µm2, N = 100, SD = 29.2 µm2). Neurons from different stripes in V2 had similar soma areas. White matter soma areas were signif-icantly larger (mean = 222.8 µm2, N = 104, SD = 52.2 µm2, P<0.001) than V1 gray matter neurons.
In parasagittal, coronal and horizontal sections dendritic field areas of V1 gray matter neurons were also measured. No cor-relation between soma area and dendritic field area could be detected.
In tangential sections, the number of cells counted in supragranular layers of V1 was three times higher in interblobs than inside blobs. However, inasmuch as the interblob
area is proportionately larger than the sum of all blob areas, there was no difference in terms of cell density inside and outside blobs. Thus, NADPHd neurons seemed to be ran-domly distributed in area 17 supragranular layers, showing no preference for blobs or interblob regions.
Discussion
NADPH-diaphorase activity in brain tis-sue reveals soluble nitric oxide synthase ac-tivity in paraformaldehyde-fixed material (4). Although particulate NOS can be demon-strated histochemically using glutaraldehyde as a fixative, this isoform seems to predomi-nate in brain regions other than the neocor-tex (22). Aoki et al. (23) provided further evidence that in V1 NADPHd and nitric
oxide synthase are co-localized in macaques. Staining for NADPHd activity in area 17 of squirrel monkeys revealed an intense neu-ropil activity that was related to cortical layers, as also observed by cytochrome oxi-dase histochemistry (17). Sandell (15) re-ported similar results for Rhesus monkeys and demonstrated that primate visual cortex presents heterogeneous metabolic activity generating modular-like structures, such as blobs and stripes. These metabolic compart-ments have been described by means of other techniques such as cytochrome oxidase and cellular uptake of tritiated neurotransmitters (17,24). Electrophysiological recordings sug-gest that units with different receptive field properties are selectively confined to zones of different metabolic activity (25). Never-theless, the functional significance of this
Figure 7 - Camera lucida draw-ings of four representative white matter neurons obtained in a tangential section.
heterogeneity is still a subject of intense debate (26).
Sandell (15) demonstrated that cyto-chrome oxidase and NADPH-diaphorase blobs coincided using NADPHd and cyto-chrome oxidase histochemistry in alternate sections in Rhesus monkey visual cortex, and aligning them using blood vessels as landmarks. We used cytochrome oxidase and NADPHd histochemistry in both alternate and single sections (double labeling; data not shown) of area 17 of the squirrel mon-key. Our results revealed that, at least in the operculum, NADPHd blobs do not always coincide perfectly with cytochrome oxidase blobs (Ref. 27 and the present paper). From a functional point of view, we do not know the significance of these results. It has been well established that NADPHd corresponds to bNOS in fixed brain tissue (4,7-10). NOS and cytochrome oxidase have markedly dif-ferent functions in the central nervous sys-tem (28,29) and their subcellular and cellu-lar distributions are also rather different. Electron microscopy of NADPHd-positive cells demonstrated that formazan is dispersed along the neuron and cannot be associated with any specific cell organelle (30). On the other hand, electron microscopic analyses have revealed a completely different distri-bution for cytochrome oxidase which is a mitochondrial enzyme (29,31,32). The mor-phological types of cytochrome oxidase-posi-tive neurons in V1 (31) and V2 (32) are different from those revealed by NADPHd histochemistry. Cytochrome oxidase stains a good part of pyramidal cells that have not been labeled so far using NADPHd. In addi-tion, most NADPHd-positive neurons were found in the white matter where cytochrome oxidase-positive neurons were not observed. Since NOS and cytochrome oxidase have different molecular roles, we may assume that they should have different topographi-cal distributions in the brain possibly deter-mined by different factors. In that case, con-gruent zones of cytochrome oxidase and
ni-tric oxide synthase blobs would be an epiphe-nomenon. On the other hand, NO has been suggested to be a retrograde messenger that stimulates glutamatergic presynaptic termi-nals creating a positive feedback loop (6). This would explain why the most metaboli-cally active (cytochrome oxidase) regions are also rich in nitric oxide synthase. NO is also a potent cerebrovasodilator. Its release from nearby NOS-positive neurons can be elicited by increased neural activity (33). Thus, NO can be the link between enhance-ment of brain metabolism and the increase in cerebral blood supply (33). This hypothesis is coherent with co-localization of cyto-chrome oxidase (a metabolic enzyme) and NOS. It should be noted that in the present study we used the malic enzyme “indirect” method to label NADPHd activity. This tech-nique requires intracellular metabolic en-zymes to reduce NADP to NADPH which is used as a cofactor for bNOS (34). Thus, we could be labeling only a subpopulation of NOS-positive neurons that are more meta-bolically active. Nevertheless, Sandell (15) obtained the same results using both “direct” and “indirect” histochemical methods. Like Sandell, we chose the “indirect” method be-cause it provides lower levels of nonspecific background activity.
by light microscopy. Subcellular distribu-tion of cytochrome oxidase is heterogeneous in most neurons (29) and this can also be true for NOS. We also do not know if the neuro-pil pattern in monkey visual cortex origi-nates from local, cortico-cortical, thalamo-cortical or different combinations of these projections. Our preliminary results with NADPHd histochemistry in the lateral gen-iculate nucleus (LGN) of squirrel and Cebus monkeys revealed a disappointingly small number of reactive neurons. This is also the case for Old World monkeys (23). No la-beled neurons are found in the LGN when NOS immunohistochemistry is performed (23). However, enzymatic activity is strongly influenced by monocular deprivation both in New World (35) and Old World monkeys (15,23). These studies demonstrated ocular dominance columns in monocular monkeys using NADPHd histochemistry (15,35) or NOS immunohistochemistry (23).
It is also still unclear whether glial cells contribute to the neuropil pattern. Glial cells present both constitutive and inductive forms of NOS (36) and may correspond to the lightly stained cell bodies observed in our material. The small faintly labeled cells de-tected with NADPHd are by far much more numerous than the intensely labeled sub-population. This is also the case for Old World monkeys (15,23). In these cells NADPHd and NOS immunoreactivity are also co-localized (23). Since histochemical procedures do not reveal their morphology in detail we are not sure if they are neural cells. Aoki et al. (23) claimed that these cells are neurons. Nevertheless, their small so-mata suggest that they may be glial elements.
Further studies using specific neuronal and/ or glial markers are needed to clarify this issue.
Although the general pattern of NADPHd histochemistry is closely similar in Old World (15,23) and New World monkeys (our re-sults), some aspects of the distribution of heavily labeled neurons seem to be different. Sandell (15) demonstrated that most neu-rons in supragranular layers were inside en-zyme-rich “patches” (blobs) (252 cells in-side blobs vs 135 cells in interblob regions).
This was not the case for squirrel monkeys in which the labeled neurons were evenly dis-tributed. In this respect, our results are simi-lar to those obtained by Aoki et al. (23). Another striking difference was the relative number of cells counted in layer 4C. This layer is typically devoid of labeled cell so-mata both in New and Old World monkeys (15,23), although it seemed to be more popu-lated in squirrel monkeys. We found that 3.3% of neurons counted in V1 (N = 2256, including white matter cells) were in layer 4C. In all other layers the ratios were similar to those reported for Rhesus monkeys (15) but in layer 4C the percentage was more than ten times greater. This can be related to the absence of ocular dominance columns in squirrel monkeys (16). Nevertheless, the functional significance of such interspecific differences is not clear and demands further investigation.
Acknowledgment
References
1. Thomas E & Pearse AGE (1964). The soli-tary active cells. Histochemical demon-stration of damage resistant nerve cells with a TPN-diaphorase reaction. Acta Neuropathologica, 3: 238-249.
2. Ferrante RJ, Kowall NW, Beal MF, Martin JB, Bird ED & Richardson EP (1987). Mor-phologic and histochemical characteristics of a spared subset of striatal neurons in Huntington’s disease. Journal of Neuro-pathology and Experimental Neurology, 46: 12-27.
3. Uemura Y, Kowall NW & Beal MF (1990). Selective sparing of NADPH-diaphorase-somatostatin-neuropeptide Y neurons in ischemic gerbil striatum. Annals of Neu-rology of the American Neurological As-sociation, 27: 620-625.
4. Matsumoto T, Nakane M, Pollock JS, Kuk JE & Förstermann U (1993). A correlation between soluble brain nitric oxide syn-thase and NADPH-diaphorase activity is only seen after exposure of the tissue to fixative. Neuroscience Letters, 155: 61-64.
5. Bredt DS & Snyder SH (1990). Isolation of nitric oxide synthase: a calmodulin requir-ing enzyme. Proceedings of the National Academy of Sciences, USA, 87: 682-685. 6. Garthwaite J (1991). Glutamate, nitric ox-ide and cell-cell signaling in the nervous system. Trends in Neurosciences, 14: 60-67.
7. Bredt DS, Glatt CE, Hwang PM, Fotuhi M, Dawson TM & Snyder SH (1991). Nitric oxide synthase protein and mRNA are dis-cretely localized in neuronal populations of the mammalian CNS together with NADPH diaphorase. Neuron,7: 615-624. 8. Dawson TM, Bredt DS, Fotuhi M, Hwang
PM & Snyder SH (1991). Nitric oxide syn-thase and neuronal NADPH diaphorase are identical in brain and peripheral tis-sues. Proceedings of the National Acade-my of Sciences, USA,88: 7797-7801. 9. Hope BT, Michael GJ, Knigge KM &
Vincent SR (1991). Neuronal NADPH-dia-phorase is a nitric oxide synthase. Pro-ceedings of the National Academy of Sci-ences, USA, 88: 2811-2814.
10. Vincent SR & Kimura H (1992). His-tochemical mapping of nitric oxide syn-thase in the rat brain. Neuroscience, 46: 755-784.
11. Franca JG & Volchan E (1995). NADPH diaphorase histochemistry as a marker for barrels in rat somatosensory cortex. Bra-zilian Journal of Medical and Biological Research,28: 787-790.
12. Picanço-Diniz CW, do Nascimento JLM & Friedlander MJ (1993). Histochemical evaluation of nitric oxide synthase levels in guinea pig visual cortex after L-nitroarginine administration in vivo and in vitro. The Association for Research in Vi-sion and Ophthalmology (ARVO) Annual Meeting, Sarasota, Florida. Investigative Ophthalmology and Visual Science, 34: 1175 (Abstract).
13. Volchan E & Franca JG (1994). Distribu-tion of NADPH-diaphorase-positive neu-rons in the opossum neocortex. Brazilian Journal of Medical and Biological Re-search, 27: 2431-2435.
14. Mizukawa K, Vincent SR, McGeer PL & McGeer EG (1988). Reduced nicotinamide adenine dinucleotide phosphate (NADPH)-diaphorase-positive neurons in cat cere-bral white matter. Brain Research, 461: 274-281.
15. Sandell JH (1986). NADPH-diaphorase his-tochemistry in the macaque striate cor-tex. Journal of Comparative Neurology, 251: 388-397.
16. Hendrickson AE, Wilson JR & Ogren MP (1978). The neuroanatomical organization of pathways between the dorsal lateral geniculate nucleus and the visual cortex in old world and new world primates. Journal of Comparative Neurology, 182: 123-136.
17. Horton JC (1984). Cytochrome oxidase patches: a new cytoarchitectonic feature of monkey visual cortex. Philosophical Transactions of the Royal Society of Lon-don, Series B,304: 199-253.
18. Martin KAC & Whitteridge D (1984). Form, function and intracortical projections of spiny neurones in the striate visual cortex of the cat. Journal of Physiology, 353: 463-504.
19. Peters A & Regidor J (1981). A reassess-ment of the forms of non-pyramidal neu-rons in area 17 of cat visual cortex. Jour-nal of Comparative Neurology,203: 685-716.
20. Fairén A, De Felipe J & Regidor J (1984). Non-pyramidal neurons - general account. In: Peters A & Jones EG (Editors), Cere-bral Cortex: Cellular Components of the Cerebral Cortex. Vol. 1. Plenum Press, New York, London, 201-253.
21. Tömböl T (1984). Layer VI cells. In: Peters A & Jones EG (Editors), Cerebral Cortex: Cellular Components of the Cerebral Cor-tex. Vol. 1. Plenum Press, New York, Lon-don, 479-519.
22. Dinerman JL, Dawson TM, Schell MJ, Snowman A & Snyder SH (1994). Endo-thelial nitric oxide synthase localized to hippocampal pyramidal cells: Implications for synaptic plasticity. Proceedings of the National Academy of Sciences, USA, 91: 4214-4218.
23. Aoki C, Fenstemaker S, Lubin M & Go C-G (1993). Nitric oxide synthase in the vi-sual cortex of monocular monkeys as re-vealed by light and electron microscopic immunocytochemistry. Brain Research, 620: 97-113.
24. Carroll EW & Wong-Riley MTT (1985). Correlation between cytochrome oxidase staining and the uptake and laminar distri-bution of tritiated aspartate, glutamate, γ -aminobutyrate and glycine in the striate cortex of the squirrel monkey. Neurosci-ence, 15: 959-976.
25. Livingstone MS & Hubel DH (1984). Ana-tomy and physiology of a color system in the primate visual cortex. Journal of Neu-roscience, 4: 309-356.
26. Allman J & Zucker S (1990). Cytochrome oxidase and functional coding in primate striate cortex: a hypothesis. Cold Spring Harbor Symposium on Quantitative Biol-ogy, 55: 979-982.
27. Picanço-Diniz CW, Martin KAC, Franca JG, Quaresma JAS, do Nascimento JLM & Friedlander MJ (1992). A new “blob” sys-tem in the visual cortex of the squirrel monkey revealed by nitric oxide synthase. Society for Neuroscience Abstracts, 18 (Part 1): 210.
28. Bredt DS & Snyder SH (1992). Nitric ox-ide: a novel neuronal messenger. Neuron, 8: 3-11.
29. Wong-Riley MTT (1989). Cytochrome oxi-dase: an endogenous metabolic marker for neuronal activity. Trends in Neuro-sciences,12: 94-101.
30. Vincent SR & Johansson O (1983). Striatal neurons containing both somatostatin and avian pancreatic polypeptide (APP)-like immunoreactives and NADPH-diaphorase activity: a light and electron microscopic study. Journal of Comparative Neurology, 217: 264-270.
32. Wong-Riley MTT & Carroll EW (1984). Quantitative light and electron microscop-ic analysis of cytochrome oxidase-rmicroscop-ich zones in VII prestriate cortex of squirrel monkeys. Journal of Comparative Neurol-ogy, 222: 18-37.
33. Iadecola C (1993). Regulation of the cere-bral microcirculation during neural activ-ity: is nitric oxide the missing link? Trends in Neurosciences, 16: 206-214.
34. Sandell JH (1985). NADPH diaphorase cells in the mammalian inner retina. Jour-nal of Comparative Neurology, 238: 466-472.
35. Do Nascimento RSV (1995). Estudo das colunas de dominância ocular do Cebus apella pela histoquímica de NADPH-diaforase. Master’s thesis, Universidade Federal do Pará/Museu Paraense Emílio Goeldi.