UNIVERSIDADE DE LISBOA
FACULDADE DE CIÊNCIAS
DEPARTAMENTO DE BIOLOGIA ANIMAL
Albatross-Cephalopod Interactions in Antarctic Ocean:
implications for albatrosses ecology and conservation
Pedro Miguel Oliveira Soromenho de Alvito
Dissertação
MESTRADO EM BIOLOGIA DA CONSERVAÇÃO
UNIVERSIDADE DE LISBOA
FACULDADE DE CIÊNCIAS
DEPARTAMENTO DE BIOLOGIA ANIMAL
Albatross-Cephalopod Interactions in Antarctic Ocean:
implications for albatrosses ecology and conservation
Pedro Miguel Oliveira Soromenho de Alvito
Dissertação
MESTRADO EM BIOLOGIA DA CONSERVAÇÃO
Orientada pelo Doutor Rui Afonso Bairrão da Rosa (CO/LMG)
E co-orientada pelo Doutor José Carlos Caetano Xavier
(IMAR-CMA)
i
Acknowledgements
The preparation of a dissertation can be compared to writing a book. On the Alexandre Dumas novel “The Three Musketeers”, D'Artagnan needed the help of his inseparable friends to attain his goals. In the same way I am very grateful to those who had helped me to organize and write this dissertation, in particular to:
Doctor José Xavier and Doctor Rui Rosa for their supervision, support, advices and help while writing the dissertation, as well as their availability and rapid answers to questions. I also thank Doctor José Xavier for the opportunity to study the spectacular life of albatrosses and the enigmatic Antarctic cephalopods, as well as his availability and transfer of knowledge during the identification of the cephalopod beaks performed at the Instituto do Mar, University of Coimbra;
My friend Miguel Guerreiro, without who I would not have been aware of this
dissertation, and for his friendship and help over the developmental and experimental work at Coimbra and Centre d ' Études biologiques of Chizé (France);
All the people from Instituto do Mar, University of Coimbra, who supported me during my experimental work: Filipe Ceia for supervising the experimental work and
contribute with data on isotopic analysis data (France), Rui Vieira for contributing with isotopic analysis data, José Seco for the help on samples screening and preparative work for isotopic analyses, Alexandra Baeta, for contributing with data on isotopic analysis data (Coimbra). And finally to Gabi, for the continuous kindness and laboratory suport.
All the people from the Centre d'Etudes biologiques of Chizé, team Ecologie et des Oiseaux Mammifères Marins (France) who supported me during my visit and particularly to Doctor Yves Cherel, for his kind reception and mainly the “hard” discussion on results on stable isotopic analyses.
My lovely family for their patience and support, specially to my parents, brother and sister and grandmother for their affection and understanding. I also thank my mother for her support throughout the process of writing the dissertation.
ii
Abstract and keywords
Albatrosses can be used as biological sampling tools to investigate poorly known organisms, such as the Southern Ocean cephalopods. The aims of the present study were to characterize the albatrosses diet, with relevance to the cephalopod component, during the reproductive period of wandering (Diomedea exulans), black-browed
(Thalassarche melanophrys) and grey-headed (Thalassarche chrysostoma) at Bird Island (South Georgia), and at the end of inter-breeding/beginning of breeding period (EIB/BB) of the last two albatross species, to assess the habitat and trophic level of key cephalopods species by stable isotopes analyses, to compare both sampled periods, to identify threats and suggest measures to reinforce these albatrosses conservation.
During the reproductive period, black-browed albatross fed mainly on fish, the grey-headed albatross on cephalopods, and the wandering albatross on both prey. The four main cephalopod species found in the albatrosses diets were Kondakovia longimana,
Martialia hyadesi, Moroteuthis knipovitchi and Galiteuthis glacialis during the
reproductive period. For the first time, black-browed and grey-headed albatrosses diets during the EIB/BB period were analyzed. K. longimana was reported as the main cephalopod species during this period and it was found that scavenging could play an important role in albatrosses diet.
Based on the stable isotopic signatures of the cephalopod lower beaks, the main species were from Antarctic and sub-Antarctic waters and could be grouped in four trophic levels.
The main threats to albatrosses included: i) interaction with fisheries, ii) the possible lower availability of krill in South Georgia region during the reproductive period, and iii) the almost absence of the M. hyadesi in grey-headed albatrosses diet. Some measures to reinforce the conservation of the three studied albatross species are related to the fishery and krill industries and by a better knowledge of southern ocean cephalopods distributions and populational trends.
iii
Resumé and keywords (in portuguese)
As populações do albatroz-viajeiro Diomedea exulans, albatroz-de-cabeça-cinzenta
Thalassarche chrysostoma e albatroz-de-sobrancelha-preta Thalassarche
melanophrys, nidificantes em Bird Island na Geórgia do Sul, são alvos de estudos
desde a década de 1960. Esta ilha subantárctica é muito importante para a sua conservação, porque nela nidificam, a nível mundial, as maiores populações do albatroz-de-cabeça-cinzenta, e importantes populações das restantes espécies estudadas. Contudo, desde finais da década de 1970 que, nesta ilha, as populações nidificantes destes albatrozes têm experimentado decréscimos populacionais,
seguindo a tendência de declínio a nível mundial. A cada ano, dezenas de milhares de albatrozes são apanhados acidentalmente em linhas de pesca. Os resíduos de
plásticos ingeridos no mar, e a introdução de espécies não-nativas nas ilhas de nidificação também apresentam riscos adicionais.
A dieta das espécies de albatrozes estudadas inclui uma importante componente de cefalópodes. Os albatrozes podem ser utilizados como ferramentas de amostragem biológicas para investigar os organismos pouco conhecidos, tais como os cefalópodes do Oceano Antárctico. Através do estudo do componente de cefalópodes na dieta dos albatrozes pode-se conhecer melhor a ecologia e dinâmica populacional dos
cefalópodes do Oceano Antárctico, que de outro modo seria muito difícil de ser obtido. Através da análise isotópica do rácio do δ13C (13C/12C) e do δ15N (15N / 14N) das
mandíbulas inferiores dos cefalópodes presentes na dieta dos albatrozes pode-se ainda revelar o habitat e o nível trófico dos cefalópodes, respectivamente. Por intermediário desta análise já foram descobertas novas relações tróficas e padrões migratórios dos cefalópodes até então desconhecidos.
Os objectivos do presente estudo consistiram em: i) caracterizar a dieta dos albatrozes, com relevância para a componente de cefalópodes, durante o período reprodutor do albatroz-viajeiro, albatroz-de-sobrancelha-preta e albatroz-de-cabeça-cinzenta, e no final do período não reprodutor /início do período reprodutor (FPNR / IR) das duas últimas espécies referidas; ii) avaliar o habitat e o nível trófico das principais espécies de cefalópodes identificadas na dieta dos albatrozes através da análise de isótopos estáveis; iii) comparar os dois períodos amostrados (reprodutor versus
iv FPNR/IR); e iv) identificar as ameaças e sugerir medidas para reforçar a conservação das espécies de albatrozes estudadas.
Os resultados relativos à dieta dos albatrozes mostraram que, durante o período reprodutor, o albatroz-de-sobrancelha-preta alimentou-se sobretudo de peixe, o albatroz-de-cabeça-cinzenta de cefalópodes, e o albatroz-viajeiro de ambos os
componentes. As quatro principais espécies de cefalópodes identificadas na dieta dos albatrozes incluíram Galiteuthis glacialis, Moroteuthis knipovitchi, Martialia hyadesi e
Kondakovia longimana para o período reprodutor. A diversidade dos cefalópodes
registada neste estudo foi menor do que a registada em anos anteriores,
correspondendo a espécies anteriormente descritas por outros autores. Não foram encontradas espécies de polvos contrariamente a outras referências nesta área. A maior diversidade de cefalópodes durante o período reprodutor foi registada na dieta do albatroz-viageiro. A sua dieta incluiu cefalópodes maiores e mais pesados do que os identificados na dieta dos restantes albatrozes, indicativo de necrofagia. O albatroz-de-sobrancelha-preta durante o período reprodutor também foi principalmente
necrófago, enquanto que o albatroz-de-cabeça-cinzenta se alimentou maioritariamente de presas vivas. Para os outros albatrozes, a diversidade de cefalópodes identificada no período reprodutor foi maior do que a encontrada durante o período FPNR/IR. Pela primeira vez, as dietas do albatroz-de-sobrancelha-preta e do albatroz-de-cabeça-cinzenta foram analisadas durante o período FPNR/IR. K. longimana foi a espécie de cefalópode mais importante para o período FPNR / IR e verificou-se que a necrofagia poderá ter um papel importante na alimentação dos albatrozes. As únicas espécies de cefalópodes comuns a ambos os períodos amostrados foram K. longimana, G.
glacialis, Gonatus antarcticus e Taonius sp.B (Voss), pelo que se sugere que possam
ter, sobretudo K. longimana, uma maior importância nos ecossistemas marinhos do que a que lhes era habitualmente atribuída.
Através da análise isotópica do rácio de δ13C (13C/12C) das mandíbulas inferiores dos cefalópodes, verificou-se que as principais espécies encontradas na dieta dos
albatrozes apresentaram assinaturas referentes a águas antárcticas e subantárticas. No primeiro caso, as espécies de cefalópodes associadas foram Batoteuthis skolops e
Psychroteuthis glacialis, e no segundo foram Chiroteuthis veranyi, Histioteuthis macrohista, Histioteuthis atlantica e Taonius sp. B (Voss). Para além destes
cefalópodes, foram identificadas outras espécies que apresentaram assinaturas isotópicas referentes às duas massas de água, antárctica e subantártica,
v nomeadamente, Histioteuthis eltaninae, Moroteuthis knipovitchi, Kondakovia
longimana, Gonatus antarcticus, Martialia hyadesi, Galiteuthis glacialis e Alluroteuthis antarcticus. Por fim, foi ainda identificada a espécie Illex argentinus com uma
assinatura referente a águas subtropicais.
Através da análise isotópica do rácio de δ15
N (15N / 14N) das mandíbulas inferiores dos cefalópodes, verificou-se que as espécies de cefalópodes poderiam ser agrupadas em quatro níveis tróficos distintos, compreendendo assinaturas entre os 2.45 a 4.40‰, 6.19 a 6.63‰, 7.15 a 8.83‰ e 9.02 a 12.18‰. No primeiro grupo referido inclui-se
Martialia hyadesi, no segundo Kondakovia longimana (com mandíbulas de tamanho
médio), no terceiro Histioteuthis eltaninae, Moroteuthis knipovitchi, Kondakovia
longimana (com mandíbulas de tamanho grande), Galiteuthis glacialis, Alluroteuthis antarcticus e Psycroteuthis glacialis, e no último Gonatus antarcticus, Chiroteuthis veranyi, Illex argentinus, Taonius sp. B (Voss), Histioteuthis macrohista, Histioteuthis atlantica e Batoteuthis skolops. A ocorrência destes níveis tróficos sugere a existência
de um continuum entre cefalópodes de níveis tróficos inferiores que se alimentam de crustáceos (como M. hyadesi) e de níveis tróficos superiores que se alimentam de peixes (como G. antarcticus). Os cefalópodes apresentaram assinaturas mais baixas do que aquelas geralmente registadas, o que poderá indicar que se alimentaram de presas que normalmente ocupam níveis tróficos inferiores. Os indivíduos de K.
longimana apresentaram um enriquecimento em N15 com o aumento do tamanho da
mandíbula inferior, como já anteriormente descrito por outros autores.
As principais ameaças identificadas para as espécies de albatrozes estudadas tendo por base as suas dietas foram: i) a interacção com a pesca, ii) a eventual baixa disponibilidade de krill Euphausia superba na Geórgia do Sul durante o período reprodutivo dos albatrozes, que afectou sobretudo o albatroz-de-sobrancelha-preta e iii) a ausência de M. hyadesi na dieta do albatroz-de-cabeça-cinzenta. A principal causa do declínio da maioria das espécies de albatrozes é conhecida ou inferida, como sendo a mortalidade acidental na pesca (bycatch), especialmente na pesca de palangre e de arrasto, onde os albatrozes são vulneráveis a anzóis, redes de arrasto e cabos de armação das mesmas. Na dieta do albatroz-viajeiro foram encontrados anzóis e linhas de pesca, incluindo uma linha de palangre. Foram ainda identificadas espécies de peixes na dieta do albatroz-viajeiro e do albatroz-de-sobrancelha-preta alvos da pesca comercial. O albatroz-de-sobrancelha-preta em anos de baixa disponibilidade de presas não altera a sua área de alimentação, pelo que uma baixa
vi disponibilidade de krill poderá ser indício de um baixo sucesso reprodutor, contribuindo para um decréscimo populacional desta espécie. Os dados da dieta do albatroz-de-cabeça-cinzenta sugerem que poderá ter enfrentado um baixo sucesso reprodutor no período avaliado devido à quase ausência de M. hyadesi, cujo consumo está
relacionado com o seu sucesso reprodutor.
Tendo por base as ameaças referidas, sugerem-se como medidas para reforçar a conservação destas três espécies de albatrozes a continuação de acções já iniciadas como: i) implementação de medidas de mitigação para reduzir as capturas acidentais de aves marinhas nas frotas de pesca, ii) combate à pesca ilegal, não declarada e não regulamentada (INN),e iii) controlo da expansão da pesca industrial do krill. Para além destas, sugere-se fortemente o desenvolvimento e aplicação de novas medidas como a protecção das zonas potenciais de alimentação dos albatrozes durante os períodos reprodutor e não reprodutor através do conhecimento das distribuições e tendências populacionais das principais espécies de cefalópodes por estes capturados.
Index
ACKNOWLEDGEMENTS ... I
ABSTRACT AND KEYWORDS ... II
RESUMÉ AND KEYWORDS (IN PORTUGUESE) ... III
1.GENERAL INTRODUCTION ... 1
1.1 Why doing research in the Antarctic?... 1
1.2 The Southern Ocean ... 1
1.3 Bird Island, South Georgia ... 2
1.4 Studied albatrosses species ... 3
1.5 Southern Ocean cephalopods ... 4
1.6 Albatross-cephalopod interactions ... 5
1.7 Albatrosses main threats ... 6
1.8 Stable isotopes: concepts and terminology ... 8
1.9 Thesis’ framework ... 8
1.10 Thesis’ objectives ... 9
2.MATERIAL AND METHODS ... 10
2.1 Sampling ... 10
2.1.1 Diet Analysis ... 11
2.2 Isotopic analysis ... 12
2.3 Statistical analysis ... 13
3.RESULTS AND DISCUSSION ... 14
3.1.1 Reproductive period ... 14
3.1.2 At the end of inter-breeding/beginning of breeding period (EIB/BB) ... 16
3.2 Grey-headed albatross ... 22
3.2.1 Reproductive period ... 22
3.2.2 At the end of inter-breeding/beginning of breeding period (EIB/BB) ... 23
3.3 Wandering albatross ... 24
3.3.1 Reproductive period ... 24
3.4 Characterization and comparison of the reproductive and EIB/BB periods ... 26
3.5 Comparison between the main cephalopod species found in albatross’ diets ... 28
3.5.1 Kondakovia longimana ... 29
3.5.2 Galiteuthis glacialis ... 29
3.5.3 Moroteuthis knipovitchi ... 30
3.5.4 Martialia hyadesi ... 30
3.6 Cephalopods habitats and trophic levels ... 30
3.6.1 Prey habitats... 31
3.6.2 Prey trophic levels ... 32
3.7. Main threats and suggested measures to reinforce these albatrosses conservation .. 33
4.CONCLUDING REMARKS ... 35
REFERENCES ... 38
1
1. General Introduction
1.1 Why doing research in the Antarctic?
Antarctica is a remarkable continent. Remote, hostile and uninhabited, Antarctica is key to understanding how our world works, and our impact upon it. For example, Antarctica is important for science because of its profound effect on the Earth's climate and ocean systems (BAS, 2012; Murphy el at. 2012). Around 30 countries operate Antarctic research stations where scientists study global environmental issues like climate change, ozone depletion and ozone hole, ocean circulation, sea level rising, and sustainable management of marine life (BAS, 2009). Locked in its four kilometre-thick ice sheet is a unique record of what our planet's climate was like over the past one million years. Antarctic science has also revealed much about the impact of human activity on the natural world. As well as being the world's most important natural laboratory, the Antarctic is a place of great beauty and wonder. However, Antarctica is fragile and increasingly vulnerable (BAS, 2012) and research is still urgently needed.
1.2 The Southern Ocean
The Southern Ocean, which surrounds the Antarctic continent, consists of a system of deep-sea basins, separated by three systems of ridges: the Macquarie Ridge (south of New Zealand and Tasmania), the Scotia Arc (between the Patagonian shelf and the Antarctic Peninsula) and the Kerguelen Ridge (Carmack, 1990). It is bounded to the north by the Antarctic Polar Front (APF) or Antarctic Convergence (Carmack, 1990) (Fig. 1). The location of the APF, where cold Antarctic surface water meets warmer sub-Antarctic water flowing southeast, varies temporally and spatially (between 47 and 63oS) and is characterized by a distinct change in temperature (2–3oC) and other oceanographic parameters (Carmack, 1990). It acts as a biological barrier, making the Southern Ocean a largely closed system. Sea ice covers large areas of the Southern Ocean; the extent varies seasonally from ~10% in summer to 50% of the total area in winter (Carmack, 1990). Within the Southern Ocean, sub-Antarctic Islands, such as South Georgia, Crozet, Kerguelen and Heard, are areas of enhanced productivity and support large populations of higher predators such as whales, seals and seabirds (Atkinson et al., 2001), as well as fisheries for toothfish, krill and icefish (Kock, 1992; Agnew, 2004).
2
1.3 Bird Island, South Georgia
The study site is Bird Island, South Georgia (54°S, 38°W), one of the islands where the three studied albatross species breed (Xavier et al., 2003a and b). South Georgia is part of the Scotia Ridge, a mainly submarine arc extending from South America to the Antarctic Peninsula, with surface extensions at Shag Rocks, South Georgia and the South Sandwich, South Orkneys and South Shetland Islands (Xavier, 2002; Foster, 1984) (Fig. 1). Located 200 km south of the Antarctic Polar Front, is it a sub-Antarctic region, although surrounding water mass and its wildlife have mainly polar origin (Orsi
et al., 1995; Peterson, 1992).
Figure 1 –Geographical location of South Georgia and a schematic representation of the surface water circulation in the study area. Arrows represent the direction and water
temperature, from blue (cold water) to red (warm water). Legend: ACC-Antarctic Circumpolar Current, APF- Antarctic Polar Front; APFZ - Antarctic Polar Front Zone, SAF - Sub-Antarctic Front, STF - Sub-Tropical Front. The position of the 1000m isobath is also presented (Orsi et al,1995; Hellmer and Bersch, 1985).
3
1.4 Studied albatrosses species
Albatrosses belong to the Diomedeidae family, a group also known as Procellariiformes or ‘tube noses’ (BAS, 2008). The three species studied here are the black- browed (Thalassarche melanophrys), grey-headed (Thalassarche chrysostoma) and wandering (Diomedea exulans) albatrosses (Fig. 2), listed the first two as Vulnerable and the latter as Endangered by the IUCN Red List of Threatened Species (IUCN 2010 a, b and c).
Black-browed albatross Grey-headed albatross Wandering albatross Thalassarche melanophrys Thalassarche chrysostoma Diomedea exulans Figure 2– The three species of albatrosses studied here (photos by José Xavier).
Albatrosses cover vast distances when foraging for food (BAS, 2008; Xavier et al. 2004). Grey-headed and black-browed albatrosses are known to forage mostly in Antarctic waters while breeding (Harrison et al., 1991; Phillips et al, 2007), whereas wandering albatrosses have a broader foraging range, between 25–64oS and 19–80oW (Prince et al., 1998). Outside the breeding season, many species (including wandering and grey-headed albatrosses from South Georgia) migrate long distances, some circumnavigate around the Antarctic continent (BAS, 2008; Croxall et al, 2005). They spend over 80% of their life at sea, visiting land only for breeding (WWF, 2012). As well as being the largest seabirds, with wing spans of up to 3.5m, albatrosses are also the longest lived, some surviving for more than 60 years. They take many years to reach sexual maturity, not breeding until they are around 10 years old (BAS, 2008). Male and female birds form a pair after ritual mating dances and this bond lasts for their lifetime (WWF, 2012).
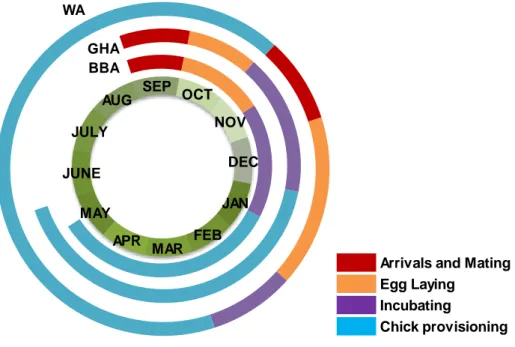
The three studied albatross species nest in colonies on sub-antarctic islands, breeding annually in the case of black -browed albatross and bi-annually in wandering and grey- headed albatrosses (Xavier et al., 2003a and b). In terms of breeding cycle, black-browed and gray-headed albatrosses have a reproductive period between September and June, while wandering albatrosses between November and November-December
4 of the following year (Xavier, 2002; see also Fig. 3). In the present dissertation, when “reproductive period” is referred it corresponds to chick provisioning, and “at the end of inter-breeding period/beginning of breeding period” correspond to the period that goes from arrivals and mating to the incubating phase (Fig. 3).
WA
Arrivals and Mating Egg Laying Incubating Chick provisioning BBA GHA JAN FEB MAR APR MAY JUNE JULY AUG SEP OCT NOV DEC
Figure 3 –Breeding cycle of the three studied albatross species (adapted from ACAP, 2009, 2010a and b). Abbreviations: BBA= black-browed albatrosses; GHA= grey-headed albatrosses; WA=wandering albatrosses; JAN=January; FEB=February; MAR=March; APR= April;
AUG=August; SEP= September; OCT= October; NOV=November; DEC=December.
1.5 Southern Ocean cephalopods
The Southern Ocean cephalopod fauna is distinctive, with high levels of squid endemism and particularly in the octopods. Loliginid squid, sepiids and sepiolids are absent from the Southern Ocean, and all the squid are oceanic species. The octopods dominate the neritic cephalopod fauna, with high levels of diversity, probably
associated with niche separation (Collins and Rodhouse, 2006). As in most temperate cephalopods, Southern Ocean species also appear to be semelparous, but growth rates are lower and longevity greater than temperate counterparts (Collins and
Rodhouse, 2006). Also, eggs are generally large and fecundity low, with putative long development times (Collins and Rodhouse, 2006). Reproduction may be seasonal in the squid but is extended in the octopods (Collins and Rodhouse, 2006). Cephalopods play an important role in the ecology of the Southern Ocean, linking the abundant mesopelagic fish and crustaceans with higher predators such as albatross, seals and
5 whales (Collins and Rodhouse, 2006). To date Southern Ocean cephalopods have not been commercially exploited, but there is potential for exploitation of Martialia hyadesi,
Kondakovia longimana, Moroteuthis knipovitchi and Gonatus antarcticus (Rodhouse
1990; Xavier et al., 2007).
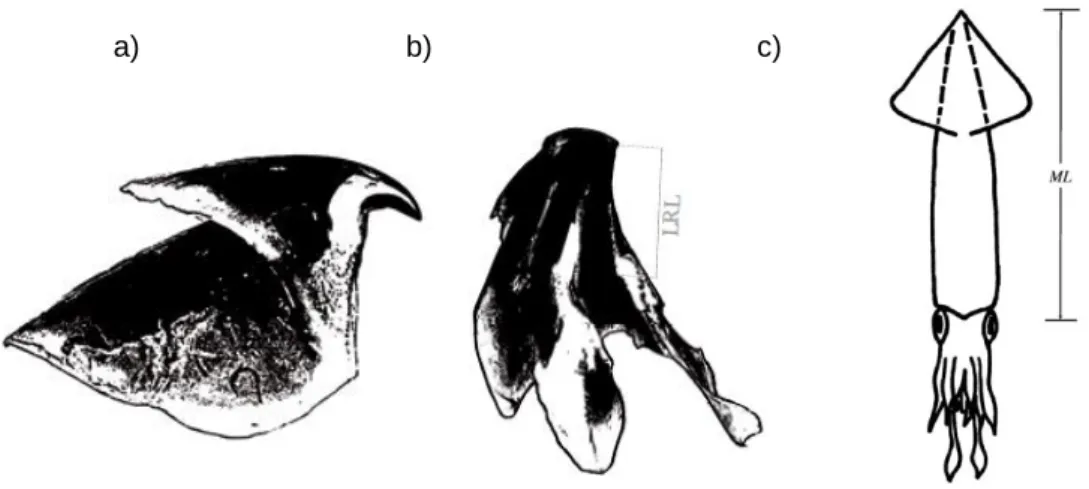
One way to determine the identity and size of these cephalopods is by analyzing their beaks present on the diet of its predators (most of the times the only way to access cephalopod material).The cephalopod beaks are divided into an upper and a lower beak (Fig. 4a and b, respectively), with its own morphology and key measurements. In this study it will be used the lower rostral length (Fig. 4 b) from the lower beak. Based on this metric length, several cephalopod characteristics can be estimated, such as dorsal mantle length (Fig. 4 c) and the original wet body mass (both variables were estimated in the present study).
Figure 4 –Cephalopod upper beak (a) and lower beak, with lower rostral length (LRL; b), adapted from Xavier and Cherel, 2009. Cephalopod dorsal mantle length (ML; c) adapted from Zeidberg, 2004.
1.6 Albatross-cephalopod interactions
Within seabirds, albatrosses play a key role in the Antarctic ecosystem as top predators, feeding on a wider diversity of prey (Xavier, 2002), including cephalopods (Xavier and Cherel, 2009).
Black-browed albatrosses during reproductive period feed mainly on crustaceans, such as Antarctic krill Euphausia superba, but also cephalopods (e.g. Martialia hyadesi) and fish, such as icefish Champsocephalus gunnari (Xavier et al., 2003 b; Prince et al., 1998). On the other hand, grey-headed albatrosses feed mainly on cephalopods, such a) b) c)
6 as Martialia hyadesi, but also feeds on other preys, such as lamprey Geotria australis (Catry el al, 2004;; Xavier et al., 2003a,b,c). Black-browed and grey-headed
albatrosses diet during non-breeding period is unknown, as they spend their time at sea (i.e. there is no possibility of collecting diet samples). During reproductive period, wandering albatrosses feed mainly cephalopods and fish, catching a varied selection of cephalopod species (ca. 50 species; mainly cranchiid and onychoteuthid squid, as
Taonius sp.B (Voss) and Kondakovia longimana, respectively) and a more restricted
range of fish (ca. 10 species) (Rodhouse et al., 1987; Croxall et al., 1988; Xavier et al., 2003a).
Wandering albatrosses feed on larger prey than smaller albatross species (Xavier and Croxall, 2007) and capture them by surface seizing while black-browed and grey-headed albatrosses can also feed by plunge diving,
Squid post-spawning death events are also likely to occur during the Antarctic winter (particularly for onychoteuthids, histioteuthids and cranchiids) and, therefore,
wandering albatrosses, as scavengers, might explore fully this type of resource (Xavier and Croxall, 2007).
Due to this predator-prey interaction, albatrosses can be used as sampling tools to investigate poorly studied organisms, such as Southern Ocean cephalopods, and in the meantime while improving our knowledge in cephalopods we improve our knowledge on the foraging and feeding behavior of these albatrosses species.
1.7 Albatrosses main threats
Long-term studies at Bird Island, South Georgia, show that numbers of wandering, black-browed and grey-headed albatrosses have been declining since the late 1970s (Poncet et al, 2006), following the global trend (IUCN 2010a,b,c). It is a huge problem to these species because South Georgia holds the largest population of grey-headed albatross Thalassarche chrysostoma, the second largest of wandering albatross
Diomedea exulans and the third largest black-browed albatross Thalassarche melanophrys in the world (Gales 1998; Robertson et al. 2003; Lawton et al. 2003).
Each year, tens of thousands of albatrosses are drowned as they scavenge behind fishing boats (BAS, 2008). Plastic waste ingested at sea, and introduction of non-native species onto breeding islands pose additional hazards (BAS, 2008). Nonetheless, the
7 main cause of the decline of most albatross species are known to be the incidental mortality in fisheries (by-catch), especially in longline (Fig. 5) and trawling (Fig. 6) fisheries, where albatrosses are vulnerable to baited hooks and trawl nets and cables (Croxall et al., 1990; Nel et al. 2000; Weimerskirch et al., 1997; Schiavini et al., 1998; Sullivan et al., 2003).
Figure 5– Longline fishing operation (FAO, 2012).
Figure 6– Bottom pair trawls (Nédélec and Prado, 1990).
Black-browed and wandering albatrosses are the ones that most interact with
commercial fishing, being possible to see huge flocks following fishing vessels (ACAP, 2009, 2010a). Grey-headed albatrosses are non-common ship followers, but the presence of some carcasses in fishing lines suggested some (small) level of interaction (ACAP, 2010b; Xavier et al, 2003c).
The implementation of mitigation measures to reduce seabirds by-catch and an effective combat to illegal, unreported and unregulated (IUU) fishing are crucial to prevent these threats to albatrosses (Small, 2005), which allied to a better knowledge
8 of southern ocean cephalopods distributions (and population trends) could enable us to protect these birds more efficiently by knowing their potential feeding zones
1.8 Stable isotopes: concepts and terminology
Each element (hydrogen, carbon, nitrogen, oxygen) occurs in nature in different forms called stable isotopes (same number of protons and different number of neutrons). Stable isotopes with less neutrons are called light elements and those with more neutrons are called heavy elements. The abundance of each form varies in a global scale due to physical and biogeochemical factors that influence fractionation
(partitioning of heavy and light isotopes between a source substrate and the product(s); Peterson and Fry, 1987; Dawson et al, 2002), allowing the creation of a fingerprint of each site based on differences of isotopic ratios (heavy element / light element; Dawson et al, 2002).
The isotope ratios of plant and animal tissues represent a temporal integration of significant physiological and ecological processes on the landscape. The timescale of this integration depends on the element turnover rate of the tissue or pool in question. In this study, stable isotope analyses were used in cephalopod lower beaks. These hard structures grow by accretion of new molecules of proteins and chitin and there is no turnover after synthesis. Consequently, cephalopod beaks retain molecules built up from early development to time of death and their isotopic signature integrates the feeding ecology of the animal over its whole life (Cherel and Hobson, 2005).
For natural abundance, the stable isotope composition of a particular material or substance is expressed as a ratio relative to an internationally accepted reference standard, as X = [(Rsample / Rstandard) -1] 1000, where X is the stable isotope of interest (13C and 15N in this study) and R is the abundance ratio of those isotopes (Dawson et al, 2002; Stowasser et al, 2012). A positive δ value therefore indicates that the sample contains more of the heavy isotope than the standard (Dawson et al, 2002).
1.9 Thesis’ framework
This thesis was conducted under the framework of the POLAR project, included in the Portuguese Polar Program (PROPOLAR) during the International Polar Year of 2007-2009, which was followed by the CEPH project. The main goal of the POLAR project was to evaluate the predator-prey interactions in the Southern Ocean in relation to
9 climate change, using new technologies applied to marine ecology, such as stable isotope signatures. POLAR was a multi-disciplinary product of an international
collaboration with the United Kingdom, France and Germany (Portal Polar, 2008). On the other hand, the CEPH project aimed to assess the importance of cephalopods in the Antarctic Ocean, particularly through diet of top predators, including albatrosses, penguins, seals and fish. This project is an international and multidisciplinary, involving several countries, and coordinated by the Institute of Marine Research, University of Coimbra and the British Antarctic Survey.
1.10 Thesis’ objectives
The aim of the present study was to investigate the albatross-cephalopod interactions in the Southern Ocean, namely by: i) Characterizing the albatrosses diet, with
relevance to the cephalopod component, during the reproductive period of wandering (Diomedea exulans), black-browed (Thalassarche melanophrys) and grey-headed (Thalassarche chrysostoma), and at the end of inter-breeding/beginning of breeding period (EIB/BB) of the last two species; ii) Assessing the habitat and trophic level of key cephalopods species found in the diet of the three studied albatross species during the studied periods using stable isotopes analyses; iii) Comparing both sampled
periods (Reproductive versus EIB/BB periods); and iv) Identifying threats and suggest measures to reinforce these albatrosses conservation.
10
2. Material and Methods
2.1 Sampling
The stomach contents were involuntarily obtained from black-browed (Thalassarche
melanophrys), grey-headed (Thalassarche chrysostoma) and wandering (Diomedea exulans) albatrosses chicks after been fed by their parents. They were randomly
collected on the colonies off Bird Island, South Georgia (54 ° 00'S, 38 ° 03 'W), from February to April 2009 for the first two albatrosses, and from May to September 2009 for wandering albatrosses (i.e during the reproductive periods). The method of
obtaining stomach contents consists on reversing the albatrosses and massage its stomach, if necessary, in order to stimulate regurgitation (Xavier et al, 2003b). Each chick was sampled only once and several colonies were analyzed in order to make considerations of the general breeding population on Bird Island. The welfare of the chicks sampled was monitored after obtaining the data and there were no differences in survival between birds sampled and not sampled.
While collecting the stomach contents of wandering albatrosses chicks, adults black-browed and grey-headed albatrosses that arrived to Bird Island to nest had started to regurgitated voluntarily indigestible items (i.e. cephalopod beaks) that could not be digested, providing an extraordinary opportunity to collect their boluses. The boluses were randomly collected near their respective nesting colonies from September to December of 2009, at the end of inter-breeding period/beginning of the breeding period. It is worth noting that the present study is the first time to analyze such data (from the end of inter-breeding period/beginning of the breeding period).
We compared the diet data from the chicks (stomach contents) and the adults
(boluses). As the chicks eat what is given by adults there is no problem of comparing the data from these two types of sampling.
A total of 80 stomach contents were collected from black-browed (n= 30), grey-headed (n= 30) and wandering albatrosses (n=20) in 2009, during reproductive period (Table 1). A total of 46 boluses were collected from black-browed (n= 14) and grey-headed albatrosses (n= 32) in 2009, during the end of inter-breeding/beginning of breeding period (Table 1).
11 Table 1– Total number of boluses during reproductive period and stomach contents during the end of inter-breeding/beginning of breeding period (EIBB/BB; samples), total number of cephalopod beaks (upper – including fresh and non-fresh beaks; and lower – fresh beaks), mean number of fresh lower beaks per sample and number of cephalopod species found in black-browed (Thalassarche melanophrys), grey-headed (Thalassarche chrysostoma) and wandering albatrosses (Diomedea exulans).
Albatross species Year Period Cephalopod beaks
Thalassarche melanophrys 2009 Reproductive 30 244 82 2,2
Thalassarche melanophrys 2009 EIB/BB 14 138 5 0,4
Thalassarche chrysostoma 2009 Reproductive 30 580 158 5,4
Thalassarche chrysostoma 2009 EIB/BB 32 346 7 0,2
Diomedea exulans 2009 Reproductive 20 872 130 6,5
10 2 15 3 Number of samples Mean number of lower beaks per sample Number of cephalopod species Upper beaks Lower beaks
6
2.1.1 Diet Analysis
All samples were frozen at -20°C (Xavier et al, 2003b; Clarke, 1986) and immediately sampled on Bird Island or two years later at the Institute of Marine Research (IMAR-CMA) of the University of Coimbra, Portugal. The stomach contents were weighted and then its components separated by categories (cephalopods, crustaceans, fish and other contents – carrion, debris, hooks and fishing lines, non-food and other food), following the methodology described by Xavier et al (2003b). The cephalopod beaks found in the boluses were also identified and counted.
The cephalopod beaks were separated into upper and lower beaks, and the former were only counted. The lower beaks were cleaned, counted and the lower rostral length (LRL) measured, using a vernier calipers of 0.1 mm (Xavier and Cherel, 2009). The lower beaks were identified, whenever possible, to the species level, according to Xavier and Cherel (2009) and reference collections at the British Antarctic Survey and at the University of Coimbra.
Allometric equations were used from LRL to estimate dorsal mantle length (ML, mm) and the original wet body mass (M, g) published by Xavier and Cherel (2009). For
?Mastigotheuthis A (Clarke) it was used Mastigoteuthis psychrophila equations,
because it had no specific equations and both are the only species in the family
Mastigoteuthidae.
To describe the diets, cephalopod beaks that were not fresh (i.e. very darkened, with abraded wings that were usually broken, and with their surfaces rounded) were
12 considered as having been captured before the time of study and thus were not
included in the analysis (Xavier et al, 2003c).
The analyses of cephalopod component in the three albatross species diet were made using fresh lower beaks data, from which were inferred the frequency of occurrence (F; number of samples with that squid species / total number of samples), total number of lower beaks per cephalopod species (N), estimated mass (M; total, mean, standard deviation (SD), and range – minima and maxima), estimated mantle lengths (ML; total, mean, standard deviation (SD), and range – minima and maxima) and lower rostral lengths (LRL; mean, standard deviation (SD), and range – minima and maxima; Xavier et al, 2003b). It was also analyzed LRL, M and ML distributions using histograms with the most important cephalopod species (Xavier et al, 2005).
Identification of different component weights in albatross species diet was presented by the total percentage of each component by solid mass ± standard deviation (SD). Finally, the role of scavenging in albatrosses was analyzed by identifying the percentages of cephalopods heavier than 500g (Croxall and Prince 1994).
2.2 Isotopic analysis
The sample sources for the isotopic measurements were: i) for black-browed and grey-headed albatrosses during the reproductive period, the chicks stomach contents mentioned above and also adult boluses ; ii) for wandering albatrosses during reproductive period, the stomach contents of chicks mentioned above and also
stomach contents from adults (that were collected during the same time period); and iii) for black-browed and grey-headed albatrosses during the end of inter-breeding
period/beginning of the breeding period, the adult boluses mentioned above . As the chicks eat what is given by adults, there is no problem of grouping the beaks from these two types of sampling to analyze their stable isotopic signature.
The cephalopod lower beaks analyzed were cleaned and kept in a 70% ethanol solution, being subsequently dried in an oven at 50 ° C for 6-24h, and reduced to fine powder in order to homogenize the sample. Part of the homogenate was then
encapsulated (0.3-0.55mg) for analysis (Cherel and Hobson, 2005). Stable isotope analyses were made only for cephalopod species with at least 6 lower beaks. There were analyzed lower beaks of different sizes of Kondakovia longimana and
13 Psychroteuthis glacialis to see if the δ15N signature increases with beak size and if it means they belonged to different squid populations, respectively.
The trophic level and habitat of the main cephalopod species in the diet of albatrosses were obtained from the ratio of δ15N (15N/14N) and δ13C (13C/12C), respectively, through a Continuous Flow Isotope Ratio Mass Spectrometer (CFIRMS). The results are presented in δ connotation as deviations to the standard references in parts per thousand (‰) according to the following equation: X = [(Rsample / Rstandard) -1] 1000, where X represents 13C or 15N and Rsample the ratios 13C/12C or 15N/14N. Rstandard
represents the international reference standard V-PDB ("Vienna" - PeeDee formation) and atmospheric N2 (AIR) for δ13C and δ15N, respectively (Dawson et al, 2002;
Stowasser et al, 2012).
Since there is no study referring the isotopic values of ocean fronts near South Georgia, especially from squid beaks data, the isotopic position of the ocean fronts presented here was based on Jaeger et al (2010) for Crozet island.
A total of 14 species of cephalopods were analyzed for stable isotopes in order to understand their habitat use (13 C) and trophic level (15 N); 9 species from black-browed albatrosses diet (out of total 5 cephalopod species presented in fresh lower beaks data (FLBD) and 4 species from non-fresh lower beaks data (NFLBD)); 13 species from grey-headed albatrosses diet (out of total 9 cephalopod species presented in FLBD and 4 species from NFLBD); and 9 species from wandering albatrosses diets (out of total 8 cephalopod species presented in FLBD and 1 specie from NFLBD; beaks from the same specie with different sizes were counted only once; see below).
2.3 Statistical analysis
One-way ANOVA or Kruskal-Wallis (p-value<0.05) were used to examine whether there were any significant differences among the LRL, M, ML and isotopic values in each albatross and cephalopod species. Tukey´s test was subsequently used (p-value<0.05). The data were analysed using Statistica version 10 and Sigmaplot 12.0.
14
3. Results and Discussion
3.1 Black-browed albatross
3.1.1 Reproductive period
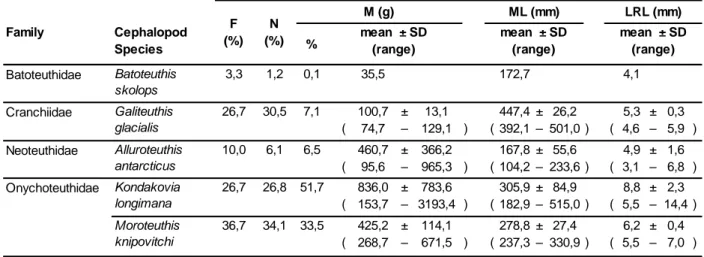
Black-browed albatrosses fed mainly, by solid mass, on fish (54 ± 36.7%), followed by cephalopods (35 ± 35.1%), crustaceans (7 ± 25.6%) and others contents (4 ± 8.4%). Within the cephalopod component, based on fresh lower beaks identification, the diversity found in stomach contents comprised 6 squid species (Table 1). The most important cephalopod species found were Kondakovia longimana (F=26.7%, N=26.8%, M=51.7%), Moroteuthis knipovitchi (F=36.7%, N=34.1%, M=33.5%) and Galiteuthis
glacialis (F=26.7%, N=30.5%, M=7.1%; Table 2).
Table 2– Cephalopod component (lower beaks) in the diet of black-browed albatrosses during the reproductive period and during the end of inter-breeding/beginning of breeding period (EIBB/BB). Abbreviations: frequency of occurrence (F); total number of lower beaks (N); estimated mass (M); estimated dorsal mantle length (ML) and lower rostral length (LRL). SD= standard deviation. Only those prey species that represented F or N ≥ 20%, M ≥ 5% or had the minimums and/or the maximums in the studied variables were included.
Batoteuthidae 3,3 1,2 0,1 35,5 172,7 4,1 Cranchiidae 26,7 30,5 7,1 100,7 ± 13,1 447,4 ± 26,2 5,3 ± 0,3 ( 74,7 – 129,1 ) ( 392,1 – 501,0 ) ( 4,6 – 5,9 ) Neoteuthidae 10,0 6,1 6,5 460,7 ± 366,2 167,8 ± 55,6 4,9 ± 1,6 ( 95,6 – 965,3 ) ( 104,2 – 233,6 ) ( 3,1 – 6,8 ) Onychoteuthidae 26,7 26,8 51,7 836,0 ± 783,6 305,9 ± 84,9 8,8 ± 2,3 ( 153,7 – 3193,4 ) ( 182,9 – 515,0 ) ( 5,5 – 14,4 ) 36,7 34,1 33,5 425,2 ± 114,1 278,8 ± 27,4 6,2 ± 0,4 ( 268,7 – 671,5 ) ( 237,3 – 330,9 ) ( 5,5 – 7,0 ) Cranchiidae 7,1 20,0 1,0 102,0 450,7 5,3 Gonatidae 7,1 20,0 1,3 134,1 183,8 5,3 Onychoteuthidae 14,3 60,0 97,7 3343,2 ± 680,2 521,3 ± 35,5 14,6 ± 1,0 ( 2666,9 – 4027,3 ) ( 485,2 – 556,1 ) ( 13,6 – 15,5 ) Alluroteuthis antarcticus Kondakovia longimana Moroteuthis knipovitchi Galiteuthis glacialis Gonatus antarcticus Kondakovia longimana Galiteuthis glacialis Batoteuthis skolops mean ± SD (range)
Black-browed albatrosses during EIB/BB period F (%) N (%) LRL (mm) % Family F (%) Cephalopod Species Family M (g) mean ± SD (range) N (%) LRL (mm) Cephalopod Species % mean ± SD (range) mean ± SD (range) ML (mm)
Black-browed albatrosses during reproductive period
mean ± SD (range) M (g) mean ± SD (range) ML (mm)
15 The lower rostral lengths (LRL) ranged from 3.1 to 14.4 mm (Fig. 7a), while the
estimated mass (M) ranged from 35.5 to 3193.4 g (Fig. 8a) and estimated mantle lengths (ML) ranged from 104.2 to 515.0 mm (Fig. 9a). The mean LRL ranged between 4.1 mm (Batoteuthis skolops) and 8.8 ± 2.3 mm (Kondakovia longimana; Table 2). Mean M ranged between 35.5 g (Batoteuthis skolops) and 836 ± 783.6 g (Kondakovia
longimana; Table 2), and mean ML ranged between 167.8 ± 55.6 mm (Alluroteuthis antarcticus) and 447.4 ± 26.2 mm (Galiteuthis glacialis; Table 2).
In terms of carbon signatures, squid lower beaks ranged from -25.44‰ (large beaks from Psychroteuthis glacialis) to -20.97‰ (Histioteuthis eltaninae; Fig. 10a), comprising 8 cephalopod species (out of total 5 cephalopod species presented in fresh lower beaks data and 3 species from non-fresh lower beaks data). The number of squid species from “Antarctic” and “sub-Antarctic” waters were 7 and 3, respectively (Fig. 10a) and the number of lower beaks per water masses were 70% and 30% (Table 3), respectively.
Table 3– Number of cephalopod lower beaks (used in isotopic analyses) per water mass in the diet of black-browed (Thalassarche melanophrys), grey-headed (Thalassarche chrysostoma) and wandering albatrosses (Diomedea exulans) during the reproductive period and for black-browed and grey-headed albatrosses during the end of inter-breeding/beginning of breeding period (EIB/BB).
Antarctic SubAntarctic Subtropical
Thalassarche melanophrys Reproductive 70,0 30,0
-Thalassarche melanophrys EIB/BB 23,8 76,2
-Thalassarche chrysostoma Reproductive 83,0 17,0
-Thalassarche chrysostoma EIB/BB 36,6 63,4
-Diomedea exulans Reproductive 32,7 59,4 7,9
Albatross species Period Number of lower beaks per water
masses (%)
In terms of nitrogen signatures, squid lower beaks ranged from 2.45‰ (Martialia
hyadesi) to 10.44‰ (Gonatus antarcticus; Fig. 11a).
Regarding the scavenging behaviour, a total of 31.7% of cephalopods was potentially scavenged by black-browed albatrosses (assuming squid heavier than 500 g were scavenged), corresponding to 64.8% of the total estimated mass of cephalopods consumed.
16
3.1.2 At the end of inter-breeding/beginning of breeding period
(EIB/BB)
The cephalopod diversity found in boluses comprised 3 squid species (based on fresh lower beaks identification; Table 1), and the most important one found was Kondakovia
longimana (F=14.3%, N=60%, M=97.7%; Table 2).
Lower rostral lengths (LRL) ranged from 5.3 to 15.5 mm (Fig. 7b), estimated mass (M) ranged from 102 to 4027.3 g (Fig. 8b) and estimated mantle lengths (ML) ranged from 183.8 to 556.1 mm (Fig. 9b).
The mean LRL of the cephalopod species ranged between 5.3 mm (Galiteuthis
glacialis and Gonatus antarcticus) and 14.6 ± 1 mm (Kondakovia longimana; Table 2).
Mean M ranged between 102 g (Galiteuthis glacialis) and 3343.2 ± 680.2 g
(Kondakovia longimana; Table 2), and mean ML ranged between 183.8 mm (Gonatus
antarcticus) and 521.3 ± 35.5 mm (Kondakovia longimana; Table 2).
In terms of carbon signatures, squid lower beaks ranged from -23.73‰ (Galiteuthis
glacialis) to -21.24‰ (Moroteuthis knipovitchi; Fig. 10b),comprising 5 cephalopod
species (out of total 3 cephalopod species presented in fresh lower beaks data and 2 species from non-fresh lower beaks data). The number of squid species from
“Antarctic” and “sub-Antarctic” waters were 1 and 4, respectively (Fig. 10b),and the number of lower beaks per water masses were 23.8% and 76.2% (Table 5),
respectively.
In terms of nitrogen signatures, squid lower beaks ranged from 8‰ (large beaks of
Kondakovia longimana) to 11.48‰ (Taonius sp. B (Voss); Fig. 11b).
Regarding the scavenging behaviour, a total of 60% of cephalopods was potentially scavenged by black-browed albatrosses, corresponding to 97.7% of the total estimated mass of cephalopods consumed.
17 Figure 7 –Number of lower beaks per consecutive and non-overlapping range of lower rostral length of the most important cephalopod species (i.e. frequency of occurrence or total number of lower beaks ≥ 20% or total estimated mass ≥ 5%; individualized) found in black-browed (BBA), gray-headed (GHA) and wandering albatrosses reproductive period and during the end of inter-breeding/beginning of breeding (EIBB/BB) period of BBA and GHA. “Others” include all cephalopod species that were not the principal squids in albatrosses diet.
18 Figure 8 – Number of lower beaks per consecutive and non-overlapping range of estimated mass of the most important cephalopod species (i.e. frequency of occurrence or total number of lower beaks ≥ 20% or total estimated mass ≥ 5%; individualized) found in black-browed (BBA), gray-headed (GHA) and wandering albatrosses reproductive period and during the end of inter-breeding/beginning of breeding (EIBB/BB) period of BBA and GHA. “Others” include all
19 Figure 9 – Number of lower beaks per consecutive and non-overlapping range of estimated mantle length of the most important cephalopod species (i.e. frequency of occurrence or total number of lower beaks ≥ 20% or total estimated mass ≥ 5%; individualized) found in black-browed (BBA), gray-headed (GHA) and wandering albatrosses reproductive period and during the end of inter-breeding/beginning of breeding (EIBB/BB) period of BBA and GHA. “Others” include all cephalopod species that were not the principal squids in albatrosses diet.
20 Figure 10 - Stable carbon isotope values (δ13
C) of lower beaks from cephalopod species (with at least 6 lower beaks) found in: a) Black-browed albatrosses during reproductive period; b) Black-browed albatrosses during the end of inter-breeding/beginning of breeding (EIBB/BB) period; c) Gray-headed albatrosses during reproductive period; d) Gray-headed albatrosses during the EIBB/BB period; e) Wandering Albatross during reproductive period. Cephalopod species were deliberately placed according to their carbon signatures, and not in taxonomic order, to illustrate the water masses to which they belonged. Abbreviations: AZ, Antarctic Zone; PF, Polar Front; SAZ, Subantarctic Zone; STF, Subtropical Front; STZ, Subtropical Zone. Fronts carbon signatures were adopted following Jaeger et al. (2010) and are represented by dashed lines.
21 Figure 11– Stable nitrogen isotope values (δ15N) of lower beaks from cephalopod species (with at least 6 lower beaks) found in: a) Black-browed albatrosses during reproductive period; b) Black-browed albatrosses the end of inter-breeding/beginning of breeding (EIBB/BB) period; c) Gray-headed albatrosses during reproductive period; d) Gray-headed albatrosses during the EIBB/BB period; e) Wandering Albatross during reproductive period. Cephalopod species were deliberately placed in trophic sequence, and not in taxonomic order, according to their nitrogen signatures to illustrate the trophic structure of the community.
22
3.2 Grey-headed albatross
3.2.1 Reproductive period
Grey-headed albatrosses fed mainly, by solid mass, on cephalopods (51 ± 35.3%), followed by fish (36 ± 32.5%), others contents (10 ± 22.9%) and crustaceans (4 ± 10.1%).
The cephalopod diversity found in stomach contents comprised 10 squid species (based on fresh lower beaks identification; Table 1). The most important squid species found were Kondakovia longimana (F=40%, N=8.9%, M=27.7%), Galiteuthis glacialis (F=50%, N=50%, M=27.3%) and Martialia hyadesi (F=40%, N=20.3%, M=15%; Table 4).
Lower rostral lengths (LRL) ranged from 1.8 to 12.3 mm (Fig. 7c), estimated mass (M) ranged from 35.5 to 1943 g (Fig. 8c) and estimated mantle lengths (ML) ranged from 72.2 to 492.6 mm (Fig. 9c). The mean LRL ranged between 3.4 ± 0.4mm (Histioteuthis
eltaninae) and 8.2 mm (Taonius sp. B (Voss); Table 4). Mean M ranged between 51.4
± 22.6 g (Chiroteuthis veranyi) and 620.3 ± 528.2 g (Kondakovia longimana; Table 4), and mean ML ranged between 78.4 ± 10.7 mm (Histioteuthis eltaninae) and 491.4 mm (Taonius sp. B (Voss); Table 4).
In terms of carbon signatures, squid lower beaks ranged from -25.36‰ (large beaks from Psychroteuthis glacialis) to -19.99‰ (Chiroteuthis veranyi; Fig. 10c), comprising 9 species (out of total 8 cephalopod species presented in fresh lower beaks data and 1 species from non-fresh lower beaks data; beaks from the same specie with different sizes were here counted only once). The number of squid species from “Antarctic” and “sub-Antarctic” waters were 9 and 2, respectively (Fig. 10c), and the number of lower beaks per water masses were 83% and 17% (Table 3), respectively.
In terms of nitrogen signatures, squid lower beaks ranged from 2.85‰ (Martialia
hyadesi) to 10.93‰ (Chiroteuthis veranyi; Fig. 11c).
In relation to scavenging, a total of 5.1% of cephalopods was potentially scavenged, corresponding to 27% of the total estimated mass of cephalopods consumed.
23 Table 4– Cephalopod component (lower beaks) in the diet of grey-headed albatrosses during the reproductive period and during the end of inter-breeding/beginning of breeding period (EIBB/BB). Abbreviations: frequency of occurrence (F); total number of lower beaks (N); estimated mass (M); estimated dorsal mantle length (ML) and lower rostral length (LRL). SD= standard deviation. Only those prey species that represented F or N ≥ 20%, M ≥ 5% or had extrema in the diets were included.
Cranchiidae 50,0 50,0 27,3 100,4 ± 12,8 446,8 ± 25,9 5,3 ± 0,3 ( 71,1 – 124,3 ) ( 383,7– 492,6 ) ( 4,5 – 5,8 ) 3,3 0,6 0,8 220,1 491,4 8,2 Histioteuthidae 10,0 2,5 0,9 62,3 ± 27,7 78,4 ± 10,7 3,4 ± 0,4 ( 46,9 – 103,7 ) ( 72,2 – 94,3 ) ( 3,1 – 4,0 ) Mastigoteuthidae 3,3 0,6 0,3 73,0 122,3 4,5 Ommastrephidae 40,0 20,3 15,0 156,1 ± 83,0 207,9 ± 29,3 3,6 ± 1,0 ( 36,1 – 305,5 ) ( 155,0– 255,2 ) ( 1,8 – 5,2 ) Onychoteuthidae 40,0 8,9 27,7 620,3 ± 528,2 277,3 ± 77,6 8,0 ± 2,1 ( 172,0 – 1943,0 ) ( 190,4– 436,7 ) ( 5,7 – 12,3 ) 20,0 5,7 12,8 413,9 ± 263,5 267,8 ± 55,7 6,0 ± 0,9 ( 201,7 – 964,4 ) ( 212,4– 374,5 ) ( 5,1 – 7,7 ) Cranchiidae 3,1 28,6 4,6 257,8 ± 137,5 519,1 ± 134,7 8,7 ± 2,2 ( 160,6 – 355,0 ) ( 423,9– 614,3 ) ( 7,1 – 10,2 ) Onychoteuthidae 6,3 71,4 95,4 2135,0 ± 366,6 449,4 ± 26,2 12,6 ± 0,7 ( 1704,8 – 2545,2 ) ( 418,0– 477,7 ) ( 11,8 – 13,4 ) M (g) % N (%) mean ± SD (range) M (g) mean ± SD (range) ML (mm) mean ± SD (range) F (%) LRL (mm) mean ± SD (range) mean ± SD (range)
Grey-headed albatrosses during reproductive period
F (%) N (%) LRL (mm) mean ± SD (range)
Grey-headed albatrosses during EIB/BB period ML (mm) Mastigoteuthis psychrophila Martialia hyadesi Kondakovia longimana Galiteuthis glacialis Taonius sp. B (Voss) Histioteuthis eltaninae Cephalopod Species Taonius sp. B (Voss) Kondakovia longimana Moroteuthis knipovitchi Family Family Cephalopod Species %
3.2.2 At the end of inter-breeding/beginning of breeding period
(EIB/BB)
During this period, the cephalopod diversity found in boluses comprised 2 squid species (Table 1), and the most important one was Kondakovia longimana (F=6.3%, N=71.4%, M=95.4%; Table 4).
Lower rostral lengths (LRL) ranged from 7.1 to 13.4 mm (Fig. 7d), estimated mass (M) ranged from 160.6 to 2545.2 g (Fig. 8d) and estimated mantle lengths (ML) ranged from 418 to 614.3 mm (Fig. 9d). The mean LRL ranged between 8.7 ± 2.2 mm
(Taonius sp. B (Voss)) and 12.6 ± 0.7 mm (Kondakovia longimana; Table 4). Mean M ranged between 257.8 ± 137.5 g (Taonius sp. B (Voss)) and 2135 ± 366.6 g
24 449.4 ± 26.2 mm (Kondakovia longimana) and 519.1 ± 134.7 mm (Taonius sp. B (Voss); Table 4).
In terms of carbon signatures, squid lower beaks ranged from -23.84‰ (Batoteuthis
skolops) to -19.60‰ (Histioteuthis macrohista; Fig.10d), comprising 8 cephalopod
species (out of total 2 cephalopod species presented in fresh lower beaks data and 6 species from non-fresh lower beaks data). The amount of squid species that were from “Antarctic” and “sub-Antarctic” waters were 3 and 5, respectively (Fig. 10d), and the number of lower beaks per water masses were 36.6% and 63.4% (Table 3),
respectively.
In terms of nitrogen signatures, squid lower beaks ranged from 4.44‰ (Martialia
hyadesi) to 10.75‰ (Gonatus antarcticus; Fig. 11d).
In terms of scavenging, a total of 71.4% of cephalopods was potentially scavenged by grey-headed albatrosses, corresponding to 95.4% of the total estimated mass of cephalopods consumed.
3.3 Wandering albatross
3.3.1 Reproductive period
Wandering albatrosses fed mainly, by solid mass, on fish (37 ± 32.6%), cephalopods
(32 ± 34.6%) and others contents (31 ± 35.8%) followed by crustaceans (0 ± 0.2%).
The cephalopod diversity found in stomach contents comprised 15 squid species
based on fresh lower beaks identification (Table 1). The most important squid species
found were Kondakovia longimana (F=60%, N=28.5%, M=73.5%), Taonius sp. B
(Voss) (F=40%, N=24.6%, M=7.3%) and Galiteuthis glacialis (F=55%, N=18.5%
25 Table 5 –Cephalopod component (lower beaks) in the diet of wandering albatrosses during the reproductive period. Abbreviations: frequency of occurrence (F); total number of lower beaks (N); estimated mass (M); estimated dorsal mantle length (ML) and lower rostral length (LRL). SD= standard deviation. Only those prey species that represented F or N ≥ 20%, M ≥ 5% or had extrema in the diets were included.
Brachioteuthidae 5,0 0,8 0,0 7,8 74,8 2,9 Cranchiidae 55,0 18,5 1,8 109,5 ± 15,4 464,4 ± 29,1 5,5 ± 0,3 ( 78,3 – 144,0 ) ( 400,5– 526,1 ) ( 4,7 – 6,2 ) 40,0 24,6 7,3 331,7 ± 70,6 591,6 ± 61,2 9,8 ± 1,0 ( 181,0 – 444,2 ) ( 448,4– 681,9 ) ( 7,5 – 11,3 ) Histioteuthidae 5,0 0,8 0,0 57,0 77,1 3,3 Neoteuthidae 15,0 3,1 1,4 507,8 ± 159,8 185,5 ± 19,5 5,4 ± 0,6 ( 367,9 – 735,4 ) ( 167,2– 212,6 ) ( 4,9 – 6,2 ) Octopoteuthidae 10,0 2,3 6,9 3311,9 ± 1599,1 461,1 ± 167,7 13,5 ± 2,2 ( 1520,5 – 4595,5 ) ( 270,5– 586,4 ) ( 11,0 – 15,2 ) Ommastrephidae 5,0 0,8 0,2 305,5 255,2 5,2 Onychoteuthidae 60,0 28,5 73,5 2881,9 ± 1966,0 470,7 ± 116,1 13,2 ± 3,1 ( 541,3 – 7524,8 ) ( 283,7– 683,0 ) ( 8,2 – 18,9 ) 25,0 6,9 2,8 456,2 ± 153,1 285,1 ± 32,9 6,3 ± 0,5 ( 268,7 – 787,6 ) ( 237,3– 349,6 ) ( 5,5 – 7,3 ) mean ± SD (range) M (g) ML (mm) mean ± SD (range) % mean ± SD (range) F (%) N (%)
Wandering albatrosses during reproductive period
LRL (mm) Taonius sp. B (Voss) Kondakovia longimana Moroteuthis knipovitchi Cephalopod Species Histioteuthis eltaninae Alluroteuthis antarcticus Taningia danae Martialia hyadesi Slosarczykovia circumantarctica Galiteuthis glacialis Family
Lower rostral lengths (LRL) ranged from 2.9 to 18.9 mm (Fig. 7e), estimated mass (M) ranged from 7.8 to 7524.8 g (Fig. 8e) and estimated mantle lengths (ML) ranged from 74.8 to 683 mm (Fig. 9e). The mean LRL ranged between 2.9 mm (Slosarczykovia
circumantarctica) and 13.5 ± 2.2 mm (Taningia danae; Table 5). The mean M ranged
between 7.8 g (Slosarczykovia circumantarctica) and 3311.9 ± 7779.7 g (Taningia
danae; Table 5), and the mean ML ranged between 74.8 mm (Slosarczykovia circumantarctica) and 591.6 ± 61.2 mm (Taonius sp. B (Voss); Table 5).
In terms of carbon signatures, squid lower beaks ranged from -24.91‰ (large beaks from Psychroteuthis glacialis) to -17.31‰ (Illex argentinus; Fig. 10e), comprising 9 cephalopod species (out of total 8 cephalopod species presented in fresh lower beaks data and 1specie from non-fresh lower beaks data; beaks from the same specie with different sizes were here counted only once). The number of squid species that were from “Antarctic”, “sub-Antarctic” and “sub-Tropical” waters were 4, 6 and 1, respectively (Fig. 10e), and the number of lower beaks per water masses were 32.7%, 59.4% and 7.9% (Table 3), respectively.
26 In terms of nitrogen signatures, squid lower beaks ranged from 6.19‰ (medium beaks of Kondakovia longimana) to 12.18‰ (Taonius sp. B (Voss); Fig. 11e).
In terms of scavenging, a total of 35.4% of cephalopods was potentially scavenged by wandering albatrosses, corresponding to 85.7% of the total estimated mass of
cephalopods consumed.
Only the wandering albatrosses showed interaction with fisheries by presenting a total of 4 hooks and 8 lines (one of itch longline) in a total of 5 chicks stomach contests (comprising all samples from August and one sample from July; which correspond to 25% of the total analyzed chick’s stomach contents from wandering albatross),
weighting this fishing gear a total of 30.2 g. Only one hook was associated to a fishing line.
3.4 Characterization and comparison of the reproductive and
EIB/BB periods
Black-browed albatrosses during reproductive period fed mainly on fish (cephalopods represented only 35 ± 35.1%), and showed the lowest cephalopod biodiversity of the albatross species studied (with 6 species). Compared with previous studies, the year 2009 was similar to 1994, 1998 and 1999 where fish was the main diet component (between 32 and 72.4%; Xavier et al, 2003b; Croxall et al. 1999).
Grey-headed albatrosses during reproductive period fed mostly on cephalopods (51 ± 35.3%), which could indicate that there were good oceanographic conditions around and north of South Georgia and no needs to switch to alternative foraging grounds in shelf and shelf-break waters around the Antarctic Peninsula. In fact, when conditions are poor in the South Georgia region, breeding grey-headed albatrosses tend to switch to alternative foraging grounds at the Antarctic Peninsula and this is accompanied by a dietary shift towards increased consumption of krill, and less of the squid Martialia
hyadesi (Xavier et al. 2003b).
Wandering albatrosses during reproductive period fed mainly on fish and cephalopods (fish and cephalopods represented 37 ± 32.6% and 32 ± 34.6%, respectively), like it has been shown in previous studies (Rodhouse et al. 1987; Xavier et al, 2003c; Xavier
et al. 2004), and presented the highest cephalopod biodiversity (with 15 species) of the
27 cephalopods than those recorded on other albatross species diets during their reproductive period (see also Xavier and Croxall, 2007).
Compared to Xavier and Croxall (2007), wandering albatrosses during reproductive period of 2009, showed similar scavenging percentages to those observed in 1989-1999. On the other hand, black-browed albatrosses showed a higher percentage of cephalopods scavenged in 2009 than in 2000 (almost reaching its double) but a similar percentage of the total estimated mass of cephalopods consumed. Last, the grey-headed albatrosses showed scavenging percentages lower in 2009 than those
recorded in 2000 (less than half 2000 percentages values). Summing up, these results showed that, during the reproductive period of 2009, the wandering and black-browed albatrosses mostly scavenge whereas grey-headed albatrosses feed more on live prey.
Moreover, during the end of interbreeding/beginning of breeding period, scavenging could play an important role in the diet black-browed and grey-headed albatrosses, as they showed higher than 60% of cephalopods potentially scavenged and more than 95% of the total estimated mass of cephalopods consumed. Black-browed and grey-headed albatrosses tended to feed on squids of similar size to those found in
wandering albatross diet (Figs. 1, 2 and 3), and grey-headed squid prey were during the reproductive period least heavier than those recorded on other albatrosses diets and during its and black-browed albatross end of interbreeding/ beginning of breeding period (Kruskal-Wallis, H = 94.2, P<0.01).
The number of cephalopod species found in grey-headed and black browed albatrosses diets were higher during reproductive period than during the end of inter-breeding period. The principal cephalopod species in albatrosses diet, chosen by their importance by number and by mass, were K. longimana, G. glacialis, M. knipovitchi and M. hyadesi during reproductive (Our results; Xavier et al, 2003b; Xavier et al, 2005) and K. longimana during the end of interbreeding/beginning of breeding period.
Only K. longimana, G.glacialis, G. antarcticus and T. sp.B (Voss) were recorded in both sampled periods, which could suggest that those cephalopod species, specially
kondakovia longimana (the most important cephalopod species by mass in 2009), may
have an even greater role in marine ecosystems than previously thought, particularly in the diet of albatrosses during the Antarctic winter.
28 According to Xavier et al (2011), to improve the assessment of the contribution of different cephalopods to predator diets, lower and upper beaks should be studied at the same time, because as the ratio of upper:lower beaks frequently differs from unity. This was observed in the present study, specially during the end of inter-breeding/beginning of breeding period for black-browed and grey-headed albatrosses. However, yet, it is worth noting that there are fewer descriptions of upper beaks morphology and even less allometric equations for estimating cephalopod mass based on upper beak measurements.
3.5 Comparison between the main cephalopod species found in
albatross’ diets
The cephalopod beaks taken by the 3 studied albatross species during their reproductive period were from species that had already been found in these albatrosses diet, and were of similar size to those taken previously in other studies (with species LRL means within ± 2 mm from the means recorded in the following studies; Clarke et al. 1981; Rodhouse et al. 1987; Imber 1992; Xavier et al, 2003a,b and c). The only cephalopod species that was outside these values was
Mesonychoteuthis hamiltoni in wandering albatrosses diet, due to one individual with a LRL of 2.3mm which was outside the LRL range recorded in Xavier et al, 2003a.
The diversity of cephalopods recorded in this study, for all albatross species studied, was lower than that recorded in previous years. There were no octopods found in their diets during both periods. It could suggest that the studied albatross species spent most of their time foraging over oceanic waters and less over neritic waters (where octopods dominate the cephalopod fauna) and that the availability / abundance of less common prey may have been lower during the sampled period and / or winds may have changed, not allowing access to certain feeding sites where those species are more probably found.
The four main cephalopod species found in the albatrosses diets were Kondakovia
longimana, Martialia hyadesi, Moroteuthis knipovitchi and Galiteuthis glacialis during
the reproductive period and K. longimana during the EIB/BB period. They were chosen because of their importance by number and by mass, representing 54.6 - 91.5% of the total fresh lower beaks found in the three albatrosses diets and 78.4 - 98.7% of the total estimated mass found per albatross species.