Relevance of the Ventricular Remodeling Pattern in the Model of
Myocardial Infarction in Rats
Marcos F. Minicucci, Paula S. Azevedo, Lidiane P. Ardisson, Katashi Okoshi, Beatriz B. Matsubara, Luiz S.
Matsubara, Sergio A. R. Paiva, Leonardo A. M. Zornoff
Faculdade de Medicina de Botucatu, Botucatu, SP - Brazil
Mailing address: Leonardo A. M. Zornoff •
Departamento de Clínica Médica - Rubião Jr - 18618-000 - Botucatu, SP - Brazil
E-mail: lzornoff@cardiol.br, lzornoff@fmb.unesp.br
Manuscript received December 09, 2009; revised manuscript received May 31, 2010; accepted June 17, 2010.
Abstract
Background: The relevance of the remodeling pattern in the model of infarcted rats is not known.
Objective: To analyze the presence of different patterns of remodeling in this model and its functional implications.
Methods: Infarcted rats (n=47) have been divided according to the geometry pattern, analyzed by echocardiogram:
normal (normal mass index and normal relative thickness), concentric remodeling (normal mass index and increased relative thickness), concentric hypertrophy (increased mass index and increased relative thickness) and eccentric hypertrophy (increased mass index and normal relative thickness). Data are median and interquartile range.
Results: Infarcted rats showed only two of the four geometric patterns: normal pattern (15%) and eccentric hypertrophy
- EH (85%). Groups of normal pattern and EH showed no differences in the values of fractional area change (Normal = 32.1 - 28.8 to 50.7; EH = 31.3 - 26.5 to 36.7; p = 0.343). Out of the infarcted animals, 34 (74%) had systolic dysfunction, detected by fractional area change. Considering these two geometry patterns, 77% of animals with eccentric hypertrophy and 57% with normal geometry presented systolic dysfunction (p=0.355). The relative wall
thickness, the geometric patterns and the body mass index were not predictors of ventricular dysfunction (p>0.05). On the other hand, infarct size was a predictive factor for ventricular dysfunction in univariate analysis (p<0.001) and multivariate analysis (p = 0.004).
Conclusion: Rats that underwent coronary occlusion showed two different patterns of remodeling, which do not constitute a predictor of ventricular dysfunction. (Arq Bras Cardiol 2010; 95(5): 635-639)
Keywords: Ventricular remodeling; myocardial infarction; rats.
Introduction
After the heart attack, molecular, cellular and interstitial heart changes may occur, which will be presented clinically by changes in architecture, size, mass, geometry and function of the heart. This phenomenon is called ventricular remodeling1-3. It is accepted that the process of remodeling
plays a fundamental role in the pathophysiology of ventricular dysfunction, since the genetic, structural and biochemical changes of this process will result in progressive deterioration of functional capacity of the heart 4-6.
In recent years, it was sought to identify variables associated with the remodeling process that could stratify the risk of cardiovascular events. Among these indices, there is the pattern of ventricular geometry.
In patients with hypertension, we observed that pressure overload may result in different geometric patterns. Using the mass index and the left ventricle (LV) relative wall thickness as echocardiographic variables, the patients were divided into four different geometry patterns: normal (normal mass index and normal relative thickness); concentric remodeling (normal mass index and increased relative thickness); concentric hypertrophy (increased mass index and increased relative thickness); and eccentric hypertrophy (increased mass index and normal relative thickness). In this model, the remodeling pattern was predictor of cardiovascular events7.
We must consider, however, that the importance of the pattern of cardiac remodeling in the model of acute myocardial infarction is not known. Hence, the purpose of this study was to analyze the presence of different patterns of remodeling in infarcted hearts of rats and their functional implications.
Methods
Animal Experimentation adopted by the Brazilian Board of Animal Experimentation.
Experimental infarction
We used male Wistar rats, weighing between 200 and 250 grams. The rats were anesthetized with ketamine (50 mg/kg) and underwent left thoracotomy. After exteriorization of the heart, the left atrium was removed, and the left coronary artery was ligated with 5-0 mononylon between the outlet of the pulmonary artery and left atrium. Then the heart was returned to the chest, the lungs were inflated with positive pressure, and the thorax was closed by sutures with cotton 108,9. In 18
animals, coronary occlusion was not performed.
The animals were kept in cages for recovery, fed with standard commercial food and free access to water, with controlled light (cycles of 12 hours), temperature of about 250 C and controlled humidity.
Echocardiographic study
After an observation period of three months, the surviving animals were anesthetized with ketamine hydrochloride (50 mg/kg) and xylazine (1 mg/kg) intramuscularly for echocardiographic studies. After shaving the anterior thorax, the animals were positioned supine in the channel especially designed and allowing a slight rotation to the left side for the examination, using Philips equipment (model HDI 5000) equipped with multifrequency electronic transducer of up to 12 MHz. The evaluation of mitral and aortic flows was performed with the same transducer, operating at 5.0 MHz. Measures of cardiac structures were performed in one-dimensional images obtained with the ultrasound beam oriented by two-dimensional image, in parasternal short axis. The image of the left ventricular cavity was obtained by positioning the M-mode cursor between the papillary muscles, just below the mitral valve plane. The images of the aorta and left atrium were obtained in parasternal short axis, with the M-mode cursor positioned at the aortic valve. The one-dimensional image (speed: 100 mm/s) was performed by printer model UP-890MD Sony Co. All measurements were conducted in accordance with the recommendations of the American Society of Echocardiography/ European Association of Echocardiography10 previously validated in
the model of infarcted rats11. The left ventricular diastolic
diameter (LVDD) and left ventricular posterior wall thickness (LVDT) were measured at the time corresponding to the maximum diameter of the cavity. The LV systolic diameter (LVSD) was measured at the maximum systolic excursion of the posterior wall of the cavity. Left ventricular diastolic areas (DA) and systolic areas (SA) were measured in two-dimensional mode, using planimetry, in the minor axis. Left ventricular systolic function was assessed by calculating the fractional area change (FAC=DA-SA/DA x 100)11. The
transmitral diastolic flow (E and A waves) was obtained with the transducer in the apical four-chamber position. For the relative thickness of the LV, the following formula was used: RWT = (2 x LVDT) / diastolic diameter. The relative thickness was considered increased when > 0.4210. For the
mass index (LVMI), we used the formula: LVMI = LVM/BW,
where LVM = { [ LVDD + (2 x LVDT)3] - LVDD3} x 1.04,
and BW is the body weight of the animal. Body mass index was considered increased if > 2.1. This corresponded to
percentile 75% found in control animals. To issue a diagnosis
on systolic ventricular dysfunction, we considered the values
below 37.2% for fractional area change. This threshold was
obtained by subtracting two standard deviations from the mean values found in control animals.
Statistic method
Comparisons between groups were performed using the Student t test when data showed normal distribution. When the data did not present normal distribution, the comparisons between the groups were made by Mann-Whitney’s U test.
Data were expressed as mean ± standard deviation or
median, with 25 and 75 percentile. Dichotomous variables
were analyzed by χ2 test. Predictive values were evaluated by
simple linear regression analysis. The significance level was 5%. Statistic analyses were made with the program SigmaStat for Windows v3.5 (SPSS Inc, Chicago, IL).
Results
After the observation period of three months, 46 infarcted animals and 18 control animals survived.
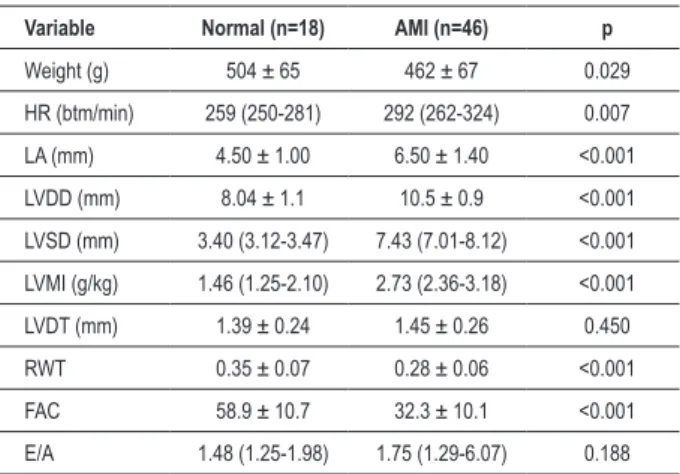
The results of echocardiographic analysis, comparing the control and infarcted animals, are shown in Table 1. The infarcted animals showed lower body weight, RWT and ACF compared to control animals. On the other hand, animals with infarction showed higher heart rate, left atrium, LVDD, LVSD and LVMI than animals without infarction. No differences were found regarding LVDT and the E/A ratio.
Considering the rats subjected to coronary occlusion we observed just two geometric patterns, in the following percentages: normal - 15% and eccentric hypertrophy - 85%.
Table 1 - Echocardiographic data of control and infracted animals Variable Normal (n=18) AMI (n=46) p
Weight (g) 504 ± 65 462 ± 67 0.029
HR (btm/min) 259 (250-281) 292 (262-324) 0.007
LA (mm) 4.50 ± 1.00 6.50 ± 1.40 <0.001
LVDD (mm) 8.04 ± 1.1 10.5 ± 0.9 <0.001
LVSD (mm) 3.40 (3.12-3.47) 7.43 (7.01-8.12) <0.001
LVMI (g/kg) 1.46 (1.25-2.10) 2.73 (2.36-3.18) <0.001
LVDT (mm) 1.39 ± 0.24 1.45 ± 0.26 0.450
RWT 0.35 ± 0.07 0.28 ± 0.06 <0.001
FAC 58.9 ± 10.7 32.3 ± 10.1 <0.001
E/A 1.48 (1.25-1.98) 1.75 (1.29-6.07) 0.188
AMI - animals infarcted; LA - left atrium diameter; LVDD - left ventricular diastolic diameter; LVSD - left ventricular systolic diameter; LVDT - left ventricular posterior
Table 2 - Echocardiographic data according to ventricular geometry in rats with myocardial infarction
Variable Normal (n=24) hypertrophy (n=15)Eccentric p
Weight (g) 523 ± 75 451 ± 60 0.008
HR (btm/min) 276 ± 27 295 ± 37 0.199
LA (mm) 6.66 ± 1.36 5.84 ± 1.59 0.162
LVDD (mm) 9.64 ± 0.8 10.6 ± 0.8 0.005
LVMI (g/kg) 1.89 (1.50-1.97) 2.80 (2.55-3.44) <0.001
LVDT (mm) 1.22 ± 0.11 1.49 ± 0.26 0.013
RWT 0.26 ± 0.03 0.28 ± 0.06 0.250
FAC 32.1 (28.8-50.7) 31.3 (26.5-36.7) 0.343
% AMI 38 ± 12 43 ± 9 0.854
E/A 2.06 (0.88-7.85) 1.73 (1.30-5.91) 0.175
LA - left atrium diameter; LVDD - left ventricular diastolic diameter; LVDT - left
ventricular posterior wall diastolic thickness; RWT - left ventricular relative wall thickness; LVMI - left ventricular mass index; FAC - fractional area change; % AMI - infarction size. E /A - ratio between the E and A waves of the transmitral diastolic low. The data are expressed in mean ± standard deviation (for normal distribution) or median with percentiles 25 and 75 (for non normal distribution).
The results of echocardiography, according to the pattern of remodeling of the infarcted animals, are shown in Table 2. Animals with eccentric hypertrophy had higher values of LVDD, LVDT and LVMI than animals with normal geometry. There were no differences in other variables.
Considering the ventricular function of infarcted animals,
34 animals (74%) had systolic dysfunction, detected by
fractional area change. Considering both geometric patterns,
77% of those with eccentric hypertrophy and 57% of those
with normal geometry had systolic dysfunction (p=0.355). As to the ventricular dysfunction prediction factors, we analyzed the influence of infarction size, mass index, relative wall thickness and the geometry pattern. In the univariate and multivariate regression analysis, the relative wall thickness, geometry patterns and mass index were not predictors of ventricular dysfunction (p>0.05). On the other hand, infarction size was predictor of ventricular dysfunction in the
univariate analysis (p<0.001) and in the multivariate analysis,
after adjustment for other factors (p=0.004).
Discussion
Some evidence suggests that the geometry may have pathophysiologic implications in some situations, for example, in hypertension. Considering the lack of information on the relevance of remodeling pattern in the model of rats exposed to acute myocardial infarction, the purpose of this study was to analyze the presence of different remodeling patterns and its relationship with ventricular functions in this model.
Regarding the variability of geometric patterns in the case of hypertension, although the mechanisms are not fully understood, the four different geometric patterns are explained by the different pathophysiological mechanisms for hypertension with different hemodynamic patterns. Thus, the
geometry would depend on the degree of vasoconstriction, the intensity of activation of neurohumoral factors and the presence of volume overload12.
In the model of myocardial infarction, however, data on the variability of geometric patterns are scarce. Verna et al13, in an
analysis of VALIANT Echocardiographic Study13, found the four
geometric patterns in patients with left ventricular dysfunction after myocardial infarction: concentric hypertrophy (12.6%); eccentric hypertrophy (18.6%); concentric remodeling (18.2%) and normal (50.6%). In this study, however, different geometric patterns are assigned to different comorbidities presented by patients, for example, hypertension14.
The first relevant information of our study was that the animals that underwent coronary occlusion, unlike the model of hypertension, presented only two of the four geometric patterns described: normal and eccentric hypertrophy. The fact that our analysis did not find the standards of hypertrophy and concentric remodeling reinforces the relevance of pressure overload to the emergence of such geometrical patterns. Additionally, we believe that our animals are of the same lineage, sex and age and are exposed to the same environmental conditions. Thus, the presence of two geometric patterns in our study suggests that in the model of experimental infarction, animals with the same characteristics may present different morphological adaptations even with the same aggression.
Regarding the relevance of patterns of ventricular remodeling in the ARIC study, the type of hypertrophy was associated with the type of ventricular dysfunction. Accordingly, eccentric hypertrophy was associated to systolic dysfunction, while concentric hypertrophy was associated to diastolic dysfunction. On the other hand, concentric remodeling was not associated with ventricular dysfunction15. In the
MESA study, however, patients with concentric remodeling presented systolic dysfunction assessed by means of magnetic resonance imaging16. Thus, the association between geometry
and ventricular dysfunction is still controversial. In our study, animals with normal geometry patterns and eccentric hypertrophy did not present differences in functional variables.
Another important aspect is that different authors have shown that the pattern of remodeling may have prognostic implications. Thus, in patients with hypertension, the pattern of concentric hypertrophy was associated with increased risk of cardiovascular events in relation to other geometry patterns17.
Other studies suggest that concentric remodeling is associated with increased risk of cardiovascular events18-22. However, the
geometry was not associated with worse prognosis in other analyses23,25 indicating that the relevance of the pattern of
remodeling in hypertensive patients remains controversial. In patients with acute myocardial infarction, the geometry pattern, the mass index and the relative wall thickness were independent predictors of death13. Worth of note is that the
relevance of ventricular geometry has been studied in different experimental models26,27.
this conclusion could have been influenced by the selection bias. However, all deaths occurred within 48 hours after AMI, preventing the development of concentric hypertrophy or remodeling. Thus, the statement that in the model of rats with coronary occlusion the pattern of remodeling has no relevance in relation to cardiac function remains valid.
Conclusion
In conclusion, rats that underwent coronary occlusion showed two different patterns of remodeling, which do not constitute a predictor of ventricular dysfunction.
Potential Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Sources of Funding
This study was funded by CNPq.
Study Association
This article is part of the thesis of doctoral submitted by Lidiane P. Ardisson, from Faculdade de Medicina de Botucatu.
References
1. Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction: experimental observations and clinical implications. Circulation. 1990; 81 (1): 1161-72.
2. Pfeffer JM, Pfeffer MA, Braunwald E. Influence of chronic captopril therapy on the infarcted left ventricle of the rat. Circ Res. 1985; 57 (1): 84-95.
3. Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling- concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. J Am Coll Cardiol. 2000; 35 (3): 569-82.
4. Zornoff LAM, Paiva SAR, Duarte DR, Spadaro J. Ventricular remodeling after myocardial infarction: concepts and clinical implications. Arq Bras Cardiol. 2009; 92 (2): 150-64.
5. Francis GS. Pathophysiology of chronic heart failure. Am J Med. 2001; 110 (Suppl. 7A): 37S-46S.
6. Swynghedauw B. Molecular mechanisms of myocardial remodeling. Physiol Rev. 1999; 79 (1): 215-62.
7. Ganau A, Devereux RB, Roman MJ, de Simone G, Pickering TG, Saba PS, et al. Patterns of left ventricular hypertrophy and geometric remodeling in essential hypertension. J Am Coll Cardiol. 1992; 19 (7): 1550-8.
8. Zornoff LAM, Matsubara BB, Matsubara LS, Paiva SAR, Spadaro J. Early rather than delayed administration of lisinopril protects the heart after myocardial infarction in rats. Basic Res Cardiol. 2000; 95 (3): 208-14.
9. Minicucci MF, Azevedo PS, Duarte DR, Matsubara BB, Matsubara LS, Campana AO, et al. Comparison of different methods to measure experimental chronic infarction size in the rat model. Arq Bras Cardiol. 2007; 89 (2): 83-7.
10. Lang RM, Bierig M, Devereaux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005; 18 (12): 1440-63.
11. Zornoff LAM, Paiva SAR, Minicucci MF, Spadaro J. Experimental myocardium infarction in rats: analysis of the model. Arq Bras Cardiol. 2009; 93 (4): 426-32, 434-40.
12. Dávila DF, Donis JH, Odreman R, Gonzalez M, Landaeta. Patterns of left ventricular hypertrophy in essential hypertension: should echocardiography guide the pharmacological treatment? Int J Cardiol. 2008; 124 (2): 134-8.
13. Verma A, Meris A, Skali H, Ghali JK, Arnold JM, Bourgoun M, et al. Prognostic implications of left ventricular mass and geometry following myocardial infarction: the VALIANT (VALsartan In Acute myocardial iNfarcTion) Echocardiographic Study. JACC Cardiovasc Imaging. 2008; 1 (5): 582-91.
14. Konstam M. Patterns of ventricular remodeling after myocardial infarction: clues toward linkage between mechanism and morbidity. JACC Cardiovasc Imaging. 2008; 1 (5): 592-4.
15. Fox E, Taylor J, Taylor H, Han H, Samdarshi T, Arnett D, et al. Left ventricular geometric pattern in the Jackson Cohort of the Atherosclerosis Risk in Communities (ARIC) Study: Clinical correlates and influences on systolic and diastolic dysfunction. Am Heart J. 2007; 153 (2): 238-44.
16. Rosen BD, Edvardsen T, Lai S, Castillo E, Pan L, Jerosch-Herold M, et al. Left ventricular concentric remodeling is associated with decreased global and regional systolic function: the Multi-Ethnic Study of Atherosclerosis. Circulation. 2005; 112 (7): 984-91.
17. Koren MJ, Devereux RB, Casale PN, Savage DD, Laragh JH. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med. 1991; 114 (5): 345-52.
18. de Simone G, Kitzman DW, Chinali M, Oberman A, Hopkins PN, Rao DC, et al. Left ventricular concentric geometry is associated with impaired relaxation in hypertension: the HyperGEN study. Eur Heart J. 2005; 26 (10): 1039-45.
19. Gardin JM, McClellan R, Kitzman D, Lima JA, Bommer W, Klopfenstein HS, et al. M-mode echocardiographic predictors of six-to-seven year incidence of coronary heart disease, stroke, congestive heart failure, and mortality in an elderly cohort. Am J Cardiol. 2001; 87 (9): 1051-7.
20. Verdecchia P, Schillaci G, Borgioni C, Ciucci A, Battistelli M, Bartoccini C, et al. Adverse prognostic significance of concentric remodeling of the left ventricle in hypertensive patients with normal left ventricular mass. J Am Coll Cardiol. 1995; 25 (4): 871-8.
21. Muiesan ML, Salvetti M, Monteduro C, Bonzi B, Paini A, Viola S, et al. Left ventricular concentric geometry during treatment adversely affects cardiovascular prognosis in hypertensive patients. Hypertension. 2004; 43 (4): 731-8.
22. Pierdomenico SD, Lapenna D, Bucci A, Manente BM, Cuccurullo F, Mezzetti A. Prognostic value of left ventricular concentric remodeling in uncomplicated mild hypertension. Am J Hypertens. 2004; 17 (11 Pt 1): 1035-9.
23. Verdecchia P, Schillaci G, Borgioni C, Ciucci A, Gattobigio R, Zampi I, et al. Prognostic value of left ventricular mass and geometry in systemic hypertension with left ventricular hypertrophy. Am J Cardiol. 1996; 78 (2): 197-202.
24. Krumholz HM, Larson M, Levy D. Prognosis of left ventricular geometric patterns in the Framingham Heart Study. J Am Coll Cardiol. 1995; 25 (4): 879-84.
25. Ghali JK, Liao Y, Cooper RS. Influence of left ventricular geometric patterns on prognosis in patients with or without coronary artery disease. J Am Coll Cardiol. 1998; 31 (7): 1635-40.
26. Azevedo PS, Minicucci MF, Matsubara BB, Matsubara LS, Duarte DR, Paiva SA, et al. Remodeling pattern and ventricular function in rats exposed to cigarette smoke. Arq Bras Cardiol. 2010; 94 (2): 209-12.