w ww . e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
Chemical
profiles
of
traditional
preparations
of
four
South
American
Passiflora
species
by
chromatographic
and
capillary
electrophoretic
techniques
Geison
Modesti
Costa
a,b,∗,
Andressa
Córneo
Gazola
a,
Silvana
Maria
Zucolotto
c,
Leonardo
Castellanos
b,
Freddy
Alejandro
Ramos
b,
Flávio
Henrique
Reginatto
a,
Eloir
Paulo
Schenkel
aaProgramadePós-graduac¸ãoemFarmácia,UniversidadeFederaldeSantaCatarina,Florianópolis,SC,Brazil
bDepartamentodeQuímica,UniversidadNacionaldeColombia,Bogotá,Colombia
cDepartamentodeFarmácia,CentrodeCiênciasdaSaúde,UniversidadeFederaldoRioGrandedoNorte,Natal,RN,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received17November2015 Accepted23February2016 Availableonline13April2016
Keywords: Passiflora
C-glycosylflavonoids Alkaloids
HPLC UPLC CE
a
b
s
t
r
a
c
t
SeveralspeciesofthegenusPassifloraaredistributedalloverSouthAmerica,andmanyofthesespecies areusedinpopularmedicine,mainlyassedativesandtranquilizers.Thisstudyanalyzesthechemical profileofextractsoffourPassifloraspeciesusedinfolkmedicine,focusingontheflavonoids,alkaloids andsaponins.Weemployedsimpleandfastfingerprintanalysismethodsbyhighperformanceliquid chromatography,ultraperformanceliquidchromatographyandcapillaryelectrophoresistechniques. TheanalysisledtothedetectionandidentificationofC-glycosylflavonoidsinalltheplantextracts,these beingthemainconstituentsinP.tripartitavar.mollissimaandP.bogotensis.Saponinswereobserved onlyinP.alataandP.quadrangularis,whileharmanealkaloidswerenotdetectedinanyoftheanalyzed extractsinconcentrationshigherthan0.0187ppm,thedetectionlimitdeterminedfortheUPLCmethod. ©2016SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Thisisanopen accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
ThegenusPassifloraisthelargestandmostimportantgenus
ofthefamilyPassifloraceae,comprisingabout500species(Lewis andElvin-Lewis, 1977).InNorth Americaand Europe,themain species,P.incarnata,ispopularlyknownaspassionfruitor passion-flower,whileinSouthAmerica,severalothersspeciesofPassiflora
are widely distributed and known by distinct names, such as
‘maracujá’, ‘curuba’, or ‘badea’, among others. (Arbelaez, 1996; Morsetal.,2000)Manyofthesespecies(P.edulisvar.edulis,P.edulis var.flavicarpa,P.tripartitavar.mollissimaandothers)arecultivated fortheirediblefruitsorforthepreparationofjuices.Infusionsof theirleavesarealsousedinpopularmedicineinmanycountries, asasedativeortranquillizer(PioCorrêa,1978;Arbelaez,1996).
Different countries in South America have registered
phar-maceuticalpreparationsthat usePassiflora speciesastheactive component.In Colombia,for example,theleavesofP.tripartita
var. mollissima are accepted as sedative and hypnotic
compo-nentinphytopharmaceuticalpreparations(Invima,2006).InBrazil,
∗ Correspondingauthor.
E-mail:gmcosta@unal.edu.co(G.M.Costa).
P.alataandP.edulisareincludedinthemostrecentversionofthe BrazilianPharmacopeia(FarmacopeiaBrasileira,2010).
Regardingtheirchemical composition, thecompoundsmore
frequently reported for the genus are flavonoids, especially C
-glycosylflavonoids, which are usually described as the main
components(Ulubelenetal.,1982;Lietal.,2011;Zucolottoetal.,
2012).Thesecompoundshaverecentlybeenassociatedwith
sev-eralpharmacologicaleffectsobservedfordistinctPassifloraspecies (Coletaetal.,2006;Santosetal.,2006;Senaetal.,2009;Zucolotto etal.,2009;Gazolaetal.,2015).Harmanealkaloidsarealso fre-quentlyassociatedwithPassifloraspecies,especially P.incarnata (LutomskiandMalek,1975;Lutomskietal.,1975).Additionally,
several saponins have been described for this genus, although
theiroccurrenceisrestrictedtocertainspecies(Orsinietal.,1985; Reginattoetal.,2001;Doyamaetal.,2005).
Aspartofourongoingstudieswithspeciesofthegenus Pas-siflora, we evaluate, in this study, the variability of metabolite presentsintheaqueousextractsoffourSouthAmericanPassiflora species:P.alata, P.quadrangularis,P.bogotensisandP.tripartita var.mollissima,focusingspecificallyontheirC-glycosylflavonoid
and alkaloid composition. The presence of saponins in these
species wasalsoevaluated.Chemicalprofileswere obtainedby
different analytical methods, such as high performance liquid
http://dx.doi.org/10.1016/j.bjp.2016.02.005
Table1
Passifloraspecies,withtheirrespectivelocalcommonname,placeofcollectionandidentification.
Species Localcommonname Placeofcollection/timeofyear Identification
P.quadrangularisLinn. Badea,
Maracujá-gigante
Rivera,Huila–Colombia (2◦59′55′′,−75◦18′16′′)/July2011
Prof.CarlosAlbertoParra/Herbariumofthe UniversidadNacionaldeColombia(COL 572634)
P.alataCurtis. Maracujá-doce Urussanga,SantaCatarina–Brazil (−28◦
32′ 9′′
,−49◦ 18′
59′′
)/January2011
Mrs.AdemarBrancher(EPAGRI/Urussanga-SC). HerbariumoftheUniversidadeFederalde SantaCatarina(FLOR37823)
P.tripartitavar. mollissima Holm-Nielsen& MüllerJörgensen
Curuba-de-Castilla SantaSofia,Boyacá–Colombia (05◦43′01′′,
−73◦36′20′′)/June2011
Prof.CarlosAlbertoParra/Herbariumofthe UniversidadNacionaldeColombia(COL 564522)
P.bogotensisBenth – Nemocón,Cundinamarca–Colombia
(4◦ 35′
60′′ ,−4◦
4′ 60′′
)/May2011
Prof.CarlosAlbertoParra/Herbariumofthe UniversidadNacionaldeColombia(COL 564523)
chromatography(HPLC),ultraperformanceliquidchromatography
(UPLC)andcapillaryelectrophoresis(CE).
Materialsandmethods
Chemicals
Acetonitrile,methanolandformicacid(HPLC-grade)were
pro-videdbyTedia®(RiodeJaneiro,Brazil).Waterwaspurifiedwith
a Milli-Q system(Millipore®, Bedford, USA). For theCE
analy-sis,stock solutionswerepreparedfromtheelectrolytessodium
tetraborate(STB)andammoniumacetate(AcNH4)at100mmol/l.
Sodiumhydroxidesolution(NaOH)at1and0.1mol/lwerealso
pre-pared.Allthesaltsusedwereofanalyticalreagentgrade,andwere
providedbySigma-Aldrich®(St.Louis,USA).Allthesolventsand
solutionswerefilteredthrougha0.22mmembranebeforeuse.
Thereferencestandardsorientin,isoorientin,vitexin,isovitexin,
vitexin-2′′-O-rhamnoside,harmol,harmaneandharmine(allwith
purity≥96%)werepurchasedfromSigma-Aldrich®.Swertisinwas
previouslyobtainedfromWilbrandeaebracteataroots,and
iden-tifiedbyNMRspectraldata(Santosetal.,1996).Thecompound
4′-methoxyluteolin-8-C-6′′-acetylglucopyranosidewaspreviously
isolatedfromP.tripartitavar.mollissimaleavesandprovidedby Prof.Dr.FreddyRamos(Ramosetal.,2010).Quadrangulosidewas previouslyisolatedfromPassifloraalataleaves(Costaetal.,2013).
Plantmaterialandpreparationofextractsandsamples
Leavesof adultindividuals of species of Passiflora were col-lectedfrom different regions of Brazil and Colombia (Table1). Leavesofthedifferentspecieswereair-driedseparatelyat40◦C,
powdered,and extracted byinfusionwithboilingwater (95◦C,
plant:solvent1:10,w/v)for10min.Theaqueousextractwasthen filtered,frozenandlyophilized.ThesamplesforHPLC,UPLCandCE analysiswerepreparedbydissolvingthelyophilizedcrude aque-ousextractsorreferencestandardsinmethanol:water(1:1,v/v)
andfilteringthrougha0.22mmembranebeforeinjection.The
concentrationofthesampleextractswas1000g/mlandforthe
referencestandards,theconcentrationwas100g/ml.
HPLCanalysis
TheHPLCanalyseswerecarriedoutinaPerkinElmer®Series200
system,equippedwithDiodeArrayDetection(DAD),quaternary
pump,on-linedegasserandautosampler.Thedatawereprocessed
usingthesoftwareChromera®Workstation.Thechromatographic
analysesfor all sampleswere performed at room temperature
(21±2◦C),withaninjectionvolumeof20
l.TheDADspectrawere
acquiredattherangeof190–450nm.Thepeaksin thesamples
werecharacterizedbycomparingtheretentiontime,UVspectra
andco-injectionwiththereferencestandards.Vertical®VertSep
C18column(250mm×4.6mmi.d.;5m)wasusedasstationary
phase.Intheanalysisofflavonoids,agradientsystemof acetoni-trile[solventA]andformicacid0.5%[solventB]wasusedasmobile phase,inasinglestep:15–35%A(0–20min).Theflowratewaskept
constantat1.2ml/minandthechromatogramswererecordedat
340nm.Foralkaloidanalysis,themobilephaseusedwascomposed
ofanaqueousbufferofsodiumphosphatedibasic(50mmol/l,pH
8.0)[A],methanol[B]andacetonitrile[C]atisocraticconditionsof 56%A:12%B:32%C(0–20min).Theflowratewaskeptat1ml/min
andtheUVdetectionat245nm.Thechromatographicconditions
fortheanalysisofsaponinswerepreviouslydescribedbyourgroup (Costaetal.,2013).
UPLCanalysis
AnUPLCWatersAcquity® HClasssystemwithDADdetector,
quaternarypump,on-linedegasserandautosamplerwasusedfor
theseanalyses.Thechromatographicparameterswereconverted
fromHPLCtoUPLCusingthesoftwareEmpower®
.Separationsof bothflavonoidsandalkaloidswerecarriedoutinaPerkinElmer®
BHE C18 column (100mm×2.9mm i.d.; 1.8m). The analyses
werealsoperformedatroomtemperature(21±2◦C),withDAD
spectra acquired at the range of 190–450nm. Flavonoid
anal-ysis used a two-steps gradient of acetonitrile [solvent A] and
formic acid 0.5% [solvent B]: 15–35%A (0–8min), followed by
35%A(8–10min).Theflowratewaskeptconstantat0.25ml/min.
Theinjectionvolumewas3l.Thechromatogramwasrecorded
at 340nm. For the alkaloids, the same mobile phase and
iso-cratic system as the HPLC was used, withan analysis time of
7min. The flow rate was determinate as 0.2ml/min, with an
injectionvolume of 2l. The UV detection was determined at
245nm.
CEanalysis
TheanalyseswereperformedonanAgilent7100capillary
elec-trophoresisinstrumentequippedwithDADdetector,temperature
controldevice,andautosampler.Foralltheexperiments,a
fused-silicacapillary (Agilent,modelG1600-61232) of60.5cm (52cm
effectivelength),with50minnerdiameterandexpanded
detec-tionwindowwasused.Thedatawereprocessedusingthesoftware Agilent ChemStation®. For the first use, the capillary was
pre-treatedwithapressureflushwith1mol/lNaOHsolution(30min). Eachday,thecapillarywasconditionedwithNaOH1mol/l(5min),
waiting time (1min), Milli-Q water (5min) and running buffer
(5min).Inbetweenruns,thecapillarywasflushedwithrunning
buffer(2min). The DAD spectrawere acquiredat the range of
200–500nm.Amethodfortheanalysisofflavonoidswas
1
500 mAU
0
45
0
120
60
0
0 200
100
50
0
0 10 20
8
P. tripartita P. bogotensis P. quadrangularis P. alata
7 5 2 1
min 100
25
10
3
2 6
5
1
6 4
∗C
∗B
∗A
∗D
∗E
∗F
∗G 20 min
mAU
2 3,4567 8 with20%ofMeOHasrunningbuffer.Thesampleswereintroduced
tothesystembyhydrodynamicinjection(50mbar/10s).All separa-tionswereperformedatavoltageof+25kV,constanttemperature of30◦C,anddirectdetectionat390nm.Apigeninwasusedas
inter-nalstandard(I.S.)inordertoalignthemigrationtime.Thealkaloid
analysiswasperformedbasedonthemethodpreviouslydescribed
byUngeretal.(1997).Briefly,AcNH4(100mmol/l;pH4.0,adjusted
withaceticacid),with50%ACNwasusedasrunningbuffer. Hydro-dynamicinjection(50mbar/5s)wasused,withseparationvoltage of+15kV,constanttemperatureof15◦C,anddetectionat245nm.
Experimentaldeterminationofthesensitivityofthemethodsfor alkaloidanalysisbyUPLCandCE
Thelimitsofquantification(LOQ)anddetection(LOD)forthe analyticalmethodsusedintheanalysisofalkaloidswere
experi-mentallydeterminedusingstandardsolutionsfromtheharmane
alkaloid,preparedindifferentconcentrationsinthematrix (sam-ples),intherangeof50–0.0187g/ml.Allsolutionswereprepared
andanalyzedintriplicatebybothtechniques.Linearitywas deter-minedusingnine-pointandfive-pointregressioncurves,forUPLC andCE,respectively.LOQwasdefinedbysignal:noiseratioof10:1 andalsobyrelativestandarddeviation(RSD>5%).LODwasdefined byasignal:noiseratioof3:1(ICH,2005).
Resultsanddiscussion
Plant extracts are often complex mixtures whose
therapeu-tic effect cannot always attributedto a single component, and
sometimes,thecomponentsresponsibleforaparticulareffectare
notknown.Asnotallthecomponentshavereferencestandards
foridentificationandquantitation,somequalitycontrolanalyses
maybeperformedbyafingerprintanalysis,inwhichthe
exper-imental datafromthechemical analysisof differentextractsis comparedwithoutaccuratequantification.Thismethodisaccepted bytheWorldHealthOrganizationforthequalitycontrolofherbal medicines(WHO,1991).
C-glycosylflavonoidanalysis
Forthedevelopmentofthechromatographicmethods,seven
authenticsamplesofC-glycosylflavonoidswereusedasreference
compounds. After evaluating several parameters, including the
distinctchromatographicsystemsdescribedinliterature,the
ini-tialconditionsusedweredeterminedasthosethatallowedgood
resolutionbetweenallreferencecompounds,andenabledthe dif-ferentiationoftheextractsanalyzed,accordingtotheflavonoid profile.Theanalyticalparameterswerethenoptimized,basedon
visualizationofthemaximumnumberofpeaks,theirresolution
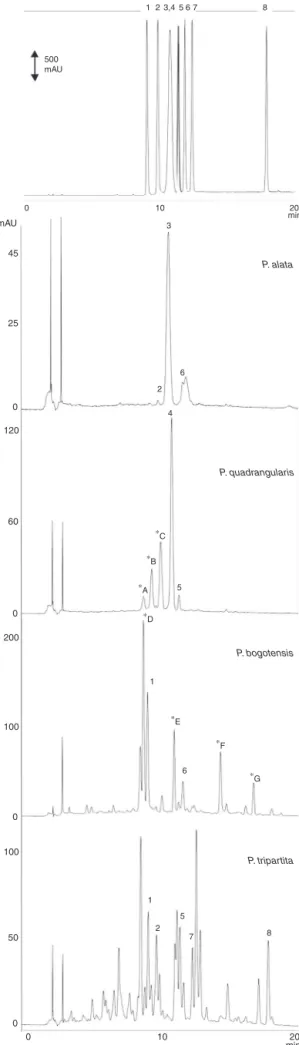
index,thetimerequiredfortheanalysis,andthesimplicityofthe method.Theflavonoidfingerprintsobtainedforeachspeciesinthe finalselectedmethodarepresentedinFig.1,andthepeak assign-mentsforeachextractaredescribedinTable2.
Amongthefouranalyzed species,P.alataand P.
quadrangu-larispresentedtheleastcomplex flavonoidprofile.It isnotable
Fig.1. HPLCchromatogramsofflavonoidsstandards(upperchromatogram; nor-malizedview)andaqueousextractsoftheleavesofPassifloraspecies.1:isoorientin, 2:orientin,3:vitexin-2′′-O-rhamnoside,4:vitexin-2′′-O-xyloside,5:vitexin,6:
isovitexin,7:swertisin,and8:4′-methoxyluteolin-8-C-6′′-acetylglucopyranoside.
*A:orientin-2′′-O-glucoside,*B:orientin-2′′-O-xyloside,*C:vitexin-2′′-O-glucoside
(identifiedbyGazola,2014),*D:isoorientin-2′′
-O-rhamnoside,*E:isovitexin-2′′
-O-rhamnoside, *F: luteolin-6-C-˛-l-rhamnopyranosyl-(1→2)-(6′′-O
-acetyl)-ˇ-d
-glucopiranoside,and*G:apigenin-6-C-˛-l-rhamnopyranosyl-(1→2)-(6′′-O
Table2
PeakassignmentsoftheaqueousextractsbyHPLC–DAD.
Flavonoids R1 R2 R3 R4 R5 Rta(min) UVmax(nm) Species
Isoorientin(1) glu H H OH OH 9.2 258,268sh,348 P:bogotensis;
P.tripartitavar.
mollissima
Orientin(2) H H glu OH OH 9.8 258,268sh,348 P.alata;
P.tripartitavar.
mollissima
Vitexin-2′′-O-rhamnoside(3) H H glu-O-rham H OH 10.5 269,335 P.alata
Vitexin-2′′-O-xiloside(4) H H glu-O-xil H OH 10.8 269,335 P.quadrangularis
Vitexin(5) H H glu H OH 11.4 269,338 P.quadrangularis;
P.tripartitavar.
mollissima
Isovitexin(6) glu H H H OH 11.7 269,338 P.alata;
P:bogotensis
Swertisin(7) glu OCH3 H H OH 12.3 270,334 P.tripartitavar.
mollissima
4′-Methoxyluteolin-8-C-6′′
-acetylglucopyranoside (8)
H H glu-O-acetyl OH OCH3 17.8 269,295,346 P.tripartitavar.
mollissima
glu,glucose;rham,rhamnose;xil,xiloside.
aPeaknumbersinFig.1.
thatthemajorflavonoidsofbothspecieshave almostthesame
retentiontimes.Thisobservationhaspreviouslybeendescribed
byourresearchgroup,thetwodistinctmajorcompoundsbeing
identifiedasvitexin-2′′-O-rhamnoside(3)(P.alata)andvitexin-2′′
-O-xyloside(4)(P.quadrangularis),proposedaschemicalmarkers for these species (Costa et al., 2013).Additionally, orientin (2) and isovitexin (6)were identified in P.alata, while vitexin (5) wasobservedonlyinP.quadrangularis.OtherC-glycosylflavonoids fromP.quadrangularis,whichhave beenisolatedand identified (Gazola,2014),werealsoassigned(Fig.1,peaks*A–*C).Someof thesecompounds(orientin-2′′-O-xyloside,vitexin-2′′-O-glucoside,
vitexin-2′′-O-xyloside)havealsobeendescribedbySakalemand
co-workers(2012).
Ahigheraccumulationofflavonoidswasobservedintheextract ofP.bogotensis,althoughonlytwoflavonoidscouldbeidentified byspikingexperimentswithcommercialstandards(isoorientin(1) andisovitexin(6)).Recently,workontheflavonoidcompositionof P.bogotensisleaveshasreportedthepresenceofthesecompounds, togetherwithotherC-glycosylflavonoids,indicatedinFig.1(peaks *D–*G)(Costaetal.,2015).
Among the extracts analyzed, P. tripartita var mollissima
displayed the most complex flavonoid profile. Isoorientin,
orientin, vitexin, swertisin and 4′-methoxyluteolin-8-C-6′′
-acetylglucopyranoside could be identified by co-injection and
UVspectra.Inapreviouswork,thesameauthorshaveevaluated
the C-glycosylflavonoid profile of P. tripartita var. mollissima leavesandpericarpbya distinctHPLCmethod(Zucolottoetal., 2012).Althoughbotharesuitablefortheirpurposes,themethod
described herein allows the great diversity of flavonoids tobe
betterobserved,withananalysistimeofonly20min.Simirgiotis andco-workers (2013)have studiedthe peeland fruit juice of thisspecies,whichalthoughdifferentpartsoftheplant,presented severalflavonoidsthatwerestructurallyrelatedtothoseobserved inourwork.
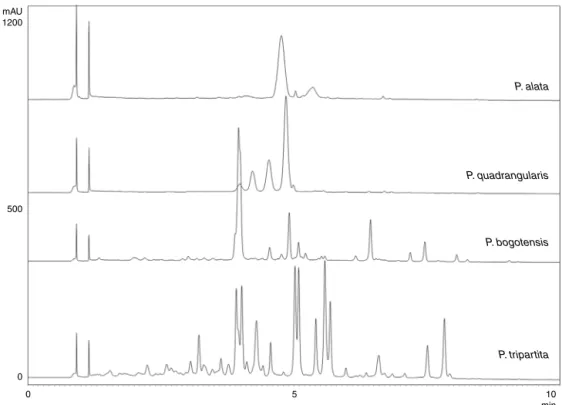
TheparametersdevelopedfortheHPLCanalysisofthe
refer-encecompounds(authenticsamplesofC-glycosylflavonoids)and
theextracts(Fig.1),weresubsequentlyusedintheUPLCanalysis, yieldingsimilarresults(Fig.2).AlthoughtheHPLCanalysisallowed rapidanalysiswithanadequateresolutionbetweenpeaks,
compar-atively,theanalysistimebyUPLCwasreducedby50%(10min),
withvirtuallyunchangedfingerprints.AsfortheHPLC analysis, theUPLCanalysisalsoenabledustodifferentiatethefourextracts, especiallyinthedifficultdistinctionofthemayorflavonoidsfrom P.alataandP.quadrangularis.
In addition to the analysis by chromatographic methods,
theflavonoid composition wasalso analyzedby capillary
elec-trophoretic(CE).Theliteraturemostlyreportstheuseofborate buffersasrunningelectrolytesintheanalysisofflavonoidsbyCE (Molnár-PerlandFüzfai,2005;Marchartetal.,2003;Rijkeetal., 2006).Consideringthatthepreviouslychromatographicanalyses haverevealedahighercomplexityofflavonoidsinP.tripartitavar. mollissima,theCEmethodwasinitiallydevelopedforthisextract.
Someparameterswereevaluatedforthechangeinelectrosmotic
flow,suchastheelectrolyteconcentration,voltage,capillary tem-peratureandinjectionvolume.Theconditionsthatprovidethebest separationforP.tripartitavar.mollissima(see‘Materialsand meth-ods’section)wereappliedtotheotherextracts(Fig.3),enablingto distinguishtheflavonoidfingerprintsforeachspecies.
Comparedwiththechromatographictechniques,theprofiles
obtainedbyCEshowedalsoashorttimeanalysis,goodpeak reso-lutionandsymmetry.Qualitatively,anothersubstantialdifference
betweenthechromatographicandelectrophoreticmethodsisthe
detectionwavelength.InHPLC,flavonoidsareusuallydetectedin
therangeof330–350nm,whichisthebandofmaximum
absorp-tionfor these compounds,providing selectivitytothe method.
mAU 1200
500
0
0 5 10
min P. tripartita P. bogotensis P. quadrangularis P. alata
Fig.2.UPLCchromatogramofflavonoidsfromaqueousextractsoftheleavesofPassifloraspecies.Fordetailsofthechromatographicmethod,seethe‘Experimental’section.
mAU
30
15
0
0 4 8 12
min I.S.
P. tripartita P. bogotensis P. quadrangularis P. alata
Fig.3. CEelectropherogramofflavonoidsfromaqueousextractsoftheleavesof
Passifloraspecies.Internalstandard(I.S.):apigenin.Fordetailsofthecapillary elec-trophoreticmethod,seethe‘Materialsandmethods’section.
Alkaloidsanalysis
Somepreviousstudiesreportthepresence of alkaloidsin P.
incarnata and P. edulis (Poethke et al., 1970; Lutomski et al., 1975).Nevertheless,morerecentworks,withmoresensitive
meth-ods,havequestionedthepresenceorlevelsofthesecompounds
(SperoniandMinghetti,1988;Rehwaldetal.,1995;Griceetal., 2001).
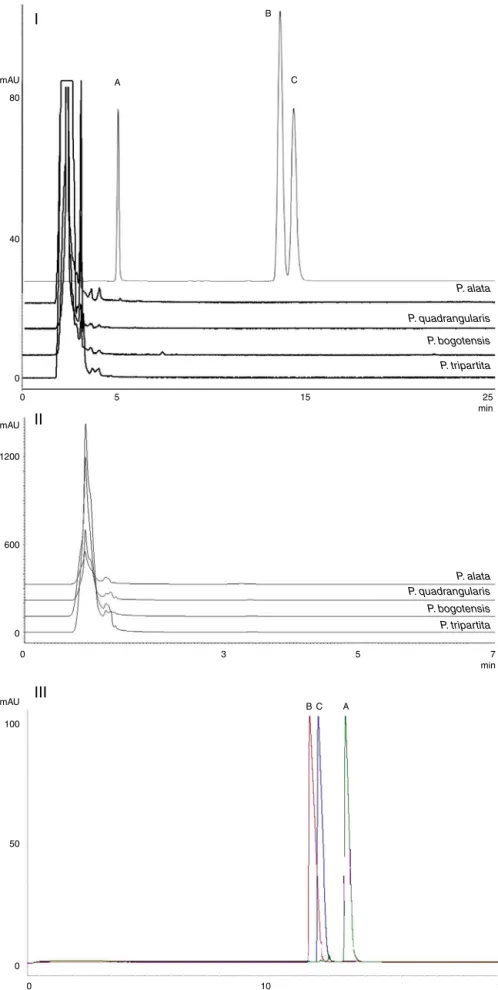
Inthealkaloidanalysispresentedinthisstudy,chromatographic
andelectrophoreticmethodsweredevelopedusingthestandards
harmol,harmaneandharmine,compoundsthathavepreviously
beendescribedforP.incarnataandP.edulis(Poethkeetal.,1970;
Lutomskietal.,1975).Qualitatively,thepresenceofthesealkaloids wasnotobservedinthecrudeaqueousextractfromtheleavesof thesespeciesbyHPLC,UPLCorCE(Fig.4).Forthisreason,a
calibra-tioncurveforharmanewasbuiltinUPLCandCE(thetechniques
withthefastestmethods)todeterminethelinearityandsensitivity ofthesemethods.TheresultsarepresentedinTable3.
The quantitative data showed an excellent linear
relation-ship betweenpeak areaand concentration (r2>0.999) for both
techniques.Aboutthesensitivityofthesemethodologies,itwas observedthatUPLCpresentedasensitivity15-foldhigherthenCEin theseconditions,whichcanbemainlyexplainedbythedifference oftheinterparticulesspacesintheUPLCcolumnandtheinternal diameterofthecapillarycolumninCE.
Considering thedetermined limitsfor thesemethods, itcan
bestatedthattheaqueousextractsanalyzedinthisworkdonot
have harmane type alkaloids at levels exceeding 0.0187g/ml
(=0.0187ppm). Thisresultis inaccordance withsomeprevious
quantitativeworksbyHPLC,whichalsodidnotdetectalkaloids
in the Passiflora species investigated, at concentrations higher than0.1ppm(Rehwaldetal.,1995).Griceandco-workers(2001) detectedalkaloidsincommercialsamplesofP.incarnataatlevels lowersthan0.018ppm,butusingafluorescencedetectorinstead ofadiodearraydetector.
Nevertheless,thenon-detectionofalkaloids,despiteappearing asanegativeresult,hasgreatrelevance.Theaqueousextracts eval-uatedwerepreparedaccordingtotheuseofPassifloraleavesin
folkmedicine.Thus,itwasdemonstratedthatthesecompounds
areabsentinthetraditionalpreparations.However,thesedatado notruleoutthepresenceofharmanealkaloidsinthesespecies,
Table3
Calibrationdataofharmane.
Technique Linearityrange(g/ml) Calibrationequationa Correlationfactor(r2) LOQb(g/ml) LODb(g/ml)
CE 50.0–1.0 y=2.4144x+1.0596 0.9997 0.5 0.25
UPLC 50.0–0.065 y=123720x−6479.9 0.9999 0.0315 0.0187
I
II
III
mAUB
A C
mAU
1200
600
0
0
100
50
0
3 5 7
min
18 min 80
0
0 5 15
BC A
10 0
mAU
25 min 40
P. tripartita P. bogotensis P. quadrangularis P. alata
P. tripartita P. bogotensis P. quadrangularis P. alata
Fig.4. Chromatograms(I:HPLC;II:UPLC)andelectropherogram(III)ofalkaloidsstandards(A:harmol;B:harmane;C:harmine)andofaqueousextractsoftheleavesof
whichmaybepresentinotherorgansoftheplant,oreveninthe leaves,inwhichaspecificalkaloidextractivemethodcouldbeused forthispurpose.
Saponinanalysis
Eventhoughmanysaponinshavebeendescribedforthegenus
Passiflora,theoccurrenceofthesemetabolitesisrestrictedtojust afewspecies.ThepresenceofsaponinsinP.alataandP. quadran-gularishasalreadybeenreportedinpreviousworksofourgroup (Reginattoetal.,2004;Birketal.,2005),ashasacomparative anal-ysisbyHPLCofthesetwospecies(Costaetal.,2013).Theadditional
resultspresentedherein,usingthesamechromatographic
condi-tions,indicatethattheextractsofP.bogotensisandP.tripartitavar.
mollissimaleavesshowednoevidenceofthesecompounds(see
supplementarymaterial).
Inconclusion,fastandsimpleanalyticalmethodsforthe finger-printingofflavonoidsandalkaloidsfromP.alata,P.quadrangularis, P.bogotensisand P.tripartita var. mollissimaextracts were suc-cessfullyestablishedbythreedifferenttechniques,showinggood
resolution and sensitivity. A wide diversity of flavonoids was
observedforthesefourspecies,whilesaponinswereaccumulated onlyinP.alataandP.quadrangularisextracts.Alkaloids,whose pres-enceiscontroversialinpreviouspapers,werenotdetectedbyanyof themethodsused.Theanalyticalmethodsandtechniquesreported hereinaresuitableforqualitycontrolanalysisofthesemetabolites inplantsamples,andwouldbeofgreathelpinfutureworkswith otherPassifloraspecies.
Ethicaldisclosures
Protectionofhumanandanimalsubjects. Theauthorsdeclare thattheproceduresfollowedwereinaccordancewiththe regula-tionsoftherelevantclinicalresearchethicscommitteeandwith thoseoftheCodeofEthicsoftheWorldMedicalAssociation (Dec-larationofHelsinki).
Confidentialityofdata. Theauthorsdeclarethattheyhave
fol-lowed theprotocolsof theirworkcenter onthe publicationof
patientdata.
Right to privacy and informed consent. The authors have obtainedthewritteninformedconsentofthepatientsorsubjects mentionedinthearticle.Thecorrespondingauthorisinpossession ofthisdocument.
Authors’contributions
GMCperformedtheextraction,analyticalwork,dataanalysis, anddraftingofthepaper.ACGcontributedtothechromatographic analysis.SMZcontributedtotheplantcollectionandextraction.LC andFARcontributedtothecollectionoftheplants,preparationof thevoucherspecimen,supervisionoflaboratorywork,andcritical readingofthemanuscript.FHRandEPSdesignedthestudy, super-visedthelaboratorywork,andcontributedtoacriticalreadingof themanuscript.Alltheauthorshavereadthefinalmanuscriptand
approvedthesubmission.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgements
Theauthorsgreatlyappreciatethefinancialsupportprovided byFondoNacionaldeFinanciamientoparalaCiencia,laTecnología
y la Innovación, FranciscoJosé de Caldas, contract No. 0459 –
2013,RedNacionalparalaBioprospeccióndeFrutas
Tropicales-RIFRUTBIO.TheauthorsE.P.SchenkelandF.H.Reginattothankthe
CNPq-NationalCouncilofScientificandTechnologyDevelopment
(Brazil)forprovidingtheresearchfellowships.
AppendixA. Supplementarydata
Supplementarydataassociatedwiththisarticlecanbefound,in theonlineversion,atdoi:10.1016/j.bjp.2016.02.005.
References
Arbelaez,E.P.,1996.PlantasutilesdeColombia.JardimBotânicoJoséCelestinoNutis, Bogotá.
Birk,C.D.,Provensi,G.,Gosmann,G.,Reginatto,F.H.,Schenkel,E.P.,2005.TLC finger-printofflavonoidsandsaponinsfromPassifloraspecies.J.Liq.Chromatogr.R.T. 28,2285–2291.
Coleta,M.,Batista,M.T.,Campos,M.G.,Carvalho,R.,Cotrim,M.D.,Lima,T.C.,Cunha, A.P.,2006.Neuropharmacologicalevaluationoftheputativeanxiolyticeffects ofPassifloraedulisSims,itssub-fractionsandflavonoidconstituents.Phytother. Res.20,1067–1073.
Costa,G.M.,Gazola,A.C.,Madóglio,F.A.,Zucolotto,S.M.,Reginatto,F.H., Castel-lanos,L.,Duque,C.,Ramos,F.A.,Schenkel,E.P.,2013.Vitexinderivativesas chemicalmarkersinthedifferentiationofthecloselyrelatedspecies Passi-floraalataCurtis.andPassifloraquadrangularisLinn.J.Liq.Chromatogr.R.T.36, 1697–1707.
Costa, G.M., Cárdenas, P.A., Gazola, A.C., Aragón, D.M., Castellanos, L., Regi-natto,F.H.,Ramos,F.A.,Schenkel,E.P.,2015.IsolationofC-glycosylflavonoids with ␣-glucosidase inhibitoryactivity from Passiflora bogotensisBenth by gradienthigh-speedcounter-currentchromatography.J.Chromatogr.B990, 104–110.
Doyama,J.T.,Rodrigues,H.G.,Novelli,E.L.B.,Cereda,E.,Vilegas,W.,2005.Chemical investigationandeffectsoftheteaofPassifloraalataonbiochemicalparameters inrats.J.Ethnopharmacol.96,371–374.
FarmacopeiaBrasileira,2010.AgênciaNacionaldeVigilânciaSanitária,Brasília,DF, 5aed,http://www.anvisa.gov.br/hotsite/cdfarmacopeia/index.htm.
Gazola,A.C.,(Ph.D.thesis)2014.Avaliac¸ãoquímicaeneurofarmacológicadeespécies dePassifloradaAméricadoSul.Florianópolis.UniversidadeFederaldeSanta Catarina,Brazil,pp.252.
Gazola,A.C.,Costa,G.M.,Castellanos,L.,Ramos,F.A.,Reginatto,F.H.,deLima,T.C.M., Schenkel,E.P.,2015.InvolvementofGABAergicpathwayinthesedativeactivity ofapigenin,themainflavonoidfromPassifloraquadrangularispericarp.Rev.Bras. Farmacogn.25,158–163.
Grice, I.D., Ferreira, L.A.,Griffiths, L.R., 2001. Identification and simultaneous analysisof hamane, harmina,harmol, isovitexin, andvitexin in Passiflora incarnataextractswith anovel HPLCmethod.J. Liq.Chromatogr. R.T.24, 2513–2523.
ICH,2005.ValidationofAnalyticalProcedures:TextandMethodology–Q2(R1). InternationalConferenceonHarmonization.ICH,London.
Invima, 2006. Normas Farmacológicas Instituto Nacional de Vigilancia de Medicamentosy Alimentos.BogotáD.C.,http://apps.who.int/medicinedocs/ documents/s18383es/s18383es.pdf.
Lewis, W.H.,Elvin-Lewis,M.P.F.,1977. MedicalBotany: PlantsAffectingMan’s Health.Wiley-Interscience,Toronto.
Li, H., Zhou, P., Yang, Q., Shen, Y., Deng, J., Li, L., Zhao, D., 2011. Com-parative studies on anxiolytic activities and flavonoid compositions of Passifloraedulis‘edulis’andPassifloraedulis‘flavicarpa’.J.Ethnopharmacol.133, 1085–1090.
Lutomski,J.,Malek,B.,1975.Pharmakochemischeuntersuchungenderdrogender gattungPassiflora.IV.Mittlg:Dervergleichdesalkaloidgehaltesin verschiede-nenharmandrogen.PlantaMed.27,381–384.
Lutomski,J.,Malek,B.,Rybacka,L.,1975.Pharmacochemicalinvestigationoftheraw materialsfromPassifloragenus.II.Thepharmacochemicalestimationofjuices fromthefruitsofPassifloraedulisandPassifloraedulisformflavicarpa.Planta Med.27,112–121.
Marchart,E.,Krenn,L.,Kopp,B.,2003.Quantificationoftheflavonoidglycosidesin Passifloraincarnatabycapillaryelectrophoresis.PlantaMed.69,452–456.
Molnár-Perl,I.,Füzfai,Z.S.,2005.Chromatographic,capillaryeletrophoreticand capillaryeletrochromatographictechniquesintheanalysisofflavonoids.J. Chro-matogr.A1073,201–227.
Mors,W.B.,Rizzini,C.T.,Pereira,N.A.,2000.MedicinalPlantsofBrazil.Reference Publications,Algonac.
Orsini,F.,Pelizzoni,F.,Verotta,L.,1985.Quadranguloside,acycloartanetriterpene glycosidefromPassifloraquadrangularis.Phytochemistry25,191–193.
Poethke,V.W.,Schwarz,C.,Gerlach,H.,1970.SubstancesofPassifloraincarnata1. (ConstituentsofPassiflorabryonioides).Alkaloids.PlantaMed.18,303–314.
Ramos,F.A.,Castellanos,L.,López,C.,Palacios,L.,Duque,C.,Pacheco,R.,Guzmán,A., 2010.AnorientinderivativeisolatedfromPassifloratripartitavar.mollissima. Lat.Am.J.Pharm.29,141–143.
Reginatto,F.H.,Kauffmann,C.,Schripsema,J.,Guillaume,D.,Gosmann,G.,Schenkel, E.P.,2001.SteroidalandtriterpenoidalglucosidesfromPassifloraalata.J.Braz. Chem.Soc.12,32–36.
Reginatto,F.H.,Gosmann,G.,Shripsema,J.,Schenkel,E.P.,2004.Assayof quadran-guloside,themajorsaponinsofleavesofPassifloraalata,byHPLC.Phytochem. Anal.15,195–197.
Rehwald,A.,Sticher,O.,Meier,B.,1995.Traceanalysisofharmanalkaloidsin Pas-sifloraincarnatabyreversed-phasehighperformanceliquidchromatography. Phytochem.Anal.6,96–100.
Rijke,E.,Out,P.,Niessen,W.M.A.,Ariese,F.,Gooijer,C.,Brinkman,U.A.T.,2006. Ana-lyticalseparationanddetectionmethodsforflavonoids.J.Chromatogr.A1112, 31–63.
Sakalem,M.E.,Negri,G.,Tabach,R.,2012.Chemicalcompositionof hydroethano-licextractsfromfivespeciesofthePassifloragenus.Rev.Bras.Farmacogn.22, 1219–1232.
Santos,R.I.,Marlise,A.,Schenkel,E.P.,1996.AnalysisoftheplantdrugWibrandia ebracteata.Int.J.Pharmacogn.34,300–330.
Santos,K.C.,Santos,C.A.M.,deOliveira,R.M.W.,2006.PassifloraactiniaHooker extractsand fractions induce catalepsy in mice.J. Ethnopharmacol. 100, 306–309.
Sena,L.M.,Zucolotto,S.M.,Reginatto,F.H.,Schenkel,E.P.,DeLima,T.C.M.,2009. Neu-ropharmacologicalactivityofthepericarpofPassifloraedulisflavicarpaDegener: putativeinvolvementofC-glycosylflavonoids.Exp.Biol.Med.234,967–975.
Simirgiotis,M.J., Schmeda-Hirschmann,G.,Bórquez,J.,Kennelly,E.J.,2013.The Passifloratripartita(bananapassion)fruit:asourceofbioactiveflavonoid C-glycosidesisolatedbyHSCCCandcharacterizedby HPLC–DAD–ESI/MS/MS. Molecules18,1672–1692.
Speroni,E.,Minghetti,A.,1988.Neuropharmacologicalactivityofextractsfrom Pas-sifloraincarnata.PlantaMed.54,488–491.
Ulubelen, A., Oksuz, S., Mabry, T.J., Dellamonica, G., Chopin, J., 1982. C-glycosylflavonoidsfromPassiflorapittieri,P.alata,P.ambiguaandaAdeniamanii. J.Nat.Prod.45,783.
Unger,M.,Stockigt,D.,Belder,D.,Stockigt,J.,1997.Generalapproachforthe anal-ysisofvariousalkaloidclassesusingcapillaryelectrophoresisandcapillary electrophoresis–massspectrometry.J.Chromatogr.A767,263–276.
WHO,1991.GuidelinesfortheAssessmentofHerbalMedicines.WorldHealth Orga-nization,Geneva,Munich.
Zucolotto,S.M.,Goulart,S.,Montanher,A.B.,Reginatto,F.H.,Schenkel,E.P.,Fröde,T.S., 2009.Bioassay-guidedisolationofanti-inflammatoryC-glycosylflavonesfrom Passifloraedulis.PlantaMed.75,1–6.