Biochemical Profile, Biological Activities, and
Toxic Effects of Proteins in the Rhinella
schneideri Parotoid Gland Secretion
Article
in Journal of Experimental Zoology Part A Ecological Genetics and Physiology · August 2016
DOI: 10.1002/jez.2035CITATIONS
0
READS
58
13 authors
, including:
Some of the authors of this publication are also working on these related projects:
Mass Spectrometry to Answer Clinical Questions: Insights in Oncology and Health Science Research
View project
Neurological complications of inflammatory bowel disease
View project
Cleverson Diniz Teixeira de Freitas
Universidade Federal do Ceará
35 PUBLICATIONS473
CITATIONSSEE PROFILE
Ronaldo de A Ribeiro
Universidade Federal do Ceará
147 PUBLICATIONS2,176
CITATIONSSEE PROFILE
Marcellus Souza
Universidade Federal do Ceará
119 PUBLICATIONS1,317
CITATIONSSEE PROFILE
André Luiz dos Reis Barbosa
Universidade Federal do Piauí
42 PUBLICATIONS278
CITATIONSSEE PROFILE
All content following this page was uploaded by Renan Oliveira Silva on 02 September 2016.
Biochemical Proile, Biological
Activities, and Toxic Effects of
Proteins in the
Rhinella schneideri
Parotoid Gland Secretion
LUIS M. SOUSA-FILHO
1
,
CLEVERSON D. T. FREITAS
2
,
MARINA D. P. LOBO
3
,
ANA C. O. MONTEIRO-MOREIRA
3
,
RENAN O. SILVA
4
, LUCAS A. B. SANTANA
1
,
RONALDO A. RIBEIRO
4
,
MARCELLUS H. L. P. SOUZA
4
,
GUSTAVO P. FERREIRA
1
,
ANNA C. T. C. PEREIRA
1
,
ANDRÉ L. R. BARBOSA
5
,
MAURO S. C. S. LIMA
6
,
AND
JEFFERSON S. OLIVEIRA
1∗
1Departamento de Biomedicina, Campus Ministro Reis Velloso, Universidade Federal do Piauí,
Parnaíba, Piauí, Brasil
2Departamento de Bioquímica e Biologia Molecular da Universidade Federal do Ceará, Campus do
Pici, Fortaleza, Ceará, Brasil
3Centro de Ciências da Saúde, Universidade de Fortaleza, Unifor, Fortaleza, Ceará, Brasil 4Departamento de Fisiologia e Farmacologia, Universidade Federal do Ceará, Fortaleza, Ceará,
Brasil
5Departamento de Fisioterapia, Campus Ministro Reis Velloso, Universidade Federal do Piauí,
Parnaíba, Piauí, Brasil
6Departamento de Biologia, Campus Almicar Ferreira Sobral, Universidade Federal do Piauí,
Floriano, Piauí, Brasil
Parotoid glands of amphibians are known for the production of several biologically active
com-pounds having pharmacological and toxic effects in mammals. In the present work, a protein
ABSTRACT
Conlict of interest: None.
Grant sponsor: Conselho Nacional de Desenvolvimento Cientíico e Tecnológico–CNPq; Grant number: 446497/2014-2; Grant sponsor: Fundação de Amparo à Pesquisa do Estado do Piauí–FAPEPI; Grant number: 004/2011.
Additional Supporting Information may be found in the online version of this article.
∗Correspondence to: Jefferson S. Oliveira, Departamento de Biomedicina, Campus Ministro Reis Velloso, Universidade Federal do Piauí, Parnaíba, Piauí,
Brasil.
E-mail: jsoliveira@ufpi.edu.br
Received 5 April 2016; Revised 1 August 2016; Accepted 5 August 2016 DOI: 10.1002/jez.2035
fraction obtained from
Rhinella schneideri
parotoid gland (RsPP) was characterized to study its
biological and toxic effects.
Rhinella schneideri
parotoid secretion is composed of up to 30% (w/w)
of soluble proteins. Tandem mass spectrometric analysis of the RsPP identiied 104 proteins,
in-cluding actin, beta-actin, ribosomal proteins, catalase, galectin, and uncharacterized proteins;
however, no peptidases were found, and this result was reinforced by the absence of proteolytic
activity. In addition, RsPP did not exhibit pro-coagulant or antibacterial effects. However,
pre-treatment of mice with different doses of RsPP intraperitoneally inhibited carrageenan-induced
paw edema and increased tissue myeloperoxidase activity. RsPP also reduced interleukin 1
β
lev-els in the peritoneal cavities and cell migration in the peritoneal cavities of an animal model of
carrageenan-induced peritonitis. Subchronic treatment of animals with RsPP for 7 consecutive
days did not alter the serum biochemical, renal, or liver parameters. However, a signiicant
re-duction in blood leukocyte count was observed. Our results showed that
R. schneideri
parotoid
secretion contains proteins with anti-inlammatory and slight toxic effects.
J. Exp. Zool. 00:1–13,
2016.
C2016 Wiley Periodicals, Inc.
How to cite this article: Sousa-Filho LM, Freitas CDT, Lobo MDP, Monteiro-Moreira ACO,
Silva RO, Santana LAB, Ribeiro RA, Souza MHLP, Ferreira GP, Pereira ACTC, Barbosa ALR, Lima
MSCS, and Oliveira JS. 2016. Biochemical proile, biological activities, and toxic effects of
proteins in the
Rhinella schneideri parotoid gland secretion. J. Exp. Zool. 00:1–13
J. Exp. Zool. 00:1–13, 2016
Amphibians are found in a diverse range of environments world-wide. Their skins are usually moist to facilitate respiration (Tempone et al., 2008). Consequently, they may be confronted with the risk of being infected by a broad range of microor-ganisms (Daly et al., ’87; Ferreira et al., 2013). Therefore, am-phibians have developed protective mechanisms against these potential risks; the glands in their skins produce a chemical ar-senal of noxious and toxic substances, such as biogenic amines, alkaloids, peptides, and proteins to ight infections (Clarke, ’97; Meng et al., 2012).
Parotoid glands are special type of glands in the amphibian skins. These glands are large and protrude from the shoulders and postorbital positions in various Anura species (Jared et al., 2009). The glands differ in shape and size depending on the species in which they are present, such as those of the genus Rhinella. These glands produce secretions that are very toxic to other animals (Abdel-rahman et al., 2010). When in contact with the buccal mucosa or when ingested, the secretions can cause salivation, excitation, paralysis, trembling, and convul-sions, often leading to death (Garrett and Boyer, ’93). To re-lease these secretions, parotoid glands have to be mechanically pressed, for example, by the bite of a predator (Habermehl, ’81). Although parotoid secretions are commonly reported as sources of toxic compounds, some studies have reported the presence of molecules having pharmacological activities. The action against cardiovascular disorders, cancer, microbial infections, diabetes, and inlammation are examples of activities related to bio-genic amines, cardiotonic steroids, and alkaloids (Meng et al., 2012; Abraham et al., 2014). However, information on purii-cation, biochemical characterization, and biological activities of
proteins and peptides from these glands is very limited in the literature.
Rhinella schneideriis a toad belonging to the Bufonidae fam-ily. This species is found in South America and is character-ized by the presence of parotoid glands in the postorbital region (Jared et al., 2009). Few studies have described the isolation and biological and toxic effects of molecules from the parotoid se-cretion ofR. schneideri(Cunha-Filho et al., 2010; Anjolette et al., 2011). In the literature, only one study has described the peptide proile of this secretion (Sciani et al., 2013). Another important aspect that necessitates the investigation of peptides and pro-teins from frog skin is their structural similarity to vertebrate proteins, explaining their potential for use in pharmacology and medicine (Lee et al., 2005; Garg et al., 2008). Therefore, the main goal of the present work was to characterize and identify new proteins isolated from theR. schneideriparotoid secretion with different biological activities.
MATERIALS AND METHODS
Extraction of the Parotoid Gland Secretion
Rhinella schneideri (Anura: Bufonidae) toads were cap-tured around the city of Floriano, Piauí, Brazil (06°46’01"S
43°01’22"O). Secretions from the parotoid gland of 16 adult
8,000 Da cut off. The dialyzed material was called proteins from R. schneideriparotoid gland (RsPP).
Treatment of RsPP with Pronase
RsPP was digested with pronase (an unspeciic proteinase from Streptomyces griseus, SIGMA P-5147) at 37°C in 100 mM
phos-phate buffer saline at pH 7.4. To 1 mg of RsPP, 0.098 U of en-zyme was added and the reaction was terminated after 12 hr. The samples were lyophilized (RsPP pronase) and the proteolysis was checked by electrophoresis.
Protein Determination
Secretions from the parotoid gland (nondialyzed crude mate-rial, 1 mg mL−1) were dissolved in water or in different buffers
at 50 mM: glycine-HCl (pH 2.0–3.0), sodium acetate (pH 4.0– 5.0), sodium phosphate (pH 6.0–7.0), Tris-HCl (pH 8.0–9.0), and glycine-NaOH (pH 10.0). The suspensions were centrifuged at 10,000×gfor 10 min and soluble proteins were measured ac-cording to Bradford’s procedure using bovine serum albumin as standard (Bradford, ’76).
Protein Proile
Polyacrylamide gel electrophoresis in the presence of dodecyl sulphate sodium (SDS) was performed as described by Laemmli (’70), with slight modiications. Parotoid secretions (nondialyzed crude material) were dissolved in 62.5 mM Tris-HCl buffer (pH 6.8) with 2% SDS and without beta-mercaptoethanol. Runs were performed at 20 mA per gel at 25°C for 2 hr. Gels were stained
with 0.1% Coomassie Brilliant Blue (R-350) solution. The molec-ular weight markers used were as follows: 97.0 kDa phosphory-lase b, 66.0 kDa albumin, 45.0 kDa ovalbumin, 30.0 kDa carbonic anhydrase, 20.1 kDa trypsin inhibitor, and 14.4 kDa lactalbumin.
Protein Identiication through Tandem Mass Spectrometry)
RsPP were digested by trypsin and analyzed using a Synapt HDMS mass spectrometer (Waters, Manchester, UK) coupled to a 2D NanoUPLC-ESI system. The analyses were per-formed using nanoelectrospray ionization in the positive ion mode nano-ESI (+). Proteins were identiied using the NCBI database and MASCOT (Matrix Science Ltd., London, UK; http://www.matrixscience.com) search engine.
Proteolytic Activity
Proteolytic activity was assayed using azocasein, BANA and BApNA as substrates, and with zymogram containing 0.1% gelatin, as described by Freitas et al. (2007). RsPP (2 mg mL−1)
was dissolved in different buffers (pH 2.0–10.0) and incubated with each substrate at 37°C for 30 min or 1 hr. One unit of
ac-tivity was deined as the amount of enzyme required to increase the absorbance by 0.01.
Clotting Activity
Human Plasma Samples. Samples of healthy human blood cer-tiied for transfusion were obtained from the Center of Hematol-ogy and Hemotherapy of the State of Ceará, Brazil (HEMOCE). The blood was mixed with 0.11 M tri-sodium citrate buffer in the ratio of 9:1 (v/v) and centrifuged at 500×gfor 15 min at 25°C to obtain platelet poor plasma (PPP).
Plasma Clotting and Fibrinogenolytic Activities. The coagulant activity was determined as described by Condrea et al. (’83). In order to evaluate the effect of the RsPP on the human plasma clotting time, samples (25, 50, and 100µg) dissolved in 30µL
10 mM Tris-HCl (pH 7.5) were incubated with 300µL PPP for
1 min at 37°C. The clot formation was initiated by adding 30µL
of 0.25 M CaCl2. Fibrinogenolytic activities of RsPP were also
in-vestigated with polymerization assays in agarose diffusion plate, spectrophotometrically and electrophoresis, as described previ-ously (Viana et al., 2013). Thrombin, trypsin, and papain were used as positive controls and Tris-HCl (pH 7.5) buffer as a neg-ative control.
Antibacterial Activity. RsPP and nondialyzed crude material were prepared by dissolving 10 mg of the samples in 1 mL dimethyl sulfoxide and diluted up to 1,024µg mL−1 in
ster-ile water. Minimal inhibitory concentrations (MICs) were deter-mined with the microdilution assay in 10% brain–heart infusion medium with different bacterial suspensions of 105CFU mL−1
(Staphylococcus aureus, Escherichia coli,Pseudomonas aerugi-nosa, Candida albicans,andCandida krusei).
Hemagglutinating Activity. RsPP was dissolved in 150 mM NaCl (1 mg mL−1), and then diluted serially (twofold) in a 96-well
microtiter U-bottom plate. The dilutions were mixed with 100µL
2% type A, AB, B, or O human erythrocyte suspension. The plate was left at 25°C for 12 hr, and the protein concentration
show-ing the minimum level of hemagglutination was determined. The hemagglutinating activity was expressed as hemagglutinating units (HU) per microgram of protein (HU/µg protein).
Anti-Inlammatory Assays
Animals. Female Swiss mice (25–30 g) were housed in
temperature-controlled rooms (25±1°C) and provided with food
and waterad libitumuntil experiments, which were conducted in accordance with the currently established principles for the care and use of research animals approved under No. 020/12 (2012) by the Ethics Committee of the Federal University of Piauí, Piauí, Brazil.
Carrageenan-Induced Paw Edema. The edema was induced by the injection of 50µL carrageenan (500µg/paw) in 0.9% sterile
saline into the right hind paw. Mice were pretreated intraperi-toneally (i.p.) with either 0.9% NaCl, 10 mg kg−1indomethacin,
RsPP (1.0, 2.5, or 5.0 mg kg−1) or RsPP pronase (2.5 mg kg−1).
2, 3, and 4 hr after carrageenan treatment (Vt) using a plethys-mometer (Panlab, Barcelona, Spain), as previously described by Winter et al. (’62). The effect of pretreatment was calculated as inhibition of the edema in relation to the paw volume of the saline-treated animals by using the following formula: % inhibi-tion of edema=[(Vt –Vo) “Control” – (Vt –Vo) “Treated”)]/[(Vt –Vo) “Control”]×100.
Determination of Myeloperoxidase Activity. Myeloperoxidase (MPO) is an enzyme found in the neutrophils and the reduc-tion of its activity provides informareduc-tion on the inhibireduc-tion of iniltration of these cells into the tissues (Bradley et al., ’82). In order to evaluate the involvement of cell inhibition on the anti-inlammatory activity displayed by RsPP, MPO activity was measured in the paw of animals injected with carrageenan and pretreated with RsPP (2.5 mg kg−1). After 4 hr of inlammatory
stimulus, 50–100 mg of tissue from the hind paws was collected and MPO activity was determined as previously described by Bradley et al. (’82). Data were presented as unit of MPO per mil-ligram of tissue (UMPO/mg of tissue).
Peritonitis Model in Mice. Mice received (i.p.) sterile saline or RsPP (2.5 mg kg−1), and after 1 hr, the animals were injected
with 250 µL carrageenan (500 µg/cavity) into the peritoneal
cavity. Four hours later, mice were euthanized and the peri-toneal cavity was washed with 1.5 mL heparinized phosphate buffered saline to harvest the peritoneal luid contained in cells (Souza and Ferreira, ’85). Total cell was measured in a Neubauer chamber, and differential cell counts (100 cells total) were per-formed on cytocentrifuge slides stained with hematoxylin and eosin. The results are presented as the number of total leucocyte and neutrophils per milliliter of peritoneal exudates. After the peritonitis assay, samples of peritoneal luids were collected and the level of interleukin-1β(IL-1β) was evaluated using
sand-wich enzyme-linked immunoabsorbent assay (ELISA). ELISA kits for IL-1βwere from the National Institute for Biological
Stan-dards and Control (Potters Bar, UK). This ELISA method has been consistently used to detect IL-1βlevel over 4,000 pg mL−1, and
it does not cross-react with other cytokines. The results were expressed as pictogram per milligram of cytokine per peritoneal cavity.
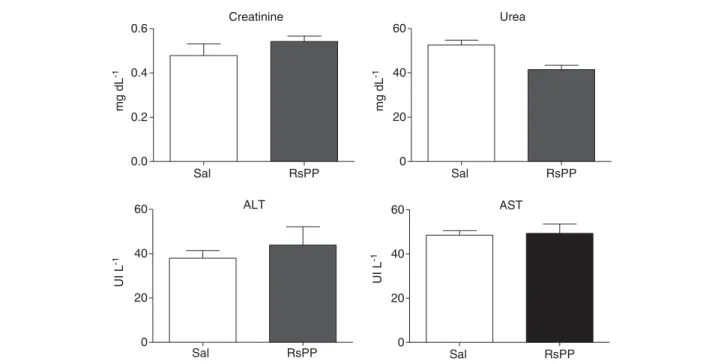
Subchronic Toxicity of RsPP. Body mass loss, organ weight alteration, and blood biochemical parameters (alanine amino-transferase [AST], aspartate aminoamino-transferase [ALT], creatinine, and urea) were evaluated after subchronic treatment with single doses of RsPP (2.5 mg kg−1; i.p.) or saline for 7 consecutive days.
After treatment, mice were weighted and peripheral blood was collected for hematological analysis and biochemical dosage (de-termined with enzymatic and colorimetric tests-LABTEST). After sacriicing the animal, the liver, kidney, and heart were removed and weighted.
Hematological Parameters of Animals Submitted to Subchronic Toxicity Assay. Several hematological parameters (red blood cells count, hemoglobin concentration, hematocrit, mean cor-puscular volume, mean corcor-puscular hemoglobin, platelet, and total and differential leukocytes counts) were performed from the blood collected from each animal through standard manual procedures using light microscopy (Coura et al., 2012).
Statistical Analysis
The results were given as the means±SEM. For the peritonitis model experiments and cytokine measurements, the statistical analysis was performed through ANOVA followed by Bonfer-roni’s test. In all other experiments, the statistical analysis was performed through ANOVA followed by Newman–Keuls tests. P<0.05 was deined as statistically signiicant.
RESULTS
Protein Content
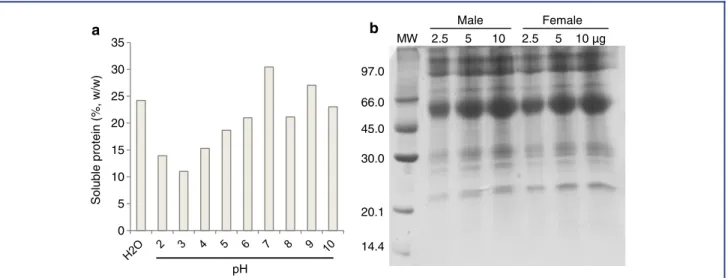
The concentration of soluble proteins in the parotoid gland se-cretion (nondialyzed crude material) ranged from 11 to 30% (w/w), depending on the solution assayed (Fig. 1a). The best so-lution for assay was 50 mM Tris–HCl buffer (pH 7.0). The frac-tion dissolved in distilled water had a protein content of 24.2% (w/w), and the protein content obtained from males and females toads did not differ signiicantly (P>0.05; data not shown). In
addition, the parotoid gland secretions of males and females exhibited the same protein proiles upon electrophoresis, with molecular masses ranging from 20 to 100 kDa, and predom-inant protein bands between 45 and 66 kDa (Fig. 1b). There-fore, fractions from both males and females toads were gathered and dialyzed against distilled water (using 8-kDa cut-off dialysis membrane) to aggregate the proteins and eliminate small toxic molecules such as biogenic amines and alkaloids. This fraction was termed proteins fromR. schneideri parotoid gland (RsPP) and was used in further experiments.
Protein Identiication
Male Female
MW 2.5 5 10 2.5 5 10 µg
0 5 10 15 20 25 30 35
Soluble protein (%, w/w)
pH
14.4 20.1 30.0 45.0 66.0 97.0
a
b
Figure 1. Protein content in nondialyzed crude material ofRhinella schneideriparotoid gland (male) evaluated using Bradford’s method (a) and polyacrylamide gel electrophoresis (12.5%) in the presence of SDS (b). Proteins at different concentrations were added in each well. Proteins were stained using 0.1% Coomassie Brilliant Blue (R-350) solution.
Carbohydrate-binding protein; 1
Lipid metabolism; 1
Anti-oxidative enzymes; 6
Carbohydrate metabolism; 9
Protein metabolism; 21
Uncharacterized; 23
Cell matrix; 43
Figure 2. Cellular functions of proteins fromRhinella schneideri
parotoid gland (RsPP) identiied through mass spectrometry. The number of proteins is labeled for each pie slice. A total of 104 proteins were identiied.
Proteolytic Activity
To evaluate the presence of proteolytic enzymes in RsPP, colori-metric assays were performed using different substrates at dif-ferent pH intervals and also using a zymogram. However, re-gardless of the sample concentration, buffer used, and assayed
pH, no proteolytic activity was detected using azocasein, BANA, and BApNA as substrates, or using a zymogram containing 0.1% gelatin.
Pro-Coagulant Activity
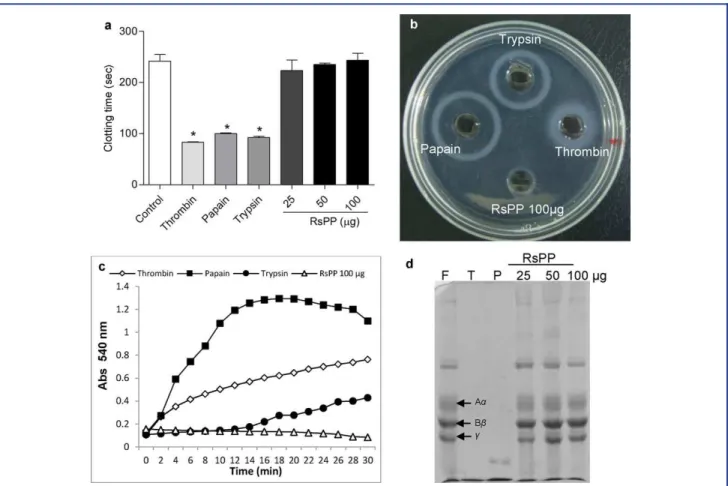
As shown in Fig. 3a, RsPP did not reduce the plasma clotting time (P>0.05), even when using 100µg of the protein. In addition,
RsPP did not hydrolyze human ibrinogen in the ibrinogen– agarose polymerization assay, spectrophotometrically, and elec-trophoresis (Fig. 3b–d). All these results conirmed the absence of pro-coagulant activity in RsPP. As expected, the proteolytic enzymes used as positive control (thrombin, bovine trypsin, and papain) signiicantly reduced the plasma clotting time compared to saline control and were able to hydrolyze human ibrinogen (Fig. 3).
Hemagglutination Activity
Once a lectin (galectin) was identiied in RsPP through mass spectrometry, its presence was conirmed with the hemagglu-tination assay using human red blood cells. RsPP was able to agglutinate all types of human erythrocytes. The speciic hemag-glutination activities (HA) were 0.58 HAµg−1 for A, B, and O
blood types, and 0.15 HAµg−1for AB blood type.
Antibacterial Activity
Figure 3. Pro-coagulant activity of proteins fromRhinella schneideriparotoid gland (RsPP). (a) Clotting time. Human plasma (300µL) was incubated with RsPP, thrombin, papain, and trypsin and the time taken for clot formation was recorded. (b) Fibrinogenolytic activity evaluated through ibrinogen–agarose polymerization assay was observed by the appearance of a turbid ring around the well. (c) Spec-trophotometric analysis of ibrin clot formation and its hydrolysis by RsPP and puriied enzymes. Turbidity was recorded by measuring absorbance at 540 nm. (d) Electrophoretic assay to determine ibrinogenolytic activity of RsPP, thrombin (T), and papain (P) on human ibrinogen. Arrows indicate the main chains of ibrinogen (F, control).∗Statistical difference (P<0.05) with the control group (ANOVA
followed by Tukey’s posttest).
µg mL−1, and
ࣙ1,000µg mL−1, respectively (Dall’Agnol et al.,
2003). The RsPP did not inhibit the growth of any tested bacteria, even at a concentration of 2,000µg mL−1.
Anti-inlammatory Effect in the Mice Paw Edema Model
The anti-inlammatory activity of RsPP was evaluated using a model of carrageenan-induced paw edema (Table 1). The car-rageenan group showed intense paw edema compared to the saline group (P < 0.05), with an inlammatory peak 3 hr
af-ter the inlammatory stimulus. However, injection of different doses of RsPP prior to the inlammatory stimulus was capable of signiicantly inhibiting the edema during all evaluated peri-ods. At the carrageenan inlammatory peak (3 hr), an inhibition rate of 54.4, 76.5, and 51.4% was observed for doses of 1, 2.5,
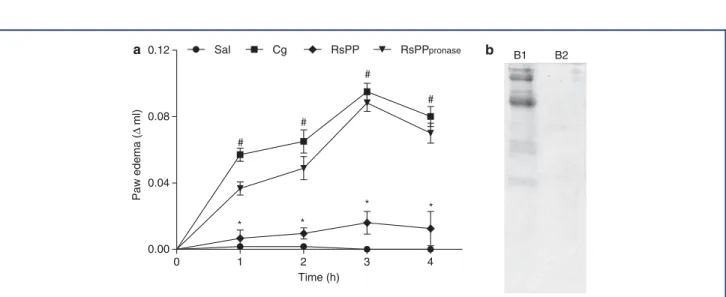
and 5 mg kg−1, respectively. The RsPP were completely degraded
when exposed to 12-hr digestion with pronase and were found to lose their anti-inlammatory properties (Fig. 4a and b). Although no statistical difference was observed among the concentrations evaluated (P> 0.05), the dose of 2.5 mg kg−1 presented the
highest inhibitory rate.
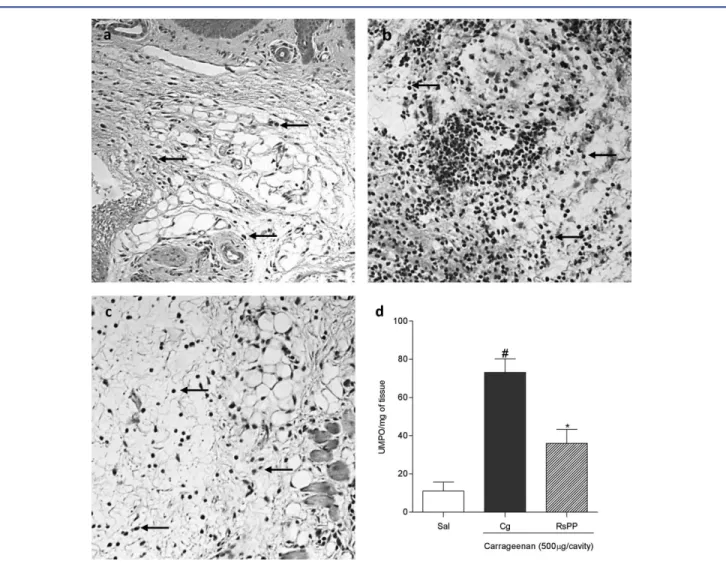
Paw Edema Histological Analysis and MPO Activity
The evaluation of histological sections of mice paw tissues pre-treated with 2.5 mg kg−1 RsPP revealed intense inhibition of
Table 1. The anti-inlammatory activity of proteins fromRhinella schneideriparotoid gland (RsPP) on paw edema induced by carrageenan
Paw edema (mL, inhibition rate)
Treatment Dose (mg kg−1) 1 hr 2 hr 3 hr 4 hr
Animals treated with carrageenan (500µg/paw)
Saline – 0.000±0.000 0.000±0.000 0.000±0.000 0.000±0.000 Control (Cg) – 0.026±0.003# 0.038±0.006# 0.068±0.005# 0.057±0.004#
RsPP 1
0.011±0.004∗
(57.7%)
0.011±0.004∗
(71.0%)
0.031±0.013∗
(54.4%)
0.035±0.011∗
(38.6%)
2.5
0.006±0.004∗
(76.9%)
0.006±0.003∗
(84.2%)
0.016±0.006∗
(76.5%)
0.022±0.010∗
(61.4%)
5
0.006±0.003∗
(76.9%)
0.015±0.005∗
(60.5%)
0.033±0.007∗
(51.5%)
0.045±0.004 (21.0%)
The values are given as the mean±SEM (n=5).
#,∗Statistical difference (P<0.05) compared to the saline group (sal) and carrageenan treatment (Cg), respectively (ANOVA followed by Neuman–Keuls
posttest).
0 1 2 3 4
0.00 0.04 0.08 0.12
* *
* *
#
#
#
# RsPPpronase
Sal Cg RsPP
Time (h)
Paw edema (
Δ
ml)
a b
B1 B2
Figure 4. The effect of pronase treatment on the anti-inlammatory activity of proteins fromRhinella schneideriparotoid gland (RsPP) in carrageenan-induced paw edema. (a) A dose of 2.5 mg kg−1; i.p. of RsPP or RsPP digested with pronase (RsPP pronase) was administered 1 hr before the application of inlammatory stimulus. Values are given as the mean±SEM. (n=5). #,∗Statistical difference (P<0.05)
Figure 5. Effects of proteins fromRhinella schneideriparotoid gland (RsPP) on carrageenan-induced edema revealed by histological sections (a–c) and myeloperoxidase activity (d) in the paw tissues of mice. A: saline group; B: carrageenan group; and C: RsPP (2.5 mg kg−1; i.p.) group. Arrows indicate the presence of neutrophils. #,∗Statistical difference (P<0.05) between the saline and the carrageenan
groups, respectively (ANOVA followed by Newman–Keuls posttest).
tissue, whereas that measured in carrageenan-treated mice was 73.11±7.06 UMPO mg−1of tissue.
Anti-Inlammatory Effect in the Mice Peritonitis Model
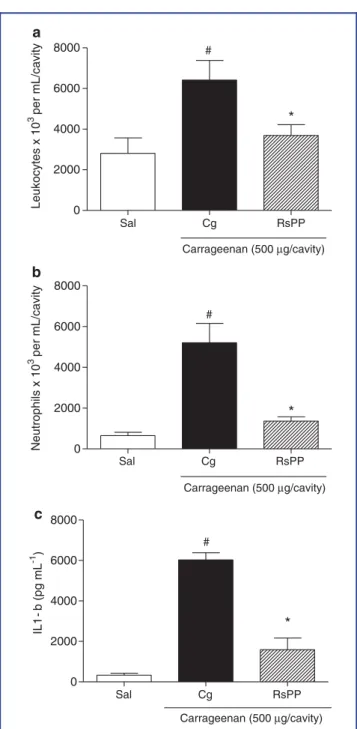
The ability of RsPP to inhibit cell migration was also evaluated in a model of carrageenan-induced peritonitis. The pretreatment of animals with RsPP (2.5 mg kg−1) signiicantly decreased the
inlux of total leukocytes (3,692± 533.1 cells×103/mL) and neutrophils (1,365±212.6 cells×103/mL) to the peritoneal cav-ity when compared to that of the carrageenan group (6,417 ± 968.3 leukocyte×103/mL; 5,213±944.4 neutrophil×103/mL) (Fig. 6a and b). Moreover, inhibition of cell migration was fol-lowed by changes in IL-1βlevels in the peritoneal luid of the
mice (Fig. 6c). The level of this cytokine in animals that re-ceived RsPP was 1,590 ±581.3 pg mL−1, whereas its level in
carrageenan-treated animals was 6,029±355.8 pg mL−1.
Subchronic Toxicity
To evaluate the possible toxicological effect promoted by the administration of RsPP, a group of mice was subjected to a daily treatment with RsPP (i.p.) at a single dose of 2.5 mg kg−1for 7
Sal Cg RsPP
0 2000 4000 6000
8000 #
*
Carrageenan (500 µg/cavity)
Leukocytes x 10
3 per mL/cavity
Sal Cg RsPP
0 2000 4000 6000 8000
#
*
Carrageenan (500 µg/cavity)
Neutrophils x 10
3 per mL/cavity
Sal Cg RsPP
0 2000 4000 6000 8000
Carrageenan (500 µg/cavity)
#
*
IL1 -
b
(pg
mL
-1 )
a
b
c
Figure 6. The inhibitory effect of proteins fromRhinella schneideri
parotoid gland (RsPP) on cell migration and cytokine production in the carrageenan-induced peritonitis model. The total leukocytes (a), neutrophil migration (b), and IL-1βlevels (c) were measured in the mice peritoneal luid. RsPP (2.5 mg kg−1; i.p.) was administered 1 hr before the application of inlammatory stimulus. Values are given as the mean±SEM (n=5). #,∗Statistical difference (P<
0.05) between the saline and the carrageenan groups, respectively (ANOVA followed by Newman–Keuls posttest).
in the body masses and organ weights of the mice subjected to RsPP treatment and the control group (Table 2).
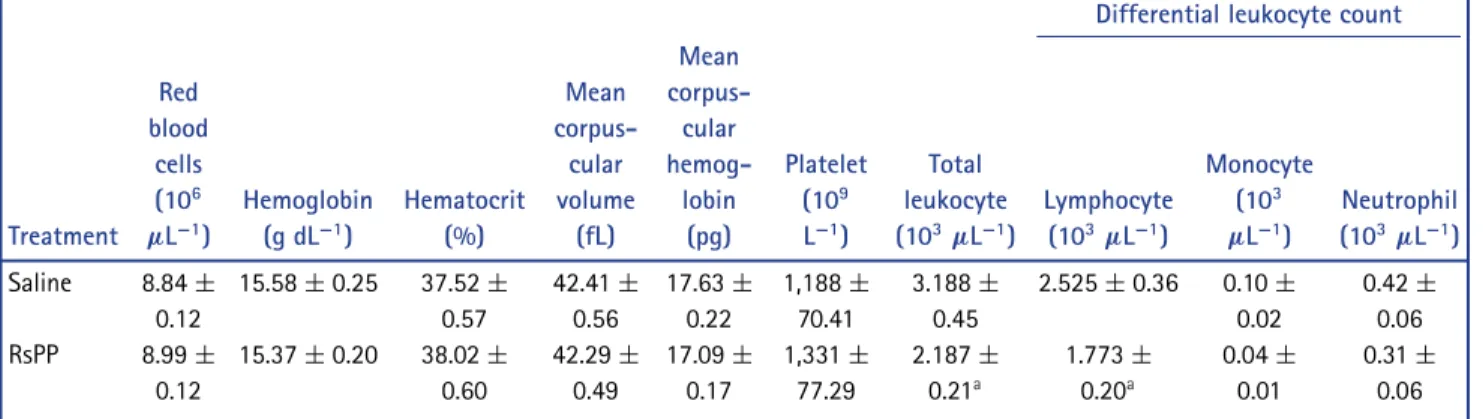
Analysis of the cellular proile of peripheral blood obtained from control and treated mice revealed that subchronic treat-ment induced a signiicant reduction in the total leukocyte and lymphocyte count (P< 0.05) (Table 3). The other leukocytes
(neutrophils and monocytes) and other cellular parameters re-mained unaltered between the groups (data not shown).
DISCUSSION
Most of the studies dealing with toad parotoid gland and skin se-cretions focus on the biological activities of biogenic amines, al-kaloids, and peptides. Therefore, these molecules and their phar-macological and toxic effects are well characterized (Abraham et al., 2014). However, there is limited information regarding the content and identiication of proteins from toad parotoid glands, as well as their pharmacological and toxic properties. In the present work, a protein fraction (>8 kDa) obtained from the R. schneideriparotoid gland secretion was the target for protein identiication and evaluation of biological activities and toxic effects.
Herein, theR. schneideriparotoid secretion was found to com-prise up to 30% (w/w) of soluble proteins, and these proteins of the genusRhinellaare known to be one of the major constituents of the parotoid glands (Mailho-Fontana et al., 2013; Sciani et al., 2013). However, skin secretions of other amphibian genera such asPhyllomedusa(Conceição et al., 2007) andLeptodactylus (Limaverde et al., 2009) contain lower protein contents. On com-paring the electrophoretic protein proile of the parotoid glands obtained fromR. schneideri with those obtained from differ-ent species of the genusRhinella(Mailho-Fontana et al., 2013; Sciani et al., 2013), we found a similar pattern, especially for the high molecular weight proteins (>55 kDa). However, unique
bands were observed for each secretion (30–55 kDa). These ob-servations show that despite similarities among different am-phibian parotoid glands belonging to the same genus, their se-cretions can be sources of different proteins.
Although toad parotoid glands are characterized by the re-lease of molecules involved in the defense response, the tandem mass spectrometry (MS/MS) approach led to the identiication of proteins mainly associated with common cell metabolism. More-over, around 23% proteins remained uncharacterized. This could be due to limited proteomic information available forR. schnei-deri.A research performed in NCBI revealed that only 25 pro-tein sequences fromR. schneideriwere deposited. Besides, the majority of the studies performed on frog skin secretions and toad parotoid glands investigated only their peptide contents (Al-Ghaferi et al., 2010).
Galectin, a member of the highly conserved family of β
Table 2. Effects of the daily administration of proteins fromRhinella schneideriparotoid gland (RsPP) on the weight of mice organs
Sample Liver (g) Kidney (g) Spleen (g) Animal (g)
Saline 0.041 ± 0.000 0.006± 0.000 0.004 ± 0.000 26.67± 0.56
RsPP 0.044 ± 0.000 0.006± 0.000 0.005 ± 0.000 25.17± 0.58
Values are given as the means±SEM of 12 animals, as analyzed by Student’st-test. Animals received doses of 2.5 mg kg−1; i.p of RsPP. No statistical differences were observed between the saline and RsPP treatment groups.
Table 3. Complete blood count of animals treated with a daily dose of proteins fromRhinella schneideriparotoid gland (RsPP)
Differential leukocyte count
Treatment Red blood
cells (106
µL−1)
Hemoglobin (g dL−1)
Hematocrit (%) Mean corpus-cular volume (fL) Mean corpus-cular hemog-lobin (pg) Platelet (109
L−1)
Total leukocyte (103
µL−1)
Lymphocyte (103
µL−1)
Monocyte (103
µL−1)
Neutrophil (103
µL−1)
Saline 8.84±
0.12
15.58±0.25 37.52±
0.57 42.41± 0.56 17.63± 0.22 1,188± 70.41 3.188± 0.45
2.525±0.36 0.10±
0.02
0.42±
0.06 RsPP 8.99±
0.12
15.37±0.20 38.02±
0.60 42.29± 0.49 17.09± 0.17 1,331± 77.29 2.187± 0.21a 1.773± 0.20a 0.04± 0.01 0.31± 0.06
Data are expressed as means±SEM of 12 animals. Animals received doses of 2.5 mg kg−1; i.p of RsPP. aSigniicant difference compared to the saline group (P<0.05) as analyzed by Student’st-test.
hemagglutination assays. Toad lectins were puriied from dif-ferent tissues including gonads, skin, and granular glands of amphibians belonging to various species (Ahmed et al., ’96; Uchiyama et al., ’97). The identiication of galectin in skin and granular gland secretions indicates that it might be involved in defense mechanisms (Riera et al., 2003). In the present study, RsPP did not present antibacterial activity against any of the bacteria tested, even at a concentration of 2 mg mL−1.
The structural correspondence between mammalian and am-phibian galectins has been demonstrated previously (Ahmed et al., ’96). Mammalian galectins have been involved in several physiological processes requiring carbohydrate recog-nition, such as innate and adaptive immune responses (Barrionuevo et al., 2007). These proteins can inhibit the pro-duction of pro-inlammatory cytokines, stimulate the produc-tion of anti-inlammatory cytokines, and inluence the release and migration of leukocytes (Rabinovich et al., ’99; La et al., 2003). Thus, RsPP was challenged to inhibit an inlammatory response induced by carrageenan in two experimental mod-els. The pretreatment of mice with proteins from the paro-toid secretion exhibited an antiedematogenic effect and reduced
MPO activity in animal paw tissues. RsPP also inhibited neu-trophil migration and decreased IL-1β levels in the peritoneal
cavity of the peritonitis model mice. The inlammatory re-sponse induced by carrageenan was characterized by the release of several inlammatory mediators, production of pro-inlammatory cytokines (TNF-αand IL-1β), and intense
migra-tion of neutrophils into the inlammatory site (Thorlacius et al., ’97; Kulinsky, 2007). In addition, galectin presented an anti-inlammatory activity by inhibiting neutrophil migration and release/production of pro-inlammatory cytokines. The loss of such anti-inlammatory activity after treatment with pronase reinforced the protein nature of molecules involved in such activity. However, further studies are required to clarify the role of galectin in the anti-inlammatory activity observed in RsPP.
Creatinine
Sal RsPP
0.0 0.2 0.4 0.6
mg dL
-1
Urea
Sal RsPP
0 20 40 60
mg dL
-1
ALT
Sal RsPP
0 20 40 60
UI L
-1
AST
Sal RsPP
0 20 40 60
UI L
-1
Figure 7. Effects of the daily administration of proteins fromRhinella schneideriparotoid gland (RsPP) on the plasma levels of biochemical parameters in mice. Mice received a dose of 2.5 mg kg−1of RsPP, i.p. No statistical differences were observed between the saline and the RsPP-treatment groups (Student’st-test).
showed that RsPP have no pro-coagulant activity. These nega-tive results may be related to the absence of peptidases in RsPP, as conirmed by colorimetric assays, zymography, and MS/MS analysis.
Proteolytic enzymes, phospholipase A2, and proteinase
in-hibitors are examples of proteins found in frog and toad skin secretions, which are known for presenting toxic effects in mam-mals (Conceição et al., 2007; Bhattacharjee et al., 2011). The MS/MS procedure was not able to detect the proteins, previ-ously described as toxic, in RsPP. However, animals subjected to subchronic treatment with RsPP (2.5 mg kg−1; i.p.) presented
a signiicant reduction in lymphocyte count compared to un-treated animals. Although other hematological parameters such as red blood cells, hemoglobin, platelets, and neutrophils and renal and liver functions were not altered, the results indicated the presence of toxic proteins in the parotoid secretion of R. schneideri. Similar to other studies, our study detected unidenti-ied proteins with molecular weights higher than 12 kDa in toad skin and gland secretions with toxic effects (Limaverde et al., 2009).
CONCLUSION
Our study was the irst proteomic analysis performed on the parotoid secretion ofR. schneideri. Most of the proteins
iden-tiied were uncharacterized or related to the basal cellular metabolism. However, a new protein, galectin, was identiied for the irst time in theR. schneideri parotoid gland. Unlike secretions of other toad species, R. schneideri secretion lacks proteolytic enzymes and pro-coagulant and antibacterial activ-ities. Interestingly, this investigation revealed thatR. schneideri parotoid secretion presents proteins with anti-inlammatory ac-tivity and slight toxic effects in mice. Further assays need to be performed to isolate the identiied galectin and evaluate its anti-inlammatory activity.
LITERATURE CITED
Abdel-Rahman MA, Ahmed SH, Nabil ZI. 2010.In vitrocardiotoxicity and mechanism of action of the Egyptian green toadBufo viridis
skin secretions. Toxicol in Vitro 24:480–485.
Abraham P, George S, Kumar KS. 2014. Novel antibacterial peptides from the skin secretion of the Indian bicoloured frogClinotarsus
curtipes. Biochimie 97:144–151.
Al-Ghaferi N, Kolodziejek J, Nowotny N, Coquet L, Jouenne T, Leprince J, Vaudry H, King JD, Conlon JM. 2010. Antimicrobial peptides from the skin secretions of the South-East Asian frogHylarana erythraea
(Ranidae). Peptides 31:548–554.
Anjolette FAP, Pereira JC, Azzolini AEC, Sampaio SV, Assis-Pandochi AI, Arantes EC, Pereira-Crott LS. 2011. Complement system modulation by components fromRhinella schneideripoison. Mol Immunol 48:1694–1695.
Baldo ECF, Silva CP, Sampaio SV, Arantes EC. 2012. Evaluation of the cytotoxic activity ofRhinella schneideritoad poison on tumor cells and on healthy mononuclear cells. Toxicon 60:103.
Barrionuevo P, Beigier-Bompadre M, Ilarregui JM. 2007. A novel functionfor galectin-1 at the crossroad of innate and adap-tive immunity: galectin-1regulates monocyte/macrophage physi-ology through a nonapoptotic ERK-dependent pathway. J Immunol 178:436–45.
Berkner KL. 2001. Blood Clotting: General Pathway. eLS.
Bhattacharjee P, Giri B, Gomes A. 2011. Apoptogenic activity and tox-icity studies of a cytotoxic protein (BMP1) from the aqueous ex-tract of common Indian toad (Bufo melanostictusSchneider) skin. Toxicon 57:225–236.
Bradford MM. 1976. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of dye binding. Anal Biochem 72:248–254.
Bradley PP, Priebat DA, Christenses RD, Rothstein G. 1982. Measure-ment of cutaneous inlammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol 78:206–209.
Clarke BT. 1997. The natural history of amphibian skin secretions, their normal functioning and potential medical applications. Biol Rev Camb Philos Soc 72:365–379.
Conceição K, Bruni FM, Antoniazzi MM, Jared C, Camargo ACM, Lopes-Ferreira M, Pimenta DC. 2007. Major biological effects induced by the skin secretion of the tree frog Phyllomedusa
hypochondrialis. Toxicon 49:1054–1062.
Condrea E, Yang CC, Rosenberg P. 1983. Anticoagulant activity and plasma phosphatidylserine hydrolysis by snake venom phospholi-pase A2. Thromb Haemost 49:151.
Coura CO, Arafljo IWF, Vanderlei ESO, Rodrigues JAG, Quinder ALG, Fontes BP, Queiroz INL, Menezes DB, Bezerra MM, Silva AAR, Chaves HV, Jorge RJB, Evangelista JSAM, Benevides NMB. 2012. Antinociceptive and anti-inlammatory activities of sul-phated polysaccharides from the red seaweedGracilaria cornea. Basic Clin Pharmacol Toxicol 110:335–341.
Cunha-Filho GA, Resck IS, Cavalcanti BC, Pessoa CO, Moraes MO, Fer-reira JRO, Rodrigues FAR, Santos ML. 2010. Cytotoxic proile of nat-ural and some modiied bufadienolides from toadRhinella schnei-deriparotoid gland secretion. Toxicon 56:339–348.
Dall’Agnol R, Ferraz A, Bernardi AP, Albring D, Nör C, Sarmento L, Lamb L, Hass M, Von-Poser G, Schapoval EES. 2003. Antimicro-bial activity of some Hypericum species. Phytomedicine 10:511– 516.
Daly JW, Myers CW, Whittaker N. 1987. Further classiication of skin alkaloids from neotropical poison frogs (Dendrobatidae), with a general survey of toxic/noxious substances in the amphibia. Toxi-con 25:1023–1095.
Ferreira PMP, Lima DJB, Debiasi BW, Soares BM, Machado KC, Noronha JC, Rodrigues DJ, Sinhorin AP, Pessoa C, Vieira-Júnior GM. 2013. Antiproliferative activity ofRhinella marinaandRhaebo
guttatus venom extracts from Southern Amazon. Toxicon 72:
43–51.
Freitas CDT, Oliveira JS, Miranda MRA, Macedo NMR, Sales MP, Villas-Boas LA, Ramos MV. 2007. Enzymatic activities and protein proile of latex fromCalotropis procera. Plant Physiol Biochem 45:781– 789.
Garg AD, Hippargi RV, Gandhare AN. 2008. Toad skin-secretions: po-tent source of pharmacologically and therapeutically signiicant compounds. Internet J Pharmacol 5:17.
Garrett CM, Boyer DM. 1993.Bufo marinus(Cane Toad). Predation. Herpetol Rev 24:148.
Habermehl GG. 1981. Venomous animals and their toxins. Berlin: Springer Verlag.
Jared C, Antoniazzi MM, Jordão AEC, Silva JRMC, Greven H, Ro-drigues MT. 2009. Parotoid macroglands in toad (Rhinella jimi): their structure and functioning in passive defence. Toxicon 54:197– 207.
Kulinsky VI. 2007. Biochemical aspects of inlammation. Biochem 72:733–746.
La M, Cao TV, Cerchiaro G, Chilton K, Hirabayashi J, Kasai K, Oliani SM, Chernajovsky Y, Perretti M. 2003. A novel biological activ-ity for galectin-1: inhibition of leukocyte–endothelial cell inter-actions in experimental inlammation. Am J Pathol 163:1505– 1515.
Laemmli UK. 1970. Cleavage of structural proteins during the assem-bly of the bacteriophage T4. Nature 227:680–685.
Lee WH, Liu SB, Shen JH, Jin Y, Lai R, Zhang Y. 2005. Identiica-tion and molecular cloning of a novel neuromedin U analog from the skin secretions of toad Bombina maxima. Regul Pept 129: 43–47.
Limaverde PT, Nascimento NR, Evangelista JS, Tomé AR, Fonte-les MC, Santos CF, Cardi BA, Carvalho KM. 2009. Isolation and pharmacological effects of leptoxin, a novel proteic toxin
fromLeptodactylus pentadactylusskin secretion. Toxicon 54:531–
538.
Mailho-Fontana PL, Antoniazzi MM, Toledo LF, Verdade VK, Sciani JM, Barbaro KC, Pimenta DC, Rodrigues MT, Jared C. 2013. Passive and active defense in toads: the parotoid macroglands inRhinella
marinaandRhaebo guttatus. J Exp Zool 321:65–77.
Meng P, Wei L, Yang S, Liu H, Liu R, Lai R. 2012. A novel frog skin peptide containing function to induce muscle relaxation. Biochim 94:2508–2513.
matrix and proinlammatory cytokine secretion by human recom-binant galectin-1. Immunology 97:100–106.
Riera AS, Daud A, Gallo A, Genta S, Aybar M, Sánchez S. 2003. An-tibacterial activity of lactose-binding lectins from Bufo arenarum skin. Biocell 27:37–46.
Sciani JM, Angeli CB, Antoniazzi MM, Jared C, Pimenta DC. 2013. Differences and similarities among parotoid macrogland secretions in South American toads: a preliminary biochemical delineatione. Sci World J 2013:1–13.
Souza GEP, Ferreira SH. 1985. Blockade by antimacrophage serum of the migration of PMN neutrophils into the inlammed peritoneal cavity. Agents Actions 17:1–5.
Tempone AG, Pimenta DC, Lebrun I, Sartorelli P, Taniwaki NN, Andrade-Junior HF, Antoniazzi MM, Jared C. 2008. Antileishmanial and antitrypanosomal activity of bufadienolides isolated from the
toadRhinella jimiparotoid macrogland secretion. Toxicon 52:13–
21.
Thorlacius H, Lindbom L, Raud J. 1997. Cytokine-induced leukocyte rolling in mouse cremaster muscle arterioles in P-selectin depen-dent. Am J Physiol 272:1725–1729.
Uchiyama H, Komazaki S, Oyama M, Matsui T, Ozeki Y. 1997. Distri-bution and localization of galectin puriied fromRana catesbiana
oocytes. Glycobiology 7:1159–1165.
Viana CA, Oliveira JS, Freitas CDT, Alencar NMN, Carvalho CPS, Nishi BC, Ramos MV. 2013. Thrombin and plasmin-like activities in the latices ofCryptostegia grandiloraandPlumeria rubra. Blood Co-agul Fibrinolysis 24:386–392.
Winter CA, Risley EA, Nuss, GW. 1962. Carrageenin-induced edema in hind paw of the rat as an assay for antiinlammatory drugs. Proc Soc Exp Biol Med 111:544–547.