0103 - 5053 $6.00+0.00
A
r
ti
c
le
* e-mail: pvolivei@iq.usp.br
W+Rh as Permanent Chemical Modifier in Simultaneous Atomic Absorption Spectrometry:
Interference Studies on As, Cd, Pb and Se Determination
Cassiana S. Nomura, Paulo R. M. Correia, Pedro V. Oliveira* and Elisabeth Oliveira
Instituto de Química, Universidade de São Paulo, CP 26077, 05513-970 São Paulo - SP, Brazil
Um estudo sistemático para verificar as interferências provocadas por Na+, K+, Ca2+, Mg2+, Cl-,
NO3-, SO 4
2- e PO 4
3- nos processos de atomização simultânea de As, Cd, Pb e Se foi realizado
utilizando um espectrômetro de absorção atômica com atomização eletrotérmica. A mistura de 250 µg W + 250 µg Rh foi termicamente depositada sobre a plataforma do tubo de grafite e utilizada como modificador químico permanente. O ajuste compromissado dos parâmetros do programa de aquecimento (Tp = 650 oC e Ta = 2200 oC) diminuiu a eficiência de decomposição e volatilização
térmica de alguns interferentes durante a etapa de pirólise, tornando a atomização simultânea de As, Cd, Pb e Se mais suscetível à interferências. Os ânions Cl- e SO
4
2- causaram as interferências mais
severas, enquanto NO3- somente afetou a atomização dos elementos estudados quando presente em
elevadas quantidades (> 27 µg). O ânion PO43- provocou uma forte interferência negativa sobre o Se.
As interferências causadas pelos cátions Na+, K+, Mg2+, Ca2+ afetaram principalmente o Cd, sendo
que os cloretos dessas espécies provocaram interferências mais intensas do que os nitratos.
A systematic study of Na+, K+, Ca2+, Mg2+, Cl-, NO 3
-, SO 4
2- and PO 4
3- interference on the
simultaneous atomization of As, Cd, Pb and Se was carried out using an electrothermal atomic absorption spectrometer. The mixture 250 µg W + 250 µg Rh was thermally deposited on the graphite tube platform and used as permanent chemical modifier. The compromised heating program adjustments (Tp = 650 oC and Ta = 2200 oC) diminished the thermal decomposition and volatilization
efficiency of the interfering species during the pyrolysis step. The Cl- and SO 4
2- anions caused the
most severe interference, while NO3- only affected the analytes’ atomization when high amounts
(> 27 µg) were used. The anion PO43- produced a strong negative interference on the Se absorbance
signals. The presence of Na+, K+, Ca2+ and Mg2+ prejudiced mainly Cd, and the interference effects
of Cl- salts were more pronounced than NO 3
- salts.
Keywords: simultaneous atomic absorption spectrometry, interference, permanent chemical modifier
Introduction
Among the instrumental techniques available for trace and ultra-trace element determinations, atomic absorption spectrometry (AAS) occupies an outstanding position due to its high specificity, selectivity, and sensitivity, low spectral interference, and ease of operation. Despite of these attributes, its conventional mono-element operation mode restrains the analytical frequency and can be considered as the main drawback of this technique.1 This shortcoming
is aggravated for electrothermal atomic absorption spectrometry (ETAAS) due to the long extent of the heating program atomizer, typically 1 to 3 min.
Multi-element atomic absorption researches have aroused interest since the first AAS stage in order to conceive a spectrometer able to determine several elements simultaneously or quasi simultaneously. As result of these efforts, the simultaneous atomic absorption spectrometry (SIMAAS) technique was proposed.2-4 Commercially
At the present, the most updated commercial instru-mentation is a line-source simultaneous spectrometer,1
equipped with transversely heated graphite atomizer with integrated platform, Zeeman-effect background corrector, and solid-state detector, making possible the operation under STPF conditions.5 This instrument allows simultaneous
determinations up to six elements.1
SIMAAS has been used for various multi-element determinations with acceptable performance6-26 (Table 1),
keeping the main features of ETAAS: high sensitivity, low detection limits, reduced sample requirements, and the possibility to carry out in situ sample decomposition inside the graphite furnace. Although the increase number of multi-element procedures, reports dealing with interference studies for SIMAAS are scare so far.12
The mixture palladium and magnesium nitrate has been widely used for multi-element determinations by SIMAAS.6,8-11,14,15,20,23,25 It is claimed as universal chemical
modifier due to the thermal stability improvement for 21 elements.27 Although this mixture seems to be the most
suitable choice, other alternatives as rhodium-based chemical modifiers can also be explored for multi-element determinations.
The rhodium-based chemical modifiers have been successfully adopted for mono-element determinations by ETAAS. The mixture of 250 µg W + 200 µg Rh was proposed as permanent chemical modifier for mono-element determinations.28-31 The results indicated its
effectiveness for As, Cd, Cu, Pb and Se, and its performance is equal or superior than that verified for the universal chemical modifier.
The use of permanent chemical modifiers allows increase the graphite tube lifetime, eliminate volatile impurities during the thermal coating process, decrease the detection limits, reduce the total heating cycle time, and minimize the high purity chemical consumption.28
Considering these favorable characteristics, the aim of this work is to investigate the performance of the permanent chemical modifier 250 µg W + 250 µg Rh to minimize interferences of Na+, K+, Ca2+, Mg2+, NO
3
-, Cl-, SO 4
2- and
PO43 on the simultaneous atomization of As, Cd, Pb and
Se. Additionally, determinations of As, Cd, Pb and Se in non-potable water using this chemical modifier are proposed.
Experimental
Apparatus
Measurements were carried out by using a SIMAA-6000 electrothermal atomic absorption spectrometer with
a longitudinal Zeeman-effect background correction system, Echelle optical arrangement, solid state detector and standard THGA tube with pyrolytic coated integrated platform (Perkin-Elmer, Norwalk, CT, USA).
The spectrometer was operated in 4-element simul-taneous mode using electrodeless discharge lamps for arsenic (λ=193.7 nm, i=380 mA), cadmium (λ=228.8 nm, i=200 mA), lead (λ=283.3 nm, i=450 mA), and selenium (λ=196.0 nm, i=290 mA). Solutions were delivered into the graphite tube by means of an AS-72 autosampler.
Argon 99.999% v/v (Air Liquide Brasil S/A, São Paulo, SP, Brazil) was used as protective and purge gas.
Reagent and reference solutions
All solutions were prepared with high purity de-ionized water (18.2 MΩ cm) obtained from a Milli-Q water purification system (Millipore Corp., Bedford, MA, USA). Analytical reagent-grade HNO3 (Synth, Diadema, SP, Brazil) was distilled in quartz sub-boiling stills (Marconi, Piracicaba, SP, Brazil). High purity standard reference solutions of arsenic (As2O3), cadmium (CdCl2), lead [Pb(NO3)2] and selenium (SeO2) from Tritisol (Merck, Darmstadt, Germany) were used to prepare the reference analytical solutions. The chemical modifier solutions were obtained from Na2WO4.2H2O (Merck) and RhCl3 (Aldrich). Cation and anion interference effects were investigated with solutions containing up to 10000 mg L-1 of Na+
[NaNO3, NaCl, Na3PO4 or Na2SO4 (Merck)], 10000 mg L-1
of K+ [KNO
3 or KCl (Merck)], 5000 mg L
-1 of Ca2+ [Ca(NO 3)2
or CaCl2 (Merck)] and 5000 mg L-1 of Mg2+ [Mg(NO 3)2 or
MgCl2 (Merck)] in 0.1% v/v HNO3.
Arsenic, Cd, Pb and Se were simultaneously determined in non-potable water samples with high saline content from Quality Technologies Pty Ltd (Mount Isa, Australia) using 250 µg W + 250 µg Rh as permanent chemical modifier. The samples were diluted 1:10 or 1:20 with 0.1% v/v HNO3 for the multi-element determinations. Addition and recovery tests were performed to verify the reliability of the obtained results for As, Cd, Pb and Se determination.
Procedure
All glassware, polypropylene flasks and bottles (Nalge Company, Rochester, USA) were cleaned with detergent solution, soaking in 10% v/v HNO3 for 24 h, rinsed with Milli-Q water and stored into a closed polypropylene container.
Table 1. Multi-element analytical procedures developed by SIMAAS
Element Sample Chemical modifier LD (mg L-1) m
0 (pg) Ref.
Mn Serum Pd + Mg 0.65 6.0 6
Se 5.0 46
Cu Serum - 4.0 26 7
Fe 2.2 16
Zn 0.4 2.7
As Drinking water Pd + Mg 0.7 39 8
Cu 0.2 17
Mn 0.6 60
Sb 0.3 43
Se 0.9 45
Al Fuel ethanol Pd + Mg 1.2 37 9
As 2.5 73
Cu 0.2 31
Fe 1.6 16
Mn 0.2 45
Ni 1.1 9.0
Bi Wine Pd + Mg - - 10
Pb 0.9 45
Cu Sea water Pd + Mg and 5% H2 0.4 - 11
Mn 0.7
-Mo 1.2
-Cr Urine Mg 0.08 7.8 13
Mn 0.16 4.6
Cd Wine Pd + Mg 0.03 0.6 14
Pb 0.8 33
As Atmosferic particulate matter Pd + Mg 0.26 37 15
Cd 0.02 2.2
Ni 0.97 28
Pb 4.3 1400
Al Urine Pd + 5% H2 0.06 - 16
Cr 0.05
-Cu 0.08
-Mn 0.06
-Cr Bismuth tellurite optical crystals - 1.1 7.5 17
Mo 4.9 20
V 6.7 58
Cd Foodstuffs NH4H2PO4 + Mg 0.04 1.5 18
Pb 0.93 37
As Water Ir 0.82 177 19
Bi 0.04 91
Sb 0.26 107
Se 0.29 90
Te -
-Cd Potable and surface water Pd + Mg 0.1 - 20
Cr 1
-Cu 1
-Ni 1
-Pb 1
-Mo Seawater NH4H2PO4 + Mg 0.35 - 21
V 0.32
-Cr Serum - 0.05 - 22
Ni 0.2
-Cd Blood NH4H2PO4 0.2 - 22
Pb 4.5
-Cd Urine NH4H2PO4 0.06 - 22
Pb 2.6
-Cu Seawater Pd + Mg 1.5 23
Mn 0.07 0.10 37
Cd Soil and sediment - 0.001 0.44 24
Pb 0.1 22
Co 0.05 22
Cu 0.02 35
Cd Whole blood Pd + Mg - - 25
Pb -
-Co Urine Mg 0.18 - 26
-2.5 µg L-1 of Cd2+ + 50 µg L-1 of As3+, Pb2+ and Se4+ in
0.1% v/v HNO3, with and without the concomitant species, were used for the heating program optimization and for the interference studies. These solutions were prepared directly in the autosampler cups by dilution (1:1) of the stock reference solution, containing 5.0 µg L-1 of Cd2+ +
100 µg L-1 of As3+, Pb2+ and Se4+ in 0.1% v/v HNO 3, with
blank or interfering solutions.
The permanent chemical modifier 250 µg W + 250 µg Rh was obtained according to the thermal coating process described in the literature.28 The heating program
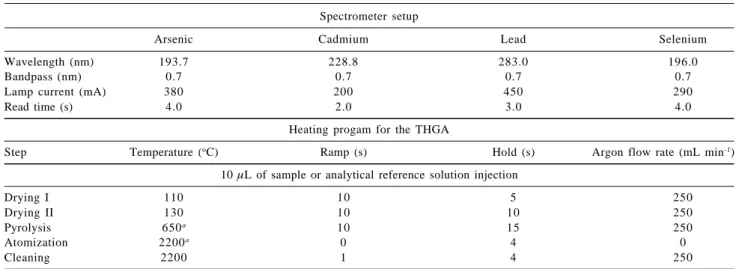
was optimized with pyrolysis and atomization temperature curves in absence and presence of the mixture 250 µg W + 250 µg Rh as chemical modifier. The instrumental adjustments and the heating program for the graphite tube are showed in Table 2.
The influence of increasing amounts of Na+, K+, Mg2+,
Ca2+, Cl-, NO 3
-, SO 4
2- and PO 4
3- in the analytes’ absorbance
signals was verified. The concomitant amounts (cations and anions) added to the analytical reference solutions were based on cation concentration, ranging from 50 to 5000 mg L-1. The interference studies of cations were
executed using NO3- and Cl- salts, while for anions were
used only Na+ salts.
Experimental measurements were made with at least three replicates and based on integrated absorbance throughout this work.
Results and Discussion
When ETAAS is used for mono-element determinations, all experimental and instrumental parameters are optimized for only one analyte. Consequently, the best optimized
pyrolysis and atomization temperatures are used in the heating program, minimizing condensed- and gas-phase interference.3 On the other hand, the adoption of
compromised conditions for multi-element determinations by SIMAAS are mandatory. The heating program temperatures and chemical modifier must be carefully selected to achieve the best atomization efficiency for all the analytes. In general, the most volatile analyte determines the pyrolysis temperature while the least volatile analyte determines the atomization temperature. Despite of these adverse temperatures, the use of a transversely heated atomizer with integrated platform offers optimum conditions for both volatile and non-volatile elements.3 Add to that, STPF conditions adoption and the
use of an appropriate chemical modifier have guaranteed similar performance of SIMAAS in comparison to mono-element ETAAS. This fact reinforces that the success of any atomic absorption spectrometer for simultaneous determination depends on using STPF conditions.
Simultaneous heating program optimization
The characteristic masses (mo) and the best pyrolysis and atomization temperatures for As, Cd, Pb and Se obtained by SIMAAS in absence and presence of the 250 µg W + 250 µg Rh are presented in Table 3 and compared with mono-element ETAAS data indicated in the literature.29,30 It can be observed an agreement between
the results obtained by SIMAAS with those obtained by mono-element ETAAS (Table 3). The higher pyrolysis temperatures achieved for Cd, Pb and Se using SIMAAS were probably due to the higher Rh mass deposited on the graphite tube surface (250 µg). However, this data are in
Table 2. Spectrometer settings and heating program parameters for the multi-element detection of As, Cd, Pb and Se
Spectrometer setup
Arsenic Cadmium Lead Selenium
Wavelength (nm) 193.7 228.8 283.0 196.0
Bandpass (nm) 0.7 0.7 0.7 0.7
Lamp current (mA) 380 200 450 290
Read time (s) 4.0 2.0 3.0 4.0
Heating progam for the THGA
Step Temperature (oC) Ramp (s) Hold (s) Argon flow rate (mL min-1)
10 µL of sample or analytical reference solution injection
Drying I 110 10 5 250
Drying II 130 10 10 250
Pyrolysis 650a 10 15 250
Atomization 2200a 0 4 0
Cleaning 2200 1 4 250
aOptimized condition for As, Cd, Pb and Se in presence of 250 µg W + 250 µg Rh as permanent chemical modifier; Total program time: 69s;
discordance with previous work, which indicates that masses higher than 200 µg are not recommended due to the sensitivity decrease (about 15% for each analyte).28
The higher characteristic masses for Cd, Pb and Se in comparison with mono-element ETAAS (Table 3), can be related to the compromised conditions adopted for the simultaneous determination. The necessity to consider compromised adjustments imposes 650 oC as pyrolysis
temperature, due to the low thermal stability achieved for Cd in presence of W+Rh.
The atomization temperatures for all analytes were also increased in presence of the W+Rh permanent modifier (Table 3), for Cd (1400 oC) and Pb (1600 oC) were lower
than those verified for As (2200 oC) and Se (2000 oC). The
atomization temperature selection for the simultaneous heating program (2200 oC) was based on the As thermal
behavior. This is also the maximum temperature recommended for the heating program in order to avoid loss of deposited Rh by vaporization.28 The adoption of
higher temperatures for the atomization and/or cleaning steps impairs the W+Rh coating lifetime.
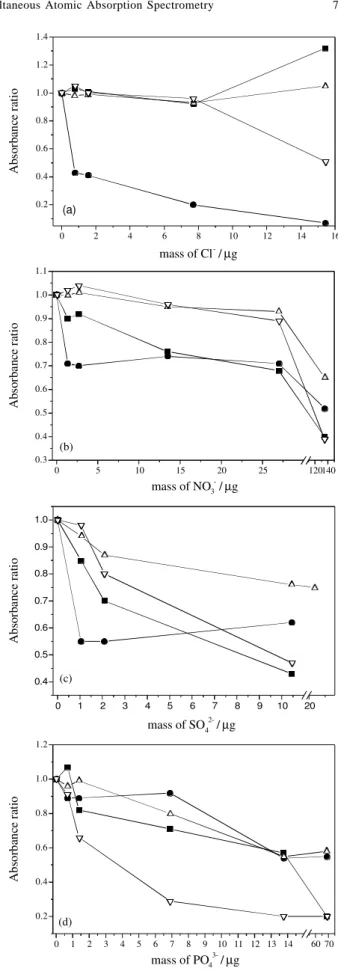
Anion interference
The effect of Cl-, NO 3
-, SO 4
2- and PO 4
3- on the
simultaneous atomization of As, Cd, Pb and Se are showed in Figure 1. The results were expressed as absorbance ratios calculated between absorbance signals with and without the anions.
The interference effect of Cl- in ETAAS has been largely
Table 3. Characteristic masses (m0), pyrolysis (Tp) and atomization
(Ta) temperatures for 25 pg Cd + 500 pg As, Pb and Se in 0.1% v/v HNO3 in absence and presence of 250 µg W + 250 µg Rh as
perma-nent chemical modifier
Chemical modifier
Analyte Parameter None W+Rha W+Rhb
m0 (pg) 74 35 39
As Tp (
oC) 500 1500 1200
Ta (
oC) 2100 2200 2100
m0 (pg) 1.2 1.8 1.1
Cd Tp (
oC) 300 650 500
Ta (
oC) 1200 1400 1200
m0 (pg) 25 43 30
Pb Tp (
oC) 500 900 800
Ta (oC) 1200 1600 1800
m0 (pg) 87 68 42
Se Tp (
oC) 400 1300 1200
Ta (oC) 1800 2000 2000
aResults for the multi-element determination of As, Cd, Pb and Se by
SIMAAS; bResults for the mono-element detemination As, Cd, Pb
and Se(29,30).
0 1 2 3 4 5 6 7 8 9 10 20 0.4 0.5 0.6 0.7 0.8 0.9 1.0 (c)
mass of SO42- / µg
A b so rb a n ce r a ti o
0 5 10 15 20 25 120140
0.3 0.4 0.5 0.6 0.7 0.8 0.9 1.0 1.1 (b)
mass of NO3- / µg
A b so rb a n ce r ati o
0 2 4 6 8 10 12 14 16
0.2 0.4 0.6 0.8 1.0 1.2 1.4 (a)
mass of Cl- / µg
A b so rb an ce r ati o A b so rb an ce r ati o
0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 60 70
0.2 0.4 0.6 0.8 1.0 1.2 (d)
mass of PO43- / µg
Figure 1. Interference effect of (a) NaNO3 (b) NaCl, (c) Na2SO4 and
Table 4. Absorbance ratios for 25 pg Cd + 500 pg As, Pb and Se in presence of Na+, K+, Mg2+, Ca2+ as chloride and nitrate salts
Absorbance ratio
Mass (µg) Cl- salt NO
3 - salt
As Cd Pb Se As Cd Pb Se
0.5 1.0 0.43 0.98 1.0 0.90 0.71 1.0 1.0
1 1.0 0.41 0.99 1.0 0.92 0.70 1.0 1.0
Na+ 5 0.92 0.20 0.93 0.96 0.76 0.74 0.95 0.96
10 1.3 0.07 1.0 0.51 0.68 0.71 0.93 0.89
50 - - - - 0.40 0.52 0.65 0.39
0.5 1.1 0.39 0.86 0.95 1.0 0.79 0.99 1.0
1 1.1 0.36 0.85 0.95 1.0 0.77 0.97 0.99
K+ 5 1.0 0.36 0.69 0.86 1.0 0.68 0.92 0.96
10 0.94 0.12 0.64 0.71 0.91 0.50 0.84 0.92
50 - - - - 0.56 0.67 0.46 0.37
0.5 0.97 0.89 0.96 0.89 1.0 0.93 0.99 0.98
1 0.99 0.79 0.89 0.96 1.1 0.96 1.0 1.0
Mg2+ 5 1.0 0.70 0.78 1.0 1.0 0.95 0.96 1.0
10 1.1 0.38 0.62 1.0 0.98 0.84 0.95 0.98
25 1.1 0.05 0.40 1.0 0.87 0.86 0.95 0.88
0.5 0.92 0.89 0.86 0.89 0.92 0.91 1.0 0.96
1 0.87 0.82 0.84 0.89 1.0 0.90 1.0 1.0
Ca2+ 5 0.85 0.66 0.82 0.87 1.1 0.77 1.1 1.0
10 0.71 0.44 0.71 0.78 0.93 0.53 0.89 0.91
25 0.62 0.40 0.71 0.77 0.87 0.64 0.93 0.91
investigated.3 The use of chemical modifier is important to
avoid loss of volatile chlorides during pyrolysis step. In this work, the pronounced effect observed from 0.8 µg Cl
-on Cd absorbance signal can be attributed to the volatilization of cadmium chloride during the pyrolysis step at 650 oC (Figure 1a). Nevertheless, Cd loss was not observed
when lower pyrolysis temperatures (< 500 oC) were used.
Probably, the formation and volatilization of CdCl2 occurred before the interaction between Cd and Rh on the graphite tube surface. It can be concluded that Cl- ions, even in low
amounts, damaged the thermal stabilization obtained for Cd in presence of the W+Rh as permanent chemical modifier. Similar behavior was observed for low masses of NO3- (1.3
mg) and SO42- (1.0 mg), which also reduced the Cd
absorbance signal (Figures 1b-1c). The increase of Cl-, NO 3
-,
SO42- masses did not cause any expressive change in the Cd
absorbance ratios. On the contrary, the initial decrease of the Cd ratio was not observed in presence of PO43- (Figure
1d), probably due to the increase in the thermal stabilization of this element. The synergistic effect between W+Rh and PO43- was efficient to stabilize Cd at 650 oC. Cadmium and
Pb form very stable oxyphosphorous compounds3 and, for
this reason, NH4H2PO4 is usually used as chemical modifier for these elements.
The effect of SO42- on As and Se was more pronounced
than on Pb atomization (Figure 1c). For the latter, the effect of Na2SO4 is aggravated in strong acid solutions.32 The
lower integrated absorbance observed for Se can be explained by molecular interference, which occurs due to the vaporization of SO42- decomposition products, such as
SO3, SO2, and SO.33-35 Arsenic exhibited strong signal
suppression in presence of the SO42- (Figure 1c). This effects
can be attributed to the interactions between As and Na2SO4 and the resulting decomposition products, particularly sulfide species.36
Selenium suffered more strong interference in presence of PO43- (Figure 1d). Additionally, As was the most affected
analyte by NO3- (Figure 1b). It can be supposed that the
low pyrolysis temperature adopted for the simultaneous heating program (650 oC), impaired the adequate formation
of the atomic precursors for As.
Cation interference
The performance of 250 µg W + 250 µg Rh to overcome the interference caused by Na+, K+, Mg2+ and Ca2+ on the
simultaneous atomization of As, Cd, Pb and Se atomization are showed in Table 4. The results were expressed as absorbance ratios calculated between the absorbance signal with and without the cations.
presence of Mg2+ as nitrate salt. For this reason, Mg(NO 3)2
is usually used as chemical modifier alone or combined with other substances. When magnesium chloride was tested, the interfering effect was more pronounced for all analytes (Table 4). These results highlighted the interfering effect of chloride anion.
Non-potable water analysis and figures of merit
Two non-potable water samples from Quality Technologies Pty Ltd (Mount Isa, Australia) used for interlaboratorial calibration were analyzed using the optimized heating program (Table 2) and the mixture of 250 µg W + 250 µg Rh as permanent chemical modifier. The results of the simultaneous determination of As, Cd, Pb and Se (Table 5) are in agreement with the interlaboratorial values (95% confidence level). Addition and recovery tests showed results between 85% and 108% for all analytes. It was possible to determine As, Cd, Pb and Se simultaneously in non-potable water samples considering 1:10 and 1:20 as dilution factor.
Detection limits for As (3.2 µg L-1), Cd (0.03 µg L-1), Pb
(0.70 µg L-1), and Se (3.0 µg L-1) were calculated based on
the variability of the 10 blank solution measurements according to 3 Sblk/m, where S corresponds to the blank measurement standard deviation and m is the calibration curve slope. Characteristic masses obtained for As, Cd, Pb and Se were 35 pg, 1.8 pg, 43 pg, and 68 pg, respectively. Under these optimized conditions, the performance of the W+Rh permanent chemical modifier was acceptable until 250 heating cycles, when the loss of sensitivity for all analytes indicated the W+Rh coating degradation. The same graphite tube was re-coated twice and their lifetime was increase to 750 heating cycles.
Conclusions
According to this study, the simultaneous deter-mination of As, Cd, Pb and Se with the mixture of 250 µg W + 250 µg Rh as permanent chemical can be subject to interference effects in high saline matrices. The volatile
analytes, such as Cd, imposes low pyrolysis temperatures (< 650 oC) when multi-element determinations are
executed by SIMAAS. The decomposition and volatilization of Na+, K+, Ca2+ and Mg2+ salts only occur
when higher pyrolysis temperatures are adopted. With the pyrolysis temperature used in the heating program (650
oC) concomitants are volatilized with analytes, increasing
the interference processes. The negative interference observed for As, Cd, Pb and Se can be related to losses by volatilization, due to the inappropriate formation of intermetallic compounds and/or solid solution between the analytes and the permanent chemical modifier.
Acknowledgments
The authors thank Fundação Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for C. S. Nomura´s fellowship and Fundação de Amparo à Pesquisa do Estado de São Paulo for the financial support (FAPESP Processo 2001/07048-1) and for P. R. M. Correia´s fellowship (FAPESP Processo 2001/02590-2).
References
1. Erickson, B.E.; Anal. Chem. 2000, 72, 543A.
2. Sneddon, J.; Farah, B.D.; Farah, K.S.; Microchem. J. 1993,
48, 318.
3. Welz, B.; Sperling, M.; Atomic Absorption Spectrometry, 3rd
ed., Wiley-VCH: Weinheim, FRG, 1999.
4. Farah, K.S.; Sneddon, J.; Appl. Spectros. Reviews 1995, 30,
351.
5. Slavin, W.; Manning, D.C.; Carnrick, G.R.; Atom. Spectros.
1981, 2, 137.
6. Correia, P. R. M.; Oliveira, E.; Oliveira, P. V.; Talanta 2002,
57, 527.
7. Correia, P. R. M.; Oliveira, E.; Oliveira, P. V.; Anal. Chim. Acta
2002, 458, 321.
8. A. Filho, V. R.; Fernandes, K. G.; Moraes, M.; Neto, J. A G.;
Atom. Spectros. 2002, 23, 7.
9. Oliveira, A P.; Moraes, M.; Neto, J. A G.; Lima, E. C.; Atom.
Spectros. 2002, 23, 39.
Table 5. Analysis and recovery test results for the simultaneous determination of As, Cd, Pb and Se in non-potable water samples
Concentration (µg L-1)
As Cd Pb Se
Dilution 1:10 Sample A 23.3 ± 10 61.8 ± 10 382 ± 11
-Ref. Value 21.6 ± 3.2 58.9 ± 13.6 296 ± 51
-Recovery (%) 91 85 86 105
Dilution 1:20 Sample B 260 ± 21 291 ± 21 501 ± 81 181 ± 20
Ref. Value 226 ± 7 235 ± 5 507 ± 9 190 ± 15
10. Fernandes, K. G.; Moraes, M.; Neto, J. A G.; Nóbrega, J. A.;
Analyst 2002, 127, 157.
11. Chen, C.; Danadurai, K. S. K.; Huang, S.; J. Anal. Atom.
Spectrom. 2001, 16, 404.
12. Volynsky, A. B.; Wennrich, R.; J. Anal. Atom. Spectrom. 2001,
16, 179.
13. Oliveira, P. V.; Oliveira, E.; Fresenius J. Anal. Chem. 2001,
371, 909.
14. Freschi, G. P. G.; Dakuzaku, C. S.; Moraes, M.; Nóbrega, J. A;
Neto, J. A G.; Spectrochim. Acta 2001, 56B, 1987.
15. Thomaidis, N. S.; Manalis, N.; Viras, L.; Lekkas, T. D.; Int. J.
Environ. Anal. Chem. 2001, 79, 121.
16. Lin, T.; Huang, S.; Anal. Chem. 2001, 73, 4319.
17. Bencs, L.; Szakács, O.; Kántor, T.; Varga, I.; Bozsai, G.;
Spectrochim. Acta 2000, 55B, 883.
18. Correia, P. R. M.; Oliveira, E.; Oliveira, P. V.; Anal. Chim. Acta
2000, 405, 205.
19. Murphy, J.; Schlemmer, G.; Shuttler, I. L.; Jones, P.; Hill, S. J.;
J. Anal. Atom. Spectrom. 1999, 14, 1593.
20. Feuerstein M.; Schlemmer, G.; Atom. Spectros. 1999, 20, 149.
21. Su, P.; Huang, S.; J. Anal. Atom. Spectrom. 1998, 13, 641.
22. White, M. A; Panati, A.; Atom. Spectros. 1998, 19, 89.
23. Su, P.; Huang, S.; Spectrochim. Acta 1998, 53B, 699.
24. Hoenig, M.; Cilissen A.; Spectrochim. Acta 1997, 52B, 1443.
25. Viksna, A; Lindgren, E. S.; Anal. Chim. Acta 1997, 353, 307.
26. Iversen, B. S.; Panayi, A.; Camblor, J. P.; Sabbioni, E.; J. Anal.
Atom. Spectrom. 1996, 11, 591.
27. Welz, B.; Schelemmer, G.; Mudakavi, J. R.; J. Anal. Atom.
Spectrom. 1992, 7, 1257.
28. Lima, E. C.; Krug, F. J.; Jackson, K.W.; Spectrochim. Acta
1998, 53B, 1791.
29. Barbosa Jr, F.; Lima, E. C.; Krug, F. J.; Analyst 2000, 125,
2079.
30. Lima, E. C.; Barbosa Jr, F.; Krug., F. J.; Tavares A.; Talanta
2002, 57, 177.
31. Zanao, R. A.; Barbosa F.; Souza, S. S.; Krug, F. J.; Abdalla, A
L.; Spectrochim. Acta 2002, 57B, 291.
32. Cabon, J. Y.; Le Bihan, A.; Spectrochim. Acta 1996, 51B, 619.
33. Cabon, J. Y.; Le Bihan, A.; Anal. Chim. Acta 1999, 402, 327.
34. Ming, N. Z.; Bin, H.; Bin, H. H.; Spectrochim. Acta 1994,
49B, 947.
35. Cabon, J. Y.; Fresenius J Anal Chem 2000, 367, 714.
36. Welz, B.; Schlemmer G.; J. Anal. Atom. Spectrom. 1986, 1,
119.
Received: February 20, 2003
Published on the web: January 19, 2004