Anti-Inflammatory and Antinociceptive Effects
of
Sterculia striata
A. St.-Hil. & Naudin (Malvaceae) in Rodents
Francilene V. Silva,
1Irisdalva S. Oliveira,
1Kayo A. Figueiredo,
1Francisco B. Melo Ju´nior,
1Danielly A. Costa,
2Mariana H. Chaves,
3Maurı´cio P.M. Amaral,
1,4Fernanda R.C. Almeida,
1,4Francisco A. Oliveira,
1,4and Rita C.M. Oliveira,
1,51Medicinal Plants Research Center, Federal University of Piauı´, Teresina, PI, Brazil. 2Health Care Academic Unit, Federal University of Campina Grande, Cuite´, PB, Brazil.
Departments of3Chemistry,4Biochemistry and Pharmacology, and5Biophysics and Physiology,
Federal University of Piauı´, Teresina, PI, Brazil.
ABSTRACT The present work reports the anti-inflammatory and antinociceptive activities of the ethanol extract obtained from the stem bark ofSterculia striataA. St.-Hil. & Naudin (Ss-EtOH) in the experimental models of edema induced by carrageenan, dextran, or histamin and nociception induced by chemical stimuli, such as acetic acid, formalin, capsaicin, or glutamate. The Ss-EtOH (50 mg/kg) promoted a marked inhibition on the hind paw edema induced by carrageenan or dextran (30% and 73%, respectively). Besides, Ss-EtOH (25 mg/kg) exhibited a slight activity (30%) on the hind paw edema induced by histamin. The Ss-EtOH (12.5 and 25 mg/kg) showed the antinociceptive activity on chemical stimuli induced by acetic acid (65.59% and 38.37%, respectively), formalin, in the initial (35.08% and 31.5%, respectively) and late phases (44.09% and 83.57%, respectively), capsaicin (43.77% and 51.31%, respectively), or glutamate (36.6% and 52.12%, respectively). Re-garding the possible mechanism involved in the antinociceptive effect, Ss-EtOH (12.5 mg/kg) showed a decrease in the antinociceptive effect (65.8%) in the acetic acid model after pretreatment with naloxone. Thus, opioid mechanisms might be underlying this response.
KEY WORDS:anti-inflammatoryantinociceptivecarrageenanopioidpaw edemaSterculia striata
INTRODUCTION
I
n folk medicine, plants have played an important role in human life since ancient times, not only as a food source but also in the treatment of various diseases. Hence, several herbs have been used as a form of therapy for pain relief along the history. The study of plants used in traditional medicine as anti-inflammatory or analgesic has represented an important research strategy in the development for newanalgesics and anti-inflammatory drugs.1
Some species of Northeastern Brazil have demonstrated their anti-inflammatory activity in animal models, such as
the essential oil of Lippia sidoides Cham. (Verbenaceae),
known as ‘‘alecrim pimenta,’’2 and antioxidant and
orofacial antinociceptive properties of aqueous extract of
leaves of Hyptis pectinata L. Poit (Lamiaceae), popularly
known as ‘‘sambacaita´’’ and found in the states of Sergipe
and Alagoas (Brazil).3
Species from the Malvaceae family have been investigated due to their providing of several secondary metabolites, such as
triterpenes,4 flavonoids,5 alkaloids,6 and coumarins.7 Some
species of the genusSterculiahave several constituents and
present several biological activities, as Sterculia foetida L.
whose anti-inflammatory and central nervous system
depres-sant activities have been reported.8Beyond the
pharmacolog-ical activities, there is also pharmaceutpharmacolog-ical interest in the biotechnological application of natural gums derived from the
family Malvaceae exudates, such as gum karaya (Sterculia
urens)9–11and chicha gum (Sterculia striata),12,13in stabilizing or improving the dissolution of formulas and the release of active ingredients.
S. striata A. St.-Hil. & Naudin is popularly known as ‘‘chicha´,’’ ‘‘Pau-rei,’’ ‘‘Mendubi-guac¸u,’’ ‘‘Arachacha´,’’ ‘‘Checha´-do-norte,’’ and ‘‘Castanheiro-do-mato.’’ It is a large and widespread tree spread from the Amazon Region toward Piauı´, Mato Grosso, Minas Gerais, and Rio Grande
do Sul, whose seeds are consumed by humans.14 In folk
medicine, the leaves have been used topically with hot
butter or olive oil for the treatment of boils.15
Preliminary phytochemical investigations of the oil
ob-tained from seeds of S. striata detected the presence of
Manuscript received 1 March 2013. Revision accepted 6 November 2013.
Address correspondence to: Rita de Ca´ssia Meneses Oliveira, PhD, Medicinal Plants Research Center, Federal University of Piauıı´, Campus Ministro Petroˆnio Portella, SG-15, Ininga, CEP 64049-550 Teresina, PI, Brazil, E-mail:menesesoliveira@gmail.com
#Mary Ann Liebert, Inc., and Korean Society of Food Science and Nutrition DOI: 10.1089/jmf.2013.0062
cyclopropenoid fatty acids,16 and the ethanol extract
ob-tained from stem barks ofS. striata (Ss-EtOH) provided a
mixture of sitosterol and stigmasterol (0.8%),
sitosterol-3-O-b-D-glucopyranoside (0.24%), and four pentacyclic
triterpenoids: lupeol (5.2%), 3-b-O-acyl-lupeol (0.04%), lupenone (0.32%), and betulinic acid (0.09%). Lupeol is the
major chemical constituent obtained.17 In the literature,
there are reports of anti-inflammatory and gastroprotective
activities for some of these chemical constituents.18–20
A previous study has demonstrated the Ss-EtOH-induced gastroprotective activity in different models of gastric
mu-cosal injury.21 In this context, several studies concerning
plants-derived products and their anti-inflammatory and
gastroprotective properties have been reported.22–25
Toxicological results demonstrate that the stratum is safe. Oral administration of Ss-EtOH (2000 mg/kg) and intra-peritoneal administration (1000 mg/kg) did not result in the death of any animal nor did it cause damage to internal
organs.21
Thus, the data presented about the Malvaceae family and the reported phytochemical and pharmacological profiles of S. striatalead us to investigate the anti-inflammatory and antinociceptive effects of the ethanol extract from stem
barks ofS. striata(Ss-EtOH) in rodents.
MATERIALS AND METHODS
Plant material and extraction
The species was collected in the city of Teresina (-520
45.5100, -4247018.4200), state of Piauı´, Brazil, determined,
and deposited in the Herbarium Graziela Barroso from the Federal University of Piauı´ (voucher specimen No. TEPB 13,870). Stem barks were dried at room temperature and then powdered (1800 g). The powder was exhaustively extracted in 95% ethanol under Ultrasonic apparatus for 45 min. This procedure was repeated four times. The ethanol
solution was concentrated in rotary evaporator at 60C
yielding 66.9 g (yield 3.71%, w/w) of crude ethanol extract (Ss-EtOH).
Animals
Male Swiss mice (25–30 g) and Wistar rats (180–220 g)
were housed at 24C–2C under a 12 h light–12 h dark
cycle, and they had free access to standard pellet diet and water. All experiments followed experimental protocols submitted to and approved by the Animal Research Ethics Committee of the Federal University of Piauı´ (no. 12/2008).
Drugs and reagents
Dexamethasone, dextran, cyproheptadine, histamine,
capsaicin,k-carrageenan, indomethacin, MK801, naloxone,
and Tween80
were purchased from Sigma-Aldrich (St. Louis, MO, USA), and morphine was obtained from Cris-ta´lia (Sa˜o Paulo, SP, Brazil).The Ss-EtOH extract was pre-viously solubilized in 1.0% Tween 80 and then diluted in saline solution (0.9% NaCl). Other drugs were dissolved either in saline solution or in distilled water. Ss-EtOH
ex-tract and drug concentrations were adjusted for oral treat-ment to 1 mL/100 g body weight.
Anti-inflammatory activity
Paw edema induced by carrageenan. Male Wistar rats
(n=8) were orally pretreated with vehicle (1 mL/100 g),
Ss-EtOH (25, 50, or 100 mg/kg), and indomethacin (5 mg/kg). Paw edema was induced by subplantar injection of 1.0%
carrageenan (0.1 mL, i.pl.) into the right hind paw.26 Their
right hind paws were measured with a pachymeter (in mil-limeters) before (Ti) and every 60 min for 6 h after induction of edema. The difference in the paw edema diameter was calculated for each group and compared with the control group.
Paw edema induced by dextran or histamine. Male
Swiss mice (n=7) were pretreated with vehicle (0.05 mL/
paw, i.pl.) in the left paw and with dextran or histamine
(0.05 mL/paw, i.pl.) in the right paw.27 After 60 min, the
animals were orally treated with vehicle, cyproheptadine (10 mg/kg), or Ss-EtOH (25, 50, or 100 mg/kg). Then, after 1 or 2 h of edema induction, the animals were euthanized by cervical dislocation and their paws were removed for the measurement of edema (in grams).
Antinociceptive activity
Writhing induced by acetic acid. In this experiment,
male Swiss mice (n=8) were orally pretreated with vehicle
or Ss-EtOH (6.25, 12.5, or 25 mg/kg) 60 min before the in-traperitoneal administration of acetic acid (0.75%, i.p.). Then, the total number of writhings was counted during
20 min.28The elicited antinociceptive effect was compared
with the response induced by morphine (2.5 mg/kg, i.p.) administered 30 min before the acetic acid injection.
Formalin test. Nociception was induced by the injection of formalin (2%, 20 (L, i.pl.) in the right hind paw of male
Swiss mice (n=8). Ss-EtOH (6.25, 12.5, and 25 mg/kg,
p.o.), morphine (5 mg/kg, p.o.), and vehicle were orally administered 30 or 60 min before formalin. The time during which the animal licked the paw that received the stimula-tion for 0–5 min (acute pain) and 15–30 min later (inflam-matory pain) was measured and compared between the
groups.29,30
Capsaicin test. In this experiment, male Swiss mice
(n=8) were orally treated with vehicle, Ss-EtOH (6.25,
12.5, and 25 mg/kg, p.o), or morphine (2.5 mg/kg, p.o.). One hour after those treatments, the right hind paw was injected with capsaicin (20 (L, 2 (g/paw), and nociception was ob-served and measured by paw biting or licking time during
5 min, with further comparison between the groups.31,32
Glutamate test. Glutamate-induced nociceptive re-sponse is promoted by an injection of the amino acid
glu-tamate (20lL, 10lmol/paw, i.pl.) on the ventral surface of
Ss-EtOH (6.25 or 12.5 mg/kg p.o.), or MK801 (0.03 mg/kg, i.p.) were administered. Then, 30 or 60 min later, they re-ceived glutamate. The number of times in which the animals licked the stimulated paw was quantified during 5 min.
Investigation of possible mechanism involved in Ss-EtOH-induced antinociceptive effect
To investigate the possible participation of the opioid system on the antinociceptive effect, male Swiss mice
(n=8) were subcutaneously pretreated with naloxone (2 mg/
kg, s.c.) 15 min before the treatment with Ss-EtOH (12.5 mg/kg, p.o.) or morphine (2.5 mg/kg, i.p.). Then,
ani-mals were subjected to the already-described writhing test.34
Data analysis
The results were analyzed by one-way ANOVA followed by Tukey’s, Dunnett’s, or Bonferroni’s test and are
ex-pressed as mean–SEM. The results were considered
sig-nificant when P<.05. All analyses were performed using
GraphPad Prism software, version 5.0 (GraphPad
Soft-ware, Inc., San Diego, CA, USA).
RESULTS
Anti-inflammatory activity
Paw edema induced by carrageenan. Ss-EtOH at a dose of 50 mg/kg was able to reduce the paw edema induced by carrageenan in rats only at the third and fifth hour when compared with the vehicle group and indomethacin group. This response was more effective at the third hour (30%). The same result was not observed at a dose of 25 mg/kg (Fig. 1).
Paw edema induced by dextran or histamine. The paw edema induced by dextran was decreased by 73% after oral treatment with Ss-EtOH (50 mg/kg). Besides, Ss-EtOH at a dose of 25 mg/kg decreased by 30% the paw edema induced by histamine. All results were compared with the vehicle group. Cyproheptadine (10 mg/kg) significantly inhibited the edema formation (56%) when compared with the vehicle group (Figs. 2 and 3).
Antinociceptive activity
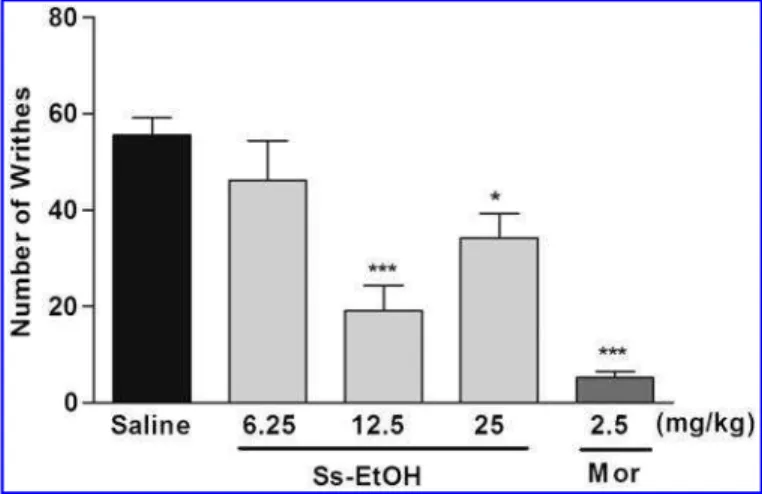
Writhing induced by acetic acid. In the writhing test, Ss-EtOH (12.5 or 25 mg/kg) reduced the number of writhes by 65.59% and 38.37%, respectively, when compared with the vehicle group. Morphine (2.5 mg/kg) markedly reduced the number of writhes (90.55%) when compared with the vehicle group (Fig. 4).
FIG. 1. Effect of ethanol extract fromSterculia striata(Ss-EtOH) and indomethacin (Indo) on the paw edema induced by carrageenan (1%, 0.1 mL/paw, i.pl) as a function of time in rats. Data were ex-pressed as mean–SEM. One-way ANOVA followed by Tukey’s
test.*P<.05, **P<.01, and ***P<.001 versus the control group.
FIG. 2. Effect of ethanol extract fromSterculia striata(Ss-EtOH) and cyproheptadine (Cypro) on the paw edema induced by dextran (0.05 mL/paw, i.pl.) in mice. Data were expressed as mean–SEM.
One-way ANOVA followed by Tukey’s test. *P<.05 versus the
control group.
FIG. 3. Effect of ethanol extract fromSterculia striata(Ss-EtOH) and cyproheptadine (Cypro) on the paw edema induced by histamine (0.05 mL/paw, i.pl.) in mice. Data were expressed as mean–SEM.
One-way ANOVA followed by Tukey’s test. *P<.05 and ***P<.001
Formalin test. Ss-EtOH (12.5 and 25 mg/kg) signifi-cantly decreased (35.08% and 31.5%, respectively) the hind paw licking time during the early phase (Fig. 5A). During the late phase, Ss-EtOH (12.5 and 25 mg/kg) also exhibited a dose-dependent effect (44.09% and 83.57%, respectively) due to a marked decreasing of the hind paw licking time (Fig. 5B).
Capsaicin test. On capsaicin-induced neurogenic noci-ception, Ss-EtOH at doses of 12.5 and 25 mg/kg promoted a significant inhibition by 43.77% and 51.31%, respectively (Fig. 6).
Glutamate test. In the glutamate test, Ss-EtOH at doses of 6.25 and 12.5 mg/kg significantly inhibited the number of times the animal lifted the paw stimulated with glutamate by 36.6% and 52.12%, respectively (Fig. 7).
Investigation of possible mechanisms involved in Ss-EtOH-induced antinociceptive effect
When used alone, naloxone (2 mg/kg, i.p.), an opioid-receptor antagonist, failed to modify the acetic acid-induced
nociceptive responses. However, naloxone was able to antagonize the Ss-EtOH-induced (12.5 mg/kg, p.o.) and morphine-induced (2.5 mg/kg, i.p) antinociceptive effect by 65.8% and 85.3%, respectively (Fig. 8).
DISCUSSION
Recent studies have shown that natural products, such as medicinal plants from Northeastern Brazil, represent an important source for the development of new therapeutic alternatives. Furthermore, novel studies regarding essential oils and herbal extracts with antimicrobial and
anti-nociceptive activities have been increasing.35–37
Several natural products that possess anti-inflammatory and antinociceptive activities have been identified and iso-lated from plants, whereas some of them are currently synthesized and available for commercial use. Hence, the major goal of this work is to report the effects of Ss-EtOH on experimental models of edema and nociception since data
on the bioactivity regarding theS. striataspecies are scarce.
Inflammation is a protective and defense mechanism of the body and occurs in two distinct phases. An acute phase characterized by local vasodilatation, increased capillary permeability, and release of inflammatory mediators such as histamine, serotonin, and prostaglandins. A chronic phase is characterized by infiltration of leukocytes and phagocytic
cells.38
Carrageenan-induced hind paw edema is the standard experimental model of acute inflammation, and it has been
increasingly used to test new anti-inflammatory drugs.39
That model is characterized by the production of a biphasic edema. In the relatively rapid early phase (1–2 h), edema formation is mediated by histamine and serotonin (5-HT), and in the late phase (3–4 h), kinins and prostaglandins
contribute to the edema.40Ss-EtOH inhibited the paw edema
between the first and fifth hour and then demonstrated the action on inflammatory mediators released during this pe-riod (e.g., histamine, 5-HT, or prostaglandins). Therefore, the positive results of the effect of Ss-EtOH on experimental models of edema induced by dextran or histamine (which were used as complementary tests to the first phase of edema triggered by carrageenan) are consistent with such data.
Histamine promotes a vasodilator response and then an increase in vascular permeability, promoting an edema FIG. 4. Effect of ethanol extract fromSterculia striata(Ss-EtOH)
and morphine (Mor) on nociception induced by acetic acid (0.75%, i.p.) in mice (n=7). Values are expressed as mean–SEM. One-way
ANOVA followed by Tukey’s test. *P<.05 and ***P<.001 versus
the control group.
FIG. 5. Effect of ethanol extract from Sterculia striata(Ss-EtOH) and morphine (Mor) on nociception induced during the first(A)and second(B) phases of the formalin 2% (20 mL/paw) in mice (n=8). Values
are expressed as mean–SEM. One-way ANOVA,
Bonferroni’s test, *P<.05, **P<.01, and ***P<.001
formation in minutes.40 Dextran is a polysaccharide that promoted the release of histamine and serotonin from mast
cells during the formation of edema,41,42 interacting with
their respective receptors (H1/H2 and 5-HT2, respectively)
on endothelium layer of microvessels. Therefore, it is rea-sonable that antiedematogenic or anti-inflammatory effect of Ss-EtOH probably involves the action of Ss-EtOH com-pounds on the release of histamine and/or inhibition of its binding to its pharmacological receptors as a competitive or noncompetitive antagonism.
The acetic acid-induced writhing reaction in mice is de-scribed as a typical model for inflammatory pain. Therefore, it is used as a screening tool for the assessment of analgesic
or anti-inflammatory properties of new agents27and, in most
cases, as a model to study the peripheral antinociceptive effect of chemical compounds. Such a model of nociception is suggested to represent the stimulation of peripheral mechanism since the administration of phlogogen leads to
an increase in the levels of cyclooxygenase (COX) and
li-pooxygenase,43 and then, several endogenous nociceptive
mediators (e.g., prostanoids of the PGE2and PGF2atypes,
serotonin, histamine, cytokines, and eicosanoids) are pro-duced and released.
The positive effect of Ss-EtOH in reducing the number of contortions may indicate its ability to inhibit the action of inflammatory mediators released by COX. Accordingly, these data are consistent with those observed in the second phase of paw edema induced by carrageenan. Furthermore, the model demonstrated that the Ss-EtOH induces the an-tinociceptive activity as well, due to nociception induced by acetic acid, besides involving inflammatory mediators and the direct stimulation of the low pH of nociceptive nerve
fibers.44
The stimulation of nociceptive nerve endings by C-fibers
or A-fibers activation transmits the painful stimuli.45Then,
signals transmitted through nociceptors (or ‘‘pain recep-tors’’) are interpreted as pain in the cognitive centers of the brain. The brain and spinal cord play a major role in central
pain mechanisms.46The capsaicin test involves the
partici-pation of afferent C-fibers, which trigger neuropathic pain when activated. This information suggests, as a response to the positive effects of Ss-EtOH about this experimental model of nociception, the involvement of these fibers and vanilloid receptors (TRPV1), a nonselective ligand-gated
ion channel,47,48that lead to membrane depolarization and
increased cation influx and then triggering the noxious
stimulus.49
Another experimental model of nociception that has been widely used to further support the evaluation of the anti-nociceptive effect induced by several new compounds is the
formalin test.50Subcutaneous injection of formalin induces
a distinct biphasic nociception. The first phase immediately begins after injection and lasts for 5 min. This phase reflects FIG. 6. Effect of ethanol extract fromSterculia striata(Ss-EtOH)
and morphine (Mor) on nociception induced by capsaicin (20mL, 2 mg/paw) in mice (n=8), 60 and 30 min before stimulation. Values are
expressed as mean–SEM. One-way ANOVA, Dunnett’s test,
***P<.001 versus the control group.
FIG. 7. Effect of ethanol extract fromSterculia striata(Ss-EtOH) and MK801 on nociception induced by glutamate (20mL, 10mmol/
paw, i.pl.) in Wistar rats (n=8). Values are expressed as mean–
SEM. One-way ANOVA, Dunnett’s test, ***P<.001 versus the
control group.
FIG. 8. Effect of pretreatment naloxone (2 mg/kg, s.c.) on the an-tinociceptive profiles of Ss-EtOH (12.5 mg/kg, p.o.) and morphine (2.5 mg/kg, i.p) on acetic acid (0.75%, i.p.) in male mice (n=6).
Values are expressed as mean–SEM. One-way ANOVA,
Bonferro-ni’s test, ***P<.001 versus the control group; #P<.001 compared
a direct effect of formalin on nociceptors (neurogenic pain). The second phase is marked by a return to high levels of nociception beginning 15–20 min after administration of formalin and may continue up to 60 min, characterized as an
inflammatory pain.51The Ss-EtOH inhibited both phases of
formalin-induced nociception, probably acting on a central level of nociception, a mechanism characteristic of opioid drugs (e.g., morphine), whereas drugs acting peripherally (e.g., nonsteroidal anti-inflammatory drugs) inhibit only
the late phase.16,50,51 Likewise, the possible participation
of opioid mechanism underlying the Ss-EtOH-induced antinociceptive effect might be reasonable, considering that naloxone, a nonspecific opioid antagonist, was able to
reverse its effect.52
In the experimental model of glutamate-induced noci-ception, Ss-EtOH also induced a significant nociceptive response, probably involving the activation of receptors at the peripheral level, especially NMDA receptor, with
subsequent release of nitric oxide.53 Thus, these previous
findings might indicate that the Ss-EtOH-induced anti-nociceptive effect could be due to a possible interaction with the glutamatergic system and the inhibition of NO production.
Hence, the Ss-EtOH possesses the antiedematogenic ac-tivity, probably due to the presence of bioactive compounds that seem to interfere with the release of inflammatory mediators. Besides, the Ss-EtOH also induces a significant antinociceptive response in chemical-induced nociception models, probably underlying opioid participation and inhi-bition of glutamatergic and vanilloid receptors, although further studies are necessary to elucidate additional mech-anisms involved in the observed effects. This work raises the
knowledge about S. striata as a rich source of bioactive
compounds and reinforces the development of new natural-based pharmaceutical products.
ACKNOWLEDGMENTS
This study was supported by Coordenac¸a˜o de Aperfei-c¸oamento de Pessoal de Nı´vel Superior (CAPES, Brazil), Conselho Nacional de Desenvolvimento Cientifico e Tec-nolo´gico (CNPq, Brazil), Fundac¸a˜o de Amparo a` Pesquisa do Estado do Piauı´ (FAPEPI, Brazil). We also thank Prof. Daniel Dias Rufino Arcanjo, MSc, and Prof. Abı´lio Antonio Borghi for the language proofreading.
AUTHOR DISCLOSURE STATEMENT
The authors declare that they have no conflicts of interest.
REFERENCES
1. Calixto JB, Beirith A, Ferreira J, Santos AR, Filho VC, Yunes RA: Naturally occurring antinociceptive substances from plants.
Phytother Res2000;14:401–418.
2. Veras HNH, Araruna MKA, Costa JGM, Coutinho HDM, Ker-ntopf MR, Botelho MA, Menezes IRA: Topical antiinflammatory activity of essential oil of Lippia sidoides cham: possible mechanism of action.Phytother Res2013;27:179–185.
3. Paixa˜o MS, Melo MS, Oliveira MGB, Santana MTS, Lima ACB, Damascena NP, Dias AS, Araujo BS, Estevam SC, Botelho MA, Quintans-Ju´nior LJ: Hyptis pectinata: redox protection and orofacial antinociception.Phytother Res2013;27:1328–1333. 4. Chang YS, Ku YR, Lin JH, Lu KL, Ho LK: Analysis of three
lupane type triterpenoids in Helicteres angustifolia by high-performance liquid chromatography.J Pharm Biom Anal2001; 26:846–855.
5. Arlorio M, Coisson JD, Travaglia F, Varsaldi F, Miglio G, Lombardi G, Martelli A: Antioxidant and biological activity of phenolic pigments fromTheobroma cacaohulls extracted with supercritical CO2.Food Res Int2005;38:1009–1014.
6. Hoelzel SCSM, Vieira ER, Giacomelli SR, Dalcol II, Zanatta N, Morel AF: An unusual quinolinone alkaloid from Waltheria douradinha. Phytochemistry2005;66:1163–1167.
7. Vardamides JC, Azebaze AGB, Nkengfack AE, Van Heerden FR, Fomum ZT, Ngando TM, Conrad J, Vogler B, Kraus W: Sca-phopetalone and scaphopetalumate, a lignan and a triterpene ester fromScaphopetalum thonneri. Phytochemistry2003;62:647–650. 8. Naik DG, Mujumdar AM, Waghole RJ, Misar AV, Annie Bligh SW, Bashall A, Crowder J: Taraxer-14-en-3b-ol, an Anti-Inflammatory Compound fromSterculia foetida L. Planta Med
2004;70:68–69.
9. Nagpal M, Rajera R, Nagpal K, Rakha P, Singh S, Mishra D: Dissolution enhancement of glimepiride using modified gum karaya as a carrier.Int J Pharm Investig2012;2:42–47. 10. Foster KA, Morgen M, Murri B, Yates I, Fancher RM, Ehrmann
J, Gudmundsson OS, Hageman MJ: Utility of in situ sodium alginate/karaya gum gels to facilitate gastric retention in rodents.
Int J Pharm2012;15:406–412.
11. Lankalapalli S, Kolapalli RM: Biopharmaceutical evaluation of diclofenac sodium controlled release tablets prepared from gum karaya—chitosan polyelectrolyte complexes.Drug Dev Ind Pharm2012;38:815–824.
12. Eiras C, Santos AC, Zampa MF, de Brito AC, Leopoldo Con-stantino CJ, Zucolotto V, dos Santos JR Jr.: Natural poly-saccharides as active biomaterials in nanostructured films for sensing.J Biomater Sci Polym2010;21:11.
13. Zampa MF, de Brito AC, Kitagawa IL, Constantino CJ, Oliveira ON Jr., da Cunha HN, Zucolotto V, dos Santos JR Jr., Eiras C: Natural gum-assisted phthalocyanine immobilization in electro-active nanocomposites: physicochemical characterization and sensing applications.Biomacromolecules2007;8:3408–3413. 14. Correˆa MP, Penna L:Diciona´rio das plantas u´ teis do Brasil e das
exo´ ticas cultivadas. Rio de Janeiro: Instituto Brasileiro de Defesa Florestal, 1984, 4:172.
15. Agra MF, Freitas PF, Barbosa-Filho JM: Synopsis of the plants known as medicinal and poisonous in Northeast of Brazil. Rev Bras Farmacogn2007;17:114–140.
16. Chaves MH, Barbosa AS, Moita Neto JM, Aued-Pimentel S, Lago JHG: Caracterizac¸a˜o quı´mica do o´leo da ameˆndoa de
Sterculia striataSt.Hil. Naud.Quim Nova2004;27:404–408. 17. Costa DA, Chaves MH, Silva WCS, Sousa CMM, Costa CLS:
Constituintes Sterculia striata St. Hil. et Naudin. Acta Amaz
2010;40:207–212.
18. Sa´nchez-Mendoza ME, Reyes-Trejo B, Sa´nchez-Go´mez P, Rodrı´guez-Silverio J, Castillo HC, Cervantes-Cuevas H, Arrieta J: Bioassay-guided isolation of an anti-ulcer chromene from
19. Lira SRS, Rao VS, Carvalho ACS, Guedes MM, Morais TC, Souza AL, Revisan MTS, Lima AF, Chaves MH, Santos FA: Gastroprotective effect of lupeol on ethanol-induced gastric damage and the underlying mechanism.Inflammopharmacology
2009;17:221–228.
20. Navarrete A, Trejo-Miranda JL, Reyes-Trejo L: Principles of root bark ofHippocratea excels (Hippocrataceae) with gastro-protective activity.J Ethnopharmacol2002;79:383–388. 21. Sousa AJ, Oliveira IS, Silva FV, Costa DA, Chaves MH, Oliveira
FA, Nunes PHM, Oliveira RCM: Gastroprotective activity of
Sterculia striataA. St. Hil. & Naudin (Malvaceae) in rodents.Z Naturforsch2012;67c:163–171.
22. Bansal VK, Goel RK: Gastroprotective effect ofAcacia nilotica
young seedless pod extract: role of polyphenolic constituents.
Asian Pac J Trop Med2012;7:523–528.
23. Lima DK, Ballico LJ, Rocha Lapa F, Gonc¸alves HP, de Souza LM, Iacomini M, Werner MF, Baggio CH, Pereira IT, da Silva LM, Facundo VA, Santos AR: Evaluation of the antinociceptive, anti-inflammatory and gastric antiulcer activities of the essential oil fromPiper aleyreanumC.DC in rodents.J Ethnopharmacol
2012;1:274–282.
24. Guilhon-Simplicio F, Pinheiro CC, Conrado GG, Barbosa G dos S, Santos PA, Pereira M de M, Lima ES: Anti-inflammatory, anti-hyperalgesic, antiplatelet and antiulcer activities of By-rsonima japurensisA. Juss. (Malpighiaceae).J Ethnopharmacol
2012;2:282–286.
25. Silva FV, Guimara˜es AG, Silva ER, Sousa-Neto BP, Machado FD, Quintans-Ju´nior LJ, Arcanjo DDR, Oliveira FA, Oliveira RC: Anti-inflammatory and anti-ulcer activities of carvacrol, a monoterpene present in the essential oil of oregano.J Med Food
2012;11:984–991.
26. Winter CA, Risley EA, Nuss GW: Carrageenan-induced oedema in hind paw of the rat as an assay for anti-inflammatory drugs.
Proc Soc Exp Biol Med1962;111:544–547
27. Yesilada E, Kupeli E: Berberis cratageina DC. Root exhibits potent anti-inflammatory, analgesic and febrifuge effects in mice and rats.J Ethnopharmacol2002;79:237–248.
28. Collier HO, Dinneen LC, Johnson CA, Schneider C: The ab-dominal constriction response and its suppression by analgesic drugs in the mouse.Br J Pharmacol1968;32:295–310. 29. Tjolsen A, Berge OG, Hunskaar S, Rosland JH, Hole K: The
formalin test: an evaluation of the method.Pain1992;51:5–17. 30. Hunskaar S, Hole K: The formalin test in mice: dissociation
between inflammatory and non-inflammatory pain. Pain 1987; 30:103–114.
31. Santos ARS, Calixto JB: Further evidence for the involvement of tachykinin receptor subtypes in formalin and capsaicin models of pain in mice.Neuropeptides1997;31:381–389.
32. Sakurada T, Katsumata K, Tan-No K, Sakurada S, Kisara K: The capsaicin test in mice for evaluating tachykinin antagonists in the spinal cord.Neuropharmacology1992;31:1279–1285.
33. Beirith A, Santos AR, Calixto JB: Mechanisms underlying the nociception and paw oedema caused by injection of glutamate into the mouse paw.Brain Res2002;924:219–228.
34. Hochberg MC, Silman AJ, Smolen JS, Weinblatt ME, Weisman MH:Rheumatology2008;4:751–915.
35. Veras HN, Rodrigues FF, Colares AV, Menezes IR, Coutinho HD, Botelho MA, Costa JG: Synergistic antibiotic activity of volatile compounds from the essential oil ofLippia sidoidesand thymol.Fitoterapia2012;3:508–512.
36. Bonjardim LR, Silva AM, Oliveira MG, Guimara˜es AG, Anto-niolli AR, Santana MF, Serafini MR, Santos RC, Arau´jo AA, Estevam CS, Santos MR, Lyra A, Carvalho R, Quintans-Ju´nior LJ, Azevedo EG, Botelho MA: Sida cordifolialeaf extract re-duces the orofacial nociceptive response in mice.Phytother Res
2011;8:1236–1241.
37. Franco CR, Antoniolli AR, Guimara˜es AG, Andrade DM, Jesus HC, Alves PB, Bannet LE, Patrus AH, Azevedo EG, Queiroz DB, Quintans-Ju´nior LJ, Botelho MA: Bioassay-guided evaluation of antinociceptive properties and chemical variability of the essential oil ofHyptis fruticosa. Phytother Res2011;11:1693–1699. 38. Posadas I, Bucci M, Roviezzo F, Rossi A, Parente L, Sautebin L,
Cirino G: Carrageenan-induced paw oedema is biphasic, age-weight dependent and displays differential nitric oxide cycloox-ygenase-2 expression.Br J Pharmacol2004;142:331–338. 39. Di Rosa M, Giroud JP, Willoughby DA: Studies on the mediators
of the acute inflammatory response induced in rats in different sites by carrageenan and turpentine.J Pathol1971;104:15–29. 40. Brand C, Townley SL, Finlay-Jones JJ, Hart PH: Tea tree oil
reduces histamine-induced edema in murine ears. Inflamm Res
2002;51:283–289.
41. Carvalho JC, Ferreira LP, Santos LS, Correˆa MJ, Oliveira Campos LM, Bastos JK: Anti-inflammatory activity of flavone and some of its derivates fromVirola michelli Heckel.J Eth-nopharmacol1999;64:173–177.
42. Andrade SF, Cardoso LGV, Carvalho JCT, Bastos JK: Antiin-flammatory and antinociceptive activities of extract, fractions and populnoic acid from bark wood ofAustroplenckia populnea. J Ethnopharmacol2007;109:464–471.
43. Ikeda Y, Ueno A, Naraba H, Oh-Ishi S: Involvement of vanilloid receptor VR1 and prostanoids in the acid-induced writhing re-sponses of mice.Life Sci2001;69:2911–2919.
44. Deraet R, Jougney S, Delevalcee F, Falhout M: Release of prostaglandin E and F in an algogenic reaction and its inhibition.
Eur J Pharmacol1980;51:17–24.
45. Besson JM: The neurobiology of pain.Lancet1999;353:1610– 1615.
46. Christopher RM, Stephen SS: Analgesic substances derived from natural products (natureceuticals).Life Sci2005;78:476–484. 47. Caterina MJ, Schumarcher MA, Tominaga M, Rosen TA, Levine
JD, Julius D: The capsaicin receptor: a heat-activated ion channel in the pain pathway.Nature1997;389:816–824.
48. Szallasi A, Blumberg PM: Mechanisms and therapeutic potencial of vanilloids (capsaicin-like molecules). Adv Pharmacol 1993; 24:123–155.
49. Julius D, Basbaum AI: Molecular mechanisms of nociception.
Nature2001;413:203–210.
50. Dubuisson D, Dennis SG: The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats.Pain1977;4:161–174.
51. Olajide OA, Awe SO, Makinde JM, Ekhelar AI, Olusola A, Morebise O, Okpako DT: Studies on the anti-inflammatory, an-tipyretic and the analgesic properties of theAlstonia booneistem bark.J Ethnopharmacol2000;71:179–186.
52. Liu J: Pharmacology of oleanolic acid and ursolic acid.J Eth-nopharmacol1995;49:57–68.