w ww.e l s e v i e r . c o m / l o c a t e / b j p
Review
Natural
products
assessed
in
animal
models
for
orofacial
pain
–
a
systematic
review
Pollyana
S.
Siqueira-Lima
a,b,
Juliane
C.
Silva
a,c,
Jullyana
S.S.
Quintans
a,
Angelo
R.
Antoniolli
a,
Saravanan
Shanmugam
a,
Rosana
S.S.
Barreto
a,
Márcio
R.V.
Santos
a,
Jackson
R.G.S.
Almeida
b,c,
Leonardo
R.
Bonjardim
d,
Irwin
R.A.
Menezes
e,
Lucindo
J.
Quintans-Júnior
a,∗aLaboratóriodeNeurociênciaseEnsaiosFarmacológicos,UniversidadeFederaldeSergipe,SãoCristóvão,SE,Brazil bDepartamentodeFarmácia,UniversidadeEstadualdeFeiradeSantana,FeiradeSantana,BA,Brazil
cColegiadodeCiênciasFarmacêuticas,UniversidadeFederaldoValedoSãoFrancisco,Petrolina,PE,Brazil dFaculdadedeOdontologiadeBaurú,UniversidadedeSãoPaulo,Baurú,SP,Brazil
eDepartamentodeQuimica-Biologica,FederalUniversityofCariri,Crato,CE,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received10February2016 Accepted27June2016
Keywords: Medicinalplants Naturalproducts Orofacialpain Pain Animalmodels
a
b
s
t
r
a
c
t
Orofacialpainisrelatedtotissuesofthehead,face,neckandalltheintraoralstructures;itisrather debil-itatingtothepatientandalsodifficulttotreat.Therearerelativelyfewstudiesdedicatedtotheuseof naturalproductstoalleviateorofacialpaininpreclinicalexperimentmodels(performedin experimen-talanimalswhichprovidesupportforclinicaltrials).Mainobjectivesofthepresentsystematicreview summarizethestudiesonnaturalproductsassessedinanimalmodelsfororofacialpainseekingtogive evidencetofuturedevelopmentofnewpharmaceuticalproductstomanagetheorofacialpain.Ourreview includesathoroughsearchofliteratureusingthetermsoforofacialpain,facialpain,medicinalplants andnaturalproducts.ThissearchwasperformedusingtoretrieveEnglishlanguagearticlesin Medline-PubMed,ScopusandWebofScience.Atotalofeighteenstudieswereincludedinoursurveyforthe inclu-sioncriteria.Firstly,thisreviewidentified210citationsfromelectronicsearch,afterremovalofduplicates andscreeningforrelevanttitlesandabstracts,atotalofeighteenarticleswereselectedtotheinclusion criteriaestablished.Ourfindingssuggestthatnaturalproductscanbeapromisingoratrumptoolforthe developmentofnewdrugstotreatorofacialpainconditions,buttheresearchersthatdealwith experi-mentalpreclinicaltrialsofnewdrugs(includingnaturalproductsorsyntheticdrugs)fororofacialpain conditionsurgentlyneedtoshowtranslationalevidence(withclinicalapproach)ofthesecompounds.
©2016SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Thisisanopen accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Orofacialpainisrelatedtotissuesofthehead,face,neckand alltheintraoralstructuresdeterminedbytheAmericanAcademy ofOrofacialPain(AAOP).Thispainconditionrepresentsahighly prevalent spectrum of disorders with pain intensity involving anatomical,biochemicalandmolecularaspectswhichare associ-atedwithpsychosocialcomponents(Hargreaves,2011;Fanetal., 2012).Theorofacialregionisoneofthemostdenselyinnervated areasofthebody, andthetrigeminalnerve playsanimportant roleinthisprocess.Anorofacialpainencompassesawiderange ofconditionsincludingtemporomandibularjointdisorders, peri-odontalpain, trigeminalneuralgia, atypical facial pain, burning
∗ Correspondingauthor.
E-mail:lucindo@pq.cnpq.br(L.J.Quintans-Júnior).
mouthsyndrome,dentalsurgicalpain,headandneckcancerpain, painduetooralinfections,andotherneuropathicand inflamma-torypain(Gilbertetal.,2001).Itisalsothesiteoffrequentchronic post-herpetical neuralgia,migraine and referredpains(Pelissier etal.,2002;RaboissonandDallel,2004).Therefore,orofacialpainis oftenratherdebilitatingtothepatientandmanydifficultiesinthe managementofacuteandchronicorofacialpainconditionsstem fromalackofrecognitionandunderstandingofpainmechanisms (Mirandaetal.,2009;KrzyzanowskaandAvenda ˜no,2012).
Uptothepresentdate,severalanimalmodelshavebeenused tostudyorofacialpain.Theseincludeinfraorbitalnerveligationor axotomy,injectionofinflammatoryagentsintothevibrissalpads, temporomandibularjointormassetermuscle,aswellasintradental orduralapplicationofirritants(RenandDubner,1999).Clavelou etal.(1989)reportedthattheadaptationoftheformalinmodel ofpainfortheorofacialregionprovidesanimportant contribu-tiontothemechanismsoftrigeminalpainandanalgesicresponse
http://dx.doi.org/10.1016/j.bjp.2016.06.005
(Mittal et al., 2009). Other orofacial pain animal models have beenmorerecentlyproposedandhavecontributedwithclinical studies,suchastheassessmentofneuropathicandinflammatory pain (Krzyzanowska and Avenda ˜no, 2012).Despite the impor-tanceoftheseanimalmodels,accordingtoKhanandHargreaves (2010),theyposesomelimitationsthatshouldalwaysbetakeninto accountinthechoiceofmodelsforthetestingofnewdrugs.Thus, theseanimalmodelshavegreatanimportancetothestudy,such asabetterunderstanding,developmentoftherapeuticproposals andadvancesinthetreatmentoforofacialpain.
The management of pain is still a major challenge to both the medical practitioners and the patients (Krzyzanowska and Avenda ˜no, 2012).Opioids,antidepressants, anticonvulsantsand non-steroidalanti-inflammatorydrugs(NSAID)remainasthemain agentsusedtorelieveacuteandchronicpain,includingorofacial pain(Paceetal.,2006),butthewiderangeofside-effectsaligned withlow adherencehasbeenastimulusintheconstantsearch fornewdrugstotreatorofacialpain(Quintans-Júnioretal.,2010). Anactualapproachistodevelopanewbiologicalcompoundthat inhibitspainfromnaturalproducts(NP),suchasmedicinalplants ortheirsecondarymetabolites,whichcouldenhanceefficacy, pro-duceminimalsideeffectsandoperateinunusualwayscompared tothesyntheticdrugs(Holanda-Pintoetal.,2008;Venâncioetal., 2011;Oliveiraetal.,2012).
The present systematic review was designed to summarize thecurrentstudiesonNPtestedinanimalmodelsfor attenuat-ingtheorofacialpain.Ourmainfocusaimedtomakethereader aware,throughasystematicway,ofwhichthemainaspectsofNP researchedtoorofacialpainare,inexperimentalprotocols.
Methods
Searchstrategy
Three internet sources were used to search for appropriate papersthatmetthestudypurpose.TheseincludedtheNational LibraryofMedicine,Washington,D.C.(Medline-PubMed),Scopus and,WebofScienceusingdifferentcombinationsofthe follow-ingkeywords:Orofacialpain,Facialpain,Medicinalplants,natural products.Thedatabasesweresearchedforstudiesconductedinthe perioduptoandincludingApril30,2015.Thestructuredsearch strategywasdesignedtoincludeallpublishedpapersthat evalu-atedtheuseofnaturalproductsinorofacialpain.Citationswere limitedtoanimalstudies.Additionalpaperswereincludedinour studyaftertheanalysesofallreferencesfromtheselectedarticles. Wedidnotcontactinvestigatorsanddidnotattempttoidentify unpublisheddata.
Studyselection
Allelectronicsearchtitles,selectedabstracts,andfull-text arti-cleswereindependentlyreviewedbyaminimumoftworeviewers (JSSQandPSSL).Disagreementsinstudyinclusion/exclusionwere resolved with a consensus meeting with more reviewers. The followinginclusioncriteria wereapplied:orofacialpainstudies, animalmodelsandtheuseofNPforreducingnociception. Stud-ieswereexcludedaccordingtothefollowingexclusion criteria: studiesinhumans,review articles,meta-analyses,abstractsand conferenceproceedings.
Dataextraction
Datawereextractedbyonereviewerusingstandardizedforms and were checked for completenessand accuracy by a second reviewer.Informationcollectedincludeddataregardingthestudy
Search pubmed (88), Scopus (68), Web of science (54) databases
210 original articles
53 articles deemed
potentially relevant 157 citations excluded:
not relevant by title
review
33 citations excluded:
not relevant by abstract
review 20 articles deemed
relevant by title and abstract or needed full
text to make
determination
18 articles included 4 citations excluded:
not relevant by full text
review
Inclusion of 2 articles
from reference list
Fig.1. Flowdiagramforliteraturesearchingandscreening.
substance(naturalproducts),animal,dose(route),model, recep-tors/mediators,antagonistsandresults.
Methodologicalqualityassessmentofstudies
Theriskofbiasandqualityofthestudydesignwereassessed using a 12-point checklist. The use of these questions has enabled theassessmentof importantaspectsthat contributeto the quality of the study, such as randomization of the treat-ment allocation, blinded drug administration, blindedoutcome assessment and outcome measurements. Studies that reported randomizationof animals,blindingandoutcomemeasurements were considered of higher methodological quality (Zeng et al., 2015).
Resultsanddiscussion
Thepreliminaryreviewofthepresentstudyidentified210 cita-tionsfromelectronicsearch,with88fromPubmed,68fromScopus, 54WebofScience.Afterremovalofduplicatesandscreeningfor rel-evanttitlesandabstracts,atotalofeighteenarticleswereselected totheinclusionandexclusioncriteriaestablished(Fig.1).
Curiously, fromeighteen final studiesselected, mostof that research,particularlyNP,wasconductedinBrazil(88%)(Table1) and 12% in India and USA. Detailsof theincluded studies are described inTable1.Thatcouldbebecausewelimitour inclu-sion criteria to orofacial pain animal models. However, if this surveysoughtclinicalstudiesand/orethnopharmacologicalsearch, it probably showed a wider range of countries (Tapsoba and Deschamps,2006;Taheri etal.,2011).Anotherfactormayhave contributedtothisfindingbecauseBrazilhastheextensiveethical, culturalandgreatestbiodiversityintheworld,whichhas histor-icallybeenasourceofexplorationandstudy(Macieletal.,2002; Oliveiraetal.,2012).TheresultshowsthescientificgrowthofBrazil in theareaofNP corroboratingwiththepotential ofbiological diversity(PeixotoandMorim,2003)thathasabout19%ofearth’s totaldiversity(Giuliettietal.,2005).Also,itisestimatedthat49,000 plantspecieshavealreadybeendescribed(Shepherd,2002).
Table1
Characteristicsofstudiesinsertedinthereview.
Authors,year, country
Substance/chemical group/(natural product)
Animals (strain/sex)
Dose(route) Methodofinductionof orofacialpain
Parametersassessed R B
Behavior Biochemical/ molecular
Gilbertetal.,2001, USA
Epibatidine Rats(SD/M) 1–5g/kg 50lsubcutaneous injectionofformalin(5%) intoonevibrissalpad
Grooming, rubbingand/or scratchingface
– N Y
Mittaletal.,2009, India
Curcumin (Curcumalonga)
Rats(W/M) 1–600mg/kg (i.p.)
50lsubcutaneous injectionofformalin(5%) intoonevibrissalpad
Grooming, and/or scratchingface
– N N
Holanda-Pinto etal.,2008,Brazil
␣,-amyrin (Protium heptaphyllum)
Rats(W/M) 10–100mg/kg (i.p.)
20lsubcutaneous injectionofformalin (1.5%),capsaicin(1.5g) intoonevibrissalpad
Face-rubbing – N N
Siqueiraetal., 2010,Brazil
Atranorin (AT)/Liquen (Cladinakalbii Ahti)
Mice(S/M) 100–400mg/kg (i.p.)
20lsubcutaneous injectionofformalin (2%),capsaicin(2.5g) intotherightupperlip (perinasalarea)
Face-rubbing – N N
Quintans-Júnior etal.,2010,Brazil
Citronellal (Cymbopogon genus)
Mice(S/M) 50–200mg/kg (i.p.)
20lsubcutaneous injectionofformalin (2%),capsaicin(2.5g), 40lglutamate(25M) intotherightupperlip (perinasalarea)
Face-rubbing Singlesucrosegap electrophysiologic assays
Y N
Bonjardimetal., 2011,Brazil
Ethanol, chloroform, methanolextract fractions(S. cordifolia)
Mice(S/M) 100–400mg/kg (p.o.)
20lsubcutaneous injectionofformalin (2%),40lglutamate (25M)intotheright upperlip(perinasalarea)
Face-rubbing – N N
Venancioetal., 2011,Brazil
(Ocimum Basilicum)and (−)-linalool
Mice(S/M) 50–200mg/kg (i.p.)
20lsubcutaneous injectionofformalin (2%),capsaicin(2.5g), 40lglutamate(25M) intotherightupperlip (perinasalarea)
Face-rubbing Fieldpotential recordings. electrophysiologic assays
N N
Santanaetal., 2011,Brazil
p-Cymene Mice(S/M) 25–100mg/kg (i.p.)
20lsubcutaneous injectionofformalin (2%),capsaicin(2.5g), 40lglutamate(25M) intotherightupperlip (perinasalarea)
Face-rubbing – N N
Britoetal.,2013, Brazil
Citronellol (Cymbopogon citrates,C. winterianus)
Mice(S/M) 25–100mg/kg (i.p.)
20lsubcutaneous injectionofformalin (2%),capsaicin(2.5g), 40lglutamate(25M) intotherightupperlip (perinasalarea)
Face-rubbing Immunofluorescence Y N
Guimarãesetal., 2012,Brazil
Carvacrol Mice(S/M) 25–100mg/kg (i.p.)
20lsubcutaneous injectionofformalin (2%),capsaicin(2.5g), 40lglutamate(25M) intotherightupperlip (perinasalarea)
Face-rubbing – Y N
Paixãoetal.,2013, Brazil
Aqueousextract (Hyptispectinata)
Mice(S/M) 100–400mg/kg (p.o.)
20lsubcutaneous injectionofformalin (2%),capsaicin(2.5g), 40lglutamate(25M) intotherightupperlip (perinasalarea)
Face-rubbing TBARS DPPH
Y N
Limaetal.,2013b, Brazil
Hydroethanol extract(Hyptis fruticosa)
Mice(S/M) 50–200mg/kg (p.o.)
20lsubcutaneous injectionofformalin (2%),capsaicin(2.5g), 40lglutamate(25M) intotherightupperlip (perinasalarea)
Face-rubbing TBARS Y N
Nomuraetal., 2013,Brazil
Ethanolicextract (Acmellaoleracea)
Mice(S/M) 10–100mg/kg (i.p)
20lsubcutaneous injectionofformalin (2%),capsaicin(2.5g), cinnamaldehyde (13.2g)intotheright upperlip(perinasalarea)
Table1 (Continued)
Authors,year, country
Substance/chemical group/(natural product)
Animals (strain/sex)
Dose(route) Methodofinductionof orofacialpain
Parametersassessed R B
Behavior Biochemical/ molecular
Quintansetal., 2014a,Brazil
Ethanolicextract (Syzygiumcumini)
Mice(S/M) 100–400mg/kg (p.o)
20lsubcutaneous injectionofformalin (2%),40lglutamate (25M)intotheright upperlip(perinasalarea)
Face-rubbing – Y Y
Siqueira-Lima etal.,2014,Brazil
-Cyclodextrin complex containing essentialoil (Lippiagrata)
Mice(S/M) 6–24mg/kg (p.o)
20lsubcutaneous injectionofformalin (2%),capsaicin(2.5g), 40lglutamate(25M) intotherightupperlip (perinasalarea)
Face-rubbing Immunofluorescence Y Y
Quintansetal., 2014b,Brazil
Hexanicextract (Combretum duarteanum)and friedelin
Mice(S/M) 100–400mg/kg (p.o)
20lsubcutaneous injectionofformalin (2%),capsaicin(2.5g), 40lglutamate(25M) intotherightupperlip (perinasalarea)
Face-rubbing – Y Y
Damascenaetal., 2014,Brazil
Aqueousextract (Anadenanthera colubrina)
Mice(S/M) 100–400mg/kg (p.o)
20lsubcutaneous injectionofformalin (2%),capsaicin(2.5g), 40lglutamate(25M) intotherightupperlip (perinasalarea)
Face-rubbing DPPH,MDA,TBARS, AAPH,FeSO4
Y N
Nowackietal., 2015,Brazil
Hydroalcoholic extract (Hypericum perforatum, Valeriana officinalis,Piper methysticum)
Mice(S/M) NR 20lsubcutaneous injectionofformalin (2%)intotherightupper lip(perinasalarea)
Face-rubbing Hematologicaland hepaticmarkers
N N
Animals:SD,Sprague–Dawley;W,Wistar;S,Swiss.
Parameters assessed: DPPH, 2,2-diphenyl-1-picrylhydrazyl radical; MDA, malonaldehyde; TBARS, thiobarbituric acid-reactive substances; AAPH, 2,2′
-azobis(2-methylpropionamidine)dihydrochloride;FeSO4,ferroussulphate;R,reportingofrandomization;B,reportingofblinding;Y,yes;N,no.
thedrugsusedcommerciallyandarenowadaysaroundarederived fromnaturalsources(Braz-Filho,2010).
RegardingtheNPusedforthemanagement oforofacialpain tabulatedinthepresentstudy,(Table1)showsisolatedsubstance (47.25%),extracts(47.25%)and-cyclodextrin(-CD)complexes containing essential oil(5.5%). That interprets mostreviews of isolatedcompoundsasreflectingtheimportanceofthe pharma-ceuticalindustry,sincethedrugsare,almostentirely,asingleactive ingredient which is responsible for its pharmacological effect. Neverthless,theplantextractsconsistofmulticomponentactive mixtures, partially active and inactive substances, which often workindifferentpharmacologicaltargets(FerreiraandPinto,2010; Ulrich-Merzenichetal.,2010).
Moreover,our findingsdemonstrated that it is necessaryto increase thenumberof studieswithNP toorofacialpain mod-elsbecause100% oftheincludedstudies inthepresent review usemainlychemicalstimulitoinducepain(formalin,glutamate, capsaicinorcinnamaldehyde)intothevibrissalpad.These charac-teristicsofthestudiesfoundbecomeobviouslimitationsbecause orofacialpainis derivedfrommany uniquetargettissues, such asthemeninges,cornea,toothpulp,oral/nasalmucosaand tem-poromandibularjoint(Hargreaves,2011).Further,thereareother orofacialpain animal modelsalso established in theliterature, such as orofacial neurophatic pain or chronic pain model in temporomandibularjoint,whichhavenotbeenexploredin arti-clesinserted in this systematic review. AccordingtoRaboisson andDallel(2004),themechanismsunderlyingorofacialpainare still poorly understood, partly due to the relative scarcity of
investigationdevotedtothefaceandthemouth,whencompared withpaininbody.Inparticular,therearerelativelyfewbehavioral modelsinlaboratoryanimalsdedicatedtothestudypaininthe trigeminalregion.
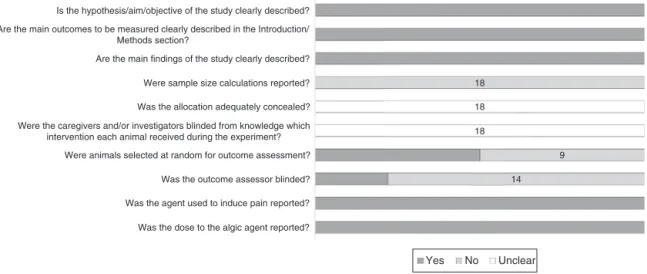
Regardingtheanalysisof qualityof thestudiesinserted,we found that 50% of studies (9) mentioned randomization in the allocationofanimalsfortesting.However,theydidnotdescribe how theyperformedrandomization of animals.Moreover,only fourstudies(22%)wereperformedbydouble-blindmanner,asit canbeseeninFig.2.Thatlimitstheinterpretationoftheresults obtainedinourreview,whatshowsalowqualityinthestudies found.
To better describe and understand the results, we summa-rizedthemasisolatedcompounds,plantspecies,essentialoilsand extracts,whicharedescribedbelow.
Isolatedsubstance
Epibatidine(1)
Is the hypothesis/aim/objective of the study clearly described?
Are the main outcomes to be measured clearly described in the Introduction/ Methods section?
Are the main findings of the study clearly described?
Were sample size calculations reported?
Was the allocation adequately concealed?
Were the caregivers and/or investigators blinded from knowledge which intervention each animal received during the experiment?
Were animals selected at random for outcome assessment?
Was the outcome assessor blinded?
Was the agent used to induce pain reported?
Was the dose to the algic agent reported?
Yes
14 9 18
18 18
No Unclear
Fig.2.Methodologicalqualityofincludedstudies.Darkgraybarsindicatetheproportionofarticlesthatmeteachcriterion;lightgraybarsindicatetheproportionofstudies thatdidnotandwhitebarsindicatetheproportionofstudieswithunclearanswers.
Curcumin(2)
Themajorbioactive constituentof theoleoresinof turmeric (Curcuma longa L., Zingiberaceae) demonstrated a significant reduction in grooming behavior induced with formalin in a dose-dependent manner in both phases. Curcumin has proven anti-inflammatoryeffectdemonstratingitsbeneficialroleforthis pain condition (Mittal et al., 2009). Kim et al. (2003) explains theanti-inflammatoryactivityofcurcuminduetoitsinhibitory actiononJanuskinase(JAK)– STATsignalingpathway inbrain microglialcells, whichcontributes totheattenuationof inflam-matoryresponse.Anti-inflammatoryactivityofcurcuminmayalso berelatedtoitsabilitytodirectlysuppressnuclearfactor-kappaB (NF-B),leadingtotheinhibitionofcellularCOX-2gene expres-sion.CurcuminalsocausesanindirectsuppressionoftheNF-Ban activitybyinhibitingthedegradationoftheinhibitoryunitI-B␣, whichhamperssubsequentnucleartranslocationofthe function-allyactivesubunitofNF-B(Surhetal.,2001).Also,ithasbeen showntooverturnthesynthesisofprostaglandinE2(PGE2),which isknowntobeinvolvedinmediating acuteinflammation(Goel etal., 2001).Thereare alsoevidencesof theinhibition of pro-inflammatorycytokineproduction(TNF-␣,IL-1,IL-8)bycurcumin (Xuetal., 1997–1998;Banerjeeetal.,2003; Jacobet al.,2007). Anotherstudyexplainsthattheoxygenradicalscavengingactivity ofcurcuminhasalsobeenimplicatedtoanti-inflammatoryeffects (KunchandyandRao,1990).Inadditiontotheanti-inflammatory profile,Sharmaetal.(2006, 2007)demonstratedtheefficacyof curcuminintheattenuationofdiabeticneuropathicpain. Accord-ingtoMittaletal.(2009),oneneedstoexplainthemechanism of curcumin in the first phase of formalin, which occurs as a directactivationofA-deltaandC-nociceptorsaswellas trigem-inaland spinalnociceptiveneurons,whereasanti-inflammatory actionoverthevariousmechanismsmentionedabovemightbe responsibleforreducingthegroomingbehaviorduringthetonic phase. Banafsheet al. (2014) explainsthat this antinociceptive effectmaybeduetotheactivationoftheopioidsystem.Based
onthesereports, the compoundcurcumin representsa natural sourceforthetreatmentoforofacialandattenuatingother pain-andinflammation-relateddisorders,whichallneedfutureclinical investigations.
˛,ˇ-Amyrin
Atranorin(3)
Siqueiraet al. (2010)evaluatedthe antinociceptiveeffect of atranorin onformalin- and capsaicin-induced orofacial pain in mice.Theirresultsshowedthatacuteadministrationofatranorin (400mg/kg,i.p.)informalin-inducedorofacialpainresultsina pro-nouncedanti-nociceptionasevidencedbydecreasedface-rubbing nociceptivebehaviorinthefirstandsecondphasesoftheformalin test.Alldosesofatranorinsignificantlyinhibitedtheface-rubbing behavioratthesecondphaseof theformalintest justasinthe capsaicintest.Therefore,thestudysuggestedapossible antinoci-ceptiveeffectofthisimportantlichenmetaboliteextractedfrom CladinakalbiiAhti,inunspecifictests(Meloetal.,2008).Thiseffect mayhaveresultedfromtheinhibitionoftheSubstancePreleaseor duetoadirectblockingactiononitsneurokinin-1receptor(NK-1) (Holanda-Pintoetal.,2008).Meloetal.(2008)suggesteda pos-sibleantinociceptiveeffectofatranorintoactperipherallyonthe inflammatorymediators,especiallyonprostaglandins.Bugnietal. (2009)showedthatapartoftheantinociceptiveeffectobtained withatranorinmaybeduetotheCOXinhibition.Additionally,it wasdemonstratedthatatranorineffectivelyinhibitedthe biosyn-thesisofleukotrieneB4inbovinepolymorphonuclearleukocytes, whichcouldalsoleadtoananti-inflammatoryeffect(Kumarand Muller,1999).Theprecisemechanismsthroughwhichatranorin exertsitsactionarecurrentlyunderinvestigation,buttheycould possiblyberelatedtothearachidonicacidcascadeand/or mod-ulationofpro-inflammatorymoleculeproduction(Siqueiraetal., 2010).
Citronella
ReportsfromQuintans-Júnioretal.,2010,revealedtheeffects ofcitronellal,amonoterpenefromtheoilsofCorymbiacitriodora (Hook.)K.D.Hill&L.A.S.Johnson,Myrtaceae,Cymbopogonnardus (L.)Rendle,C.citratus(DC.)StapfandC.winterianusJowittexBor, Poaceae,inthepossibleanti-nociceptiveactivitybyusingorofacial nociceptive tests (formalin-, capsaicin-, and glutamate-induced orofacialnociception)andinvestigatedwhethersucheffectsmight causea changetoneuralexcitability.Theirresultsshowedthat an acute administration of citronellal caused pronounced anti-nociceptionasevidencedbydecreasedface-rubbingbehaviorin thefirstandsecondphasesoftheformalintest,suggestingthat citronellalhasacentralanalgesiceffect.Toconfirmsuchaneffect, theblockingeffectofnaloxone,aspecificantagonistof morphi-nomimeticreceptors,wastestedonbothphasesoftheformalin test(Belvisietal.,1998).Itsantagonisteffectssuggestedthe par-ticipationoftheopioidsysteminthemodulationofnociception inducedbycitronellal.Citronellalalsoinhibitednociceptive behav-ior inducedby the capsaicininjection intothe right upperlip. Theinhibitoryeffectobservedwithcitronellaloncapsaicin-and formalin-inducedface-rubbingbehaviorseemssimilarlyactto␣, -amyrin,whichcaninvolvetheinhibitionoftheSubstancePrelease ortheactiononreceptorneurokinin-1(NK-1)(Holanda-Pintoetal., 2008).Theresultsalsoshowedthattheintraperitoneal adminis-trationofthecitronellalproduceda significantinhibitionofthe nociceptiveresponseinducedbytherightupperinjectionof glu-tamateintomice.Thatnociceptiveresponseinducedbyglutamate seemstoinvolveperipheral,spinalandsupraspinal sitesandits action ismediated byNMDAand non-NMDA receptors(Beirith
etal.,2002).Thus,thesuppressionofglutamate-induced nocicep-tionbycitronellaltreatmentcanbeassociatedwiththeinteraction ofcitronellalwiththeglutamatergicsystem(Ferreiraetal.,1999). Thepresent studyusedthesinglesucrosegapmethodtoshow that citronellal couldreduce the excitability of isolated nerves throughadiminutionofamplitudeofcompoundactionpotential (CAP).It is possible thatthe antinociceptiveeffects of citronel-lalintheexperimentalmodelsofnociceptioncouldbeinvolved in voltage-gatedNa+ channel blocking,and mayalsohave CNS effects(Quintans-Júnioretal.,2010).Quintans-Júnioretal.(2010, 2011) alsoproposed thatthe orofacialantinociceptive effectof citronellalcouldberelatedtotheantioxidantprofilesincethese monoterpenesexhibitedasignificantantioxidantactivityasseen inTRAP/TARassays,andscavengeNOandsuperoxidemolecules whicharewidelyacceptedmethodstoreliablyestablishthe abil-ity of isolatedmolecules toact as generalantioxidantsinvitro (Salveminietal.,2006;Guimarãesetal.,2010).Thus,citronellal whenclinicallytestedcouldformapromisingsourceofdrugfor treatingorofacialpaindisorders.
p-Cymene
Santanaetal.(2011)investigated theantinociceptive poten-tialofp-cymene,amonoterpenebiologicalprecursorofcarvacrol and one of themain constituentsof theessential oil obtained fromspeciesofProtium.p-Cymenesignificantlyreducedthe noci-ceptive behavior in the first and second phases of orofacial nociceptioninallmodelsinducedbyformalin,capsaicinand glu-tamate,suggestinganactioninbothneurogenicandinflammatory pain.Recently,Santanaetal.(2015)demonstratethatp-cymene anti-hyperalgesic shows an effect against carrageenan, TNF-␣, dopamineandPGE2tests.Theanti-hyperalgesiceffectseemsto berelatedtothecapacityofp-cymenetodecreasetotalleukocyte migration,TNF-␣levels,actinopioidreceptorsandincreased num-berofc-Fos-immunoreactiveneuronsinperiaqueductalgray(PAG) area.Quintansetal.(2013)suggestedthatp-cymenecouldhaveits analgesicandanti-inflammatoryeffectenhancedbycomplexation withcyclodextrinsprobablybyincreasingthebioavailabilityofthe terpenoid.
Citronellol
ofperipheralmediators,aswellastheactivation,directorindirect, ofCNSregions.
Carvacrol
Thismonoterpeneispredominantinmanyessentialoilsfrom thefamilyLamiaceae,includingtheOriganumandSaturejaspecies. Guimarãesetal.(2012)reportedthatacutetreatmentwith car-vacrolplaysaprotective roleinreducing behavioralpain when screenedfor formalin-, capsaicin-,and glutamate-induced orof-acialnociceptioninmice.Accordingtotheauthors,carvacrolseems toproduceanti-nociceptionintheorofacialformalin-, capsaicin-andglutamate-testsduetoitseffectsinthereductionofthe neu-ronalexcitabilityandvoltage-gatedNa+channel(NaV)inhibition inperipheralneurons(Jocaetal.,2015)ortoproduceanalgesicand anti-inflammationeffectsbythemodulationofcytokines,suchas IL-10,IL-1orTNF-␣(Guimarãesetal.,2014,2015).Additionally, thecarvacrol effects ontheCNSwere assessedthrough immu-nofluorescencefor Fosprotein. Furthermore,moleculardocking studieswereperformedtoevaluateintermolecularinteractionsof thecarvacrolandmuscimol,asligandsofIL-10andGABAA recep-tors,whichshowedtheneuromodulatorypropertiesofcarvacrol inthebrainareasthatcomprisethedescending-painpathway con-trol,suchasPAG,nucleusraphemagnus(NRM)andlocusceruleus (LC).Inaddition,carvacrolcomplexedin-cyclodextrin(-CD)had prolongedanalgesiceffectandactedondescending-paininhibitory pathwaywithoutshowingtoleranceandina smallerdosethan thecarvacrolisolatedastheywererenderedhighlybioavailable (Barretoetal.,2014;Guimarãesetal.,2014,2015).
Additionally, Lima et al. (2013a) evaluated the contribution of thecytokine modulation tothe anti-inflammatoryeffects of carvacrol in complete Freund’s adjuvant (CFA) model. Hence, theadministrationofthissubstanceproducedanti-inflammatory effectsandattenuatedthepawedemaandreducedtheIL-1and PGE2,butnotTNF-␣,locallevels.Thateffectsuggestedthat car-vacrolcausedanti-inflammatoryeffectsbyreducingtheproduction ofinflammatorymediators,suchasIL-1andprostanoids, possi-blythroughtheinductionoftheIL-10release.Banjietal.(2014) usedthesamemodel,CFA,asLimaetal.(2013a),butBanjietal. usedacombinationofcarvacrolwithmethotrexateobservingthat thisassociationenrichesthetherapeuticbenefitenhancingits anti-arthriticactionandminimizingitstoxicity.
Plantspecies,essentialoilsandextracts
SidacordifoliaL.,Malvaceae
ThisplantisanativespeciesofNortheasternBrazil,popularly knownas“malva-branca”.Pharmacologicalactivityoftheextracts and fractions from S. cordifolia in two animal models of orof-acialnociceptionhasbeenpreviouslyreported(Bonjardimetal., 2011).Administrationofethanolextract(EE),chloroform(CF)and methanol(MF)fractionsfromS.cordifoliaproducedareductionin thefacerubbingbehaviorinducedbyformalin.Alldosestested sig-nificantlyincreasedanti-nociceptionbothinthefirstandsecond phasecomparedwiththecontrol(vehicle).Morphine(MOR)was abletoreducenociceptivebehaviorinbothphases.TheeffectsofEE, CF,MFandMORwereantagonizedbynaloxone.Thetreatmentwith CFandMFsignificantlyreducedthenociceptivebehaviorinduced byglutamateinrelationtothecontrolgroup.Thus,itissuggested thattheconstituentsofthisfractioncouldinterfereinthe gluta-matergicsystem,throughtheactivationofNMDAreceptors,which wouldlimittheproductionofNOandotherinflammatory media-tors(Ribasetal.,2008).Henceforth,theantinociceptiveeffectofthe ethanolextractfromS.cordifoliaanditschloroformandmethanol fractionsisprobablyduetothepresenceofalkaloidsandflavones. Additionally,itseemsatleastinpartthatthisantinociceptiveaction
ofEE,CFandMFinvolvestheopioidandtheglutamatergicsystems (Bonjardimetal.,2011).
Hyptispectinata(L.)Poit.,Lamiaceae
Paixãoetal.(2013,2015)investigatedtheeffectofthe aque-ousextractfromH.pectinata(AEPH)ontheorofacialnociceptive modelsandits antioxidantpotential.Theresultsofthepresent studyshowedthatAEPHisactiveagainstneurogenicand inflam-matorypain sinceit inhibitsthemice face-rubbingnociceptive behavior in both phases. In addition, when the mechanism of AEPHactionwasinvestigatedusingnaloxone,itseffectwasnot reversed,suggestingthatitsanalgesicactivitydoesnotinvolve opi-oidreceptors.Bispo etal.(2001)foundthatnaloxone(5mg/kg, i.p.)markedlyreversedtheantinociceptiveeffectoftheaqueous extract(200mg/kg)andofmorphine(10mg/kg)inthehot-plate test.Inordertobetterassessthisaspect,Lisboaetal.(2006) eval-uatedthenon-selectiveopioidantagonistnaloxone(5mg/kg,i.p.), whichwasco-administratedwithchloroform(200mg/kg),ethyl acetate(100mg/kg)andhexaneextracts(100mg/kg),since nalox-onereversedtheantinociceptiveeffect.Thismechanismissimilar tothatfoundinthevolatileoilofH.pectinata,inwhichthe admin-istration(i.p.)of theopioid agonistnaxolone(5mg/kgbodywt) completelyreversedtheantinociceptiveeffectofthevolatileoils ofallgenotypesonthehot-platetest,suggestingthatopioid recep-torsareinvolvedinitsantinociceptiveaction(Arrigoni-Blanketal., 2008;Raymundoetal.,2011).Othermechanismsofvolatileoilof H.pectinatawerealsoinvestigated.l-NAMEsignificantlyreversed theeffectsoftheEOintheaceticacid-inducedcontortionsand hot-platemodelsand atropine completelyreversed in all mod-els.Besides,theEOinhibitedtheinflammatoryprocessinduced bysubcutaneouscarrageenaninjectionbyreducingcellmigration, exudatevolume,proteinconcentrationandinflammatory media-tors(nitricoxide,PGE2,IL-6,andTNF-␣)(Raymundoetal.,2011).
To further investigate the mechanism through which AEPH actsagainstorofacialpain,capsaicinwasused,and therewasa reductioninthecapsaicinnociceptiveeffectinadose-dependent manner,whichindicatesAEPHisactingasanantagonistof vanil-loidreceptors,possiblybyinhibitingtheinitialreleaseofsubstance Porboundingintoitsneurokinin1receptor.AEPHexhibited signif-icantantinociceptiveactivitywhentheglutamatemodelwasused. Therefore,itcanbesuggestedthatitinterfereswiththe glutamater-gicsystem.InvitrostudiesofthisstudyshowedthatAEPHhasan importantneurogenicandinflammatoryorofacialantinociceptive effect,withoutinterferenceinthemotorperformance(Paixãoetal., 2013).
Recently,theliteraturehasshownthattheanalgesicand anti-inflammatory effects of H.pectinata can be improvedwiththe incorporationintocomplexingsystems(ascyclodextrins)or vec-torizationofdrugs,whichcanbringbenefitstophysicochemical orpharmacologicalaspectsofpharmaceuticalpreparations con-tainingextractsoressentialoilsofmedicinalplants(Oliveiraetal., 2015;Britoetal.,2015;Paixãoetal.,2015;Menezesetal.,2015; Quintans-Júnioretal.,2016).
HyptisfruticosaSalzm.exBenth.,Lamiaceae
duetothevarietyofvolatilecompoundsfoundintheleavessuch as,␣-pinene,-pinene,1,8-cineoleandlimonene.
TheresultsofLimaetal.(2013b)showedthatthehydroethanol extractoftheH.fruticosaleaves(CHEE)inhibitedthenociception causedbyglutamatewithsignificantresultsforthehighest concen-trationused(200mg/kg),suggestingthattheextractcanbeactive ininhibitingthepainfulstimulusinducedbythisaminoacid.CHEE at100and200mg/kgwasabletoreducethecapsaicinnociceptive effect,suggestingthatvanilloidreceptorscanbeinvolvedinthe CHEEaction.
OcimumbasilicumL.,Lamiaceae,andlinalool
Venâncioetal.(2011)evaluatedearliertheeffectofO.basilicum. Lamiaceae.Theleafessential oilfromO.basilicum (LEO)andits majorchemicalconstituentlinalool(LIN)informalin-, glutamate-and capsaicin-induced orofacial nociception in mice and were investigatedforwhetherthesesubstancescouldalsointerferewith thehippocampalneuronalexcitability.Intheformalintest,there was a reduction in face rubbing after intraperitoneal injection of LEOor linalool(LIN). Every doseof linalooltested produced significantantinociceptive action in the first and second phase comparedtocontrolgroup.LEOmanifesteditseffectonlyinthe highdose, whilemorphinedecreased thepainbehavior inboth phases.Incapsaicintest,LINorLEOdiminishedthenociception evidencedbythesuppressionoftheface-rubbingbehavior.When assessedtoglutamatetest,LEOandLINproducedmarked analge-sia,but onlyinhigherdoses.Batistaetal.(2008)demonstrated that the antinociceptive effect of LIN may have a relationship withglutamatereceptors,namely␣ -amino-3-hydroxy-5-methyl-4-isoxasolepropionic acid (AMPA), NMDA and kainate. These resultsconfirmedthishypothesis,sincepretreatmentwithLIN sig-nificantlyprotectedagainsttheorofacialformalintest.Asimilar resultwasobtainedbyacuteadministrationofLEO.Itissuggestthat LEOandLINcanbeconsideredasnon-NMDAglutamateantagonists (AMPAorKainateblockers).Hence,thesebehavioralexperiments suggestthatmostoftheanalgesiceffectsofLEOcouldbeattributed toLIN,itsmajorcomponent.Thestimulationofthehylarregion of thedentategyrus(antidromic stimulation)generated a field potentialresponsein thegranularlayer,which is characterized byamajornegativecomponent(populationspike)followedbya smallpositivephase.Sincethisresponseisaconsequenceof acti-vationofvoltage-dependentsodiumchannelsintheaxonsofthe granularcells(Andersenetal.,1971),antagonistsofthesechannels wereexpectedtoblocktheresponse.Similareffectswereseenfor LEOandLIN,whichinhibitedthefieldpotentialsactivatedbythe antidromicstimulationofthehylus.
Acmellaoleracea(L.)R.K.Jansen,Asteraceae
Nomuraet al.(2013) examinedtheantinociceptive effectof theethanolicextractobtainedfromtheflowersofAcmella oler-acea (EEAO) and their results revealed that EEAO (10, 30 and 100mg/kg)reducedbothphasesoftheformalin-inducedorofacial nociception.Besides,theresultsdecreasedthepainin capsaicin-andcinnamaldehyde-inducedorofacialnociception.Furthermore, thestudyalsosuggestedthattheanalgesiceffectofEEAOonthe orofacialnociceptionmediatedbycapsaicinandcinammaldehyde couldberelatedtoTRPV1andTRPA1receptormodulationand/or blockade.
Syzygiumcumini(L.)Skeels,Myrtaceae
Quintansetal.(2014a)evaluatedtheantinociceptiveeffectof theethanolextract(EE)fromS.cuminileavesonorofacial nocicep-tionthatproducedsignificantanti-nociceptionintheinflammatory phase of theformalin and glutamate test. Whenglibenclamide andl-NOARGwereadministrated,theantinociceptioncausedby EEwasreversed. However,pretreatmentwithnaloxonedidnot
change theantinociceptive action. Muruganandanet al. (2001) investigatedtheethanolicextractofthebarkofS.cuminiforits anti-inflammatoryactivityandtheirresultsshowthatthisextract hasapowerfulanti-inflammatoryactionagainstdistinctphases of inflammationwithoutanyside effectonthegastricmucosa. Theanti-inflammatoryactivityofthis specieswaseven studied byMachadoetal.(2013),whoinvestigatedtheanti-inflammatory andapoptoticactivityoftheessentialoilofS.cuminishowing rel-evantanti-inflammatoryactivityinvivo,possiblyduetothehigh percentageof-caryophylleneidentified.
LippiagrataSchauer,Verbenaceae
Siqueira-Limaetal.(2014)investigatedthepossible antinoci-ceptiveactivityof-cyclodextrincomplexcontainingLippiagrata leafessentialoil(-CD/EO) inanimalmodelsespeciallyin orof-acial pain.The resultsdemonstrated that -CD/EO wascapable ofreducingthenociceptiveface-rubbingbehaviorinbothphases oftheformalin,glutamateandcapsaicininduction,possiblydue totherelationshipwithvanilloid,opioidandcannabinoid recep-tors.-CD/EOappearstobeveryeffectiveinactivateddescending pain-inhibitory mechanisms without untoward effects already described for morphine-like drugs (Siqueira-Lima et al., 2014, 2016).Additionally,this studywasthefirsttoshowthe chemi-calandpharmacologicalbenefitscausedbythecomplexationin cyclodextrins,which seemstobean advantageousapproach to essentialoilsandnon-polarcompounds(Oliveiraetal.,2015;Brito et al.,2015).The cyclodextrins(CD)uses toenhance biological andchemicalcharacteristicsofterpenesandrelatedcompounds, such asessential oils,have beenwidely explored in the study ofnewdrugstotreatpain,inflammationandcancerbecauseCD maypromoteincreasedbioavailabilityandpharmacological effi-cacy(Siqueira-Limaetal.,2016).
CombretumduarteanumCambess.,Combretaceae
Quintansetal.(2014b)demonstratedthatthehexanicextract andfriedelinmainbeacompoundfromtheCombretumduartenum -reducednociceptionofformalin(inbothphases).Glutamateand capsaicin-induced orofacialnociceptiontests suggest a possible interactionwiththeglutamatergicsystemandapossibleinhibition ofthesubstancePreleaseorduetoadirectblockofits neurokinin-1receptor(NK-1).Theseresultsaresupportedbypreviousfindings ofAntonisamyetal.(2011)regardingfriedelin,whoattributedthe analgesicprofileduetothecentralandperipheraleffects.However, noneofthestudiesprovidemolecularevaluationsasfriedelincan actbycentralandperipheralways.
Anadenantheracolubrina(Vell.)Brenan,Fabaceae
A.colubrina,popularlyknownas‘angico-branco’,isnativetothe BrazilianCaatingabiomeandusedbythepeopletotreatrespiratory conditions(asthma,bronchitis,coughandflu),asaskin-healing agent,analgesicandagainstinflammatoryprocesses(Damascena et al., 2014)due to its biological properties, Damascena et al. (2014)soughttoassesswhethertheA.colubrinastembarkaqueous extractcaninhibitorofacialpaininducedbychemicalagents (for-malin,capsaicinandglutamate)andinvestigatedtheantioxidant potential.Thus,theA.colubrinastembarkaqueousextractreduced orofacialpaininallanimalmodelsassessedandthiseffectappears tobeassociatedwithitsredox-activeradicalscavengingactivities.
HypericumperforatumL.Hypericaceae,ValerianaofficinalisL., Caprifoliceae,andPipermethysticumG.Forst.,Piperaceae
hepatic,hematologicandbiochemicalalterationsinducedby reg-ularadministrationoftheseextracts.Theauthorsfoundthatthe compositionofthesethreemedicinalplantsproducedsignificant analgesicprofileinformalin-inducedorofacialpaininrodents,and alsoproducedanoutstandinghepatoprotectiveeffect.
Animalmodelsoforofacialpain
Theimportanceinthefieldof orofacialpain assessmentcan besuggestedbythesharpincreaseinpublicationsovertherecent decades(Hargreaves,2011).Thisfacthasalsobeenaccompaniedby theincreasednumberofstudiesusingorofacialpainanimal mod-els,whichhavesoughttobetterunderstandthepathophysiology oforofacialpainandtoassessnewdrugstotreatit(Raboissonand Dallel,2004).Therearerelativelyfewbehavioralmodelsin labora-toryanimalsdedicatedtothestudyofnociceptioninthetrigeminal region(RaboissonandDallel,2004).Thus,theanimalmodelsof orofacialpainhadsoughttomimicsymptomsorthediseaseperse (KhanandHargreaves,2010).However,theorofacialpainis mul-tifactorialandtoocomplexfor asinglemodeltoincorporateall thenuances,sothereisanincreasingnumberofdifferenttypesof animalmodels.
Asinoursurveymoststudieshavesoughtaccesstoorofacial painbychemicalstimuli,wemadehereabriefcommentonthe orofacialpainanimalmodelsinducedbychemicalstimuli,suchas formalin,capsaicinandglutamate(animalmodelsarebasedonthe inductionofpainbyadministrationofalgogenintothevibrissal padofrodents).
AsdescribedbyHargreaves(2011),thistypeofanimalmodel canbringlimitationsfortheanalysisoftheresultsbecausethe orof-acialpainconditionisnotderivedfromauniquetargettissue,but fromavarietyofsensitivetissuessuchasthemeninges,cornea, toothpulp,oral/nasalmucosaandtemporomandibularjoint.Thus, thechoiceoftheappropriatemethodforinvestigatingthe phys-iologicalorpharmacologicaleventisextremelyimportantinthe studydesignbytheresearcher.
Fortheformalintest,probablythebestestablishedandmost reliableexperimentalprotocoltoassessquicklyorofacialpain con-dition(itisknowntobethesubcutaneous,s.c.,injectionofdilute formalin),whichcausesindeedtissueinjuriesandgenerates behav-ioralaswellaselectrophysiologicalresponsesthatlastfromseveral minutesuptomorethan1h(RaboissonandDallel,2004). Simi-larly,asithappenswiththeadministrationofformalinintothe rodentspaw,theinjectionofformalininthevibrissapad(intothe upperlip)producesabiphasicpainstage(Luccarinietal.,2006).It isamodelthatevaluatesdrugsthatactcentrallyorbyperipheral mechanismsmediatedbylocalinflammationinducedbyformalin (RaboissonandDallel,2004;HunskaarandHole,1987).However,it isnotthebestanimalmodeltoevaluatepainfuloranalgesicroutes. Recently,twootheranimalmodelshavebeenwidelyusedto assessdrugswithpotentialanalgesicprofiletoorofacialpain: cap-saicinandglutamate-inducedorofacialpaintests.Theapplication ofcapsaicin,theprototypicaltransientreceptorpotentialvanilloid 1(TRPV1)agonist,evokesneuropeptidereleaseandinduces pri-maryandsecondaryhyperalgesia(KhanandHargreaves,2010).The administrationofcapsaicinintothevibrissapadofmiceinduces significantnociceptivebehaviorsexpressedasheadflinchingand rubbingoftheorofacialtissuesduringatleast1h(Holanda-Pinto et al.,2008).On the otherhand, glutamate is one of themost important excitatory neurotransmitters in the production and managementofpain(Mogil,2009).Moreover,glutamateispresent inbothcentralandperipheralterminalsoftrigeminalanddorsal rootganglionneurons(KeastandStephensen,2000).Accordingto Quintans-Júnioretal.(2010),theinjectionofglutamate(volume of20l)intotherightupperlip(perinasalarea),usinga27-gauge needle,causedapersistentnociceptivebehaviorcharacterizedby
strongrubbingoftheorofacialfaceandheadflinching.Therefore, drugsthatinhibitthesebehaviors,afteradministrationofcapsaicin orglutamate,arepotentiallyusefulasanalgesics(Quintans-Júnior etal.,2010).
Nowwebrieflymentionthemostcommonanimalmodelsto assessorofacialpaininducedbychemicalstimuli.More informa-tionaboutanimalmodelstostudyorofacialpaincanbeassessedin theexcellencereviewspublishedbyRaboissonandDallel(2004), Mogil(2009),KhanandHargreaves(2010)andHargreaves(2011).
Conclusion
Thissystematicreview suggeststhatthenaturalcompounds haveapotentialforthetreatmentofpainconditionsinorofacial region,hopingtodiscovernewbiologicallyactivesubstancesthat mayofferanewpossibilityforthemostappropriatetreatmentof orofacialpain,sincethereisnopharmacologicaltreatmentswhich mayresultinclinicalimprovementwithoutsignificantsideeffects inthesepathologicalconditions.Paradoxically,thestudiesfound inoursurveyareverylead-off(basicallyscreenings),whicharenot directlyrelatedtothepossibleclinicalapplicability.Therefore,it couldbebelievedthatpharmacokineticstudies,incorporationinto modernpharmaceuticalformulationsandbiotechnological prod-uctdevelopmentseemtobeadistanthorizonineventheselected studies.Thus,ouropinionisthattheevidencefoundinthestudies showedapossibleuseoftheseNPinthemanagementoforofacial painconditions.However,newstudiesareneededtoprovetheir underlyingmechanismsandeffectsonthemolecularlevelandin clinicalresearchinanearfuture.
Authors’contributions
PSSL,JCS,JSSQ,RSSBandJRGSAcontributedwithboththesurvey ofthearticlesandthepreparationofthemanuscript.SS,MRVS,LRB, JSSQ,ARA,IRAMandLJQJparticipatedinthedrafting,correction andwiththeirexpertiseinthediscussionandfinalizationofthe manuscript.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgements
ThisworkwassupportedbygrantsfromFAPITEC-SE, CAPES andCNPq(allfromBrazil).WethankteacherAbilioBorghiforthe grammarreviewofthemanuscript.
References
Andersen,P.,Bliss,T.V.,Skrede,K.K.,1971.Unitanalysisofhippocampalpopulation spikes.Exp.BrainRes.13,208–221.
Antonisamy,P.,Duraipandiyan,V.,Ignacimuthu,S.,2011.Anti-inflammatory, anal-gesicandantipyreticeffectsoffriedelinisolatedfromAzimatetracanthaLam.in mouseandratmodels.J.Pharm.Pharmacol.63,1070–1077.
Arrigoni-Blank,M.F.,Antoniolli,A.R.,Caetano,L.C.,Campos,D.A.,Blank,A.F.,Alves, P.B.,2008.AntinociceptiveactivityofthevolatileoilsofHyptispectinataL.Poit. (Lamiaceae)genotypes.Phytomedicine15,334–339.
Banafshe,H.R., Hamidi, G.A.,Noureddini, M., Mirhashemi,S.M., Mokhtari,R., Shoferpour,M.,2014.Effectofcurcuminondiabeticperipheralneuropathic pain:possibleinvolvementofopioidsystem.Eur.J.Pharmacol.723,202–206. Banerjee,M.,Tripathi,L.M.,Srivastava,V.M.,Puri,A.,Shukla,R.,2003.Modulationof
inflammatorymediatorsbyibuprofenandcurcumintreatmentduringchronic inflammationinrat.Immunopharmacol.Immunotoxicol.25,213–224. Banji,O.J.,Banji,D.,Soumya,N.,Chilipi,K.K.,Kalpana,C.H.,KranthiKumar,C.H.,
Annamalai,A.R.,2014.Combinationofcarvacrolwithmethotrexatesuppresses CompleteFreund’sAdjuvantinducedsynovialinflammationwithreduced hep-atotoxicityinrats.Eur.J.Pharmacol.723,91–98.
producesantinociceptiveeffectsuperiortothatofcarvacrolinorofacialpain models.FASEBJ.28(1),Supplement(657.15).
Batista,P.A.,Werner,M.F.P.,Oliveira,E.C.,Burgos,L.,Pereira,P.,Brum,L.F.,Santos, A.R.,2008.Evidencefortheinvolvementofionotropicglutamatergic recep-torsontheanti-nociceptiveeffectof(−)-linaloolinmice.Neurosci.Lett.440, 299–303.
Beirith,A.,Santos,A.R.S.,Calixto,J.B.,2002.Mechanismsunderlyingthenociception andpawoedemacausedbyinjectionofglutamateintothemousepaw.Brain Res.924,219–228.
Belvisi,M.G.,Chung,D.M.,Barnes,P.J.,1998.Opioidmodulationofnon-cholinergic neuralbronchoconstrictioninguinea-piginvivo.Br.J.Pharmacol.95,413–418. Bispo,M.D.,Mourão,R.H.,Franzotti,E.M.,Bomfim,K.B.,Arrigoni-Blank,M.F.,Moreno, M.P.,Marchioro,M.,Antoniolli,A.R.,2001.Antinociceptiveand antiedemato-geniceffectsoftheaqueousextractofHyptispectinataleavesinexperimental animals.J.Ethnopharmacol.76,81–86.
Bonjardim, L.R., Silva, A.M., Oliveira, M.G.B.,Guimarães,A.G., Antoniolli,A.R., Santana,M.F.,Serafini,M.R.,Santos,R.C.,Araújo,A.A.,Estevam,C.S.,Santos,M.R., Lyra,A.,Carvalho,R.,Quintans-Júnior,L.J.,Azevedo,E.G.,Botelho,M.A.,2011. Sidacordifolialeafextractreducestheorofacialnociceptiveresponseinmice. Phytother.Res.25,1236–1241.
Braz-Filho,R.,2010.Contribuic¸ãodafitoquímicaparaodesenvolvimentodeumpaís emergente.Quim.Nov.33,229–239.
Brito,R.G.,Araújo,A.A.S.,Quintans,J.S.S.,Sluka,K.A.,Quintans-Júnior,L.J.,2015. Enhancedanalgesicactivitybycyclodextrins–asystematicreviewand meta-analysis.ExpertOpin.DrugDeliv.12,1677–1688.
Brito,R.G.,Santos,P.L.,Prado,D.S.,Santana,M.T.,Araújo,A.A.,Bonjardim,L.R.,Santos, M.R.,deLuccaJúnior,W.,Oliveira,A.P.,Quintans-Júnior,L.J.,2013. Citronel-lolreducesorofacialnociceptivebehaviourinmice–evidenceofinvolvement ofretrosplenialcortexandperiaqueductalgreyareas.BasicClin.Pharmacol. Toxicol.112,215–221.
Bugni,T.S.,Andjelic,C.D.,Pole,A.R.,Rai,P.,Ireland,C.M.,Barrows,L.R.,2009. Biologi-callyactivecomponentsofaPapuaNewGuineaanalgesicandanti-inflammatory lichenpreparation.Fitoterapia80,270–273.
Butler,D.,2008.Translationalresearch:crossingthevalleyofdeath.Nature453, 840–852.
Cechinel-Filho,V.,Yunes,R.A.,1998.Estratégiasparaaobtenc¸ãodecompostos farmacologicamenteativosapartirde plantasmedicinais.Conceitos sobre modificac¸ãoestruturalparaotimizac¸ãodaatividade.Quim.Nov.21,99–105. Clavelou,P.,Pajot,J.,Dallel,R.,Raboisson,P.,1989.Applicationoftheformalintest
tothestudyoforofacialpainintherat.Neurosci.Lett.103,349–353. Damascena,N.P.,Souza,M.T.,Almeida,A.F.,Cunha,R.S.,Damascena,N.P.,Curvello,
R.L.,Lima,A.C.,Almeida,E.C.,Santos,C.C.,Dias,A.S.,Paixão,M.S.,Souza,L.M., QuintansJúnior,L.J.,Estevam,C.S.,Araujo,B.S.,2014.Antioxidantandorofacial anti-nociceptiveactivitiesofthestembarkaqueousextractofAnadenanthera colubrina(Velloso)Brenan(Fabaceae).Nat.Prod.Res.28,753–756.
DeSouza, D.P.,Gonc¸alves,J.C.,Quintans-Júnior,L.J.,Cruz, J.S.,Araújo,D.A.,de Almeida,R.N.,2006.Studyofanticonvulsanteffectofcitronellol,amonoterpene alcohol,inrodents.Neurosci.Lett.401,231–235.
Fan,W.,Huang,F.,Wua,Z.,Zhu,X.,Li,D.,He,H.,2012.Theroleofnitricoxidein orofacialpain.NitricOxide26,32–37.
Ferreira,J., Santos,A.R.S.,Calixto, J.B.,1999. Therole ofsystemic,spinaland supraspinal l-argininenitric oxide-cGMP pathway in thermal hyperalgesia causedbyintrathecalinjectionofglutamateinmice.Neuropharmacology38, 835–842.
Ferreira,V.F.,Pinto,A.C.,2010.Afitoterapianomundoatual.Quim.Nov.33,1829. Fitch,R.W.,Spande,T.F.,Garraffo,H.M.,Yeh,H.J.C.,Daly,J.W.,2010.Phantasmidine:
anepibatidinecongenerfromtheEcuadorianpoisonfrogEpipedobatesanthonyi. J.Nat.Prod.73,331–337.
Franco,C.R.P.,Alves,P.B.,Andrade,D.M.,Jesus,H.C.R.,Silva,E.J.S.,Santos,E.A.B., Anto-niolli,A.R.,Quintans-Júnior,L.J.,2011.Essentialoilcompositionandvariability inHyptisfruticosaSalzm.exBenth.,Lamiaceae.Rev.Bras.Farmacogn.21,24–32. Gilbert,S.D.,Clark,T.M.,Flores,C.M.,2001.Antihyperalgesicactivityofepibatidine
intheformalinmodeloffacialpain.Pain89,159–165.
Giulietti,A.M.,Harley,R.M.,Queiroz,L.P.,Wanderley,M.G.L.,Berg,C.V.D.,2005. BiodiversityandconservationofplantsinBrazil.Conserv.Biol.19,632–639. Goel,A.,Boland,C.R.,Chauhan,D.P.,2001.Specificinhibitionofcyclooxygenase-2
(COX-2)expressionbydietarycurcumininHT-29humancoloncancercells. CancerLett.172,111–118.
Gonc¸alves,J.C.R.,Oliveira,F.S.,Benedito,R.B.,Sousa,D.P.,Almeida,R.N.,Araújo, D.A.,2008.Antinociceptiveactivityof(−)-carvone:evidenceofassociationwith decreasedperipheralnerveexcitability.Biol.Pharm.Bull.31,1017–1020. Guimarães,A.G.,Oliveira,M.A.,Alves,R.S.,Menezes,P.P.,Serafini,M.R.,Araújo,A.A.,
Bezerra,D.P.,QuintansJúnior,L.J.,2015.Encapsulationofcarvacrol,a monoter-penepresentintheessentialoiloforegano,with-cyclodextrin,improvesthe pharmacologicalresponseoncancerpainexperimentalprotocols.Chem.Biol. Interact.227,69–76.
Guimarães,A.G.,Scotti,L.,Scotti,M.T.,Mendonc¸aJúnior,F.J.,Melo,N.S.,Alves,R.S.,De LuccaJúnior,W.,Bezerra,D.P.,Gelain,D.P.,QuintansJúnior,L.J.,2014.Evidence fortheinvolvementofdescendingpain-inhibitorymechanismsinthe attenu-ationofcancerpainbycarvacrolaidedthroughadockingstudy.LifeSci.116, 8–15.
Guimarães,A.G.,Silva,F.V.,Xavier,M.A.,Santos,M.R.,Oliveira,R.C.,Oliveira,M.G., Oliveira,A.P.,DeSouza,C.C.,Quintans-Júnior,L.J.,2012.Orofacialanalgesic-like activityofcarvacrolinrodents.Z.Naturforsch.C67C,481–485.
Guimarães,A.G.,Oliveira,G.F.,Melo,M.S.,Cavalcanti,S.C.,Antoniolli,A.R., Bon-jardim,L.R.,Silva,F.A.,Santos,J.P.,Rocha,R.F.,Moreira,J.C.,Araújo,A.A.,Gelain,
D.P.,Quintans-Júnior, L.J.,2010. Bioassay-guidedevaluation of antioxidant andantinociceptiveactivitiesofcarvacrol.BasicClin.Pharmacol.Toxicol.107, 949–957.
Hargreaves,K.M.,2011.Orofacialpain.Pain152,S25–S32.
Henry,J.L.,Yashpal,K.,Pitcher,G.M.,Chabot,J.,Coderre,T.J.,1999.Anevidencefor tonicactivationofNK-1receptorsduringthesecondphaseoftheformalintest intherat.J.Neurosci.19,6588–6598.
Holanda-Pinto,S.A.,Pinto,L.M.S.,Guedes,M.A.,Cunha,G.M.,Chaves,M.H., San-tos,F.A.,Rao,V.S.,2008.Antinociceptiveeffectoftriterpenoid␣,-amyrinin ratsonorofacialpaininducedbyformalinandcapsaicin.Phytomedicine15, 630–634.
Hunskaar,S.,Hole,K.,1987.Theformalintestinmice:dissociationbetween inflam-matoryandnoninflammatorypain.Pain30,103–114.
Jacob,A.,Wu,R.,Zhou,M.,Wang,P.,2007.Mechanismoftheanti-inflammatory effectofcurcumin:PPAR-␥ activation.PPARRes., 89369,http://dx.doi.org/ 10.1155/2007/89369.
Joca,H.C.,Vieira,D.C.,Vasconcelos,A.P.,Araújo,D.A.,Cruz,J.S.,2015.Carvacrol mod-ulatesvoltage-gatedsodiumchannelskineticsindorsalrootganglia.Eur.J. Pharmacol.756,22–29.
Keast,J.R.,Stephensen,T.M.,2000.Glutamateandaspartateimmunorreactivityin dorsalrootganglioncellssupplyingvisceralandsomatictargetsandevidence forperipheralaxonaltransport.J.Comp.Neurol.424,577–587.
Khan,A.,Hargreaves,K.M.,2010.Animalmodelsoforofacialpain.MethodsMol.Biol. 617,93–104.
Kim,H.Y.,Park,E.J.,Joe,E.H.,Jou,I.,2003.CurcuminsuppressesJanuskinase-STAT inflammatory signaling through activation of Src homology 2 domain-containing tyrosine phosphatase 2 in brain microglia. J. Immunol. 171, 6072–6079.
Krzyzanowska,A.,Avenda ˜no,C.,2012.Behavioraltestinginrodentmodelsof orof-acialneuropathicandinflammatorypain.BrainBehav.2,678–697.
Kumar, K.C., Muller,K.,1999. Lichenmetabolites. 1. Inhibitoryaction against leukotrieneB4 biosynthesisby a non-redox mechanism. J. Nat.Prod. 62, 817–820.
Kumari,A.,Kumar,V.,Yadav,S.K.,2012.Nanotechnology:atooltoenhancevalues ofnaturalproducts.TrendsMed.Res.7,24–42.
Kunchandy,E.,Rao,M.N.A.,1990.Oxygenradicalscavengingactivityofcurcumin. Int.J.Pharm.58,237–240.
Lima,M.S.,Quintans-Júnior,L.J.,deSantana,W.A.,MartinsKaneto,C.,PereiraSoares, M.B.,Villarreal,C.F.,2013a.Anti-inflammatoryeffectsofcarvacrol:evidencefor akeyroleofinterleukin-10.Eur.J.Pharmacol.699,112–117.
Lima,A.C.,Paixão,M.S.,Melo,M.,deSantana,M.T.,Damascena,N.P.,Dias,A.S.,Porto, Y.C.,Fernandes,X.A.,Santos,C.C.,Lima,C.A.,QuintansJúnior,L.J.,Estevam,C.S., Araújo,B.S.,2013b.Orofacialantinociceptiveeffectandantioxidantpropertiesof thehydroethanolextractofHyptisfruticosasalmzexBenth.J.Ethnopharmacol. 146,192–197.
Lima-Júnior,R.C.P.,Sousa,D.I.M.,Brito,G.A.C.,Cunha,G.M.,Chaves,M.H.,Rao,V.S., Santos,F.A.,2007.Modulationofacutevisceralnociceptionandbladder inflam-mationbyplanttriterpene,␣,-amyrininamousemodelofcystitis:roleof tachykininNK1-receptors,andK+ATPchannels.Inflamm.Res.56,1–8. Lisboa,A.C.,Mello,I.C.,Nunes,R.S.,DosSantos,M.A.,Antoniolli,A.R.,Marc¸al,R.M.,
Cavalcanti,S.C.,2006.AntinociceptiveeffectofHyptispectinataleavesextracts. Fitoterapia77,439–442.
Luccarini,P.,Childeric,A.,Gaydier,A.,Voisin,D.,Dallel,R.,2006.Theorofacial formalintestinthemouse:abehavioralmodelforstudyingphysiologyand modulationoftrigeminalnociception.J.Pain7,908–914.
Luccarini,P.,Henry,M.,Alvarez,P.,Gaydier,A.M.,Dallel,R.,2003.Contribution ofneurokinin1receptorsinthecutaneousorofacialcutaneouspain.Naunyn SchmiedebergsArch.Pharmacol.368,320–323.
Machado,R.R.P.,Jardim,D.F.,Souza,A.R.,Scio,E.,Fabri,R.L.,Carpanez,A.G.,Grazul, R.M.,Mendonc¸a,J.P.R.F.,Lesche,B.,Aarestrup,F.M.,2013.Theeffectofessential oilofSyzygiumcuminionthedevelopmentofgranulomatousinflammationin mice.Rev.Bras.Farmacogn.23,488–496.
Maciel,M.A.M.,Pinto,A.C.,Veiga-Júnior,V.F.,Grynberg,N.F.,Echevarria,A.,2002. Plantasmedicinais:anecessidadedeestudosmultidisciplinares.Quim.Nov.25, 429–438.
Melo,M.G.D.,Araujo,A.A.S.,Rocha,C.P.L.,Almeida,E.M.,Siqueira,R.S.,Bonjardim, L.R.,Quintans-Junior,L.J.,2008.Purification,physicochemicalproperties, ther-malanalysisandantinociceptiveeffectofatranorinextractedfromCladinakalbii. Biol.Pharm.Bull.31,1977–1980.
Menezes,I.A.C.,Marques,M.S.,Santos,T.C.,Dias,K.S.,Silva,A.B.L.,Mello,I.C.M., Lisboa,A.C.C.D.,Alves,P.B.,Cavalcanti,S.C.H.,Marc¸al, R.M.,Antoniolli,A.R., 2007.AntinociceptiveeffectandacutetoxicityoftheessentialoilofHyptis fruticosainmice.Fitoterapia78,192–195.
Menezes,P.P.,Araujo,A.A.,Doria,G.A.,Quintans-Junior,L.J.,deOliveira,M.G.,dos Santos,M.R.,deOliveira,J.F.,Matos,J.R.,Carvalho,F.M.,Alves,P.B.,deMatos,I.L., dosSantos,D.A.,Marreto,R.N.,daSilva,G.F.,Serafini,M.R.,2015. Physicochem-icalcharacterizationandanalgesiceffectofinclusioncomplexesofessentialoil fromHyptispectinataL.Poitleaveswith-cyclodextrin.Curr.Pharm.Biotechnol. 16,440–450.
Miranda,H.F.,Sierralta,F.,Prieto,J.C.,2009.SynergismbetweenNSAIDsinthe orof-acialformalintestinmice.Pharmacol.Biochem.Behav.92,314–318. Mittal,N.,Joshi,R.,Hota,D.,Chakrabarti,A.,2009.Evaluationofantihyperalgesic
effectofcurcuminonformalin-inducedorofacialpaininrat.Phytother.Res.23, 507–512.
Muruganandan,S.,Srinivasan,K.,Chandra,S.,Tandan,S.K.,Lal,J.,Raviprakash, V.,2001.Anti-inflammatoryactivityofSyzygiumcuminibark.Fitoterapia72, 369–375.
Nomura,E.C.O.,Rodrigues,M.R.A.,Silva,C.F.,Hamm,L.A.,Nascimento,A.M.,deSouza, L.M.,Cipriani,T.R.,Baggio,C.H.,Werner,M.F.,2013.Antinociceptiveeffectsof ethanolicextractfromtheflowersofAcmellaoleracea(L.)R.K.Janseninmice.J. Ethnopharmacol.150,583–589.
Nowacki,L.C.,Worfel,P.R.,Martins,P.F.A.,Santos,R.S.,Stechman-Neto,J.,Souza, W.M.,2015.AnalgesiceffectofHypericumperforatum,Valerianaofficinalisand
Pipermethysticumfororofacialpain.Braz.J.OralSci.14,60–65.
Oliveira,M.G.,Guimarães,A.G.,Araújo,A.A.S.,Quintans,J.S.,Santos,M.R., Quintans-Júnior,L.J.,2015.Cyclodextrins:improvingthetherapeuticresponseofanalgesic drugs:apatentreview.ExpertOpin.Ther.Patents25,897–907.
Oliveira,S.G.,deMoura,F.R.,Demarco,F.F.,Nascente,P.S.,Pino,F.A.,Lund,R.G.,2012. Anethnomedicinalsurveyonphytotherapywithprofessionalsandpatients fromBasicCareUnitsintheBrazilianUnifiedHealthSystem.J.Ethnopharmacol. 140,428–437.
Pace,M.C.,Mazzariello,L.,Passavanti,M.B.,Sansone,P.,Barbarisi,M.,Aurilio,C., 2006.Neurobiologyofpain.J.CellPhysiol.209,8–12.
Paixão,M.S.,Melo,M.S.,Damascena,N.P.,Araújo,A.A.S.,Soares,A.F.,Oliveira,D.V.A., Oliveira,J.S.,Almeida,F.T.C.,Amaral,F.S.,Araújo,B.S.,Estevam,C.S.,Botelho, M.A.,Quintans-Júnior,L.J.,2015.Hyptispectinatagelpreventsalveolarbone resorptioninexperimental periodontitisinrats.Rev.Bras.Farmacogn.25, 35–41.
Paixão,M.S.,Melo,M.S.,Oliveira,M.G.B.,Santana,M.T.,Lima,A.C.,Damascena,N.P., Dias,A.S.,Araujo,B.S.,Estevam,C.S.,Botelho,M.A.,Quintans-Junior,L.J.,2013.
Hyptispectinata:redoxprotectionandorofacialantinociception.Phytother.Res. 27,1328–1333.
Peixoto,A.L.,Morim,M.P.,2003.Colec¸õesbotânicas:documentac¸ãoda biodiversi-dadebrasileira.Cien.Cult.55,21–24.
Pelissier,T.,Pajot,J.,Dallel,R.,2002.Theorofacialcapsaicintestinrats:effectsof differentcapsaicinconcentrationsandmorphine.Pain96,81–87.
Quintans-Júnior,L.J.,Araújo,A.A.S.,Brito,R.G.,Santos,P.L.,Quintans,J.S.S.,Menezes, P.P.,Serafini,M.R.V.,Silva,G.F.,Carvalho,F.M.,Brogden,N.K.,Sluka,K.A.,2016. -Caryophyllene,adietarycannabinoid,complexedwith-cyclodextrinproduced anti-hyperalgesiceffectinvolvingtheinhibitionofFosexpressioninsuperficial dorsalhorn.LifeSci.149,34–41.
Quintans,J.S.S.,Brito,R.G.,Aquino,P.G.V.,Franc¸a,P.H.,Siqueira-Lima,P.S.,Santana, A.E.,Ribeiro,E.A.,Salvador,M.J.,Araújo-Júnior,J.X.,Quintans-Júnior,L.J.,2014a. AntinociceptiveactivityofSyzygiumcuminileavesethanolextractonorofacial nociceptionprotocolsinrodents.Pharm.Biol.52,762–766.
Quintans,J.S.S.,Costa,E.V.,Tavares,J.F.,Souza,T.T.,Araújo,S.S.,Estevam,C.S.,Barison, A.,Cabral,A.G.S.,Silva,M.S.,Serafini,M.R.,Quintans-Júnior,L.J.,2014b. Phy-tochemicalstudyandantinociceptiveeffectofthehexanicextractofleaves fromCombretumduarteanum andfriedelin, atriterpene isolatedfrom the hexanicextract,inorofacialnociceptiveprotocols.Rev.Bras.Farmacogn.24, 60–66.
Quintans,J.S.S.,Menezes,P.P.,Santos,M.R.,Bonjardim,L.R.,Almeida,J.R.,Gelain, D.P., Araújo, A.A., Quintans-Júnior, L.J., 2013. Improvement of p-cymene antinociceptiveandanti-inflammatoryeffectsbyinclusionin-cyclodextrin. Phytomedicine20,436–440.
Quintans-Júnior,L.J.,DaRocha,R.F.,Caregnato,F.F.,Moreira,J.C.,daSilva,F.A.,Araújo, A.A.,dosSantos,J.P.,Melo,M.S.,deSousa,D.P.,Bonjardim,L.R.,Gelain,D.P., 2011.Antinociceptiveactionandredoxpropertiesofcitronellal,anessential oilpresentinLemongrass.J.Med.Food14,630–639.
Quintans-Júnior,L.J.,Melo,M.S.,DeSousa,D.P.,Araujo,A.A.,Onofre,A.C.,Gelain,D.P., Gonc¸alves,J.C.,Araújo,D.A.,Almeida,J.R.G.S.,Bonjardim,L.R.,2010. Antinocicep-tiveeffectsofcitronellalinformalin-,capsaicin,andglutamate-inducedorofacial nociceptioninrodentsanditsactiononnerveexcitability.J.Orofac.Pain24, 305–312.
Raboisson,P.,Dallel,R.,2004.Theorofacialformalintest.Neurosci.Biobehav.Rev. 28,219–226.
Raymundo,L.J.,Guilhon,C.C.,Alviano,D.S.,Matheus,M.E.,Antoniolli,A.R., Caval-canti,S.C.,Alves,P.B.,Alviano,C.S.,Fernandes,P.D.,2011.Characterisationofthe anti-inflammatoryandantinociceptiveactivitiesoftheHyptispectinata(L.)Poit essentialoil.J.Ethnopharmacol.134,725–732.
Ren,K.,Dubner,R.,1999.Inflammatorymodelsofpainandhyperalgesia.ILARJ.40, 111–118.
Ribas,C.M.,Meotti,F.C.,Nascimento,F.P.,Jacques,A.V.,Dafre,A.L.,Rodrigues,A.L., Farina,M.,Soldi,C.,Mendes,B.G.,Pizzolatti,M.G.,Santos,A.R.,2008. Antinoci-ceptiveeffectofthePolygalasabulosahydroalcoholicextractinmice.BasicClin. Pharmacol.Toxicol.103,43–47.
Salvemini,D.,Doyle,T.M.,Cuzzocrea,S.,2006.Superoxide,peroxynitriteand oxida-tive/nitrativestressininflammation.Biochem.Soc.Trans.34,965–970. Santana,M.F.,Quintans-Júnior,L.J.,Cavalcanti,S.C.H.,Oliveira,M.G.B.,Guimarães,
A.G.,Cunha,E.S.,etal.,2011.p-cymenereducesorofacialnociceptiveresponse inmice.RevistaBrasileriaFarmacogen21,1138–1143.
Santana,M.F.,Guimarães,A.G.,Chaves,D.O.,Silva,J.C.,Bonjardim,L.R.,deLucca Júnior,W.,Ferro,J.N.,Barreto,E.O.,dosSantos,F.E.,Soares,M.B.,Villarreal, C.F.,Quintans,J.S.,Quintans-Júnior,L.J.,2015.Theanti-hyperalgesicand anti-inflammatoryprofilesofp-cymene:evidencefortheinvolvementofopioid systemandcytokines.Pharm.Biol.53,1583–1590.
Sharma,S.,Chopra,K.,Kulkarni,S.K.,2007.Effectofinsulinanditscombinationwith resveratrolorcurcumininattenuationofdiabeticneuropathicpain: participa-tionofnitricoxideandTNF-alpha.Phytother.Res.21,278–283.
Sharma,S.,Kulkarni,S.K.,Agrewala,J.N.,Chopra,K.,2006.Curcuminattenuates thermalhyperalgesiainadiabeticmousemodelofneuropathicpain.Eur.J. Pharmacol.536,256–261.
Shepherd,G.,2002.ConhecimentodediversidadedeplantasterrestresdoBrasil.In: Lewinsohn,T.M.,Prado,P.I.(Eds.),BiodiversidadeBrasileira:SíntesedoEstado AtualdoConhecimento.Contexto,SãoPaulo.
Silva,K.A.,Paszcuk,A.F.,Passos,G.F.,Silva,E.S.,Bento,A.F.,Meotti,F.C.,Calixto,J.B., 2011.Activationofcannabinoidreceptorsbythepentacyclictriterpene␣, -amyrininhibitsinflammatoryandneuropathicpersistentpaininmice.Pain152, 1872–1887.
Siqueira,R.S.,Bonjardim,L.R.,Araújo,A.A.S.,Araújo,B.E.,Melo,M.G.,Oliveira,M.G., Gelain,D.P.,Silva,F.A.,DeSantana,J.M.,Albuquerque-Júnior,R.L.,Rocha,R.F., Moreira,J.C.,Antoniolli,A.R.,Quintans-Júnior,L.J.,2010.Antinociceptiveactivity ofatranorininmiceorofacialnociceptiontests.Z.Naturforsch.C65C,551–561. Siqueira-Lima,P.S.,Araújo,A.A.S.,Lucchese,A.M.,Quintans,J.S.,Menezes,P.P.,Alves, P.B.,LuccaJúnior,W.,Santos,M.R.,Bonjardim,L.R.,Quintans-Júnior,L.J.,2014. -cyclodextrincomplexcontainingLippiagrataleafessentialoilreducesorofacial nociceptioninmice–evidenceofpossibleinvolvementofdescendinginhibitory painmodulationpathway.BasicClin.Pharmacol.Toxicol.114,188–196. Siqueira-Lima, P.S., Lucchese, A.M., Araújo-Filho, H.G., Menezes, P.P., Araújo,
A.A.S.,Quintans-Júnior,L.J., Quintans,J.S.S.,2016. Inclusionof terpenesin cyclodextrins:preparation,characterizationandpharmacologicalapproaches. Carbohydr.Polym.151,965–987.
Surh,Y.J.,Chun,K.S.,Cha,H.H.,Han,S.S.,Keum,Y.S.,Park,K.K.,Lee,S.S.,2001. Molec-ularmechanismunderlyingchemopreventiveactivitiesofanti-inflammatory phytochemicals:downregulationofCOX-2andiNOSthroughsuppressionof NF-kappaBactivation.Mutat.Res.480,243–268.
Taheri,J.B.,Azimi,S.,Rafieian,N.,Zanjani,H.A.,2011.Herbsindentistry.Int.Dent. J.61,287–296.
Tapsoba,H.,Deschamps,J.P.,2006.Useofmedicinalplantsforthetreatmentoforal diseasesinBurkinaFaso.J.Ethnopharmacol.104,68–78.
Ulrich-Merzenich,G.,Panek,D.,Zeitler,H.,Vetter,H.,Wagner,H.,2010.Drug devel-opmentfromnaturalproducts:exploitingsynergisticeffects.IndianJ.Exp.Biol. 58,208–219.
Venâncio,A.M.,Marchioro,M.,Estavam,C.S.,Melo,M.S.,Santana,M.T.,Onofre, A.S.,Guimarães,A.G.,Oliveira,M.G.,Alves,P.B.,Pimentel,H.C.,2011.Ocimum basilicumleafessential oiland(−)-linalool reduceorofacialnociceptionin rodents:Abehavioralandelectrophysiologicalapproach.Rev.Bras.Farmacogn. 21,1043–1051,http://dx.doi.org/10.1590/S0102-695X2011005000147. Xu,Y.X.,Pindolia,K.R.,Janakiraman,N.,Chapman,R.A.,Gautam,S.C.,1997–1998.
CurcumininhibitsIL1alphaandTNF-alphainductionofAP-1andNF-B DNA-bindingactivityinbonemarrowstromalcells.Hematopathol.Mol.Hematol.11, 49–62.