www.jped.com.br
ORIGINAL
ARTICLE
Risk
factors
associated
with
growth
failure
in
the
follow-up
of
very
low
birth
weight
newborns
夽
Milene
M.S.
Rover
a,∗,
Cláudia
S.
Viera
a,
Rita
C.
Silveira
b,
Ana
T.B.
Guimarães
a,
Sabrina
Grassiolli
aaUniversidadeEstadualdoOestedoParaná(UNIOESTE),Cascavel,PR,Brazil
bDepartmentofPediatrics,UniversidadeFederaldoRioGrandedoSul(UFRGS),PortoAlegre,RS,Brazil
Received18May2015;accepted2September2015 Availableonline6February2016
KEYWORDS
Preterminfant; Growth; Verylowbirth weight; Riskfactors
Abstract
Objective: Todeterminerisk factorsduringneonatalhospitalstay andfollow-upassociated withfailuretothriveinthefirstyearoflifeofverylowbirthweightnewborns.
Methods: Studyofpreterm verylowbirthweightnewbornsfollowedfrom2006to2013ina publicinstitutionalhospitalprogram.Thestudyincludednewbornsthatattendedatleastone appointmentineachofthethreeperiods:PeriodI,upto3monthsofcorrectedage(CA);Period II,4---6monthsofCA;andPeriodIII,7---12monthsofCA.Thevariableswereanalyzedbylogistic regressionwithXLSTAT2014software(Microsoft®,WA,USA).Failuretothrive(Z-scorebelow −2SD)wasclassifiedasadichotomousdependentvariable(0---failure/1---success),whilethe othervariableswereclassifiedasexplanatoryvariablesforthehospitalizationperiodsandfor eachofthefollow-upperiods(I,II,andIII).
Results: ChildrenbornadequateforgestationalageincreasedthechanceofZ-scoreforweight atdischarge>−2SD(OR=10.217;95%CI:1.117---93.436).Metabolicbonediseaseand retinopa-thy ofprematurity inPeriod I, as well ashospital readmissions inPeriods II and IIIduring follow-upincreasedthechanceofZ-score<−2SD.
Conclusion: Failuretothriveisinfluencedbyintrauterinefactorsand,subsequently,byseveral morbidities,bothinthebirthandhospitalizationperiod,aswellasinthepost-dischargeperiod andthus,suchvariablesshouldbeprioritizedinthefollow-up.
©2016SociedadeBrasileiradePediatria.PublishedbyElsevierEditoraLtda.Allrightsreserved.
夽
Pleasecitethisarticleas:RoverMM,VieraCS,SilveiraRC,GuimarãesAT,GrassiolliS.Riskfactorsassociatedwithgrowthfailureinthe
follow-upofverylowbirthweightnewborns.JPediatr(RioJ).2016;92:307---13.
∗Correspondingauthor.
E-mails:mmsrover@hotmail.com,milenerover@uol.com.br(M.M.S.Rover). http://dx.doi.org/10.1016/j.jped.2015.09.006
PALAVRAS-CHAVE
Prematuro; Crescimento; Recém-nascidode muitobaixopeso; Fatoresderisco
Fatoresderiscoassociadosàfalhadecrescimentonoseguimentoderecém-nascidos demuitobaixopeso
Resumo
Objetivo: Determinar fatores de risco do período de internac¸ão neonatale do seguimento ambulatorial associados àfalhadecrescimento noprimeiro ano de vidaderecém-nascidos demuitobaixopeso.
Métodos: Estudocomcrianc¸asnascidasprematurasdemuitobaixopesoemacompanhamento de2006a2013em Ambulatóriode AltoRisco deumHospital Escola.Incluídas aquelasque realizarampeloumaconsultaemcadaumdostrêsperíodosassimdeterminados:PeríodoI ---até3mesesdeIdadeCorrigida(IC);PeríodoII---entre4a6mesesdeICePeríodoIII---entre7a 12mesesdeIC.AsvariáveisforamanalisadasporregressãologísticacomprogramaXLStat2014 (Microsoft®,WA,EUA).Afalhadecrescimento(escorezabaixode−2DP)classificadacomo variáveldependentedotipodicotômica(0--- falha/1--- sucesso)edemaisvariáveisclassificadas como variáveisexplicativaspara osperíodosdeinternac¸ãoepara cadaumdosperíodos de seguimento(I,IIeIII).
Resultados: NascerAdequadoparaaIdadeGestacionalaumentaachancedeapresentarescore Z do peso na alta hospitalar acima de −2 DP (OR=10,217; IC95% 1117---93,436). Doenc¸a MetabólicaÓssea eRetinopatia daPrematuridade, duranteoPeríodo Iereinternac¸ões nos PeríodosIIeIIIdeseguimentoaumentamachancedeescoreZabaixode−2DP.
Conclusão: Afalhadecrescimentoéinfluenciadaporfatoresintrauterinoseposteriormentepor diversasmorbidades,tantonoperíododainternac¸ãocomonopós-alta,taisvariáveisestudadas deveriamserpriorizadasnoseguimento.
©2016SociedadeBrasileiradePediatria.PublicadoporElsevierEditoraLtda.Todososdireitos reservados.
Introduction
Failure tothrive during early childhoodcan have perma-nentharmfuleffects,especiallyinpreterminfants(PI)1as growth, mainly in those born with very low birth weight (VLBW),is influencedbyintrauterineandbirth factors,as well as variables during hospitalization and post-hospital discharge,2causingfutureproblemssuchas neurodevelop-mentalalterations3,4andmetabolicsyndrome.5,6
Studies have addressed the influence of the hospital-ization period and the first years of life after discharge onthe growthofPI,7---10 demonstratingthatbirth (weight, weight/GAratio)andhospitalization(hospitallengthofstay, presenceof hyalinemembrane disease)variables have an effectongrowthintheshort-andlongterm.11
During hospitalization, the VLBW preterm infant has restricted growth, with significantly lower rates than the intrauterine rates. Most of these PI are born weighing betweenthe10thand90thpercentilesoftheintrauterine growth curve, considered adequate for gestational age ---(AGA).However,atthedischargefromtheneonatal inten-sivecare unit(NICU)or at36 weekspost-conceptionage, theyarebelowthe10thpercentileofthesamecurve, char-acterizingextrauterinegrowthrestriction(EUGR).
ThissituationinfluencestheprognosisofPI,bothin rela-tionto growth and todevelopment, leadingto failure to thrivein childhood,stunting,and underweight, with con-sequencesin adulthood.3,12 Factors associated withEUGR include:nutritionalpractice,malegender, needfor venti-lationonthefirstdayoflife,useofmechanicalventilation forlongperiods,hospitallengthofstay,andcomplications inherent to premature birth, such as bronchopulmonary
dysplasia (BPD), necrotizing enterocolitis (NEC), and late sepsis.13
The post-hospital discharge and outpatient follow-up periods can also be accompanied by several complications,suchasBPD,whichfavorsfrequent respira-tory complications, resulting in recurrent hospitalizations early in life; gastroesophageal reflux; presence of visual and auditory deficits;psychomotor developmentaldelays; andcerebralpalsy.14,15
Giventhe current survivalrates of PI, especiallythose withVLBW, itbecomesprudenttosearchfor better long-termresults,withgrowthconstitutingthecriticalpointto beemphasizedinthecareofprematureinfants.Thus,the authorsemphasizetheneedtoidentifycomplicationsduring thehospitalstayandpost-dischargeperiodstounderstand the growthdynamicsof the PI dischargedfromthe NICU. Hence,theaimofthisstudywastoassessvariablesduring the NICU hospitalizationand outpatientfollow-up periods thatcaninfluencefailuretothriveinVLBWPI.
Methods
This study was carried out based on medical records of pretermchildrenbornwithVLBW,followedattheHigh-Risk OutpatientClinicofateachinghospitallocatedinthe West-ernregionofthestateofParaná,Brazil.Thisservicetreated 305childrenfromtheNICUduringthestudyperiod;ofthis total,101wereVLBWPI,thesubjectofthepresentstudy.
first12monthsoflife,withatleastoneconsultationineach periodofthestudy,asfollows:PeriodI,upto3months cor-rectedage(CA);periodII,between4and6monthsCA;and periodIII,between7and12monthsCA.TheCAwas consid-eredasthechronologicalageminustheweeksofgestational ageatbirth,subtractedfrom40weeks.
Patientswithseverecongenitalmalformations,thosenot hospitalizedatbirthintheNICUoftheresearchhospital,or thosewhodiedduringfollow-upwereexcluded.Ofthetotal of101VLBWPI,therewasalossof30patients,ofwhich22 (73%)hadfewerappointmentsoratdifferentperiodsthan thosedeterminedbythestudytoassessthePI.Therefore, thestudysamplecomprised71VLBWPI,withthepowerof analysisof0.84calculatedbyGPowersoftwareversion3.1, available at: http://www.gpower.hhu.de/en.html, consid-eringinthelogisticregressionanerrortype1of0.1;error type2of0.2,andsignificantrelativeriskof2.
To correlate weight/GA and calculate the Z-score of the anthropometric variables weight, height, and head circumference (HC) at birth and at the time of hospital discharge, the Fenton and Kim curve1 was used with the help of Fenton Growth Chart Cal-culator, available at: http://www.ucalgary.ca/fenton/. The Z-score of the anthropometric variables of the follow-up period was calculated using the anthropomet-ric calculator of the Anthro program (2011), available at: http://www.who.int/childgrowth/software/en/. When thereweremoreappointmentsduringtheassessedperiods, theZ-scorewascalculatedforeachappointmentandthen theaveragewasobtainedforeachfollow-upperiod.
Data were entered into Microsoft Excel® 2010 (Microsoft®, WA, USA), using the CA, and descriptive statistics were performed (minimum, maximum, mean, standard deviation, relative frequency). The variables were analyzed regarding the distribution pattern using the Shapiro---Wilkstest, followed by thehomogeneity test of variance through the F-test. The variables that were in accordance with the assumptions of normality and homoscedasticity were analyzed between groups children thatmettheinclusioncriteriawiththosewhowerenotin accordancewiththecriteria(losses),usingStudent’st-test forindependentsamples.Othervariablesthatwerenotin accordancewiththestatistical assumptionswereassessed usingthenonparametricMann---WhitneyU-test.
Thevariableswerethenanalyzedbylogisticregression. Failuretothrive(Z-score<−2)wasclassifiedasa dichoto-mousdependentvariable(0---failure/1---success)andthe othervariableswereclassifiedasexplanatoryvariablesfor the periodsof hospitalization andfor each of the follow-upperiods(I,II,andIII).The explanatoryvariablesof the hospitalizationperiod were:gender, weight/GA classifica-tion;timeofbirth weightrecovery;percentage ofweight lostduringhospitalization;hospitallengthofstay.
Forthefollow-upperiod,theexplanatoryvariableswere: gastroesophageal reflux (GER) --- (considered as the pres-ence of abundant and frequent vomiting after feedings); retinopathy of prematurity (ROP --- considering stages 3, 4, and 5); BPD (defined as the use of oxygen at 28 days of life); use of oxygen at hospital discharge; metabolic bone disease (MBD) --- (regarded as alkaline phosphatase serial measurements>900mg/dL, calcium, and phospho-rus associated with clinical and radiological criteria),
and rehospitalization during the post-discharge follow-up period.
Therefore, modelswere created for the anthropomet-ricparameters for the time of discharge, as well as one modelfor each follow-up periodafterdischarge fromthe NICU. The explanatory admission and postnatal variables wereappliedtoeachmodelseparately.Thelogitmodelwas usedfor the purpose of logistic regression analysis,using the stepwise-forward method with binary response. The receiveroperatingcharacteristic(ROC)curvewasadjusted usingtheHosmerandLemeshowmodel.Attheendofthe adjustment, the sensitivity (the proportion of true posi-tives)andspecificity(theproportionoftruenegatives)were calculated,aswell asthe areaunderthe ROCcurve that representsthemodeladjustmentexplicability,showinghow themodeldiscriminatestheoutcome(growth).All statisti-calanalyses were performed using XLStat software, 2014 version,availableat:https://www.xlstat.com/en/.
The study was approved by the Ethics Committee on Human Research of Universidade Estadual do Oeste do Paraná(UNIOESTE),opinionNo.385.407.
Results
A total of 71 VLBW PI was evaluated, of which 36 were males,withmost childrenborn by cesareansection (59%) and mean GA of 29.4±2.8 weeks, with 70% of children classified asAGA. At discharge, 68 children (95.8%) were belowthe 10th percentileof the Fenton andKim curve.1 Atadmission,43 (61%)usedparenteral nutrition(PN), for ameanof21.38±13.90days.Thepercentageoflostbirth weightaveraged 13.64±6.18%. This weight loss occurred within5.27±2.60daysandtheVLBWPItookanaverageof 14.96±5.82daystorecoverthebirthweight.Thelengthof stay was 68.73±27.26 days. At hospital discharge, mean
Z-scores for weight, length, and HC were: −3.05±1.21;
−2.23±1.14,and−1.5±1.45.
The30VLBWPInotincludedinthestudy,astheydidnot meettheestablishedcriteria,hadmeanGAof29.33±2.77 weeks (p=0.895); 23 (73%) were AGA (p=0.541), mean birth weight 1154.16±274.08g (p=0.167); had length of 36.83±2.47cm(p=0.557),HCof 27±1.62cm(p=0.288), and birthweight Z-score of −1.0±0.9 (p=0.074). Signifi-cantly similar values were observed when comparing the excludedchildrenwiththoseincludedinthestudy.
For the 71 VLBW PI, mean Z-scores of the follow-up periodsI,II,andIIIofeachanthropometricvariableshowed thatweight ranged from−2.4±1.3to −1.2±1.3, length variedfrom−2.5±1.5to−1.1±1.4,andHCranged from
−1.1±1.6to−0.5±1.5betweenthefirstandthirdperiods. Atbirth,12(17%)VLBWPIwerebelow−2SDforweight, 29%forheightand13%forHC.Atdischarge,57(80%)were below−2SDforweight.Duringthefollow-upfromPeriodI untilPeriodIII,therewasadecreaseinthepercentageofPI belowtheZ-scores<−2SD;regardingweight,itdecreased from49%to27%,heightfrom61%to25%,andHCfrom22% to14%inPeriodIII.
Table1 Hospitaladmissionvariablesaandgrowthfailureinthefollow-upofverylowbirthweightpreterminfants.
Variable Period Source OR 95%CI p-Value
Weight Discharge Numberofdaysofhospitalization 0.973 0.946---1.001 0.057
AGA 10.217 1.117---93.436 0.040
Follow-upI Numberofdaysofhospitalization 0.979 0.961---0.998 0.030
Follow-upII AGA 4.024 1.210---13.383 0.023
Follow-upIII Lostweight 0.885 0.794---0.987 0.028
AGA 5.060 1.290---19.848 0.020
Height Follow-upI Numberofdaysofhospitalization 0.975 0.956---0.994 0.012
HC Follow-upI Numberofdaysofhospitalization 0.975 0.954---0.997 0.023
OR,oddsratio;95%CI,95%confidenceinterval;PeriodI,upto3monthscorrectedage;PeriodII,4---6monthscorrectedage;PeriodIII,
7---12monthscorrectedage;AGA,adequateforgestationalage.
aExplanatoryvariablesofthehospitalizationperiod:gender,weight/GAratio,birthweightrecoverytime,percentageofweightlost
duringhospitalizationintheNICU,andhospitallengthofstay.
main causeof rehospitalizationwasrespiratory problems, withpneumoniabeingthemostcommon.
Whenevaluatingthepredictivemodels,itwasobserved thatthefactofbeingbornAGA madechildren10.3times more likely to have a weight Z-score at discharge>−2 (OR=10.217, 95% CI: 1.117---93.436; p=0.04), in addi-tion to increasing by 4.024- and 5.060-fold the chance of the weight Z-score>−2 during Periods II (OR=4.024, 95% CI: 1.210---13.383, p=0.023) and III (OR=5.060, 95% CI: 1.290---19.848; p=0.020) of the outpatient follow-up, respectively(Table1).
Longer hospital length of stay of the VLBW PI was associated with a 1.027-fold increased chance of weight at discharge<−2 SD (OR=0.973, 95% CI 0.946---1.001;
p=0.057). A similar result wasobserved for the Z-scores ofweight,height,andHCintheoutpatientperiod.Higher percentageofweightlostduringhospitalizationintheNICU wasassociated witha 1.129-fold higherchance tofail at weight gain in Period III of follow-up (OR=0.885, 95% CI 0.794---0.987;p=0.028;Table1).
The presence of MBD in Period I increased by more than10-foldtherisktoobtainascore<−2SDinthesame period for all assessed anthropometric parameters. Also,
duringPeriodII,therewasa4.608higherchanceofweight being<−2SD.
The occurrence of ROP in Period I increased by 7.194-fold the risk of failure to thrive for weight (OR=0.139;95%CI:0.027---0.723;p=0.019)andby 14.925-foldforheight(OR=0.067,95%CI:0.008---0.571;p=0.013) (Table2).
Re-hospitalizationinPeriodIIincreasedby7.692-foldthe chanceofHCbeing<−2SDinPeriodIII(OR=0.130,95%CI: 0.030---0.563; p=0.006). Likewise, rehospitalization in the Period IIIincreasedby 6.622-foldthe riskof Z-scores<−2 SDforHC(OR=0.151,95%CI:0.036---0.641;p=0.010).
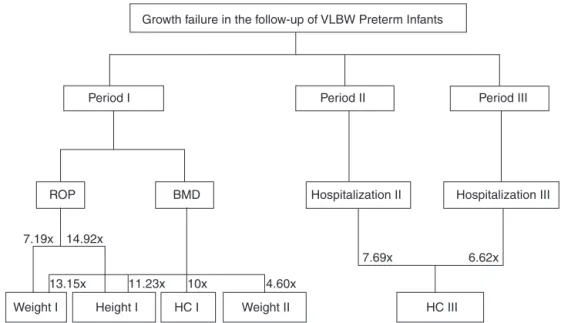
Fig.1showsaflowchartfromTable2,demonstratingthe complicationsforeachfollow-upperiodandfailuresintheir respectiveanthropometricvariables.
BasedontheROCcurve,thespecificity,sensitivity,and explanatorypowerofthevariablesofhospitalstayandthe three follow-up periods were determined. The weight at dischargeappearstobeagoodpredictoramongthe varia-blesthatconstitutethemodelofneonatalhospitalization, whereas in the three periods of outpatient follow-up, Z -scorevariationsofheadcircumference(HC)showedbetter explanatorypower(Table3).
Table2 Outpatientfollow-upvariablesaandgrowthfailureinthefollow-upofverylowbirthweightpreterminfants.
Variable Period Source OR 95%CI p-Value
PeriodI Weight Follow-upI ROP 0.139 0.027---0.723 0.019
MBD 0.076 0.009---0.653 0.019
Follow-upII MBD 0.217 0.059---0.794 0.021
Height Follow-upI ROP 0.067 0.008---0.571 0.013
MBD 0.089 0.010---0.783 0.029
HC Follow-upI MBD 0.100 0.026---0.383 0.001
PeriodII HC Follow-upIII InternII 0.130 0.030---0.563 0.006
PeriodIII HC Follow-upIII InternIII 0.151 0.036---0.641 0.010
OR,oddsratio;95%CI,95%confidenceinterval;PeriodI,upto3monthscorrectedage;PeriodII,4---6monthscorrectedage;PeriodIII,
7---12monthscorrectedage;MBD,metabolicbonedisease;ROP,retinopathyofprematurity;GER,gastroesophagealreflux.
aExplanatoryvariablesoftheoutpatientfollow-upperiodupto12monthsofcorrectedage:GER,ROP,BPD,oxygenatthetimeof
Growth failure in the follow-up of VLBW Preterm Infants
Period I
BMD Hospitalization II Hospitalization III ROP
Weight I Height I HC I Weight II HC III
Period II Period III
14.92x 7.19x
13.15x 11.23x 10x 4.60x
7.69x 6.62x
Figure1 Riskfactorsassociatedwithgrowthfailureinthefollow-upofverylowbirthweightpreterminfants.
Table3 Specificity,sensitivity,andexplanatorypowerof variablesduringthehospitalstayandfollow-upperiods.
Specificity (%)
Sensitivity (%)
Explanatory power(%)
Hospitalizationperiodmodels
WeightZdischarge 100 0.00 79.71
WeightZI 58.82 65.71 62.32
WeightZII 29.63 85.71 63.77 WeightZIII 21.05 94.00 73.91
HeightZI 66.67 63.64 65.22
HCZI 12.50 98.11 78.26
Follow-upperiodImodels
WeightZI---I 52.78 91.43 71.83 WeightZII---I 32.14 90.70 78.87 HeightZI---I 52.63 93.94 71.83
HCZI---I 50.00 90.91 81.69
HCZIII---I 0.00 100 80.28
Follow-upperiodIImodels
HCZIII---II 0.00 100 85.92
Follow-upperiodIIImodels
HCZIII---III 0.00 100 85.92
PeriodI,upto3monthscorrectedage;PeriodII,4---6months
correctedage;PeriodIII,7---12monthscorrectedage.
Discussion
ThemainvariablethatinfluencedfailuretothriveinVLBW PI duringhospitalstay and over the12 monthsof CAwas SGA,whilethosebornAGAhad10.3timesmorechanceof havingaZ-scoreforweightatdischarge>−2SD.The pres-enceofMBDandROPpredictsriskforfailuretothriveinthe firstandsecondtrimestersoflifeofVLBWPI.Additionally, rehospitalization inthe second andthird trimestersof CA increasedthechancesoffailuretothrivefortheHCby 7.7-and6.6-fold,respectively.
EUGRis alwaysconsidered tobearisk situationinthe NICU,withvariablefrequenciesinstudiescarriedoutin sev-eralcountries:63%inIndia,1457%inNorway,1663.5%inthe stateofRiodeJaneiro, Brazil,7and39.1%in astudy con-ductedinthesamecity,butapproximatelyadecadelater.17 Amongtheassessedpreterminfants,itwasfoundthat95.6% werebelowthe10thpercentile,amuchhigherEUGRrate thanthosefoundintheabovementionedstudies.OfthePI thathadEUGR,12.6%hadheight<−2SDinchildhood,which wasmorecommoninthemalegender.18
AlthoughtheZ-scoresofbirthweightweresimilar8atthe timeof hospitaldischarge, there wasa significant reduc-tionwhencomparedtotheZ-scoreatdischargeinthesame studycarried outin Southeastern Brazil(3.05 vs. −1.79). The present study includes children born before 2010, a periodwhenthereweredifferentroutinesregarding nutri-tion.Standardizedcarepracticesandroutinesofaggressive PNandearlyenteralnutritionarenecessarytotrytoavoid or minimize EUGR, which may reduce by up to2.17-fold theriskofZ-score≤−2SD.7Supplementationwithcholine, Uridine,anddocosahexaenoicacidisbeinginvestigated aim-ingtoimprovegrowthinhigh-risknewborns.19Additionally, thefactthatthereisamultidisciplinaryteaminparenteral nutritionfornutritionalmanagementreducestheincidence ofEUGRfrom62.6%to44%.20
BeingbornSGA andhaveinadequategrowthinthefirst year of life are risk factors for growth alterations at 24 monthsofCA.21Still,similartootherstudies,inwhichbeing bornSGAincreasedby12.19timestheZ-score≤−2SD7at termandincreasedby3.41times theriskof Z-score≤−2 SDatdischarge,17beingbornSGAalsorepresentedan addi-tionalriskforEUGR.
follow-upthatshowedaninfluenceonfailuretothrivewere MBDandthepresenceofROP.Similarresultsshowedthere wasno association between failure tothrive and chronic pulmonarydisease;however,it wasstronglyinfluencedby severeROP.23
In a study22 with extremely low birth weight PI, fol-loweduntil20months ofCA,the hospitalizationratewas 40% during the entire follow-up period, with no statisti-caldifferencebetweenthosewhoshowedfailuretothrive (n=62; 40%) and those who did not (n=92; 60%). The presentstudy’srehospitalizationratewashigher(52%)and the occurrence of hospitalization during Periods II and III increased the chance of failure to thrive for the HC in PeriodIII.Thisaspectisparticularlyrelevantbecauseinthis phaseoflife,appropriateHCgrowthisessentialfornormal development.24
Differences in thegrowth patternsafter the discharge ofVLBWPI shouldbeconsidered.The findingsshownhere allowustoverifyprogressiveincreaseinthevalueoftheZ -scoreforweight,height,andHCthroughouttheoutpatient follow-up.Althoughat12monthsofCAtheobtainedrates oftheseanthropometricparameterswerelowerthanthose found in Southern Brazil,10 the growth rates were higher than thosein an Indian study.14 Thus, it is observed that oneshouldconsider,inadditiontoneonatalandfollow-up complicationsinherent topretermbirth, theenvironment wherethechildlives,aswellassocioeconomic,nutritional, educational,andregionalculturalfactors.2Manymodelsof anthropometricvariablesconsideredsignificant,definedin theneonatalperiodandPeriods I,II, andIII offollow-up, showed high sensitivity and intermediate specificity, i.e.
theydefine high probability of detecting cases of growth success,andmoderatelytheprobabilityofdetectingcases of failure to thrive. HC showed high sensitivity, but zero specificity, exceptfor the neonatalperiod. The definition of three periods for critical evaluation of the PI growth, allowsacloserlookbytheprofessionaltodetectearly fail-uresandpromoteappropriategrowthpatternsduringthose moments.
Based on these findings, it can be concluded that the main factor that influenced the VLBW PI throughout the hospitalstaywasSGA,whichduringhospitaldischargeand alsooverthe12monthsofCA,increasedfailuretothrive. ThepresenceofMBDandROPsignificantlyinfluencegrowth inthe firstquarter ofthe first12 monthsof life ofVLBW PI.
Althoughgrowthisalsoinfluencedbymaternaland fam-ilysocioeconomicfactors,25itwasnotpossibletoverifythis influencein the present study,asit wasretrospective, in additiontothefactthatthisinformationwasnotincludedin thereviewedmedicalrecords,significantlylimitingthe find-ings.Similarly,thenumberofPIthatwereexcludedfornot havingtheminimumnumberofthreeappointmentsduring thefirstyearoflifeconstitutedalimitingfactortoextend theresultsofthisstudy.Thedifficultytoperformlong-term follow-upisalsofoundinothercontexts.26,27
The growth of VLBW PI during the first 12 months of life is influenced by several factors, from the nutritional statusin utero, nutritionalpractices in the NICU andthe follow-upperiod,aswellasregional,cultural,and environ-mentalaspects,whichneedtobeclarifiedinfurtherstudies designedforthispurpose.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
References
1.FentonTR,KimJH.Asystematicreviewandmeta-analysisto revisetheFentongrowthchartforpreterminfants.BMCPediatr. 2013;13:59.
2.Cardoso-DemartiniADA,BagatinAC,PaulaR,VieiraG,Cristina M. Crescimento de crianc¸as nascidas prematuras. Arq Bras EndocrinolMetab.2011;55:534---40.
3.EhrenkranzR,DusickAM,VohrBR,WrightLL,WrageL,Poole WK.Growthintheneonatalintensivecareunitinfluences neu-rodevelopmentand growth outcomesof extremely lowbirth weightinfants.Pediatrics.2006;117:1253---61.
4.SenterreT,RigoJ.Optimizingearlynutritionalsupportbasedon recentrecommendationsinVLBWinfantsandpostnatalgrowth restriction.JPediatrGastroenterolNutr.2011;53:536---42.
5.Lapillone A, Griffin IJ. Feeding preterm infants today for later metabolic and cardiovascular outcomes. J Pediatr. 2013;162:S7---16.
6.Jong M, Lafeber HN, Cranendonk A, van Weissenbruch MM. Components of the metabolic syndrome in early child-hood invery-low-birth-weight infants. Horm ResPædiatrics. 2014;81:43---9.
7.GianiniN,VieiraA,MoreiraM.Avaliac¸ãodosfatoresassociados aoestadonutricionalnaidadecorrigidadetermoem recém-nascidosdemuitobaixopeso.JPediatr(RioJ).2005;81:34---40.
8.LimaPA,CarvalhoMd,CostaAC,MoreiraME. Variables asso-ciatedwithextrauterinegrowthrestrictioninverylowbirth weightinfants.JPediatr(RioJ).2014;90:22---7.
9.GoulartAL,MoraisMB,KopelnBI.Impactodosfatoresperinatais nosdéficitsdecrescimentodeprematuros.RevdaAssocMedica Bras.2011;57:272---9.
10.OliveiraMG,SilveiraRC,ProcianoyRS.Growthofverylowbirth weightinfantsat12monthscorrectedageinsouthernBrazil.J TropPediatr.2008;54:36---42.
11.SilveiraRC,ProcianoyRS.Crescimentonosprimeiros anosde vidaderecém-nascidosdemuitobaixopeso.In:ProcianoyRS, LeoneCR,editors.Programadeatualizac¸ãoemneonatologia. PortoAlegre:Artmed;2010.p.49---86.
12.Martin CR, Brown YF, Ehrenkranz R, O’SheaTM, Allred EM, BelfortMB,etal.Nutritionalpracticesandgrouwthvelocityin firstmonthoflifeinextremelyprematureinfants.Pediatrics. 2009;124:649---57.
13.EhrenkranzR.Extrauterinegrowthrestriction:isitpreventable? JPediatr(RioJ).2014;90:1---3.
14.MukhopadhyayK,MahajanR,LouisD,Narang A.Longitudinal growthofverylowbirthweightneonatesduringfirstyearof lifeand riskfactors formalnutritioninadevelopingcountry. ActaPaediatr.2013;102:278---81.
15.SharmaPK,SankarMJ,SapraS,SaxenaR,KarthikeyanCV, Deo-rariA,et al.Growth andneurosensory outcomesofpreterm verylowbirthweightinfantsat18monthsofcorrectedage. IndianJPediatr.2011;78:1485---90.
16.WesterbergAC,HenriksenC,EllingvagA,VeierodMB,Júlíusson PB,Nakstad B,etal.Firstyeargrowthamongverylowbirth weigthinfants.ActaPaediatr.2010;99:556---62.
17.LimaPA, CarvalhoM, Costa AC,Moreira ME. Author’s reply:
Z-score: Fenton 2013. Ten-year update. J Peditr (Rio J). 2014;90:427---8.
19.AndrewMJ,ParrJR, Montague-JohnsonC,Braddick O, Laler K,WilliamsN, etal. Optimisingnutritionto improvegrowth andreduceneurodisabilitiesinneonatesatriskofneurological impairment,andchildrenwithsuspectedorconfirmedcerebral palsy.BMCPediatr.2015;15:22.
20.ShanHM,CaiW,CaoY,FangBH,FengY.Extrauterinegrowth retardationinprematureinfantsinShangai:amulticenter ret-rospectivereview.EurJPediatr.2009;168:1055---9.
21.KiyAM,RugoloLM,DeLucaAK,CorrenteJE.Growthofpreterm lowbirthweightinfantsuntil24monthscorrectedage:effect ofmaternalhypertension.JPediatr(RioJ).2015;91:256---62.
22.SicesL,Wilson-CostelloD,MinichN,FriedmanH,HackM. Post-discharge growth failure among extremely low birth weight infants:Correlatesand consequences.PaediatrChild Health. 2007;12:22---8.
23.Griffin IJ, Tancredi DJ, Bertino E, Lee HC, Pro J. Postnatal growthfailure invery lowbirthweightinfants bornbetween
2005 and 2012. Arch Dis Child Fetal Neonatal. 2016;101: 50---5.
24.Franz AR, Pohlandt F, Bode H, Mihatsch W, Sander S, Kron M, et al. Intrauterine, early neonatal, and postdischarge growthandneurodevelopmentalnutritionalsupport.Pediatrics. 2009;123:e101---9.
25.SwamyG,OsstbyeT,SkjaervenR.Associationofpretermbirth with long-term survival, reproduction, and next-generation pretermbirth.JAMA.2008;299:1429---36.
26.Mukhopadhyay K, Louis D, Mahajan R, Mahajan R. Longi-tudinal growth and post-discharge mortality and morbidity amongextremelylowbirthweightneonates.IndianPediatrics. 2014;51:723---6.