w w w . j c o l . o r g . b r
Journal
of
Coloproctology
Original
Article
Inflammatory
bowel
and
oxidative
stress
changes
in
an
experimental
model
of
portal
hypertension:
action
of
N-acetylcysteine
Francielli
Licks
a,∗,
Renata
Minuzzo
Hartmann
b,
Elizângela
Schemitt
b,
Josieli
Raskopf
Colares
d,
Lúcio
Sarubbi
Fillmann
c,
Henrique
Fillmann
c,
Norma
Possa
Marroni
a,b,daUniversidadeFederaldoRioGrandedoSul(UFRGS),ProgramadePósGraduac¸ãoemCiênciasBiológicas,PortoAlegre,RS,Brazil bUniversidadeFederaldoRioGrandedoSul(UFRGS),ProgramadePósGraduac¸ãoemMedicina,PortoAlegre,RS,Brazil
cPontifíciaUniversidadeCatólicadoRioGrandedoSul(PUCRS),PortoAlegre,RS,Brazil
dUniversidadeLuteranadoBrasil(ULBRA),ProgramadePósGraduac¸ãoemBioSaúde,Canoas,RS,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received28April2016
Accepted7May2016
Availableonline6July2016
Keywords: N-acetylcysteine
Portalhypertension
Intestine
Oxidativestress
Inflammation
a
b
s
t
r
a
c
t
Introduction:Portalhypertension(PH)ischaracterizedbyvasodilatationintheportalsystem
andthebowelisoneoftheseverelyaffectedorgans.N-acetylcysteine(NAC)isamolecule
withimportantpropertiesandwidelyusedinclinicalpractice.
Objective:ToevaluateNACactioninthebowelofanimalssubmittedtotheanimalmodelof
partialportalveinligation(PPVL).
Methods:18maleWistarratsweredividedintothreeexperimentalgroups(n=6):
sham-operated (SO), PPVL, and PPVL+NAC. On the 8th day after surgery, N-acetylcysteine
(10mg/kg,ip)wasadministereddailyfor7days.Onthe15thdaytheanimals’bowelwas
collectedforoxidativestressanalysis,immunohistochemistryandWesternblot.We
evalu-atedtheexpressionofNF-KBandTNF-␣byimmunohistochemistryandofiNOSbyWestern
blot.LipidperoxidationwasassessedbyTBARStechnique,andtheactivitiesofantioxidant
enzymessuperoxidedismutase(SOD)andglutationperoxidase(GPx)werechecked.
Results:WeobservedanincreasedexpressionofNF-KBandTNF-␣inPPVLgroup,andan
increasediNOSexpressionassessedbyWesternblot.NACreducedtheexpressionofall
proteinsevaluated.Wealsoobservedanincreaseinoxidativestressinthebowelofmice
PPVLgroupcomparedtocontrols(SO),andNACwaseffectiveinreducingthesevaluesin
PPVL+NACgroup.Also,areductionintheactivityofSODandGPxenzymeswasobserved
inthediseasedgroup,andNACwasabletorestoretheactivityoftheenzymesassessed.
Conclusion: Wesuggesttheanti-inflammatoryandantioxidantactionofNACinthebowel
ofanimalssubmittedtoPPVLmodel.
©2016SociedadeBrasileiradeColoproctologia.PublishedbyElsevierEditoraLtda.This
isanopenaccessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/
licenses/by-nc-nd/4.0/).
∗ Correspondingauthor.
E-mail:francielli.licks@gmail.com.br(F.Licks).
http://dx.doi.org/10.1016/j.jcol.2016.05.005
2237-9363/©2016SociedadeBrasileiradeColoproctologia.PublishedbyElsevierEditoraLtda.ThisisanopenaccessarticleundertheCC
Alterac¸ões
intestinais
inflamatórias
e
de
estresse
oxidativo
em
modelo
experimental
de
hipertensão
portal:
ac¸ão
da
N-acetilcisteína
Palavras-chave: N-Acetilcisteína
HipertensãoPortal
Intestino
EstresseOxidativo
Inflamac¸ão
r
e
s
u
m
o
Introduc¸ão: AHipertensãoPortal(HP)écaracterizadaporumavasodilatac¸ãonosistema
portal,eointestinoéumdosórgãosgravementeacometidos.AN-acetilcisteína(NAC)é
umamoléculacomimportantespropriedades,amplamenteutilizadanaclínica.
Objetivo: Avaliaraac¸ãodaNACnointestinodeanimaissubmetidosaomodeloanimalde
ligaduraparcialdaveiaporta(LPVP).
Métodos: Foramutilizados18ratosmachosWistardivididosemtrêsgruposexperimentais
(n=6):Sham-operated(SO),LPVP,LPVP+NAC.No8◦diaapósacirurgia,aN-acetilcisteína
(10mg/kg,ip)foiadministradadiariamentedurante7dias.No15◦diafoicoletadoointestino
dosanimaisparaanálisesdeestresseoxidativo,imunohistoquímicaeWesternblot.Nós
avaliamosaexpressãodoNF-kbeTNF-␣porimunohistoquímicaedaiNOSporWestern
blot.Alipoperoxidac¸ãofoiavaliadapelatécnicadeTBARS,easatividadesdasenzimas
antioxidantesSuperóxidoDismutase(SOD)eGlutationaPeroxidase(GPx)foramverificadas.
Resultados: Observamosumaumentoda expressãodoNF-kbeTNF-␣nogrupoLPVP,e
aumentonaexpressãodaiNOSavaliadaporWesternblot.ANACreduziuaexpressãode
todasasproteínasavaliadas.Observamosumaumentodoestresseoxidativonointestino
dosratosdogrupoLPVPcomrelac¸ãoaoscontroles(SO),sendoaNACeficaznareduc¸ão
dessesvaloresnogrupoLPVP+NAC.Ainda,umareduc¸ãonaatividadedasenzimasSODe
GPxnogrupodoente,sendoaNACcapazderestauraraatividadedasenzimasavaliadas.
Conclusão: Sugerimosaac¸ãoanti-inflamatóriaeantioxidantedaNACnointestinode
ani-maissubmetidosaomodeloLPVP.
©2016SociedadeBrasileiradeColoproctologia.PublicadoporElsevierEditoraLtda.Este
´eumartigoOpenAccesssobumalicenc¸aCCBY-NC-ND(http://creativecommons.org/
licenses/by-nc-nd/4.0/).
Introduction
Portalhypertension(PH)isasyndromewhoseclinicalpicture
isestablishedbytheemergenceofananatomicalobstaclein
theportalsystem.Thisobstacle,whichblocksthebloodflow,
causesblooddammingatthesiteofobstruction.The
compen-satorymechanismofdecompressionintheportalsystemis
thedevelopmentofanimportantvasodilationinthe
splanch-nicterritory;inturn,this eventisresponsibleforthemain
complicationsoftheportalhypertensionsyndrome.1
Wecancorrelatethedevelopmentofahyperdynamic
col-lateralcirculationwithoneofthemajorcomplicationsfrom
PH:thebleedingfromgastrointestinalvarices,aneventthat
is triggeredwhen the portal pressure gradient rises above
12mmHg.2Theprogressivevasodilationinthesplanchnic
ter-ritoryisresponsiblefortheappearanceofthesevaricoseveins,
themostimportantbeingthoselocatedinthestomachand
bowel.These conditionsare known asPortal Hypertension
Gastropathy(PHG)andPortalHypertensiveColopathy(PHC),
respectively,andthefirstoftheseconditionsisalreadywell
establishedintheliterature.3
IntestinalchangespresentinPHarestillbeingelucidated
andwere graduallyidentifiedover thelastdecade asbeing
mainlyoneofthecausesoffatalgastrointestinalbleedingin
patientswithPH.4ThepatternoflesionsincasesofPHcan
befoundinotherpartsofthegastrointestinaltract,including
theintestine,5duetomucosaledema,inflammatorydiseases,
andectopicandanorectalvarices.6
The experimental model of Partial Portal Vein Ligation
(PPVL)hasbeenusedbymanyauthorstostudythe
molecu-larchangesinpre-hepaticportalhypertension.7–9Inrats,the
hemodynamicchangespresentinPHshowuparoundtheday
14aftersurgery,andhyperdynamiccirculationand
splanch-nicvasodilationareprevailingconditionsinanimalssubjected
toaPPVLmodel.10Inaddition,PHCandencephalopathyare
among the most important manifestations resulting from
thisexperimentalmodel,andinflammatorymechanismsare
aggravatingfactorsinbothmanifestations.11
Inflammationisaneventoftenassociatedwithinjuriesof
different origins.In the caseofPH, systemic and
splanch-nic vascularresponsesappeartoplayanimportantrolein
the pathogenesisofhyperdynamiccirculationand are very
similar tothose producedinthe post-traumatic
inflamma-toryresponse.Themechanicalstresscausedbytheincreased
bloodflowinthesplanchnicterritorystimulatesthe
endothe-liumtosecretevasoactivesubstances,cytokinesandgrowth
factors,andthisisatriggeringfactorforlocalorgeneralized
inflammation.12
With respect to local inflammation, it is important to
mention that the mucosa ofthe gastrointestinal tract isa
major reservoir ofmacrophagesand mast cells,and these
cellslocatedintheintestineareconsideredaseffectorcells
that participate in the first line of defense of our body.13
Inthecaseofinflammation,theintestinalmucosaacquires
a phenotypic pro-inflammatory profile, secreting cytokines
that can amplify the systemic inflammatory vascular
Amongthecytokinessecretedbytheintestineafterthe
ini-tialstimulation,onemustmentionthetumornecrosisfactor
(TNF-␣),releasedbymastcells oftheintestineand
mesen-tericlymphnodes.Thisproinflammatorycytokineisprobably
relatednotonlytotheinflammatoryprocesspresentinPHbut
alsotothedevelopmentofhyperdynamiccirculation,sinceit
stimulatestheprimaryrouteofsplanchnicvasodilation,nitric
oxide.14 In addition, TNF-␣ is anextracellular stimulus for
releaseofanotherpro-inflammatorycytokine,NF-kB.This
fac-torinducesphosphorylationinIkB,thecytoplasmicinhibitory
proteinthatpreventsthenucleartranslocationofNF-kB.15
The activation of nitric oxide (NO) is the main event
proposedasatriggering factorforthe developmentof
col-lateralcirculationincasesofPH.NOissynthesizedbynitric
oxidesynthase(NOS),including,amongitsmajorisoforms,
endothelialnitricoxidesynthase(eNOS),neuronalnitricoxide
synthase(nNOS),andinduciblenitricoxidesynthase(iNOS).
This latter isoform is associated with increased levels of
NOproduction.16iNOS ismainlyexpressed inmacrophages
and smooth muscle cells, especially after the stimulation
by lipopolysaccharides, or inflammatory cytokines.17 This
enzymeismodulatedbytranscriptionfactors,amongwhich
NF-kBisconsidered theprimary mediatorofits activation,
which,inturn,canbeactivatedbytheoxidativestress.18
Inflammatory cells are important sources for the
gen-eration ofreactive oxygen species; thus the oxidative and
inflammatory damage acts synergistically in the
develop-mentandworseningofconditionsinwhichtheseeventsare
present.19
With HP, the role of oxidative stress is associated with
the overproduction of nitric oxide, which determines the
productionofhighlyreactive species,forexample,
peroxy-nitrite(ONOO ).20 Using the PPVL model, previous studies
publishedbyourstudygrouphavealreadyshownthis
signif-icantinvolvementinthepathogenesisofpre-hepaticportal
hypertension9,21,22aswellastheparticipationof
inflamma-tioningastricinjuryinthisexperimentalmodel.23
Thisstudy aimedtoevaluatetheinvolvementof
oxida-tiveandinflammatorystressintheintestinalmucosaofrats
withpre-hepaticportalhypertension.Inaddition,weaimed
toevaluatetheantioxidantandanti-inflammatoryactionof
N-acetylcysteineinintestinalinjuriespresentinPPVLmodel.
Material
and
methods
Ethics
Theexperimentalprocedureswithanimalswerecarriedout
inaccordance withthe current Brazilian legislationin the
practiceofscientificresearch(Law11,794,OfficialGazette–
October8,2008),EuthanasiaPracticeGuidelinesofCONCEA
(2013)andBrazilianGuidelinesfortheCareandUseofAnimals
forScientificandDidacticPurposes–DBCA(2013).
Animals
TheanimalswereacquiredinthevivariumofHCPAaccording
tothespecificationsoftheAnimalExperimentationUnit(UEA)
andwere maintainedthroughoutthe experimentinplastic
cagesmeasuring47cm×34cm×18cm,linedwithwood
shav-ings, in a 12-h light/dark cycle and at a temperature of
22±4◦C.Allanimalswerefedacommerciallyavailable
ani-malfood(Purina®–Nutripal,PortoAlegre,RS,Brazil)andhad
accesstowateradlibitum.
Groupsandexperimentalprotocols
Forthisstudy,weused18maleWistarrats(±250g)whichwere
divided randomly into three groups (n=6): sham-operated
(SO),PPVL,andPPVL+NAC.
On the first day of the experiment, the animals were
weighed and anesthetized with ketamine (100mg/kg) and
xylazine(10mg/kg)intraperitoneally(IP).Weperformedlocal
asepsisandthenmadeamid-ventrallaparotomywitha
care-ful exposure of bowel loops with a gauze pad soaked in
saline.Allanimalsunderwentthesame surgicalprocedure;
however, the animalsin group SO were submitted onlyto
the manipulation ofthe portal vein. The animals of PPVL
and PPVL+NACgroupswere submittedtoanexperimental
modelofPartialPortalVeinLigature,describedbySikuleretal.
1985.7
Weuseda20Gneedletopromoteapartialobstructionof
theportalvein;forthispurpose,boththevesselandthe
nee-dle were tiedwith silk3-0. Theimmediate vasodilationof
the splanchnicterritorywas observedand thenthe needle
wasgentlywithdrawn,leavingonlytheportalveinpartially
occluded.Next,intestinalloopswerereplacedintothe
abdom-inalcavityoftheanimals,andaninfusionof10mLofsaline
wasadministered,andtheperitoneumwassuturedwith
con-tinuouspoints.Theepitheliallayerwasclosedwithindividual
sutures. Theabove-described model features apre-hepatic
portalhypertension.7
Aftercompletionofthesurgicalprocedures,theanimals
wereplacedinindividualcagesfortheirrecoveryunder
anal-gesiawithdipyrone(200mg/kg);thefirstadministrationwas
performed byintramuscular route and the remaining was
orallyadministeredwithafrequencyof8/8hduringthe
sub-sequent72h.
Sevendays aftersurgery,thetreatment wasinitiatedin
the respectivegroups.Theanimals inOSand PPVLgroups
received only the vehicle (0.9% NaCl, 0.6mL IP). On the
other hand, the animals in PPVL+NAC group received
N-acetylcysteine(SigmaChemicalCo.,St.Louis,MO,USA;CAS
registrynumber616-91-1)atadoseof10mg/kgdissolvedin
0.6mLof0.9%NaCl.Thetreatmentwascarriedoutbeginning
onthe8thday,forsevendays.
Euthanasiaandtissuecollection
Attheendoftreatmentonday15,the animalswereagain
weighedandanesthetizedusingthesameprotocoldescribed
above.Afteraninspectionoftheanimal’sstateof
anesthe-sia,anewlaparotomywasmadeforremovaloftheintestines
forsubsequentanalysis.Aportionofthecollectedmaterial
wasstoredinafreezerat−80◦C,andtheremainderwasfixed
in 10% buffered formalin for24h. After this period, 3-mm
sections oftheparaffin blockwere obtainedusinga rotary
Immunohistochemistry
The histological technique of immunohistochemistry was
usedtoevaluatetheexpressionofthenuclearfactorkappa
B(NF-kB)andtumornecrosisfactor(TNF-␣)intheanimal’s
intestine. Thereacquisitionofthe antigenwas carried out
usingabufferat100◦C;subsequently,theactivityof
endoge-nous peroxidase was blocked by incubation with absolute
methanol.Theslideswereincubatedwithrabbitpolyclonal
antibody (NF- [sc-9072], 1: 200, Santa Cruz Biotechnology,
SantaCruz,CA,USA)andgoatmonoclonalantibody(TNF-␣
[sc-1351],1:200, SantaCruz Biotechnology,Santa Cruz,CA,
USA)overnightat4◦C.Next,the materialwaswashedand
incubatedwiththesecondaryantibodygoatanti-rabbit
IgG-HRP(sc-2004)for30minatroomtemperature.Theslideswere
analyzedusingamicroscopeequippedwithadigitalcamera,
andtheimageswerecapturedusingtheImage-Plussoftware
(MediaCybernetics,Bethesda,MD,USA).Quantificationofthe
markingofbothanalyteswascarriedoutbydigitalanalysis
withAdobePhotoshop®CS3extended10.0,usingthe
count-ingofbrowncolorstainedpixels.Theexpressionlevelwas
determinedbymultiplyingtheaveragedensityoftheimage
bythepercentageofthestainedareas.24
Westernblot
The cytosolic extracts prepared on the basis of intestinal
homogenateswereusedinWesternblot,andproteinvalues
were determined bythe Bradford method.25 Then, protein
lysateswerefractionatedbypolyacrylamidegel
electrophore-sisat9–12% inan electrophoresisbuffer(25mM Tris,0.2M
glycine,3.5mM SDS,pH8.8)and thentransferredto
mem-branes of polyvinylidene fluoride (PVDF). Blocking of the
membranes was done using a 5% solution of skimmed
milk powder in PBS-Tween. Then, the PVDF membrane
was incubated overnight at 4◦C with the specific
mono-clonalprimary antibody,mouse polyclonalantibody(NOS2
[sc-7271], Santa Cruz Biotechnology, Santa Cruz,CA, USA).
Aftertheovernightincubation,themembraneswerewashed
withTTBSandincubated for1hatroom temperaturewith
the secondary antibody IgG-HRP sc-2005, anti-goat donkey
(sc-2020, Santa Cruz Biotechnology, Santa Cruz, CA, USA,
1:4000).Afterrevealed,thebandswerequantifiedusingthe
Scion Imageprogram, v. 4.02 for Windows(Scion
Corpora-tion,Frederick,USA).Theresultswereexpressedinarbitrary
units.26
Biochemicalanalyses
Homogenate
Theintestinesofthe animalswere homogenizedfor1min
withUltra-Turrax(IKA-WERK)inthepresenceofpotassium
chloride (KCl) 1.15% (5mL/g of tissue) and
phenylmethyl-sulfonyl fluoride (PMSF) at a concentration of 100mM in
isopropanol(10L/mL KCl added). Then, the homogenates
werecentrifugedfor10minat3000rpm(1110×g)ina
refrig-erated centrifuge (SORVALLRC-5B Refrigerated Superspeed
Centrifuge)andthesupernatantwasremovedandfrozenina
freezerat−80◦Cforsubsequentmeasurements.27
Proteincontent
The protein concentration in the homogenates was
deter-minedbytheBradfordmethod,withbovinealbumin(SIGMA)
usedasastandard.Thesamplesweremeasured
spectropho-tometrically at 595nm, and the values were expressed in
mg/mL. These values were used to calculate subsequently
TBA-RS and the values of antioxidant enzymes SOD and
GPx.25
Determinationofoxidativestress
For the determination of lipid peroxidation, we used the
method ofreactive substances to thiobarbituricacid
(TBA-RS).Thetechniqueconsistsinheatingthehomogenateinthe
presenceofthiobarbituricacid,withconsequent formation
ofapinkcolorproduct,measuredinaspectrophotometerat
535mm.Theappearanceofcoloroccursduetothepresence
ofmalondialdehydeandothersubstancesfromlipid
peroxida-tioninbiologicalmaterial.0.5mLofthiobarbituricacid(TBA)
0.67%, 0.25mL of distilled water, 0.75mL oftrichloroacetic
acid(TCA)10%,and0.25mLofthehomogenatewereplaced
in a test tube, in this order.TBA reacts with lipid
peroxi-dation productsforminga Schiffbase,and TCAexerts the
function ofdenaturation of the proteins present and also
acidifies thereactionmedium.Then,each tubewasstirred
and heated to100◦Cfor15min.Afterthat, thetubes were
cooled and 1.5mLof n-butylalcohol was added toextract
the pigment formed. The tubes were placed on a shaker
(Biomatic) for 45s and centrifuged for 10min at 3000rpm
(1110×g). Finally, thestained productwas takenaway and
thereadingwastakenusingaspectrophotometer (CARY3E
–UV–VisibleVarianSpectrophotometer)atawavelengthof
535nm.TheconcentrationofTBA-RSisexpressedasnmol/mg
protein.28
Theactivityofsuperoxidedismutase(SOD)isdefinedbyits
abilitytoinhibitadetectionsystemwhichreactswithO2•−.
For this purpose, adrenalineis used, which, inanalkaline
medium,turnstoadrenochrome,producingO2•−,whichitis
thesubstrateoftheenzyme.Beforeperformingthe
determi-nation withthehomogenate,measurement ofthe reaction
medium(50mMglycine-NaOH,pH9.6)wascarriedoutwith
50Lofadrenaline(60mM,pH2.0),correspondingto100%of
the reaction. Thismixturewas stirredand readat480nm.
Subsequently, different volumesof the homogenate(50L,
25L,and10L)wereadded,andtheinhibitionofthe
reac-tionwasmeasured.Theenzymaticactivitywasexpressedas
SODunits/goftissue(quantityofSODabletoinhibitin50%
theadrenalinereductionrate).29
Thedeterminationofglutathioneperoxidase(GPx)using
the Flohé–Guntzler method30 consists in measuring the
NADPHconsumptionrateinasystemcontainingGSH;the
oxi-dationisrecordedspectrophotometricallyatawavelengthof
340nm.Tothisend,2.7mLofaregulatingsolutionofNa+and
K+phosphates(100mM,pH7.0)with50LofNADPH(10mM),
150LofBOOH(10mM)and50Lofglutathionereductase
(12U/mL)wereplacedinaquartzcuvette.Themixturewas
readfor1min;atthispoint,abaselinewasestablished,and
then50LofGSH(100mM)and50Lofthehomogenatewere
A
B
C
TNF-α
120 000
SO
Expression of the positiv
e pix
els
,%
PPVL ∗
#
PPVL+NAC 100 000
80 000
60 000
40 000
20 000
0
Fig.1–ImmunohistochemistryofTNF-␣.Effectsofpartialportalveinligation(PPVL)andN-acetylcysteine(NAC)
administrationonTNF-␣expression.SO,Sham-operatedgroup;PPVL,partialportalveinligation;PPVL+NAC,partialportal veinligationtreatedwithNAC.*p<0.001,#p<0.001(n=6).
atanabsorbanceof340nm. Theactivity wasexpressed in
nmol/min/mgprotein.
Statisticalanalysis
Alldatawerepresentedasamean±standarderror.Statistical
analyseswerecalculatedusingGraphpadInstatsoftware,
ver-sion3.0forWindows.Analysisofvariance(ANOVA)andthe
Student–Newman–Keulstestwasusedformultipleanalyses,
andthesignificancelevelwassetatp<0.05(5%).
Results
Immunohistochemistry
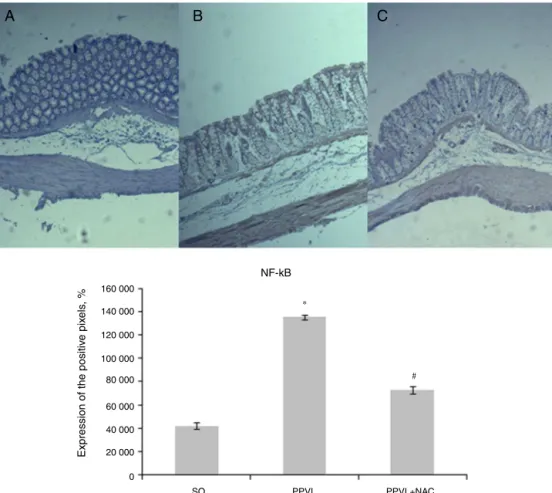
InassessingtheexpressionoftheproteinTNF-␣inthe
differ-entexperimentalgroups,weobservedasignificantincreasein
itsmarkingsinPPVLversusSOgroup(p<0.001).The
adminis-trationofN-acetylcysteineintheproposeddoseinthisstudy
was ableto reducethe expression ofTNF-␣ inPPVL+NAC
group(p<0.001)(Fig.1).
The expression of NF-kB was increased in animals
subjected to the experimental model of PPVL versus
con-trols (p<0.001), and the values of PPVL+NAC group were
significantlyreducedversusvaluesforanimalsofthediseased
group(p<0.001)(Fig.2).
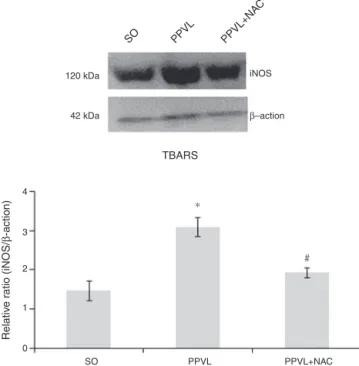
Westernblot
Using Western blot, we observed a significant increase in
iNOSexpressionintheintestineofanimalsofthediseased
group(p<0.01).TheanimalstreatedwithNAChadtheirvalues
reduced,asobservedinPPVL+NACgroup(p<0.01)(Fig.3).
Biochemicalanalyses
Anincreasewasobservedinlipidperoxidation,evaluatedby
TBA-RSinanimalsfromPPVLversusOSgroup(p<0.05),and
NACwas abletoreducetheselevels whenadministeredto
PPVL+NACgroup(p<0.01)(Fig.4).
SODactivitywasalsoevaluatedintheintestineofanimals
ofdifferentexperimentalgroups;theactivitywasreducedin
diseasedversuscontrolanimals(p<0.01),andthetreatment
hasproveneffectiveinincreasingtheactivityofthisenzyme
(p<0.05)(Fig.5).
Another antioxidant enzyme, GPx, was also evaluated
in this study; We observed a similar behavior in PPVL
(p<0.05),andinPPVL+NACgroupsversusOSgroup(p<0.01)
Discussion
Theportalhypertensionsyndromeshowsascharacteristics
the occurrence of ascites, hepatic encephalopathy, and a
hyperdynamiccollateralcirculation31;thislatteroccurrence
isconsideredthemaincauseofworseningofthegeneral
con-dition.
Thecollateral circulationdevelopsinordertodivertthe
blood flow from the obstructed territory; and the
obstruc-tion may be situatedbefore (pre-hepatic), into (hepatic) or
after(post-hepatic)theliver.32Regardlessoftheplaceofthis
obstruction,the unblocking compensatory mechanism will
eventuallyresultintheformationofvaricoseveinsdistributed
alongthedigestivetractofthepatient.
Ingeneral,varicoseveinslocatedinthecolonareprevalent
inthececumandrectosigmoidregion33andcharacterize
por-talhypertensioncolopathy(PHC).Inthisscenario,theriskof
bleedingthroughcolonicvaricesis1–8%34;ontheotherhand,
forrectalvaricoseveins,theriskisfrom44to89%incirrhotic
patients.6 Inaddition,vascularectasiaandmicrocirculatory
changesalongtheintestinalmucosadamageitsintegrityand
promotethedevelopmentofalocalinflammatoryprocess.35
Theintestinalinflammatoryconditionpresentincasesof
portalhypertensionisconsideredanaggravatingfactorinthe
pathogenesis ofthe disease.Among the pro-inflammatory
cytokines involved in the process, TNF-␣ is considered as
an importantmediator, being produced byintestinal mast
cells.36 In this study,we observeda significant increaseof
thiscytokineintheintestineofanimalsofPPVLgroup(Fig.1).
ThesamebehaviorwasobservedintheevaluationofNF-kB,
whichwasincreasedinrelationtotheanimalsofSOgroup
(Fig.2).Thesedataareconsistentwithstudiespublished,that
reportleukocyteinfiltrationandaninflammatoryprocessin
thissameexperimentalmodel.37
N-acetylcysteinewasabletoreducetheexpressionofboth
cytokinesevaluatedintheintestineofanimalsofPPVL+NAC
group. Theanti-inflammatory propertiesofNAC havebeen
previously described in inflammatory bowel diseases with
encouragingresults,38andtheseresultsmayberelatedtoits
thiol group, which isimportantto combatoxidative stress
and inflammation.39 In addition, the treatment with NAC
suppressestheactivationofNF-kBandthesubsequent
pro-duction of its cytokines and also blocks TNF-␣ activation,
causingstructuralchangesinitsreceptor.40
Theinvolvementofnitricoxideinthisexperimentalmodel
iswellestablishedintheliterature.Withtheprogressionof
thedisease,theimmunesystemisactivatedandtheinducible
formofNOS(iNOS)undergoesup-regulation.40Inthisstudy,
theanimalsofPPVLgroupdemonstratedasignificantincrease
intheexpressionofiNOSversusanimalsofOSgroupinthe
evaluation by Westernblot (Fig. 3). A previous study,
pub-lishedbyourresearchgroup,observedthesamebehaviorof
thisenzymeinthestomachofanimalssubjectedtothesame
experimentalmodelofpartialportalveinligation.23
A
NF-kB
160 000
140 000
120 000
100 000
80 000
60 000
40 000
20 000
0
SO
∗
#
Expression of the positiv
e pix
els
, %
PPVL PPVL+NAC
B
C
SO PPVL PPVL+NA C
120 kDa
42 kDa
iNOS
∗
# β–action
TBARS
4
3
2
1
0
SO PPVL PPVL+NAC
Relativ
e r
a
tio (iNOS/
β
-action)
Fig.3–WesternblotofiNOS.Effectsofpartialportalvein ligation(PPVL)andN-acetylcysteine(NAC)oniNOS expression.SO,Sham-operatedgroup;PPVL,partialportal veinligation;PPVL+NAC,partialportalveinligationtreated withNAC.*p<0.01,#p<0.01(n=6).
Inthisstudy,N-acetylcysteinewasabletoreducethe
lev-elsofiNOSintotheintestineofanimalsofPPVL+NACgroup.
Thisfindingisinagreementwithapreviouslypublishedstudy,
inwhichitwasreportedthatNACinhibitsnitricoxide
pro-ductionbycellsoftheimmunesystemandbytheinducible
isoformofNOS(iNOS).41
Theinvolvementofoxidativestressinthedevelopmentof
hyperdynamiccirculationwasinitiallyproposedbyFernand
etal.1998.42Sincethen,severalstudieshavedemonstrateda
positivecorrelationoftheexperimentalmodelofpartialportal
veinligationwiththesameoxidativeimpairments.8,9,21–23
In the present study, we observed increased levels of
thiobarbituricacid reactivesubstances (TBA-RS)inanimals
subjectedtothisexperimentalmodel(Fig.4).Furthermore,the
increaseoflipidperoxidationwasaccomplishedbyreducing
TBARS 0.8
SO PPVL PPVL+NAC
∗
#
0.7 0.6 0.5 0.4 0.3 0.2 0.1 0.0
nmoles/mgprot
Fig.4–TBA-RS.Effectsofpartialportalveinligation(PPVL) andN-acetylcysteine(NAC)onTBA-RSvalues.SO,
Sham-operatedgroup;PPVL,partialportalveinligation; PPVL+NAC,partialportalveinligationtreatedwithNAC. *p<0.05,#p<0.01(n=6).
SOD
SO PPVL PPVL+NAC
∗
#
80 70 60 50 40 30 20 10 0
USOD/mgprot
Fig.5–SOD.Effectsofpartialportalveinligation(PPVL) andN-acetylcysteine(NAC)onSODactivity.SO,
Sham-operatedgroup;PPVL,partialportalveinligation; PPVL+NAC,partialportalveinligationtreatedwithNAC. *p<0.01,#p<0.05(n=6).
the activity ofantioxidant enzymes superoxide dismutase
(SOD)(Fig.5)andglutathioneperoxidase(GPx).Thisfinding
demonstratesapotentialconditionofoxidativestressinthe
bowelofexperimentalanimals.Thisisinagreementwith
pre-viousstudiesinwhichPPVLtriggeredaconditionofoxidative
stressinotherbodyorgansstudied:stomach9andliver.8The
antioxidant action ofN-acetylcysteine revolves around the
fact thatthis moleculeisacysteine precursor forthe
syn-thesis ofGSH, and alsobyacting directly asa freeradical
scavenger.Thankstoitsantioxidantandanti-inflammatory
properties,NAChasbeenwidelystudiedinthetreatmentof
liverdiseases.40
Theauthorsofthisstudy havealreadyusedNACinthe
treatmentofexperimentalpre-hepatic portalhypertension,
withverypromisingresults.9,21Thisstudydemonstratedthe
role of this moleculeinthe bowel of animalssubjected to
thesamemodel,whichpointstoasystemicpotentialofthis
drug.NACwasabletoreducelipidperoxidationlevels,as
mea-suredbyTBA-RS,andtorestoretheactivityofbothantioxidant
enzymesstudied(SODandGPx)intheanimals’bowel.Thus,
onecan say thatN-acetylcysteine actedasanantioxidant,
reducingoxidativestressintheintestinalmucosainanimals
withPPVL.
In conclusion, we point out an anti-inflammatory and
antioxidantsynergisticeffectofN-acetylcysteineinthebowel
ofanimalswithportalhypertension.Judgingbytheresults,
NACwasabletoreducetheintestinaldamageinanimalsby
GPx
SO PPVL PPVL+NAC
∗
#
0.7 0.6 0.5 0.4 0.4 0.2 0.1 0.0
nmolmin/mgprot
Fig.6–GPx.Effectsofpartialportalveinligation(PPVL)and N-acetylcysteine(NAC)onGPxactivity.SO,Sham-operated group;PPVL,partialportalveinligation;PPVL+NAC,partial portalveinligationtreatedwithNAC.*p<0.05,#p<0.01
reducingoxidativestressandinflammation-bothbeing
con-ditionsextremelyharmfulanddeterminantintheevolution
ofthediseaseherestudied.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgements
This study received financial support from the Fundo de
IncentivoàPesquisaeEventos(FIPE ProjectNo.11-0293)of
Hospital de Clínicas de Porto Alegre (HCPA), Coordenac¸ão
de Aperfeic¸oamento de Pessoal de Nível Superior (CAPES)
andConselhoNacionaldeDesenvolvimentoCientíficoe
Tec-nológico(CNPq).Wewantalsotothankthecontributionof
the ExperimentalHepatology andGastroenterology
Labora-tory(HCPA/UFRGS)andtheOxidativeStressandAntioxidants
Laboratory(ULBRA),placesofsupportanddevelopmentofthis
study.
r
e
f
e
r
e
n
c
e
s
1. DmitryVictorovichGarbuzenko.Contemporaryconceptsof themedicaltherapyofportalhypertensionunderliver cirrhosis.WorldJGastroenterol.2015;21:6117–26.
2. BoschJ,GroszmannRJ,ShahVH.Evolutionintheunder standingofthepathophysiologicalbasisofportal hypertension:howchangesinparadigmareleadingto successfulnewtreatments.JHepatol.2015;62:S121–30.
3. GjeorgjievskiM,CappellMS.Portalhypertensivegastropathy: asystematicreviewofthepathophysiology,clinical
presentation,naturalhistoryandtherapy.WorldJHepatol. 2016;8:231–62.
4. MekaroonkamolP,CohenR,ChawlaS.Portalhypertensive enteropathy.WorldJHepatol.2015;7:127–38.
5. MisraV,MisraSP,DwivediM,GuptaSC.Histomorphometric studyofportalhypertensiveenteropathy.AmJClinPathol. 1997;108:652–7.
6. HoskingSW,SmartHL,JohnsonAG,TrigerDR.Anorectal varices,haemorrhoids,andportalhypertension.Lancet. 1989;1:349–52.
7. SikulerE,KravetzD,GroszmannRJ.Evolutionofportal hypertensionandmechanismsinvolvedinitsmaintenance inaratmodel.AmJPhysiol.1985;248:G618–25.
8. GonzalesS,PerezMJ,PerazzoJC,TomaroML.Antioxidantrole ofhemeoxygenase-1inprehepaticportalhypertensiverats. WorldJGastroenterol.2006;12:4149–55.
9. LicksF,HartmannRM,MarquesC,SchemitE,ColaresJR, SoaresMdoC,etal.N-acetylcysteinemodulatesangiogenesis andvasodilationinstomachsuchasDNAdamageinbloodof portalhypertensiverats.WorldJGastroenterol.
2015;21:12351–60.
10.GeertsAM,VanheuleE,PraetM,VanVlierbergheH,DeVosM, ColleI.Comparisonofthreeresearchmodelsofportal hypertensioninmice:macroscopic,histologicalandportal pressureevaluation.IntJExpPath.2008;89:251–63.
11.AllerMA,AriasJL,CruzA,AriasJ.Portalhypertensionand
inflammation:lessonsfromthepast.Hepatology,Research
Media,inpress.
12.AllerMA,AriasJL,CruzA,AriasJ.Inflammation:awayto understandingtheevolutionofportalhypertension.Theor BiolMedModel.2007;4:44.
13.PrietoI,AllerMA,SantamaríaL,NavaMP,MaderoR, Perez-RobledoJP,etal.Prehepaticportalhypertension producesincreasedmastcelldensityinthesmallboweland inmesentericlymphnodesintherat.JGastroenterol Hepatol.2005;20:1025–31.
14.GordonJR,GalliSJ.Mastcellasasourceofbothpreformed andimmunologicallyinducibleTNF-alpha/cachectin.Nature. 1990;346:274–6.
15.GlezerI,MarcourakisT,AvellarMCW,GorensteinC,Scavone C.TheroleofthetranscriptionfactorNF-kBinthemolecular mechanismsofactionofpsychoactivedrugs.RevBras Psiquiatr.2000;22:26–30.
16.HuLS,GeorgeJ,WangJH.Currentconceptsontheroleof nitricoxideinportalhypertension.WorldJGastroenterol. 2013;19:1707–17.
17.PautzA,ArtJ,HahnS,NowagS,VossC,KleinertH.Regulation oftheexpressionofinduciblenitricoxidesynthase.Nitric Oxide.2010;23:75–93.
18.MankanAK,LawlessMW,GraySG,KelleherD,McManusR. NF-kappaBregulation:thenuclearresponse.JCellMolMed. 2009;13:631–43.
19.CrowleySD.Thecooperativerolesofinflammationand oxidativestressinthepathogenesisofhypertension. AntioxidRedoxSignal.2014;20:102–20.
20.VercelinoR,TieppoJ,DiasAS,MarroniCA,GarciaE,MeurerL, etal.N-acetylcysteineeffectsongenotoxicandoxidative stressparametersincirrhoticratswithhepatopulmonary syndrome.BasicClinPharmacolToxicol.2008;102: 370–6.
21.LicksF,MarquesC,ZetlerC,MartinsMI,MarroniCA,Marroni NP.AntioxidanteffectofN-acetylcysteineonprehepatic portalhypertensivegastropathyinrats.AnnHepatol. 2014;13:370–7.
22.MarquesC,LicksF,ZattoniI,BorgesB,deSouzaLE,Marroni CA,etal.Antioxidantpropertiesofglutamineanditsrolein VEGF-Aktpathwaysinportalhypertensiongastropathy. WorldJGastroenterol.2013;19:4464–74.
23.MoreiraAJ,FragaC,AlonsoM,ColladoPS,ZetllerC,Marroni C,etal.QuercetinpreventsoxidativestressandNF-kB activationingastricmucosaofportalhypertensiverats. BiochemPharmacol.2004;68:1939–46.
24.GaffeyMJ,MillsSE,SwansonPE,ZarboRJ,ShahAR,WickMR. ImmunoreactivityforBER-EP4inadenocarcinomas,
adenomatoidtumors,andmalignantmesotheliomas.AmJ SurgPathol.1992;16:593–9.
25.BradfordMM.Arapidandsensitivemethodforthe
quantitationofmicrogramquantitiesofproteinutilizingthe principleofprotein-dyebinding.AnalBiochem.
1976;72:248–54.
26.TowbinH,StaehelinT,GordonJ.Electrophoretictransferof proteinsfrompolyacrylamidegelstonitrocellulosesheets: procedureandsomeapplications.ProcNatlAcadSci. 1979;76:4350–4.
27.LlesuySF,MileiJ,MolinaH,BoverisA,MileiS.Comparisonof lipidperoxidationandmyocardialdamageinducedby adriamycinand4′-epiadriamycininmice.Tumori.
1985;71:241–9.
28.BuegeJA,AustSD.Microsomallipidperoxidation.Methods Enzymol.1978;52:302–10.
29.MisraHP,FridovichI.Theroleofsuperoxideanioninthe autoxidationofepinephrineandasimpleassayfor superoxidedismutase.JBiolChem.1972;247:3170–5.
31.SauerbruchT,TrebickaJ.Futuretherapyofportal hypertensioninlivercirrhosis–aguess.F1000PrimeRep. 2014;6:95.
32.MartinelliALC.Hipertensãoportal.Medicina.2004;37:253–61.
33.SharmaM,RameshbabuCS.Collateralpathwaysinportal hypertension.JClinExpHepatol.2012;2:338–52.
34.GangulyS,SarinSK,BhatiaV,LahotiD.Theprevalenceand spectrumofcoloniclesionsinpatientswithcirrhoticand noncirrhoticportalhypertension.Hepatology.
1995;21:1226–31.
35.LuigianoC,IabichinoG,JudicaA,VirgilioC,PetaV,Abenavoli L.Roleofendoscopyinmanagementofgastrointestinal complicationsofportalhypertension.WorldJGastrointest Endosc.2015;7:1–12.
36.CoulonS,HeindryckxF,GeertsA,VanSteenkisteC,ColleI, VanVlierbergheH.Angiogenesisinchronicliverdiseaseand itscomplications.LiverInt.2011;31:146–62.
37.AllerMA,delasHerasN,NavaMP,RegaderaJ,AriasJ,Lahera V.Splanchnic-aorticinflammatoryaxisinexperimental portalhypertension.WorldJGastroenterol.2013;19: 7992–9.
38.MouraFA,deAndradeKQ,DosSantosJC,AraujoOR,Goulart MO.Antioxidanttherapyfortreatmentofinflammatory boweldisease:doesitwork.RedoxBiol.2015;6:617–39.
39.KerksickC,WilloughbyD.Theantioxidantroleofglutathione andN-acetylcysteinesupplementsandexercise-induced oxidativestress.JIntSocSportsNutr.2005;9:38–44.
40.deAndradeKQ,MouraFA,dosSantosJM,deAraújoOR,de FariasSantosJC,GoulartMO.Oxidativestressand
inflammationinhepaticdiseases:therapeuticpossibilitiesof N-acetylcysteine.IntJMolSci.2015;16:30269–308.
41.HouY,WangL,YiD,DingB,YangZ,LiJ,etal.N-acetylcysteine reducesinflammationinthesmallintestinebyregulating redox,EGFandTLR4signaling.AminoAcids.2013;45:513–22.