Expression of serum microRNA-378 and its clinical significance in renal cell
carcinoma
Lixin Shi
1, Lei Zhang
1, Chunyang Wang
1, Shengkun Sun
1, Xiyuan Cao
2and Xu Zhang
1 1Department of Urology, PLA General Hospital, Beijing, China.
2
Institute of Basic Medicine, Military Medical Science Academy of the PLA, Beijing, China.
Abstract
Studies have demonstrated that miRNA-378 is expressed in various malignant tumors. In the present study, we aimed to explore the expression of serum miRNA-378 and its clinical significance in renal cell carcinoma (RCC) pa-tients. A total of 75 RCC patients, 63 renal cysts (RC) patients and 75 healthy controls were selected. The miRNA-378 level in RCC and RC groups was significantly higher than in healthy control group, with RCC group hav-ing the highest level. The miRNA-378 levels were significantly decreased within the same group after surgery. When compared with healthy controls, RC group had higher levels but not significantly (p > 0.05) while levels in RCC group were significantly higher (p < 0.05). miRNA-378 expression was correlated with clinical stage and differentiation de-gree, but not correlated with patient’s age, gender, surgical strategy and tumor diameter. The AUC of miRNA-378 was 0.896, 95% confidence interval was 0.847 to 0.945, and AUC hypothesis testing was statistically significant (p < 0.001, RCC vs healthy control). miRNA-378 shows potential in the diagnosis and prediction of postoperative curative effect of renal cell carcinoma, but further studies with lager samples are needed.
Keywords: Renal cell carcinoma, microRNA 378, diagnosis, patients, AUC.
Received: May 05, 2016; Accepted: November 24, 2016.
Introduction
Renal cell carcinoma (RCC) is the most common space-occupying lesion in adult kidney. RCC accounts for about 3% of all tumors, and the mortality rate reaches 40% (Franceschi and Wild, 2013; Tanet al., 2015). There is no typical clinical symptom at the early stages and most RCCs are diagnosed at late stages, with symptoms including inter-mittent hematuria, low back pain, abdominal mass, large tumor area and even with distant metastasis (Golan and Eggener, 2015). RCC is not sensitive to radiotherapy and chemotherapy, and its prognosis is unfavorable (Golan and Eggener, 2015). Although surgical resection is the best treatment currently, relapse still occurs in 20 to 40% of pa-tients. Finding early tumor markers and evaluating their clinical curative effect on RCC have become extremely ur-gent and important.
MicroRNAs (miRNAs) are non-coding small RNA molecules that contain a loop-stem structure. These miRNAs regulate cell growth and differentiation, and play important roles in the process of life and the development of disease (Chenget al., 2015; Daset al., 2014; Duanet al., 2014). Studies indicate that miRNAs are closely related to the occurrence and development of tumors
(Warnecke-Eberzet al., 2015; Wonget al., 2011; Yeet al., 2013). It has been reported that miRNAs play an important role in the regulation of tumor in prostate, lung, breast and colon can-cers. In addition, miRNAs also play important roles in the pathogenesis of RCC (Fedorko et al., 2015; Lin et al., 2010). Wulfken et al. (2011) indicated that circulating miR-1233 could serve as a potential biomarker for RCC pa-tients(Wulfkenet al., 2011). MiRNA expression profiling has found a number of specific miRNAs in RCC, which mainly act through regulating the balance between cancer genes and tumor suppressor genes (Huanget al., 2014; Juan
et al., 2010).
Studies have demonstrated that miRNA-378 is ex-pressed in various malignant tumors, and is related to the survival, migration, invasion, angiogenesis and growth of tumor cells (Chenet al., 2012; Denget al., 2013; Leeet al., 2007). Reports have shown that miRNA -378 is expressed abnormally in serum of RCC patients, however, the results were inconsistent. Fedorkoet al.(2015) reported that sig-nificantly increased serum levels of miR-378 enabled to clearly distinguish RCC patients and healthy controls. Wang et al. (2015a) reported that plasma miR-378 was markedly decreased in patients with RCC, even in those with stage I disease, compared with the non-cancer controls (p < 0.01)(Fedorkoet al., 2015; Wanget al., 2015a). In this study, we detected the expression of miRNA-378 in RCC patients from North China, aiming to clarify the role of
Send correspondence to Xu Zhang. Department of Urology, PLA General Hospital, No. 28 Fuxing road, Haidian District, 100853 Beijing, China. E-mail: xuzhang779@163.com
miRNA-378 in RCC, and investigate its diagnostic feasibil-ity.
Patients and Methods
Patients
All procedures performed in human participants were in accordance with the ethical standards of the PLA Gen-eral Hospital, and complied with the Declaration of Hel-sinki and its later amendments or comparable ethical standards. All subjects signed a form of informed consent. In this study, three groups were recruited: primary re-nal cell carcinoma (RCC) patients, simple rere-nal cyst (RC) patients, and healthy controls. The RCC and RC patients were all recruited from the hospital clinic, had received sur-gical resection at our department, and their resected tissues were confirmed by pathological examination. Healthy con-trols were adults in the outpatient physical examination clinic. The age of patients and controls ranged from 18 to 68.
Blood sample collection
For RCC and RC patients, blood samples were col-lected at two time points: before surgery and six months af-ter surgery. The fasting serum samples of healthy controls were collected during their physical examination. A total of 3 mL blood samples were taken in SSTII advance tubes (Becton Dickinson, UK) and preserved at 4 °C in a refriger-ator for 1 h. The blood samples were centrifuged for 3 min at 500 rpm, the upper level of serum was transferred to a 1.5 mL Eppendorf tube without RNase. Phenol/chloroform were used for deproteinization, ethanol was used to precipi-tate RNA, and DEPC water was added to dilute the RNA. The overall quality of an RNA preparation was assessed by electrophoresis on a denaturing agarose gel, and purity of extracted RNA was tested by UV spectrophotometry. The OD260/OD280 ratio value should be greater than 1.8.
Computer tomography or ultrasonography and (or) chest X-ray examination were performed to assess tumor recurrence in postoperative RCC patients once a month for six months. The imaging or endoscopic examination indi-cated recurrence of primary tumor site; regional or distant lymph node metastasis can be considered as recurrence af-ter tumor resection. The follow-up of this study ended in April 2016.
RT-PCR for miRNA-378
The total serum miRNA was extracted by the microRNA Extraction Kit (miRNeasy Mini Kit, Qiagen, Hamburg, Germany). The reverse transcription polymerase chain reaction (RT-PCR) method was used to measure miRNA-378 levels. The RT-PCR assays were done in a TIANGEN Biotech system (Beijing) in a total volume of 20 mL composed of: 10mL 2x microRNA premix regent, 0.4 mL self-contained primer, 2mL RT reaction product, 0.4mL
reverse primer, and 7.2mL of DEPC water. As intercalating dye, 20xSYBR Green I was used (TIANGEN Biotech, Beijing, CHN) and assays were run in a CFX96 Touch Real-Time PCR Detection System (BioRad, Hercules, CA, USA) for quantitative measurement with the following conditions: 95 °C for 10 min, 95 °C for 15 s, 60 °C for 1 min (40 cycles). The relative expression of miRNA-378 was calculated according to (Schmittgen and Livak 2008): F = 2-Dct, Dct = ctmiRNA-378 - ctmiRNA-U6. MIR378 (MIMAT0000731) miRNA qPCR Primer Pairs was pur-chased from OriGene Technologies Inc (Rockville, MD, USA), with the following primer sequence: forward 5’
CTCCTGACTCCAGGTCCTGT3’, and reverse 5’
GCCTTCTGACTCCAAGTCCA 3’. U6 primers were syn-thesized by Life Technology (Shanghai, China): RT 5’CGCTTCACGAATTTGCGTGTCAT3’U, and forward 5’GCTTCGGCAGCACATATACTAAAAT3’
Statistical analysis
The relative expression levels of miRNA-378 were reported as the means and standard deviations. The data were analyzed by SPSS 17 software (IBM, Chicago, IL, USA) with Wilcoxon rank sum test and H Kruskal-Wallis rank sum test. The sensitivity and specificity of miRNA-378 were calculated by ROC curve (AUC) based on the data collected from healthy controls and RCC patients; Graphpad Prism 5.0 software (GraphPad Software, San Diego, USA) was used for ROC curve analysis. A p < 0. 05 was considered as statistically significant.
Results
Demographic data
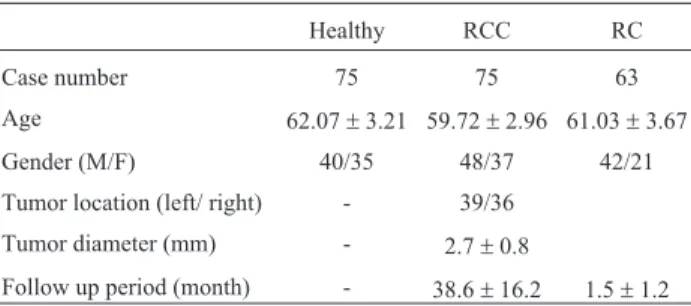
From April 2011 to December 2014, a total of 75 RCC patients and 63 RC patients were selected. In addition, blood samples from 75 healthy controls were collected. The demographic data of these patients are listed in Table 1. Six patients were lost to follow up. The follow up time of RCC patients ranged from 8 to 61 months with average of 38.6± 16.2 months. A total of 18 patients had recurrence during the follow-up, 8 of them relapsed within 1 year after sur-gery; 4 within 1 to 3 year after surgery and 6 within 3 to 5 years.
Table 1- Demographic data of selected subjects.
Healthy RCC RC
Case number 75 75 63
Age 62.07±3.21 59.72±2.96 61.03±3.67
Gender (M/F) 40/35 48/37 42/21
Tumor location (left/ right) - 39/36
Tumor diameter (mm) - 2.7±0.8
miRNA-378 expression before surgery
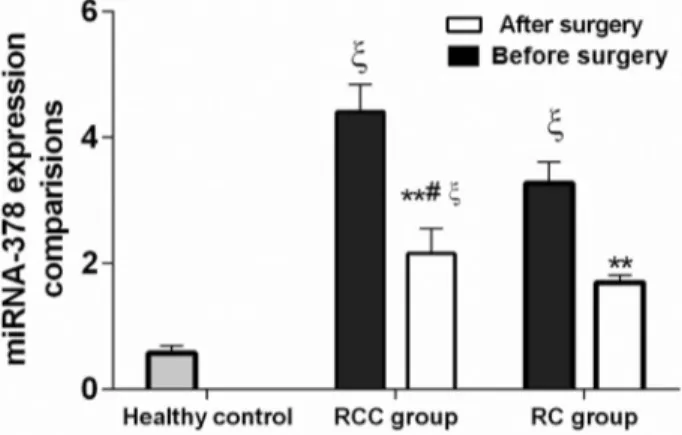
We first compared the miRNA-378 expression in healthy controls, and in RC and RCC patients. As shown in Figure 1, the expression of miRNA-378 was significantly different between the three groups; the RCC group had the highest miRNA-378 level (2.41 ± 1.43) when compared with RC (1.67±1.46) and healthy control (0.37±0.22, p < 0.05); the miRNA-378 level in RC group was clearly higher (p < 0.01) than in the healthy control. These results suggest that high expression of miRNA-378 may be associated with renal lesions.
miRNA-378 expression after surgery
Six months after surgery, the expression level of miRNA-378 in the RCC and RC groups decreased signifi-cantly when compared with same group before surgery (p < 0.05). The levels decreased 53% in the RCC group and 59% in the RC group, but were still higher than in the healthy control. When compared with healthy control, the RC group had higher levels but these were not statistically dif-ferent (p > 0.05), while the RCC group maintained signifi-cantly higher levels (p < 0.05), Figure 1.
miRNA-378 expression is correlated with clinical stage and differentiation degree
We further studied the relationship between miRNA-378 expression level and clinical pathological pa-rameters in RCC (Table 2). The results showed that miRNA-378 expression is correlated with clinical stage and differentiation degree; patients with higher clinical stage and low differentiation degrees had a much higher ex-pression level of miRNA-378 (3.23±1.44 in stage IVvs
0.76±1.16 in stage I; 3.98±1.66 in low differentiationvs
1.46±1.18 in high differentiation degree). Levels were not correlated with patient’s age (£50vs> 50), gender, surgical strategy (partial nephrectomyvsradical nephrectomy), and tumor diameter (£2 mmvs> 2 mm).
miRNA-378 in relapsed patients
According to the image or endoscopic examination results, we compared miRNA-378 expressions in patients with and without tumor recurrence (from blood samples of RCC patients six months after the surgery). Results showed that the patients with tumor recurrence had signif-icantly higher miRNA-378 levels than patients without tu-mor recurrence (3.86±2.16vs1.95±1.03,Z= 26.26, p < 0.001).
Diagnostic efficiency of serum miRNA-378 in RCC
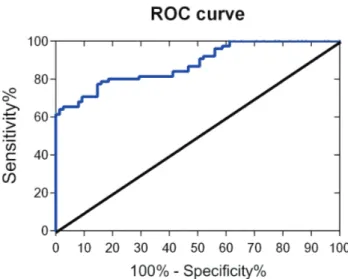
We used miRNA-378 as a biological indicator for RCC patients and healthy people. As a sensitivity curve, the closer the ROC curve is to the upper left corner of the graph, the higher is the accuracy of the tested methods; when the AUC value is between 0.7 to 0.9, the method has a certain accuracy and if > 0.9, it has high accuracy. The AUC of miRNA-378 was 0.896 with a standard error of 0.025, 95% confidence interval of 0.847 to 0.945, and the AUC hypothesis testing was statistically significant (p < 0.001), Figure 2.
Figure 1- Comparison of miRNA-378 expressions among healthy
con-trols, renal cyst (RC) and renal cell carcinoma (RCC) patients before sur-gery and six months after sursur-gery.**p < 0.01 compared with before sur-gery in same group;#p < 0.05, compared with RC group after surgery.xP < 0.01 compared with healthy subjects.
Table 2- The relationship between miRNA-378 expression level and clin-ical pathologclin-ical parameters in RCC patients (n = 75).
Pathological parameter Cases miRNA-378 (Mean±SD)
Tvalue Pvalue
Age (yr) 0.26 0.14
£50 27 2.14±0.98
> 50 48 2.56±1.22
Gender 0.133 0.07
Male 49 1.39±1.08
Female 26 2.45±1.34
Clinical stages
I 21 0.76±1.16 -2.895 0.002
II 14 1.64±1.24
III 18 2.84±1.52
IV 12 3.23±1.44
Operation mode
Partial nephrectomy 52 2.08±1.24 0.19 0.089
Radical nephrectomy 23 3.17±1.89
Differentiation degree
High 28 1.46±1.18 -0.675 0.001
Middle 30 2.42±1.24
Low 17 3.98±1.66
Tumor diameter (mm)
£2 42 2.23±1.05 2.362 0.129
Discussion
miRNA genes account for about 1% of the whole ge-nome, and they act via complementary pairing in the 3’UCR of target RNAs. miRNA binding results in the deg-radation of these mRNA and/or the inhibition of transla-tion. miRNAs and their target mRNA molecules constitute a complex regulatory network, including the biological processes of cell proliferation, apoptosis, cell differentia-tion, development, stress response and other biological ac-tivity (Romero-Cordobaet al., 2014).
miR-378 is a highly conserved miRNA that is ex-pressed in a variety of tumors, such as liver cancer (Liet al., 2013), non-small-cell lung cancer (Sunet al., 2015), colorectal cancer and others (Wanget al., 2015b). The ab-normal expression of miRNA-378 could be involved in the development of tumors, and its expression may be used as a marker in clinical diagnosis, pathological classi-fication and prognosis. Redovaet al. (2012) used gene chips to screen 15 patients with RCC and 12 healthy con-trols, and they identified the expression levels of 667 miRNAs and verified candidate miRNAs by RT-qPCR. They concluded that miRNA-378 is highly expressed in RCC and it might serve as a biomarker for early diagnosis (Redova et al., 2012). Lee et al. (2007) reported that miRNA-378 could inhibit the expression of SuFu and
Fus-1, two tumor suppressor genes, and promote cell sur-vival, tumor growth and angiogenesis.
Our study found that the expression of miRNA-378 was higher in RCC patients than in the benign tumor (RC) and healthy control groups. The relative expression of miRNA-378 in serum could effectively distinguish RCC patients and normal population, which is consistent with Redova’s report. In addition, our research demonstrated that the expression of miRNA-378 was correlated with tu-mor differentiation degree and clinical stage, but not
corre-lated with patients’ age and gender. In this study, the AUC was statistically significant, which indicates that miRNA-378 can effectively distinguish benign and malignant kid-ney lesions. Moreover, the relative expression of serum miRNA-378 in RCC and RC groups was significantly de-creased at six months after surgery, which means that miRNA-378 could be used as a biological indicator for the curative effect in kidney lesions.
Blood samples have the advantage of being easy to obtain, noninvasive and repeatable. In addition, serum miRNAs have good stability and are is not easily affected by temperature and pH. Therefore, miRNA-378 can be used as a convenient biological indicator for the diagnosis of RCC. However, it is not clear whether the high sion of miRNA-378 in plasma is consistent with its expres-sion in cancer tissues of RCC patients. Khellaet al.(2012) analyzed 10 miRNA (200c, 185, 34a, miR-142-3p, RNA, miR-155, miR-210, miR-224, miR-21, miR-592) in kidney mass tissues, and determined whether these were benign or malignant tumors. Their results showed that the miRNAs are closely related to hypoxia-induced stimulation and angiogenesis. (Youssef et al., 2011) assessed 94 RCC specimens through qPCR methods and then genotyped and compared the results with patho-logic examinations based on 900 miRNA detection results. The authors concluded that the diagnostic accuracy of miRNA in renal clear cell carcinoma was 100%, and 97% in papillary carcinoma.
On the other hand, there are also reports that serum miRNA-378 cannot be used for early diagnosis of RCC. (Hauseret al., 2012) analyzed the serum miRNA-378 ex-pression levels in RCC and normal persons through RT-PCR and concluded that circulating serum levels of miR-378 are unlikely to provide helpful diagnostic/prognostic information in RCC patients. Such inconsistent results might be caused by the following factors: inconsisten selec-tion of reference genes; use of reagents from different com-panies; individual differences in the serum levels of expres-sion of miRNA; rae, gender, and regional differences; and small numbers of patients.
Our study also has its limitations as we did not com-pare the miRNA-378 levels between serum and RCC (and RC) tissues. Furthermore, the study had a limited number of cases, and we did not perform a correlation analysis. However, our research provides useful data for diagnosis of RCC: we demonstrated that miRNA-378 is highly ex-pressed in the plasma of cancer patients, it can be used to distinguish RCC patients and healthy controls, and its higher expression is correlated with higher clinical stages and low differentiation degrees. At present, the mechanism of miRNA-378 in the occurrence and development of RCC is not clearly explained. Larger samples are needed to clar-ify the role of miRNA-378 in the diagnosis of renal cell car-cinoma, targeting therapy, curative effect evaluation and prognosis.
References
Chen LT, Xu SD, Xu H, Zhang JF, Ning JF and Wang SF (2012) MicroRNA-378 is associated with non-small cell lung can-cer brain metastasis by promoting cell migration, invasion and tumor angiogenesis. Med Oncol 29:1673-1680. Cheng L, Doecke JD, Sharples RA, Villemagne VL, Fowler CJ,
Rembach A, Martins RN, Rowe CC, Macaulay SL, Masters CL,et al.(2015) Prognostic serum miRNA biomarkers as-sociated with Alzheimer’s disease shows concordance with neuropsychological and neuroimaging assessment. Mol Psychiatry 20:1188-1196.
Das J, Podder S and Ghosh TC (2014) Insights into the miRNA regulations in human disease genes. BMC Genomics 15:1010.
Deng H, Guo Y, Song H, Xiao B, Sun W, Liu Z, Yu X, Xia T, Cui L and Guo J (2013) MicroRNA-195 and microRNA-378 mediate tumor growth suppression by epigenetical regula-tion in gastric cancer. Gene 518:351-359.
Duan L, Xiong X, Liu Y and Wang J (2014) miRNA-1: Functional roles and dysregulation in heart disease. Mol Biosyst 10:2775-2782.
Fedorko M, Stanik M, Iliev R, Redova-Lojova M, Machackova T, Svoboda M, Pacik D, Dolezel J and Slaby O (2015) Combi-nation of MiR-378 and MiR-210 serum levels enables sensi-tive detection of renal cell carcinoma. Int J Mol Sci 16:23382-23389.
Franceschi S and Wild CP (2013) Meeting the global demands of epidemiologic transition - The indispensable role of cancer prevention. Mol Oncol 7:1-13.
Golan S and Eggener S (2015) Evaluating the emerging trends in the diagnosis and treatment of kidney cancer-the renal mass global study. J Endourol 29:495-497.
Hauser S, Wulfken LM, Holdenrieder S, Moritz R, Ohlmann CH, Jung V, Becker F, Herrmann E, Walgenbach-Br1nagel G, von Ruecker A,et al.(2012) Analysis of serum microRNAs (miR-26a-2*, miR-191, miR-337-3p and miR-378) as po-tential biomarkers in renal cell carcinoma. Cancer Epidemiol 36:391-394.
Huang J, Dong B, Zhang J, Kong W, Chen Y, Xue W, Liu D and Huang Y (2014) miR-199a-3p inhibits hepatocyte growth factor/c-Met signaling in renal cancer carcinoma. Tumour Biol 35:5833-5843.
Juan D, Alexe G, Antes T, Liu H, Madabhushi A, Delisi C, Ganesan S, Bhanot G and Liou LS (2010) Identification of a microRNA panel for clear-cell kidney cancer. Urology 75:835-841.
Khella HW, White NM, Faragalla H, Gabril M, Boazak M, Dorian D, Bishoy Khalil, Antonios H, Bao TT, Pasic MD, et al. (2012) Exploring the role of miRNAs in renal cell carci-noma progression and metastasis through bioinformatic and experimental analyses. Tumour Biol 33: 131-40.
Lee DY, Deng Z, Wang CH and Yang BB (2007) MicroRNA-378 promotes cell survival, tumor growth, and angiogenesis by targeting SuFu and Fus-1 expression. Proc Natl Acad Sci U S A 104:20350-20355.
Li LH, Gao Q, Wang XY and Guo ZJ (2013) [miR-378 suppresses HBV-related hepatocellular carcinoma tumor growth by
di-rectly targeting the insulin-like growth factor 1 receptor]. Zhonghua Gan Zang Bing Za Zhi 21:609-613.
Lin J, Horikawa Y, Tamboli P, Clague J, Wood CG and Wu X (2010) Genetic variations in microRNA-related genes are associated with survival and recurrence in patients with re-nal cell carcinoma. Carcinogenesis 31:1805-1812.
Redova M, Poprach A, Nekvindova J, Iliev R, Radova L, Lakomy R, Svoboda M, Vyzula R and Slaby O (2012) Circulating miR-378 and miR-451 in serum are potential biomarkers for renal cell carcinoma. J Transl Med 10:55.
Romero-Cordoba SL, Salido-Guadarrama I, Rodriguez-Dorantes M and Hidalgo-Miranda A (2014) miRNA biogenesis: Bio-logical impact in the development of cancer. Cancer Biol Ther 15:1444-1455.
Schmittgen TD and Livak KJ (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3:1101-1108.
Sun D, Li X, Ma M, Liu J, Xu Y, Ye L, Hou H, Wang C, Li X and Jiang Y (2015) The predictive value and potential mecha-nisms of miRNA-328 and miRNA-378 for brain metastases in operable and advanced non-small-cell lung cancer. Jpn J Clin Oncol 45:464-473.
Tan HJ, Filson CP and Litwin MS (2015) Contemporary, age-based trends in the incidence and management of patients with early-stage kidney cancer. Urol Oncol 33:21.e19-21.e26.
Wang C, Hu J, Lu M, Gu H, Zhou X, Chen X, Zen K, Zhang CY, Zhang T, Ge J,et al.(2015a) A panel of five serum miRNAs as a potential diagnostic tool for early-stage renal cell carci-noma. Sci Rep 5:7610.
Wang Z, Ma B, Ji X, Deng Y, Zhang T, Zhang X, Gao H, Sun H, Wu H, Chen X, et al. (2015b) MicroRNA-378-5p sup-presses cell proliferation and induces apoptosis in colorectal cancer cells by targeting BRAF. Cancer Cell Int 15:40. Warnecke-Eberz U, Chon SH, Hölscher AH, Drebber U and
Bollschweiler E (2015) Exosomal onco-miRs from serum of patients with adenocarcinoma of the esophagus: Compari-son of miRNA profiles of exosomes and matching tumor. Tumour Biol 36:4643-4653.
Wong KY, Yu L and Chim CS (2011) DNA methylation of tumor suppressor miRNA genes: A lesson from the miR-34 family. Epigenomics 3:83-92.
Wulfken LM, Moritz R, Ohlmann C, Holdenrieder S, Jung V, Becker F, Herrmann E, Walgenbach-Br1nagel G, von Ruecker A, M1ller SC,et al.(2011) MicroRNAs in renal cell carcinoma: Diagnostic implications of serum miR-1233 levels. PLoS One 6:e25787.
Ye J, Wu X, Wu D, Wu P, Ni C, Zhang Z, Chen Z, Qiu F, Xu J and Huang J (2013) miRNA-27b targets vascular endothelial growth factor C to inhibit tumor progression and angio-genesis in colorectal cancer. PLoS One 8:e60687.
Youssef YM, White NM, Grigull J, Krizova A, Samy C, Mejia-Guerrero S, Evans A and Yousef GM (2011) Accurate mo-lecular classification of kidney cancer subtypes using microRNA signature. Eur Urol 59:721-730.
Associate Editor: Ana Maria Aranha Camargo