Patrícia Catarina Santos Rebelo
outubro de 2014
Comorbidity between experimental
osteoarthritis and mood disorders in the
rat: investigating the role of supraspinal
galanin
UMinho|20
14
Patrícia Catarina Sant
os R ebelo Comorbidity be tw een e xperiment al os teoar
thritis and mood disorder
s in t
he rat
: inves
tigating t
he role of supraspinal galanin
Universidade do Minho
Trabalho efetuado sob a orientação da
Professora Doutora Filipa Pinto-Ribeiro
e co-orientação da
Mestre Diana Amorim
Patrícia Catarina Santos Rebelo
outubro de 2014
Dissertação de Mestrado
Mestrado em Ciências da Saúde
Comorbidity between experimental
osteoarthritis and mood disorders in the
rat: investigating the role of supraspinal
galanin
Universidade do Minho
DECLARAÇÂO
Nome: Patrícia Catarina Santos Rebelo
Endereço eletrónico: patricia_gemea@hotmail.com Número do Bilhete de Identidade: 13904074
Título: Comorbidity between experimental osteoarthritis and mood disorders in the rat: investigating the role of supraspinal galanin
Orientadores: Professora Doutora Filipa Pinto-Ribeiro e Mestre Diana Amorim Ano de conclusão: 2014
Designação do Mestrado: Mestrado em Ciências da Saúde
É AUTORIZADA A REPRODUÇÃO INTEGRAL DESTA DISSERTAÇÃO APENAS PARA EFEITOS DE INVESTIGAÇÃO, MEDIANTE DECLARAÇÃO ESCRITA DO INTERESSADO, QUE A TAL SE COMPROMETE;
Universidade do Minho,
III
AGRADECIMENTOS
Esta tese de Mestrado representa um marco importante na minha vida, um longo caminho percorrido e sempre desejado. Cresci muito em termos de conhecimento e maturidade e, como tal, não podia deixar de agradecer a todas as pessoas que contribuiram para que isso fosse possível. Portanto, em homenagem a todos aqueles que sempre me apoiaram e que, direta ou indiretamente, colaboraram na realização deste trabalho, deixo aqui os meus sinceros e reconhecidos agradecimentos.
À minha orientadora, Doutora Filipa Pinto-Ribeiro, o meu maior agradecimento pela oportunidade, apoio, orientação e paciência ao longo deste ano, por toda a liberdade de ação e pelas sugestões e correções que permitiram o desenvolvimento de um trabalho melhor.
À minha co-orientadora, Mestre Diana Amorim, pela forma como me orientou no laboratório, pela sua preciosa e disponível ajuda nunca negada, pela imensa paciência e amabilidade com que sempre tentou responder às minhas questões e pelos experientes conselhos. Agradeço de coração tudo aquilo que me ensinou e todo o tempo que dedicou a este trabalho.
À Vera Cardoso, um especial agradecimento, por todas as “aulas” de microscópio, pela sua boa disposição e disponibilidade em colaborar sempre que solicitada a sua ajuda.
À Ana Pereira e Sónia Puga pela sua prontidão em ajudar.
A todos os docentes pelos conhecimentos transmitidos ao longo destes dois anos e por todos os ensinamentos de vida que contribuiram para o meu crescimento académico e pessoal.
Aos meus pais, que sem eles nada seria possível, pelo amor incondicional, pela educação e por toda a dedicação nesta minha caminhada. Serei eternamente grata por todo o esforço para que nunca me faltasse nada e por todo o apoio. A eles, que renunciaram aos seus sonhos para que eu pudesse realizar o meu, espero retribuir e compensar todo o conforto que sempre tive. Por isso, a eles dedico todo este trabalho.
Ao meu irmão por toda a amizade e apoio, e aos meus tios, primas e avó pelo incentivo recebido ao longo destes anos.
Ao meu namorado, pelo apoio e carinho diários, por toda a sua ajuda nas alturas mais difíceis, pela força que sempre me transmitiu e pela paciência com que ouviu as minhas inquietações,
IV
dúvidas e desânimos. O meu agradecimento de coração pela forma como tornou especial este ano e transformou momentos menos bons em momentos felizes.
Às meninas que levarei para sempre no meu coração:
- Cristiana, amiga e companheira de todas as horas de laboratório, pelos momentos felizes, desânimos e angústias que ultrapassamos juntas. Não esquecerei todas as nossas horas de microscópio juntas, assim como as nossas caminhadas noturnas.
- Maria João, que mesmo longe sempre deu o seu apoio e me lembrou que estava ali para tudo. Exemplo de coragem e força, agradeço toda a sua amizade verdadeira, todos os momentos divertidos e as longas conversas via skype.
- Joana, que por circunstâncias da vida nos últimos tempos nos vimos um pouco afastadas, mas que existiu sempre entre nós uma carinhosa amizade.
A todos, um carinhoso muito obrigada!
“Quando não souberes para onde ir, olha para trás e sabe pelo menos de onde vens.” (Provérbio africano)
V
Comorbidity between experimental osteoarthritis and mood disorders in the rat:
investigating the role of supraspinal galanin
ABSTRACT
Arthritis and depression are pathologies highly prevalent in society with great impact not only on National Health Systems but also on the quality of life of patients. Several studies have shown that anxiety and depression are common comorbidities of chronic pain patients while anxious and/or depressed individuals also display altered perception of pain. This interplay has been suggested to result from the fact that these pathologies share common modulatory pathways. Indeed, acute changes in the neurochemistry of brain areas, such as the amygdala (AMY), have been shown to transiently alter both mood and nociception. In chronic inflammatory pain, galanin (GAL) and its receptors have been recently proposed as potential mediators of pain-depression comorbidity as the expression of this neuropeptide is greatly increased not only in areas mediating emotions (AMY), but also in areas mediating nociception, such as the dorsomedial nucleus of the hypothalamus (DMH). Additionally, some studies using animal models of depression, have also demonstrated that the differential availability/activation of galanin receptors could induce a depressive profile in these animals. In the present work, we propose to (i) evaluate how GAL in the DMH influences anxiety- and/or depressive-like behaviour; (ii) study the role of the rostral ventromedial medulla (RVM) as a mediator of GAL effects and (iii) investigate which supraspinal areas might be involved in relaying GAL effects through the quantification of c-Fos expression. Wistar Han adult male rats were divided into two experimental groups: animals with experimental osteoarthritis (ARTH) and control animals (SHAM). In a first set of animals, emotional and nociceptive behaviours were assessed after GAL administration in the DMH. In a second set, c-Fos expression in caudal brain areas that mediate nociception was also quantified after GAL in the DMH and peripheral noxious stimulation of the right knee joint. Our results showed ARTH animals display a depressive phenotype concomitant with alterations in serotoninergic tone, a pathway mediated by GAL. Descending facilitation from the DMH after GAL microinjection appears to be mediated both by the RVM and the dorsal reticular nucleus (DRt).
VII
Comorbidade entre a osteoartrite experimental e os distúrbios do humor: avaliação
do papel da galanina
RESUMO
A artrite e a depressão são patologias muito prevalentes na sociedade com um grande impacto não só sobre os Sistemas Nacionais de Saúde mas também sobre a qualidade de vida dos pacientes. Vários estudos demonstraram que a ansiedade e a depressão são comorbidades comuns em pacientes que sofrem de dor crónica, enquanto indivíduos ansiosos e/ou deprimidos também apresentam alterações na perceção da dor. Considera-se que esta interação entre alterações emocionais e dor crónica resulta da partilha de vias moduladoras comuns entre as duas patologias. De facto, já se demonstrou que alterações agudas na neuroquímica de áreas cerebrais, como a amígdala (AMY), alteram tanto o humor como a perceção dolorosa. Na dor inflamatória crónica, a galanina (GAL) e os seus receptores foram recentemente propostos como potenciais mediadores da comorbidade dor-depressão, uma vez que a expressão deste neuropeptídeo está aumentada não apenas em áreas que medeiam as emoções (AMY), mas também em áreas que medeiam a nociceção, como o núcleo dorsomedial do hipotálamo (DMH). Paralelamente, alguns estudos realizados em modelos animais de depressão, também demonstraram que diferenças na disponibilidade/activação dos recetores de galanina promoviam um fenótipo depressivo nestes animais. Neste trabalho, propusemo-nos a (i) avaliar o efeito da GAL no DMH sobre o comportamento emocional e nocicetivo; (ii) estudar o papel da medula rostral ventromedial (RVM) como mediadora do efeito facilitador da GAL, e (iii) estudar quais as áreas supraespinhais que potencialmente medeiam a ação da GAL através da quantificação da expressão de c-Fos. Os animais, ratos machos Wistar Han adultos, foram divididos em dois grupos experimentais: animais osteoartríticos (ARTH) e animais controlo (SHAM), nos quais se avaliou o comportamento emocional e nocicetivo após a administração de GAL no DMH. Num segundo grupo avaliou-se a expressão de c-Fos em áreas cerebrais caudais após a administração de GAL no DMH e a aplicação de estímulos nóxicos periféricos. Os nossos resultados mostraram que a osteoartrite promove um fenótipo depressivo nos animais associado a alterações nas vias serotoninérgicas, as quais se sabe serem mediadas pela GAL. Por fim, verificou-se que a facilitação descendente após a ativação do DMH pela GAL é mediada pelo RVM e pelo núcleo reticular dorsal (DRt).
IX
INDEX
CHAPTER 1: INTRODUCTION 1
1.1 Pain mechanisms 3
1.1.1 The role of the nociceptors 4 1.1.2 Ascending pain pathways 6 1.1.3 Supraspinal and descending pain modulation 7 1.1.3.1 Dorsomedial nucleus of the hypothalamus (DMH) 9 1.1.3.2 Periaqueductal gray matter (PAG) 9 1.1.3.3 Dorsal raphe nucleus (DRN) 10 1.1.3.4 Locus coeruleus (LC) 10 1.1.3.5 Rostral ventromedial medulla (RVM) 11 1.1.3.6 Dorsal reticular nucleus (DRt) 12 1.2 Chronic pain 12
1.2.1 Inflammatory pain 13 1.2.1.1 Osteoarthritis (OA) 13 1.2.1.1.1 Most common comorbidities in OA 15 1.2.2 Galanin (GAL) 16 1.2.2.1 Regulation of GAL in inflammation 17 CHAPTER 2: OBJECTIVES 19
CHAPTER 3: MATERIALS AND METHODS 23
3.1 Animals and ethical considerations 25
3.2 Anaesthesia and euthanasia 25
3.3 Induction of experimental osteoarthritis 26
3.4 Drugs 26
3.5 Evaluation of nociceptive behaviour 27
3.5.1 Vocalization 27 3.5.2 Pressure application measurement (PAM) 27 3.5.3 Paw-withdrawal (PW) test 28 3.6 Chronic implantation of intracerebral cannulae 28
X
3.7 Evaluation of mood-like behaviour 29
3.7.1 Open field (OF) test 29 3.7.2 Forced swimming test (FST) 29 3.8 Evaluation of c-Fos expression 30
3.8.1 Stimulation of c-Fos 30 3.8.2 c-Fos immunohistochemistry 30 3.8.3 c-Fos quantification 31 3.9 Experimental design 32
3.9.1 Impact of GAL in the DMH upon emotional, motor and nociceptive behaviour (Experiment 1) 32 3.9.2 Evaluation of the expression of c-Fos after GAL in the DMH (Experiment 2) 33 3.10 Statistical analysis 34
CHAPTER 4: RESULTS 37
4.1 Histological confirmation of the injection sites 39
4.2 Nociceptive behaviour 40
4.2.1 Mechanical hyperalgesia 40 4.2.2 Heat hyperalgesia 40 4.2.3 Role of the RVM in behavioural hyperalgesia after GAL in the DMH 41 4.3 Locomotor activity 42
4.4 Mood-like behaviour 43
4.4.1 Anxiety-like behaviour 43 4.4.2 Depressive-like behaviour 44 4.5 c-Fos quantification 46
4.5.1 Ventrolateral periaqueductal gray matter (VLPAG) 46 4.5.2 Dorsal raphe nucleus (DRN) 47 4.5.3 Locus coeruleus (LC) 49 4.5.4 Rostral ventromedial medulla (RVM) 50 4.5.5 Dorsal Reticular Nucleus (DRt) 51 CHAPTER 5: DISCUSSION 53
XI
5.1.1 The choice of animal strain 55
5.1.2 Anaesthesia 55
5.1.3 The choice of animal model 56
5.1.4 Evaluation of nociceptive behaviour 58
5.1.4.1 Mechanical hyperalgesia 59
5.1.4.2 Heat hyperalgesia 60
5.1.5 Pharmacological studies 60
5.1.6 Evaluation of mood-like behaviour 61
5.1.6.1 Anxiety-like behaviour 61
5.1.6.2 Depressive-like behaviour 62
5.1.7 Evaluation of c-Fos expression 63
5.1.7.1 c-Fos as neuronal marker 63
5.1.7.2 c-Fos stimulation protocol 64
5.2 The role of GAL in the DMH upon behaviour 65
5.2.1 Locomotor activity 65
5.2.2 Anxiety-like behaviour 66
5.2.3 Evaluation of depressive-like behaviour 66 5.3 The RVM as a relay of the GAL pronociceptive action in the DMH 68 5.4 c-Fos expression upon key areas of nociception 69
5.4.1 Ventrolateral periaqueductal gray matter (VLPAG) 69
5.4.2 Dorsal raphe nucleus (DRN) 70
5.4.3 Locus coeruleus (LC) 71
5.4.4 Rostral ventromedial medulla (RVM) 72
5.4.5 Dorsal Reticular Nucleus (DRt) 73
CHAPTER 6: CONCLUSION AND FUTURE PERSPECTIVES 75 CHAPTER 7: REFERENCES 79
XIII
ABREVIATIONS
5-HT – serotoninABC – avidin-biotin complex AMY – amygdala
ANOVA2W – two-way analysis of variance
ARTH – osteoarthritic group
cAMP – cyclic adenosine monophosphate CGRP – calcitonin gene-related peptide CNS – central nervous system
DAB – diaminobenzine DAG – diacylglycerol
DMH – dorsomedial nucleus of the hypothalamus DNIC – diffuse noxious inhibitory controls
DRN – dorsal raphe nucleus DRt – dorsal reticular nucleus EPM – elevated plus maze FBS – fetal bovine serum FST – forced swimming test GABA – gamma-aminobutyric acid GAL – galanin
GALR – galanin receptors
GALR1 – galanin receptors type 1 GALR2 – galanin receptors type 2 GALR3 – galanin receptors type 3 GLU – glutamate
XIV K/C – kaolin/carrageenan LDB – light/dark box LC – locus coeruleus LIDO – lidocaine LWT – limb-withdrawal threshold
MAPK – mitogen-activated protein kinase MIA – monoiodoacetate
NA - noradrenaline OA – osteoarthritis OF – open field
PAG – periaqueductal gray matter
PAM – pressure measurement application PBN – parabrachial nucleus
PBS – phosphate buffer saline
pERK – phosphorylated extracellular signal-regulated kinase PFA – paraformaldehyde
PLC – phospholipase C
PVN – paraventriculat nucleus of the hypothalamus PW – paw-withdrawal
PWL – paw-withdrawal latency RVM – rostral ventromedial medulla SAL – saline
SEM – standard error SHAM – control group SP – substance P
XV
STM – peripheral noxious stimulation TF – tail-flick
VLPAG – ventrolateral periaqueductal gray matter VPL – ventral posterolateral
1
3
1. INTRODUCTION
The interest in the study of chronic pain and the search for therapies for its management has increased in the last decades (Perez, 2006). Chronic pain is a major health issue all over the world (Zhuo, 2008), affecting around 20% of the European population (McGuire and Kennedy, 2013; van Hecke et al., 2013). Unfortunately most of the available therapies have limited success, highlighting the need to better understand the mechanisms underlying the development and maintenance of chronic pain (Porreca et al., 2002).
The inability to satisfactorily treat pain has a profound economic impact on National Health Systems and in the quality of life of patients with ramifications to their families and society (Brennan et al., 2007). More importantly, prolonged longevity has also led to an increase in age-related diseases, most of which are accompanied by chronic pain, e.g. osteoarthritis and diabetes, again stressing the need for efficient therapies (Scholz and Woolf, 2002).
Pain is a multidimensional sensation that affects and is affected by emotional components such as mood (Katz, 2002), and is considered the fifth vital sign (Lynch, 2001; Morone and Weiner, 2013). According to the definition adopted by the International Association for the Study of Pain (IASP), pain is defined as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage” (Loeser and Treed, 2008). This definition clearly highlights that pain is a subjective experience and stresses the notion that pain can occur without apparent reason or visible injury (Rainville, 2002).
1.1 Pain mechanisms
Pain can be divided into acute and chronic pain. Acute pain being considered an important adaptive alarm system of short duration that usually disappears after healing, while chronic pain lasts for at least 3 months, long after its original cause has been treated (Casey et al., 2008). However, chronic pain differs from acute pain not only because of the duration of pain but, more importantly, because of the inability to restore its function to normal homeostatic levels (Loeser and Melzack, 1999).
Acute pain, defined as an adverse physiological response to a chemical, thermal or mechanical stimulus often associated with surgery, trauma or certain diseases (Carr and Goudas, 1999),
4
comprises a motor and emotional response (Brennan et al. 2007) and varies according to the intensity, type and duration of the stimulus (Voscopoulos and Lema, 2010).
Although the specific mechanisms underlying the transition from acute to chronic pain are mostly unknown (Lavand’homme, 2011; Buchheit et al., 2012), peripheral inflammation and the continuous activation of primary afferents play an important role in this process. Importantly, the persistent activation of primary afferents winds-up neuronal activity in the spinal cord leading to changes in the neurochemistry and signalling properties of neuronal networks not only within the spinal cord but also in the brain, a process designated as secondary or central sensitization (Julius and Basbaum, 2001).
Both, peripheral and central sensitizations are considered to play an important role in the transition from acute to chronic pain conditions (Apkarian et al., 2009; Voscopoulos and Lema, 2010), as they contribute to the development of chronic pain syndromes such as hyperalgesia (enhancement of the nociceptive response after a noxious stimulus), allodynia (a stimulus that previous was innocuous is now perceived as painful) and spontaneous pain (sharp pain sensation without any obvious source of stimulation) (Vadivelu et al., 2010).
Besides physical factors, life stressors and affective-cognitive factors have also been demonstrated to play an important role towards the sustainability of chronic pain (Casey et al., 2008; Kyranou and Puntillo, 2012). More recently Apkarian and colleagues (2011) proposed pain-related long-term memories remained active long after healing and contributed to the maintenance of chronic pain.
1.1.1 The role of the nociceptors
Pain is only perceived as such after the nociceptive signal is processed by the brain (Rainville, 2002), but nociceptors play an essential role in initiating it (Rainville, 2002). Nociceptors, or primary afferents, are responsible for the detection, transduction and transmission of peripheral stimuli to the spinal cord (Woolf and Ma, 2007).
Primary afferents are thus specialized receptors that detect noxious (painful) stimuli, such as extreme temperatures (heat and cold) and pressure (Dubin and Patapoutian, 2010), and
5
represent the first line of defense against any potential threats to the organism (Woolf and Ma, 2007).
Cutaneous primary afferents involved in the transmission of sensory information include three fibers types: Aβ-, A- and C-fibers (Julius and Basbaum, 2001), but only A- and C-fibers are involved in acute nociceptive transmission (D’Mello and Dickenson, 2008; Basbaum et al., 2009). A-fibers are small myelinated primary afferents responsible for fast nociceptive transmission (Basbaum et al., 2009). These fibers project to the superficial lamina I and lamina V of dorsal horn (Fig. 1B) and its activity is associated with the sensation of “first pain” (Basbaum et al., 2009; Dubin and Patapoutian, 2010).
On the other hand, C-fibers, are unmyelinated fibers and are responsible for slow conduction, whose activity is associated with “second pain” (Julius and Basbaum, 2001; Craig, 2003; Dubin and Patapoutian, 2010). Interestingly, while A-fiber activity is usually evoked by one stimulatory modality (only pressure or only temperatures), C-fibers respond simultaneously to mechanical, thermal and chemical stimuli (Julius and Basbaum, 2001), being classified as polymodal (Dubin and Patapoutian, 2010). In addition, C-fibers target mainly superficial laminae I and II (Fig. 1A) (Basbaum et al., 2009; Dubin and Patapoutian, 2010).
Figure 1 – Schematic representation of the projections of A- (B) and C-fibers (A) to the spinal cord (Dubin and Patapoutian, 2010).
B A
6
1.1.2 Ascending pain pathways
Once primary afferents synapse in the superficial dorsal horn of spinal cord, the signal is forwarded to the brain by projection neurons through multiple ascending pathways (Basbaum et al., 2009) such as the spinothalamic, spinomesencephalic, spinoreticular, spinohypothalamic and spinoreticulothalamic tracts (Lopes, 2007). These tracts relay nociceptive information to several levels (Almeida et al., 2004) with the lateral column relaying the discriminative component of pain (intensity, location, duration) while the medial column is associated with the transmission of cognitive/affective components of pain (Almeida et al., 2004; Lopes, 2007). Brain areas involved in the processing and modulation of pain are often referred to as the “pain matrix” or “homeostatic afferent processing network” (Neugebauer et al., 2009).
The spinothalamic tract is a crucial pathway for pain transmission (Willis, 1985). It is composed of a lateral component, that directly projects to the ventral posterolateral (VPL) and ventral posteromedial (VPM) nuclei of the thalamus, relaying information about sensory-discriminative aspects of pain to the somatosensory cortex (Almeida et al., 2006; Lopes, 2007). The medial component projects to the medial thalamus that conveys information to the limbic system concerning the emotional/cognitive aspects of pain (Almeida et al., 2004; Lopes, 2007).
The spinothalamic tract projects to multiple midbrain reticular areas that are involved in the descending modulation of pain (Lopes, 2007) such as the periaqueductal gray matter (PAG) (Millan, 1999), an area that plays a central role in the integration of supraspinal drives and modulation of nociception (Lemke, 2004).
The spinomesencephalic tract projects specifically to mesencephalic nucleus also involved in descending pain modulation (Lopes, 2007), namely the dorsal reticular nucleus (DRt) (Willis and Westlund, 1997) and the parabrachial nucleus (PBN) (Millan, 1999). It is also known that the stimulation of the regions innervated by the spinomesencephalic tract may evoke aversive behaviours in the presence of noxious stimulation (Dougherty et al., 1999; Almeida et al., 2004). The spinoreticular tract targets not only the lateral and medial reticular formation, involved in motor control and nociception, respectively (Millan, 1999; Almeida et al., 2004), but also the medial thalamic nucleus involved in the motivational-affective component of pain (Lopes, 2007; Almeida et al., 2004). Its importance is related to the fact that it targets brainstem structures responsible for the descending inhibition of pain (Almeida et al., 2004).
7
Finally, the spinohypothalamic tract projects to autonomic control centers in the hypothalamus, thalamus and amygdala (Lemke, 2004; Lopes, 2007). This tract is responsible for contributing to the activation of the neuroendocrine autonomic, motivational-affective responses to pain and for activating the “fight or flight” response in life threatening situations (Almeida et al., 2004; Lemke, 2004).
As evidenced by the number of areas activated during and after noxious stimulation, the transmission and modulation of nociception is not a linear process (Basbaum et al., 2009).
1.1.3 Supraspinal and descending pain modulation
The activation of areas processing nociception result in the activation of the descending pain modulatory pathways (Fig. 2) that, through brainstem relays with spinal projections, will either enhance or inhibit nociceptive transmission at the spinal cord level (Lima and Almeida, 2002). Descending pain modulation is a dynamic and plastic process (Lima and Almeida, 2002). It involves many brain regions that play an important role in the integration/processing of the emotional, cognitive and autonomic components of pain (Lopes, 2007) towards the control of the nociceptive transmission in the dorsal horn both in acute and chronic pain conditions (Millan, 1999; Heinricher et al., 2009). Literature shows that regions such as the medial and prefrontal cortex has been associated with the cognitive aspects of the modulation (Oshiro et al. 2009), the amygdala (AMY) with the emotional component processing (Neugebauer et al., 2009) and the paraventricular nucleus of the hypothalamus (PVN) (Pinto-Ribeiro et al., 2008) and dorsomedial nucleus of the hypothalamus (DMH) (Pinto-Ribeiro et al., 2013) with the autonomic and the innate responses to pain (Borszcz, 2006).
Several studies have shown that most frontal and medial brain areas present few or no projections to the spinal cord, these areas target relay nuclei such as the PAG (Fig. 2), the dorsal raphe nucleus (DRN), the locus coeruleus (LC), the rostral ventromedial medulla (RVM) and the DRt (Lemke, 2004; Tracey and Mantyh, 2007; Heinricher et al., 2009).
The PAG and the RVM, commonly known as the PAG-RVM system, (Lopes, 2007; Heinricher et al., 2009) were considered, for a long time, as the sources of descending inhibitory control (antinociception) (Heinricher et al., 2009). Since the PAG presents very few projections to the
8
spinal cord, its modulatory effects are relayed through spinal projecting RVM neurones (Hudson and Lumb, 1996).
However, it is now evident that the descending pain modulatory system, depending on the circumstances, can facilitate the spinal transmission of nociception (pronociception) (Ren and Dubner, 2002; Tracey and Mantyh, 2007; Heinricher et al., 2009).
Figure 2 – Schematic representation of the ascending and descending pain modulatory circuits (Adapted from Ossipov et al., 2010).
9
1.1.3.1 Dorsomedial nucleus of the hypothalamus (DMH)
The DMH, located in the hypothalamic region (Stotz-Potter et al., 1996), is implicated in a wide variety of functions including thermogenic responses to emotional stressors (DiMicco et al., 2002; DiMicco et al., 2006), and cardiovascular, locomotor and stress/anti-anxiety responses (Thompson et al., 1996). More importantly, the DMH is strongly involved in the “fight or flight” response as it is responsible for the activation of areas mediating acute behaviour and autonomic responses (DiMicco et al., 2002). Due to its role in acute stress and its projections to areas mediating pain (ter Horst and Luiten, 1986; Wagner et al., 2013), the DMH was first considered to inhibit nociception. Additionally, in 1996, anatomical studies by Thompson and colleagues, showed that the DMH projects to areas such as PAG (Samuels et al., 2004; Martenson et al., 2009), DRN and RVM.
However, more recently the DMH was proposed to mediate behavioural hyperalgesia (Martenson et al., 2009; Pinto-Ribeiro et al., 2013). Studies by Martenson and colleagues (2009) and Pinto-Ribeiro and colleagues (2013) showed the disinhibition of the DMH by bicucculine or its activation by glutamate (GLU), respectively, decreased tail-flick (TF) latency which was concomitant with changes in the activity of RVM pain modulatory cells towards the facilitation of nociception. Interestingly, while the behavioural effect of the DMH disinhibition/activation appears to be mediated the RVM in control animals, in an experimental model of monoarthritis it was absent (Pinto-Ribeiro et al., 2013).
Interestingly, while DMH glutamatergic projections appear to be inhibited in monoarthritic animals, galaninergic neurones remain active and the administration of galanin (GAL) in the DMH decreased paw withdrawal (PW) latency - pronociceptive affect - in normal and arthritic animals (Amorim et al., 2014).
1.1.3.2 Periaqueductal gray matter (PAG)
The PAG is a midbrain nucleus involved in several functions such as pain and analgesia, fear and anxiety, autonomic regulation and reproductive behavior (Behbehani, 1995; Linnman et al., 2012). However, it is for its role in pain modulation, both in acute and chronic conditions, that the PAG is mostly known (Waters and Lumb, 2008).
10
In 1969, Reynolds demonstrated that the electrical stimulation of the PAG evoked behavioral analgesia in rats (Loyd and Murphy, 2009), showing this area as an antinociceptive area able to inhibit nociceptive transmission in the spinal cord (Waters and Lumb, 1997; Cui et al., 1999; Loyd and Murphy, 2009). More recently, Waters and Lumb (2008) showed the neuronal activation of the PAG may selectively “modulate” the activity of A and C-fibers by suppressing the activity of latter while enhancing the activity of the former. These authors showed for the first time, that the PAG could play both an antinociceptive and a pronociceptive role.
1.1.3.3 Dorsal raphe nucleus (DRN)
The DRN is another midbrain nucleus (Michelsen et al., 2008) where approximately half of the brain’s serotonergic neurons can be found (Jacobs and Azmitia, 1992; McDevitt and Neumaier, 2011). The DRN has been strongly associated with a great variety of physiological and behavioural functions, namely pain, sleep and mood disorders, such as depression (Peyron et al., 1998; Michelsen et al., 2008).
This nucleus projects to sensory-motor areas in the rat (Kirifides et al., 2001; Lee et al., 2008) and receives projections from many limbic areas, such as the prefrontal cortex, the hypothalamus and the AMY (Nakamura, 2013). Its descending projections were shown to modulate the behavioural responses evoked by noxious stimulations and may be involved in analgesia (Wang and Nakai, 1994). It is also known that the inhibition of the activity of serotonergic neurons in the DRN reduces anxiety in animal models while increasing it enhances anxiety (Maier and Watkins, 2005).
1.1.3.4 Locus coeruleus (LC)
The LC, located in the dorsolateral pons (Liu et al., 2008) contains most of the noradrenergic neurones in the brain and is involved in several biological functions including attention, vigilance, brain plasticity, learning and memory (Berridge and Waterhouse, 2003; Liu et al., 2008). This nucleus receives projections from several areas, including the PAG and the hypothalamus, and targets the RVM and the spinal cord (Maeda et al., 2009; Ossipov et al., 2010).
11
Its role in pain modulation was first suggested by anatomical studies showing strong projections to areas such as the PAG, the RVM and the spinal cord (Maeda et al., 2009). In addition, electrophysiological studies showed an increase in the activity of LC neurones during peripheral noxious stimulation, while its activation by morphine (Jones and Gebhart, 1988) inhibited nociception (Liu et al., 2008; Maeda et al., 2009; Szabadi, 2012). Tsuruoka and Willis (1996) and Maeda and colleagues (2009) showed that during chronic inflammation, the activation of the LC decreased hyperalgesia, while LC bilateral lesion increased pain syndromes (hyperalgesia and allodynia) and the expression of c-Fos protein, a marker of cell activation (Tsuruoka et al., 2003).
1.1.3.5 Rostral ventromedial medulla (RVM)
The RVM is considered as one of the brain effectors of pain descending modulation (Millan, 2002; Chai et al., 2012) as it projects to the dorsal horn of the spinal cord, where it is able to directly and indirectly modulate the activity of primary afferents (Millan, 2002).
The RVM exerts a biphasic descending action either enhancing (facilitation) or inhibiting spinal nociceptive transmission (Carlson et al., 2007; Lopes, 2007; Tillu et al., 2008; Aicher et al., 2012), depending on the type and intensity of the initial stimulus (Urban and Gebhart, 1997; Bee and Dickenson, 2007). Interesting, the inactivation of this nucleus by lidocaine (LIDO) attenuates spinal hyperalgesia and mechanical allodynia (Géranton et al., 2010) in acute inflammation (Ambriz-Tututi et al., 2011; Cleary and Heinricher, 2013) and neuropathic pain (Pertovaara et al., 1996; Sanoja et al., 2008).
The facilitatory or inhibitory action of the RVM is associated with the activity of its heterogeneous neurones that can be divided into: ON-, OFF- and NEUTRAL-cells (Carlson et al., 2007; Khasabov et al., 2012). ON-cells are known to facilitate nociception since they increase their activity in response to noxious stimuli just prior to the motor withdrawal reflex while OFF-cells inhibit nociceptive transmission as their activity decreases immediately before the motor withdrawal response (Fields et al., 1983; Martenson et al., 2009; Khasabov et al., 2012).
By contrast, NEUTRAL-cells do not respond to noxious or innocuous peripheral stimulation, although a role in pain modulation could not be ruled out (Miki et al., 2002; Martenson, 2009). In fact, Miki and colleagues (2002) observed that, during prolonged inflammation, NEUTRAL-cells
12
shifted their response profile from NEUTRAL- to ON- or OFF-like cells and thereby also contributed to the descending modulation of pain in chronic disorders (Khasabov et al., 2012).
1.1.3.6 Dorsal reticular nucleus (DRt)
The DRt is located in the most caudal portion of the medullary dorsolateral reticular formation (Leite-Almeida et al., 2006) and is one of the few nuclei that exclusively facilitate nociception (Almeida et al., 1996; Tavares and Lima, 2007). Interestingly, this area is activated exclusively by noxious stimuli independently of the body part stimulated (Almeida et al., 1996, 1999; Lima and Almeida, 2002; Leite-Almeida et al., 2006). Anatomically, the DRt shares reciprocal projections with the spinal cord and several brainstem areas such as the RVM, PAG and LC, and forebrain areas (Lima and Almeida, 2002; Leite-Almeida et al., 2006). In addition to its activation during acute stimulation, the DRt also displayed significant activation in chronic pain states (inflammatory pain and neuropathic pain) (Pinto et al., 2006, 2008).
1.2 Chronic pain
The continuous activation of nociceptors increase synaptic excitability, decrease activation thresholds and increase responsiveness of spinal neurons (Woolf and Ma, 2007; Woolf, 2011) resulting in the plasticity of areas mediating nociception and leading to the development of pain syndromes (hyperalgesia, allodynia and spontaneous pain) (Wall, 1979; Dubin and Patapoutian, 2010; Woolf, 2011).
During central sensitization, pain is no longer proportional to the intensity and duration of the peripheral noxious stimuli (Woolf, 2011) due the occurrence of neuroplasticity in spinal and supraspinal circuits mediating pain (Besson, 1999; Loeser and Melzack, 1999). Apkarian and colleagues (2009) proposed plastic changes in the pain matrix led to “the inability to extinguish the memory evoked by the initial injury” which imbalanced the facilitatory and inhibitory descending pain drives towards the exacerbation of pain.
Chronic pain can be divided into (i) nociceptive or inflammatory, (ii) neuropathic and (iii) neurogenic pain (Carr and Goudas, 1999; Scholz and Woolf, 2002). Chronic nociceptive pain is usually associated with recurrent inflammatory processes due to tissue injury and/or the
13
activation of immune cells (Backonja, 2003), while neuropathic pain is associated with lesion of the peripheral and central nervous system (Woolf, 2001). Neurogenic pain occurs when a cause (physical) cannot be associated to pain (Bowsher, 1991).
1.2.1 Inflammatory pain
Inflammatory pain may be classified as acute or chronic (Lawrence and Gilroy, 2007) and although chronic inflammatory pain shares many characteristics with it acute counterpart, it is biologically distinct in what concerns exudation, cellular recruitment and the types of cells that prevail in the chronic inflammatory response (Wakefield and Kumar, 2001).
Chronic inflammatory pain is characterized by long-term inflammation (or recurrent episodes of prolonged inflammation) (Heap and van Heel, 2009), which induce the release of an “inflammatory soup” from the affected tissues (Julius and Basbaum, 2001). This “inflammatory soup” containing chemical mediators activates surrounding nerve endings and nociceptors evoking pain (Scholz and Woolf, 2002; Kyranou and Puntillo, 2012). Besides external causes (such as injuries) (Wakefield and Kumar, 2001), chronic inflammation might also be due to aging, as tissue degeneration occurs (Backonja, 2003).
Age associated chronic inflammatory disorders include knee osteoarthritis, multiple sclerosis and Chrohn’s disease (Wakefield and Kumar, 2001; Sommer and Kress, 2004; Heap and van Heel, 2009).
1.2.1.1 Osteoarthritis (OA)
OA is the leading cause of disability in the elderly population and affects a large proportion of society (Dieppe and Lohmander, 2005; Arendt-Nielsen et al., 2010; Egloff et al., 2012; Ferreira-Gomes et al., 2012). Epidemiological studies estimate that OA affects approximately 43 million people in the Unites States (Egloff et al., 2012) and 10% of the world’s population over 60 years (Adães et al., 2014). According to Monjardino and colleagues (2011), in Portugal 11,1% of the population suffers from OA with 5,5% suffering from knee OA.
14
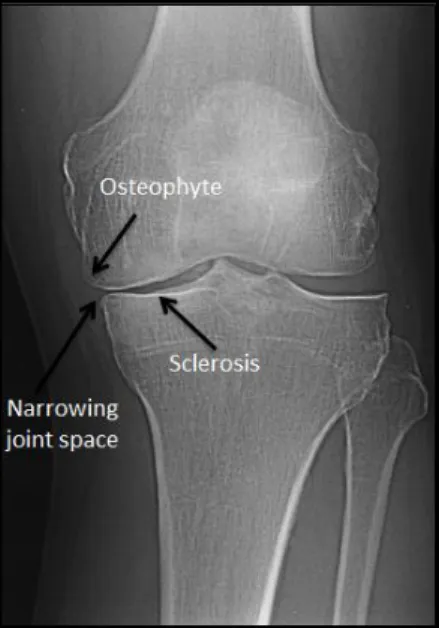
OA is a chronic multifactorial disease characterized by a progressive degradation of the articular cartilage (narrowing joint space) associated with subchondral bone remodeling, bone sclerosis, the formation of bone cysts, marginal osteophytes and secondary inflammation of synovial membranes (Fig. 3) (van Laar et al., 2012; Hawamdeh and Al-Ajlouni, 2013). It is a degenerative disease that involves nociceptive and non-nociceptive components due to peripheral inflammation and central sensitization (Woolf, 2011; Arendt-Nielsen et al., 2010).
Figure 3 – Radiograph of a knee joint from a patient suffering from knee osteoarthritis. Note the development of osteophytes, the existence of bone sclerosis and the narrowing of the space between the adjacent joints (Adapted from Dieppe and Lohmander, 2005).
Amongst the possible affected joints, the knees present the higher prevalence of OA (Arden and Nevitt, 2006; Jinks et al., 2007; Arendt-Nielsen et al., 2010). Knee OA leads to decreased knee flexibility, constant pain and joint effusion, crepitus, bone deformities and loss of function (Hawamdeh e Al-Ajlouni, 2013). OA patients display lower withdrawal thresholds during mechanical and thermal stimulation when compared to healthy controls (van Laar et al., 2012). They also display increased sensitivity to innocuous stimulation (Kosek e Ordeberg, 2000) suggesting that in this disorder pain is centrally mediated (Lee et al., 2011).
Indeed, functional magnetic resonance imaging studies allowed the identification of several brain regions involved in processing of pain in OA patients including the thalamus and AMY (Sofat et al., 2011). Interestingly, the intensity of pain reported by patients is not proportional to the extent
15
of joint damage (van Laar et al., 2012) again suggesting the occurrence of central plasticity in areas mediating pain.
The degeneration of knee structures induces a complex and dynamic cascade of biochemical and cellular inflammatory events (Ji et al., 2011) affecting the normal activity of surrounding nociceptors (Nagy et al., 2006) towards the enhancement on spinal nociceptive signaling through the excessive release of substance P (SP) (Khasabov et al., 2012), calcitonin gene related-peptide (CGRP) (Bird et al., 2006; Nagy et al., 2006; Orita et al., 2011) and GAL (Liu and Hökfelt, 2002).
1.2.1.1.1 Most common comorbidities in OA
In addition to the exacerbation of pain, the development of emotional impairments is common in OA patients. Epidemiological studies show the incidence of chronic pain enhances the development of mood disorders, such as anxiety and depression (Barton et al., 2007; Sherbourne et al., 2009; Axford et al., 2010). Similarly, patients suffering from anxiety and/or depression display changes in sensitivity and are more likely to develop chronic pain (Neugebauer et al., 2009). In the case of OA patients, depression is the most common comorbidity observed (Lin, 2008).
The comorbidity between chronic pain and emotional impairments is thought to result from the existence of pathways that modulate both pain and emotions (Krishnan et al., 2008). This theory is supported by the fact, that in elderly patients displaying both depression and arthritis, the administration of antidepressants not only improved mood but also reduced the intensity of pain contributing significantly to the improvement of the quality of life of these patients (Lin, 2008). Although the mechanisms underlying chronic pain-depression comorbidities are still poorly understood (Agarwal et al., 2013) GAL and its receptors are considered potential mediators of this interplay, at least in rodents (Kuteeva et al., 2008). GAL exerts modulatory effects in noradrenergic and serotonergic systems and Kuteeva and colleagues (2008) recently demonstrated, in an animal model of depression, that the differential activation of GAL receptors (GALR) could induce a more or less depressive-like profile. Interestingly, GAL receptors type-1 (GALR1) promoted “prodepressive”-like behaviours while GAL receptors type-2 (GALR2) promoted an “antidepressant”-like phenotype.
16
1.2.2 Galanin (GAL)
GAL is a neuropeptide consisting of 29 or 30 amino acids (Xu et al., 2000; Elliot-Hunt et al., 2004) involved in many diverse biological functions, including learning, feeding, memory, cognition, neuroendocrine modulation and nociception (Jimenez-Andrade et al., 2004; Xu et al., 2012a).
GAL is expressed widely throughout the central nervous system of various species, including the rat (Lang et al., 2007), namely in the hypothalamus (preoptic, paraventricular, periventricular and dorsomedial nuclei), DRN, LC, RVM, AMY (medial and lateral) and supraoptic nuclei (Fang et al., 2012). This neuropeptide is also co-expressed with other neurotransmitters such as serotonin (5-HT) in DR, norepinephrine (NA) in LC, SP and CGRP in dorsal root ganglia and gamma-aminobutyric acid (GABA) in the dorsal horn (Landry et al., 2005; Lang et al., 2007). GAL is released in the spinal cord predominantly by C-fiber (Liu and Hökfelt, 2002; Xu et al., 2012b) during nerve injury and peripheral inflammatory processes (Liu and Hökfelt, 2002; Hulse et al., 2012).
GAL is an important messenger for intercellular communication (Xiong et al., 2005) and its role in pain modulation is complex (Holmes et al., 2003). It’s involved in the transmission and modulation of nociception in the spinal cord (Fu et al., 2011) in a dose-dependent manner, with high concentrations promoting antinociception and low concentrations pronociception (Hulse et al., 2012).
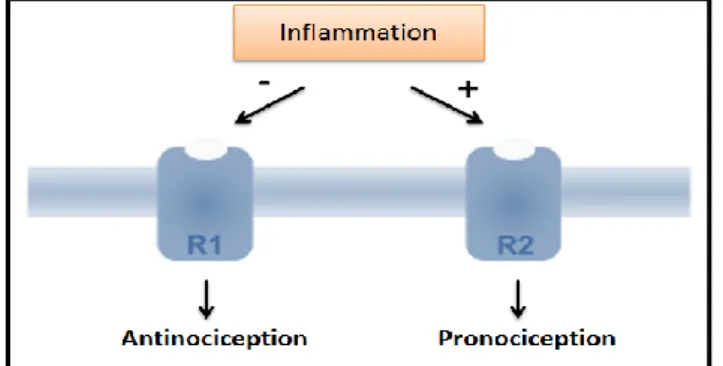
GAL effect depends on the activation of three GAL G-protein-coupled receptor subtypes present in primary afferent neurones: GALR1, GALR2 and GAL receptor type-3 (GalR3) (Fig. 4) with GALR1 and GALR3 being inhibitory and GalR2 excitatory (Xu et al., 2008, Hulse et al., 2012). The signaling properties of GalR3 are still poorly defined (Lang et al., 2007) and its expression in rodents is weak (Lu et al., 2005; Yu et al., 2013). By contrast GALR1 and GALR2 are highly expressed in both humans and rodents (Hohmann et al., 2003; Yu et al., 2013) although their expression between species varies significantly (Kuteeva et al., 2008).
In normal conditions, the activation of signaling pathways via GALR1 and GALR3 facilitate hyperpolarization through Gi/G0 type G-proteins (Liu and Hökfelt, 2002) (Fig. 4) and,
consequently, contributes to the inhibition of circuits involved in nociception in spinal cord (Landry et al., 2005). According to Landry and colleagues (2005), the activation of GALR1 and
17
GALR3 can lead to cell hyperpolarization through activation of Gi/Go proteins with a consequent decrease of cyclic adenosine monophosphate (cAMP) intracellular levels, that is a second messenger used in the ntracellular transduction, and opening of rectifying potassium (K+)
channels. GALR1 activating mitogen-activated protein kinase (MAPK) (Fig. 4), that is involved in cellular responses to stimulation, for example factors proinflammatory. Contrary, GALR2 binds to the alpha q/11 subunit and stimulate the phospholipase C (PLC), resulting in the increase of inositol (1,4,5)-triphosphate (Ins(1,4,5)P3) and intracellular calcium (Ca2+), and in the activation
of protein kinase C via diacylglycerol (DAG) (Fig. 4). So, there is an enhancement of neural excitation and the transmitter release is increased (Liu and Hökfelt, 2002; Landry et al., 2005).
Figure 4 – Transduction mechanisms of GAL receptors (K+-potassium; AC-adenylyl cyclase; ATP-adenosine
triphosphate; cAMP- cyclic adenosine monophosphate; MAPK-mitogen-activated protein kinase; PLC-phospholipase C; Ptdlns(4,5)P2-phosphatidylinositol 4,5-bisphosphate; Ins(1,4,5)P3- inositol (1,4,5)-triphosphate; Ca2+ -calcium). (Liu
and Hökfelt, 2002)
1.2.2.1 Regulation of GAL in inflammation
Several studies showed that GAL is involved in the regulation of inflammatory processes (Land and Kofler, 2011) as this neuropeptide is strongly up-regulated (10x) during inflammatory events (Liu and Hökfelt, 2002). This observation also suggested GAL could be modulating nociception at the spinal cord (Sun et al., 2003; Lang and Kofler, 2011), a fact that was confirmed when the intrathecal administration of GAL altered nociception (Lemons and Wiley, 2011). Furthermore, during inflammatory processes, the pronociceptive effect of exogenous GAL in the spinal cord
18
was associated with the activation of GALR2 while activation of GALR1 was antinociceptive (Liu and Hökfelt, 2002) (Fig. 5).
Figure 5 – Schematic representation of the effect of the activation of galanin receptors during inflammation. The pronociceptive role of GAL during inflammation is associated to a down-regulation of GALR1 and up-regulation of GALR2 in the spinal cord (Lundström et al. 2005).
Although, the action of GAL in nociceptive modulation has been intensively investigated in the spinal cord using behavioural and electrophysiological techniques (Sun et al., 2003; Gu et al., 2007), the role of GAL at the supraspinal level is still unclear (Yu et al., 2013).
In the brain, the intracerebroventricular administration of GAL activates areas that facilitate nociception, such as the DMH and the AMY (Blackshear et al., 2007) as shown by increased expression of c-Fos in these areas (Fang et al., 2012). Preliminary data from our group showed that the activation of GALR in the DMH enhance nociception in rats (data not published). In addition, Xiong and colleagues (2005) demonstrated that the intrathecal administration of GAL increased hindpaw withdrawal latencies during the application of noxious thermal and mechanical stimuli in rats with inflammation (Yu et al., 2013).
19
21
2. OBJECTIVES
OA is a chronic disease characterized by inflammation of the cartilage and adjacent structures as a result of a gradual degeneration of articular structures. The release of proinflammatory factors activates surrounding nerve endings causing pain. The prolonged activation of primary afferent increases excitability at the spinal cord level promoting sensitization of spinal neurons and supraspinal areas involved in pain modulation. These neuroplastic events lead to the development of hyperalgesia, allodynia and spontaneous pain. In addition to the exacerbation of pain, the occurrence of emotional disturbances is also common in chronic pain patients. Although the mechanisms underlying this comorbidity are poorly understood, the galaninergic pathways are one of the potential players in the correlation between these pathologies.
Taking the above mentioned into account and the fact that our group recently developed an experimental model of OA in which animals display concomitant mood disorders, the objective of this work was to evaluate the effect of the activation of DMH neurones expressing GAL receptors upon the activity of caudal brain areas involved in the processing of nociception, through the use of behavioural and molecular approaches. More specifically, our objectives were to:
1. Evaluate the effect of the activation of the DMH by GAL upon emotional, motor and nociceptive behaviour in controls and animals with experimental OA;
2. Verify if the RVM mediates descending facilitation after GAL in the DMH;
3. Investigate which supraspinal areas are activated by the intracerebral administration of GAL in the DMH through the quantification of c-Fos expression in the brain;
23
25
3. MATERIALS AND METHODS
3.1 Animals and ethical considerationsIn this work we used Wistar Han adult male rats weighing between 235 – 285g (Charles River, Barcelona, Spain) at the beginning of experiment. The animals were housed two per cage under standard conditions at a constant ambient temperature of 20-24°C, relative humidity of 55+/-10% with a 12h-12h light-dark cycle (light between 08.00h and 20.00h) and food and water available ad libitum throughout the experiment. All procedures performed in this work were approved by the European Community Council Directive 86/609/EEC and 2010/63/EU concerning the use of animals for scientific purposes and by the ICVS Ethical Commission. The experiments were designed to minimize animal suffering as well as the number of animals used. Prior to performing any procedures, all animals were submitted to daily handling sessions (10 min) and those animals used in behavioural assessments were habituated to the experimental conditions by spending 1-2 hours every day of the week preceding the test in the testing room.
3.2 Anaesthesia and euthanasia
For the induction of OA and intracerebral implantation of cannulae, the animals were anaesthetized with a mixture of ketamine (1.5 mg/kg; Imalgene®, Merial, Lisbon, Portugal) and medetomidine (1.0 mg/kg; Dorbene®, ESTEVE, Carnaxide, Portugal) administered intraperitoneally (i.p.). After the surgery, the animals were recovered through the administration of atipamezole (0.1 mL/kg i.p.; Antisedan®, Pfizer, Seixal, Portugal) and were monitored until fully awake (feeding and grooming).
In order to perform the protocol to induce c-Fos expression, the animals were anaesthetized using pentobarbitone (0.5 mL/kg; Eutasil®, CEVA, Algés, Portugal) delivered i.p. The level of anaesthesia was monitored by pinching of the tail and dilatation of the pupils and was maintained by infusing pentobarbitone diluted in saline solution (SAL; 0.25 mL/kg/h i.p.; Unither, Amiens, France; pH=7.2).
26
At the end of the behavioural period and at the end of each experimental session of the c-Fos stimulation protocol, the animals were injected with a lethal dose of pentobarbitone i.p. (Eutasil®, CEVA) and transcardially perfused with 200 mL of 4% paraformaldehyde (PFA; Panreac, Barcelona, Spain) in 0.1M phosphate buffer saline (PBS; pH=7.4) at room temperature for easier diffusion. Afterwards, the brains were excised, post-fixed in the same fixative (PFA 4%) for a week and then placed for 48 hours in a solution of 8% sucrose (Panreac). The contralateral side of the brain was marked with a short cut and the brains were then sectioned in coronal sections (50 µm of thickness) in a vibratome (Leica VT100, Freiburg, Germany). The sections were mounted in microscope slides, counterstained, dehydrated, covered in mounting media (Entellan New, Merck, Darmstadt, Germany) and cover slipped. This process was performed in order to confirm the injection sites by comparing the coronal section with plates from the rat brain atlas (Paxinos and Watson, 2007). In case of evaluation of c-Fos expression, the coronal sections were not counterstained, they were collected in 12 wells culture plates previously filled with PBS for c-Fos immunohistochemistry.
3.3 Induction of experimental osteoarthritis
The induction of osteoarthritis (OA) in rats was performed according to the protocol described previously by Pinto-Ribeiro and colleagues. (2011). Briefly, a solution of 3% kaolin (Sigma-Aldrich, St Louis, MO, USA) and 3% carrageenan (Sigma-Aldrich) dissolved in 0.9% sodium chloride (NaCl; B. Braun, Barcarena, Portugal) was injected (0.1 mL) in the synovial capsule of the right knee of animals in the osteoarthritic group (ARTH), while the control group (SHAM) animals were injected with 0.1 mL SAL in the synovial capsule of the right knee. The injection of carrageenan induces an inflammatory reaction that results in mechanical hyperalgesia, while kaolin is responsible for the mechanical damage to the knee joint structures (Okamoto et al., 2013).
3.4 Drugs
Galanin (GAL; 1.0 nmol in 0.5 µL saline; Tocris, Ellisville, MO, USA) was prepared to perform intracerebral injections in DMH. 2 % Lidocaine (LIDO; B. Braun) was used in the intracerebral injections in the RVM. These drugs were administered in the DMH and RMV using a 33-gauge injection cannula (Plastics One) connected to a syringe (5.0 µL Hamilton, Nevada, USA) with a
27
microinjection volume of 0.5 µL. The intracerebral injections lasted for 20s in order to prevent activation of the DMH and RVM by pressure. After the injections, the guide cannula was left in place for another 30s to minimize the return of the drug solution back to the injection cannula. The expected spread of injecting 0.5 µL of the drugs in brain was around +/- 1 mm in diameter (Myers, 1966). The drug doses were chosen in accordance to previous studies (Pinto-Ribeiro et al., 2008) and control injections were performed with SAL as control values to eliminate any bias that may result from injecting the solutions themselves.
All drug injections were performed taking into account that GAL and LIDO reached their peak effect between 10 and 20 min after the injection (Gillis et al., 1973; Brock et al., 2001; Wang et al., 2000; Sun and Yu, 2005; Xiong et al., 2005; Gu et al., 2007).
3.5 Evaluation of nociceptive behaviour 3.5.1 Vocalization
For each animal, the development of OA was confirmed by performing five consecutive flexion-extension movements of the injected knee joint, 3 days after the induction. All ARTH animals vocalized every time during each flexion-extension movement while SHAM animals did not vocalized during the same procedure.
3.5.2 Pressure application measurement (PAM)
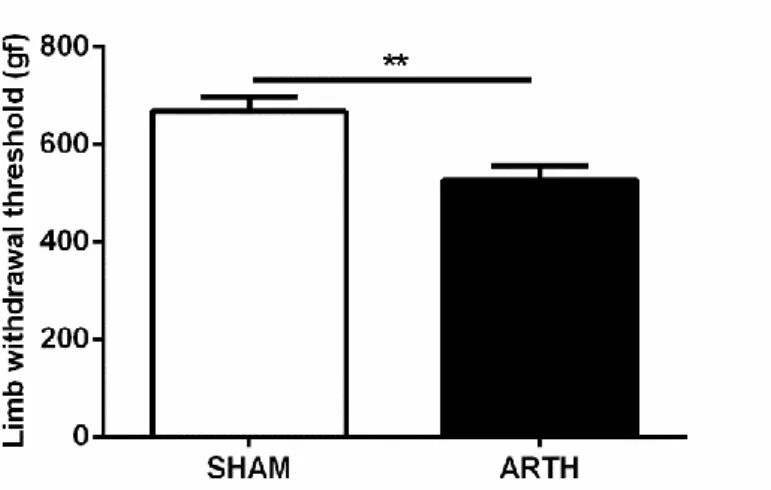
The pressure application measurement (PAM) is a novel behavioural test which allows measuring mechanical hyperalgesia in rodent’s joints by the application of a force range of 0-1500g (Barton et al., 2007). Briefly, the animals (n=19) were securely held and the force transducer was placed on one side of the animal’s knee joint using the thumb and the forefinger on the other. Afterwards, a gradually increasing force was applied across the joint until the animal showed behavioural signs of discomfort (vocalization) or withdrew the limb. The value of the peak gram force (gf) applied was recorded as the limb-withdrawal threshold (LWT). A total of two measurements were made in the ipsilateral knee joint of both SHAM and ARTH animals and the mean LWTs were calculated.
28
3.5.3 Paw-withdrawal (PW) test
The paw-withdrawal (PW) test is a tool that allows assessing thermal hyperalgesia by measuring hind paw withdrawal latency (PWL) (Ossipov et al., 1999; Saegusa et al., 2000) as described by Hargreaves and colleagues (1988). Firstly the animals (n=19) were habituated to the experimental conditions, where they were placed on the test apparatus (Plantar Test Device Model 37370, Ugo Basile, Comerio, Italy) for 30 min every day of the week preceding the test. After, for assessing nociception in unanaesthetized animals, the PWL following radiant heat stimulation onto the plantar aspect was determined before and 10, 20 and 30 min after the intracerebral administration of the drugs, SAL and/or GAL and/or LIDO administration to the DMH and/or RVM, according to the protocol for each experimental group (Fig. 7). In each animal, the measurements were repeated twice and the mean of these values was used for further calculations. A cut-off time of 14 s was used to prevent any tissue damage.
3.6 Chronic implantation of intracerebral cannulae
For drug administration, two stainless steel guide cannulae (26 gauge; Plastics One, Roanoke, VA, USA) were implanted in the brain, one in the DMH and another in the RVM as described by Pinto-Ribeiro and colleagues (2013). Briefly, the animals were anaesthetized as described in section 3.2 and in order to avoid blindness, due to dehydratation of eyes, they were protected by applying vaseline. The animals were, then, placed in a stereotaxic frame (KOPF Instruments, Tujunga, California, USA), a longitudinal incision was made with a scalpel in the skin above the skull and a sterilized stainless steel guide cannula was vertically positioned 1 mm above the desired injection site in the DMH (rostrocaudal: -3,24 mm, lateromedial: -0,4 mm; dorsoventral: -7,9 mm) and RVM (rostrocaudal: -10,92 mm; lateromedial: 0,0 mm; dorsoventral: -9,4 mm) according to the coordinates of the atlas by Paxinos and Watson (2007). The guide cannulae were firmly fixed into the skull with two anchoring screws and using dental acrylic cement. Subsequently, the skin was sutured and a dummy cannula (Plastics One) was inserted into each guide cannulae to prevent contamination and to maintain patency. At the end, the animals were placed one per cage. Before any tests were performed, they were allowed to recover for at least one week.
29
3.7 Evaluation of mood-like behaviour 3.7.1 Open field (OF) test
The open field (OF) test is used to assess anxiety-like behaviour and locomotor performance as described by Leite-Almeida and colleagues (2009). In summary, 15 min after intracerebral administration of SAL or GAL to the DMH, according to the protocol for each experimental group (Fig. 7), each animal (n=18) was placed in the centre of a squared arena (43.2 x 43.2 cm; Med Associates Inc., St. Albans, Vermont, USA) with light intensity of 240 lx in the central arena. The animal’s behaviour was recorded for 5 min using a screen monitor connected to a video camera and its exploratory activity was automatically registered by sensors. At the end of each session, the arena (inner areas, floor and walls) was cleaned with a solution of 10% alcohol. The anxiety-like behaviour was assessed by counting the number of faeces left in the arena at the end of the OF test and measuring the time spent in the center of the arena (Mesquita et al., 2006; Leite-Almeida et al., 2009; Amorim et al., 2014). The locomotor performance was assessed by measuring the total distance travelled by the animals (Mesquita et al., 2006; Leite-Almeida et al., 2009; Amorim et al., 2014).
3.7.2 Forced swimming test (FST)
The forced swimming test (FST) is a behavioural test that evaluates learned helplessness and that is widely used for assessing antidepressant effect of drugs (Bessa et al., 2009; Slattery and Cryan, 2012). This test was performed according to a report by Amorim and colleagues (2014). On day 1, the rats (n=19) were submitted to a 5 min pre-test session, where they were individually placed in transparent cylinders containing clean water, with a water depth such that animals could not touch the base with their hind limbs or tails, (25°C; depth 30 cm). On day 2, 24h later, in each animal per experimental group (SHAM and ARTH), SAL or GAL was administered to the DMH according to the protocol as shown in figure 7. Fifteen min after intracerebral administration the rats were again placed in the cylinders under the same conditions and the test session was recorded using a video camera. Posteriorly, the quantification of the latency to immobility, the time spent swimming, climbing (escape behavior) and immobile (floating) was performed for each rat using the Etholog® 2.2. Software (Ottoni, 2000). The latency to immobility corresponds to the time elapsed between placing the animal in the water
30
and observing a first immobile behaviour; swimming corresponds to active swimming motions with large forepaw movements displacing around in the cylinder, more than necessary to merely keep the head above water; climbing was defined as vigorous movements with front and hind paws directed against the wall of cylinder in an attempt to climb out, and include diving; immobile behaviour was observed when all the small movements are made only in the direction of the animal to stay at the surface (Rénéric et al., 2002; Lino-de-Oliveira et al., 2005). Learned helplessness behaviour was defined as a reduction in the latency to immobility and an increase in time of immobility (Bessa et al., 2009; Amorim et al., 2014).
3.8 Evaluation of c-Fos expression 3.8.1 Stimulation of c-Fos
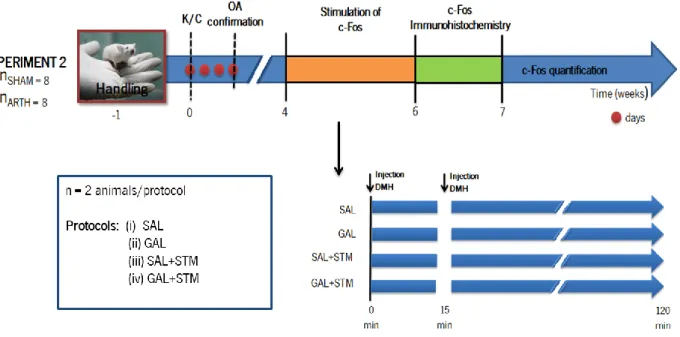
For stimulation of c-Fos, the animals (n=16) were placed in a stereotaxic frame, the skull was exposed with a scalpel and a small hole was drilled using a manual drill above the DMH (rostrocaudal: -3,24 mm, lateromedial: -0,4 mm; dorsoventral: -7,9 mm), according to the coordinates of the atlas by Paxinos and Watson (2007), for the insertion of the guide cannula (Plastics One) on the right hemisphere. The bregma was used as reference.
During 2 hours, the animals were submitted to a four stimulation protocols as shown in figure 8, where it was injected SAL or GAL in the DMH at the beginning of the protocol and after 15 min. These administrations were also accompanied by extension-flexion of the right knee 5 times every 2 minutes in two stimulation protocols.
3.8.2 c-Fos immunohistochemistry
The procedure followed to perform c-Fos immunohistochemistry was described previously by Morgado and Tavares (2007) involving the avidin-biotin-peroxidase method. First, the sections were washed twice in 0.1M PBS (pH=7.2) for 5 min each, incubated in hydrogen peroxidase (H2O2, Panreac, Barcelona, Spain) (330 µL in 10 mL PBS 0.1M) for 30 min to inhibit endogenous
peroxidase activity, followed by 2 washes of 5 min in PBS 0.1M and PBS/T (3mL Triton X 100 (Sigma-Aldrich) in 1000 mL PBS 0.1M; pH=7.2). Sections were then incubated in a blocking solution, 2.5% fetal bovine serum (FBS; Biochrom, Cambridge, United Kingdom) in PBS/T, for 2 h in order to avoid non-specific bindings followed by incubation in rabbit anti-c-Fos primary
31
antibody (Calbiochem, Merck, Algés, Portugal) (1:2000 in PBS/T and 2% FBS) overnight at room temperature on an orbital shaker. The following day, sections were again washed in PBS/T followed by incubation for 1 h in biotinylated swine anti-rabbit (Dako, Lisbon, Portugal) (1:200 in PBS/T) at room temperature. After being washed 3 times for 10 min in PBS/T, the sections were incubated in the avidin-biotin complex (ABC; Vectastain, Vector Laboratories, Peterborough, USA) diluted in 1:200 in PBS/T for 30 min at room temperature. The sections were then washed 3 times for 5 minutes in PBS/T, PBS and Tris 0.05M (Trizma® base, Sigma-Aldrich; pH=7.6), and stained with diaminobenzidine (DAB; Sigma-Aldrich; 20 mg DAB in solution of 40 mL Tris with 8 µL H2O2). Finally, the sections were washed twice in Tris and PBS to stop the staining reaction.
3.8.3 c-Fos quantification
c-Fos quantification was performed by counting the total number of c-Fos-immunoreactive neurones occurring bilaterally in the brain with the aid of a Stereo Investigator 10 Software® (MicroBrightField, Williston, VT, USA) coupled to a video camera attached to a Olympus Golgi microscope. The brain sections were outline according to Paxinos and Watson (2007) stereotaxic atlas and the quantification of c-Fos-positive neurones was performed blind. The cells in the ipsilateral side of the coronal sections were marked in red and contralateral side were marked in blue (Fig. 6).
Figure 6 – Coronal section of a SHAM animal showing c-Fos expression in the brain after the injection of GAL. The ipsilateral side was stained red and the contralateral side stained blue.
32
3.9 Experimental design
The present work was divided in two main experiments, experiment 1 and experiment 2 as shown in figures 7 and 8, respectively. The animals we handled for a week previously to the induction of OA. After this habituation period (as described in section 3.1), OA was induced (as described in section 3.3) and its development was confirmed three days after.
3.9.1 Impact of GAL in the DMH upon emotional, motor and nociceptive behaviour (Experiment 1)
In experiment 1 (Fig. 7), three weeks after the induction of OA, the animals were reanaesthetized (Section 3.2) to implant a cannula in the DMH and another in the RVM (Section 3.6) and were allowed to recover for one week. To evaluate if the acute administration of GAL in the DMH altered the performance emotional-like and nociceptive behaviours of SHAM and ARTH animals, the rats were tested in (i) the OF test (Section 3.7.1) to assess anxiety/like and motor behaviours, (ii) the FST (Section 3.7.2) to evaluate depressive-like behaviour and (iii) the PW test (Section 3.5.3) to evaluate thermal hyperalgesia. During OF and FST the animals were administered either with SAL (SHAM SAL and ARTH SAL) or GAL (SHAM GAL and ARTH GAL) in the DMH 15 min before the beginning of the test. To evaluate nociceptive behaviour, basal PW values were determined as the following protocols: (i) SAL+SAL administration in the RVM+DMH, respectively; (ii) SAL+GAL administration in the RVM+DMH, respectively; (iii) LIDO+SAL administration in the RVM+DMH, respectively; (iv) LIDO+GAL administration in the RVM+DMH, respectively. The PW assessment was repeated at 10, 20 and 30 min after drugs administration. At the end of the behavioural period the animals were sacrificed and the brains were removed for further confirmation of injection sites.
33
Figure 7 – Schematic representation of the experimental design for experiment 1. One week preceding the induction of osteoarthritis (OA) using the kaolin/carrageenan (K/C) model, the animals were habituated to the experimenter and the testing apparatus. The development of OA was confirmed three days after induction. Three weeks after OA induction, the animals were reanaesthetized to implant an intracerebral cannula in the DMH and another in the RVM. After one week of recovery, the pressure application measurement (PAM) was performed (day 1). The Open field (OF) test was performed on day 2 and the forced swimming test (FST) on days 4 and 5, 15 min after the intracerebral microinjection of the drugs. To perform these tests, the animals were divided in four experimental groups: SHAM animals that injected with SAL (SHAM-SAL) or GAL (SHAM-GAL) and ARTH animals injected with SAL (ARTH-SAL) or GAL (ARTH-GAL). Finally, the rats performed the paw-withdrawal (PW) test in which they were injected with: (i) SAL+SAL in the RVM and DMH, respectively; (ii) SAL+GAL in the RVM and DMH, respectively; (iii) LIDO+SAL in the RVM and DMH, respectively; (iv) LIDO+GAL in the RVM and DMH, respectively. In this test, the PW Latency was assessed pre-injection (PI) of drugs and 10, 20 and 30 min following intracerebral drug injection. At the end, the animals were sacrificed and brains were removed to confirm the injection sites.
3.9.2 Evaluation of the expression of c-Fos after GAL in the DMH (Experiment 2)
In experiment 2 (Fig. 8), four weeks after the induction of OA the animals were reanaesthetized (Section 3.2) to allow the evaluation of c-Fos expression induced by GAL administration in the DMH (Section 3.8.2). To evaluate which the effect of the acute administration of the DMH, SHAM and ARTH animals were placed in a stereotaxic frame (Section 3.8.1) and were submitted to one of the following stimulation protocols for 2 h: (i) SAL administration in the DMH without