w ww . e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
Amazon
emulsions
as
cavity
cleansers:
antibacterial
activity,
cytotoxicity
and
changes
in
human
tooth
color
Cristiane
Coelho
De
Bari
a,
Fábio
Sampaio
b,
Nikeila
Conde
a,
Luanny
Moura
a,
Valdir
Veiga
Júnior
c,
Gleyce
Barbosa
d,
Marne
Vasconcellos
d,
Carina
Toda
a,
Gisely
Venâncio
a,
Maria
Fulgência
Bandeira
a,∗aFaculdadedeOdontologia,UniversidadeFederaldoAmazonas,Manaus,AM,Brazil
bDepartamentodeOdontologiaClínicaeSocial,LaboratóriodeBioquímicaeMicrobiologiaOral,CentrodeCiênciasdaSaúde,UniversidadeFederaldaParaíba,JoãoPessoa,PB,Brazil
cDepartmentodeQuímica,InstitutodeCiênciasExatas,UniversidadeFederaldoAmazonas,Manaus,AM,Brazil
dFaculdadedeCiênciasFarmacêuticas,UniversidadeFederaldoAmazonas,Manaus,AM,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received10October2015 Accepted1March2016 Availableonline21May2016
Keywords:
Dentistry Emulsions Cytotoxicity Fabaceae Phytotherapy
a
b
s
t
r
a
c
t
Thecopaibaoleoresin,CopaiferamultijugaHayne,Fabaceae,isaphytotherapeuticagentwith
antimicro-bialactivity.Thisstudyevaluatedtheantibacterialactivityandcytotoxicityof,andtoothcolorchanges
causedbyfourcopaibaoilemulsions(Emulsion1,10%CM;Emulsion2,10%C.multijuga+1%biotech
product;Emulsion3,30%C.multijuga;andEmulsion4,30%C.multijuga+1%biotechproduct).The
antibac-terialactivitiesagainstmicroorganismscausingdentalcaries(StreptococcusmutansATCC25175,S.oralis
ATCC10557,S.salivariusATCC7073,andLactobacilluscaseiATCC7469)weretestedusingthree
param-eters:minimuminhibitoryconcentration,minimumbactericidalconcentration,andcellviabilityby
fluorescencemicroscopy.Theemulsionswereassessedforcytotoxicitybymeansofthehemolyticassay
andcellculture(murinefibroblastcellsNHI3T3)usingAlamarBlueTM.Thedentincolorchangecaused
bytheemulsionswasexaminedat10s,30s,and10min.Theemulsionsshowedantibacterialactivity
againstthemicroorganismstestedwithanMICof125l/ml.Theminimumbactericidalconcentration
washigherthanminimuminhibitoryconcentrationforthetestedmicroorganismandthefluorescence
confirmedthatthecellswereviableatminimuminhibitoryconcentrationvalues.Theemulsionshada
hemolyticactivityof71.16%(Emulsion3)and44.67%(Emulsion4)ataconcentrationof30l/ml.Incell
cultureassay,NHI-3T3cellstreatedwiththeemulsionsshowed6–16%viability.Emulsion1caused
clin-icallyimperceptiblecolorchangeindentinat10s(E=3.21),Emulsion2at30s(E=2.70)and10min
(E=3.08),andEmulsion4at10min(E=3.03).Emulsion3causedcolorchangeatalltimestested.This
researchdocumentedpositivedataregardingantibacterialactivity,cytotoxicity,andtoothcolorchanges
whenusingcopaibaoleoresinemulsions,showingitspotentialforuseindentistry.
©2016SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Thisisanopen
accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Copaiba(Copaifera multijuga Hayne, Fabaceae) oleoresin has greatsocialandeconomicvalueinphytotherapyespeciallyinthe Amazonregion,whereitisnativeandwidelyusedasan antibac-terial,anti-inflammatory,anesthetic,andantitumoragent,andfor healingwounds(Bandeiraetal.,1999;VeigaJunioretal.,2007; Vasconcelosetal.,2008).
Cariesisoneofthemaindentaldiseasesaffectinghumanity.It isconsideredamultifactorialdiseasethatinvolvesbehavioraland
∗ Correspondingauthor.
E-mail:fulgencia@ufam.edu.br(M.F.Bandeira).
social factorscoupledwithinteractions amongmicroorganisms, host,anddiet(FejerskovandKidd,2011).
Theprocessofcariesoccurswhenmicroorganismslike Strepto-coccusmutans,S.oralis,S.salivarius,andLactobacilluscasei,present inbiofilmordentalplaque,producemetabolitesthatcause fluctua-tionsinpH.Theresultismineraltoothlossandformationofdental cavity(Kidd,2011).
Theuseofrotarytoolsduringtherestorationproceduresand afterestablishmentofthecavitylesion,resultsintheformation ofasmearlayer.Thelayerconsistsofsaliva,blood,bacteria,and residueoilsofrotaryinstruments.Thesmearlayerisremovedby theapplicationofcleaning agent,whichsubstantially decreases the cariogenic microorganisms and prevents the recurrence of caries. The procedure reduces the occurrence of microleakage restorations and thus, reduces the possibility of postoperative
http://dx.doi.org/10.1016/j.bjp.2016.03.010
sensitivity,marginalstaining,marginalfractures,andinjuriesto thepulp–dentincomplex(ReisandLoguercio,2007).
Thegoalofthestudywastoevaluatetheuseofcopaibaoleoresin emulsionsascavity cleansers and determinetheirantibacterial activityandcytotoxicityasreportedintheliteraturefordifferent bioactiveAmazonianproductsthathaveimportantdentistry func-tionsagainstperiodontaldiseasesanddentalcaries(Souzaetal., 2011a,2011b;Pierietal.,2012).
Materialsandmethods
Collectionofcopaibaoleoresinandbiotechproduct(BP)
CopaiferamultijugaHayne,Fabaceae(CM),wascollectedatthe AdolphDuckeReserve,atManaus,Amazonasstate(Brazil),and catalogedunderN◦69bytheInstitutoNacionaldePesquisasda
Amazônia(INPA).Oncetheplantmaterialwasidentified,avoucher specimenwasdepositedintheINPAherbariumunderthe regis-trationN◦270709.Thebiotechproduct(BP)wasextractedfroma
naturalpitchoftheBurseraceaefamily.
Identificationofchemicalsandemulsionsformulation
Identificationof copaiba components wasperformedby gas chromatographycoupledwithaflameionizationdetector(GC-FID) andgaschromatographycoupledwithmassspectrometricdetector (GC-MSD)(Vasconcelosetal.,2008).
Fourtest emulsionswith differentconcentrations (Emulsion [EM]1,10%CM;EM2,10%CM+1%BP;EM3,30%CM;andEM4,30% CM+1%BP)andapH=∼4.598wereformulatedattheSchoolof Den-tistry,FederalUniversityofAmazonas,followingtherequirements oftheBrazilianPharmacopoeia(Brasil,2010).Theemulsions,which arecurrentlyunderthepatentregistrationprocess,werecomposed ofdistilledwater(DW),Tween®80(Merck,Germany),CM,andBP.
The experiments were carried out after approval from the FederalUniversity of Amazonas/Institutional EthicalCommittee (number0312.0.115.000-08/2009).
Antibacterialactivity
S. mutans (ATCC25175), S. oralis (ATCC10557), S. salivarius (ATCC7073), and L. casei (ATCC7469) were used for assessing antibacterial activity. The microorganisms were reactivated in BrainHeartInfusionbroth(BHI, Himedia,Mumbai,IN),at37◦C
for24hinaerophiliafor S.oralis, S.salivarius, andL. casei, and in a microaerophilia for S. mutans. Inoculants were standard-ized at #0.5 McFarland scale (Probac of Brazil, São Paulo, SP, BR).
Minimuminhibitoryconcentration(MIC)andminimum bactericidalconcentration(MBC)
TheMICof test emulsionswasdeterminedaccordingtothe methoddescribedbyCLSI(2007),Andrews(2001),andSampaio etal.(2009).Theemulsionsweredilutedin1:1ratiowithdimethyl sulfoxide(DMSO)(Vetec,Germany)toformastocksolution(SS). The2%chlorhexidinedigluconate(FGM–Joinville,SC,Brazil)was usedasapositivecontrolandtheemulsionvehicle(Tween®80)
wasusedasanegativecontrol.
Copaibaemulsionsweredilutedandaddedto96-wellplates with each well containing 100l of differing concentrations (400–125l/ml). A volume of inoculum (20l) standardized at 108CFU/ml and complemented with BHI was added. The
1st plate column represented the sterility test substance (medium+emulsion). The 12th column represented the bacte-ria viability (bacteria+medium). The antibacterial activity of
each emulsion was tested in triplicate. The well plates were sealed with parafilm after filling and incubated at 37◦C for
24h. After 24h, 30l of 0.01% resazurin(Sigma Aldrich, USA) was added and the plates were incubated for another 30min. Absence of color change was interpreted as microorganism sensitivity to the tested emulsion during the reading of the plates. For MBC, 100l of copaiba oil emulsions was placed in the wells at different concentrations, plated on BHI agar, and incubated at 37◦C under microaerophilic and aerophilic
conditions. Testing of microorganisms was performed after 24h.
Cellviabilityanalysisbyfluorescencetechnique
Theeffectoncellviabilityfor emulsionsthat showedbetter MBC(EM3 andEM4)wasdeterminedaccordingtofluorescence techniqueagainstS.mutans(ATCC25175)andL.casei(ATCC7469) (Filocheetal.,2007).
Initially,thedilutionofthetestemulsions(DTE)wasperformed byadding1mlofemulsion+1mlofTween® 80+1mlofDMSO.
TheDTEwasfurtherdilutedformingthreesolutions:SS1:500l ofDTE+500lofBHI;SS2:500lofDTE+2500lof BHI;and SS3:500lofDTE+4500lofBHI.TheMICofthesolutionswas between4.5and120l/ml.
The reading of cell viability was performedby fluorescence techniqueusingtheLive/Dead®BacLightTMBacterialViabilityKit
L13152(MolecularProbes,Eugene,USA).Inthissystem,viablecells withoutwalldamagearestainedgreen(ComponentA:SYTO9)and cellswithdamagetothecellmembranearestainedred (Compo-nentB:propidiumiodide).
Forthepreparationofbacterialsuspensions,tubeswereused toidentifyviableandnon-viablebacteriaand3mlofBHIbroth containingtheinoculantaccordingtothetestmicroorganismswas added.Thepreparationswerecentrifugedfor15minandthe super-natantwasremoved.Theinfranatantwasresuspendedin240l of sodiumchloride (NaCl) and homogenized. NaCl(4.8ml) and 4.8mlof70%isopropylalcoholwereaddedtothetubes contain-ingdeadbacteria.Bothtubeswereincubatedatroomtemperature for60min,stirringevery15min,andthenwerecentrifugedagain for15min.Thesupernatantwasremovedandtheinfranatantwas resuspendedin2.4mlofNaCl.
Thepatternofbacterialcellswaspreparedinproportionsof knownconcentrationsofviableandnon-viablebacteria,asfollows: 0:100;20:80;50:50;80:20,and100:0.Constructionofstandard curvesused96-wellplateswithblackcolor(Greiner-Bio-One). Con-centrationratiosofviableandnon-viablebacteria(100l)were insertedintowellsA1–A5.InwellsB1–B5wereplaced100lof theratesofMICresultsconsideringtwoconcentrationsforward andtwoafter.
EqualvolumesofSYTO9andpropidiumiodidewereprepared andhomogenizedusing3minofvortex.Themixture(30l)was addedtoafluorescenceplateandthewellswereincubatedunder lightatroomtemperaturefor15min.
Thereadingwasevaluatedasfluorescenceintensityreadusing themicroplatereadermultimodetype(FluorStarOptima,BMGLab Tech,Germany)underanexcitationfilterof485nmandemission wavelengthof520and620nmforthedetectionofgreenandred color,respectively.
CytotoxicityevaluationofC.multijugaoilemulsions
Hemolyticassay
Thetestwasperformedin96-well platesusinga 2%human erythrocytesuspensionin0.85%NaClcontaining10mMcalcium chloride(CaCl2)(Jimenezetal.,2003).ThesubstancesEM1,EM2,
EM3,EM4,freshcopaibaoleoresin,Tween®
andchlorhexidine(control)weretestedatconcentrationsranging from0.234to 30l/ml.The supernatant wasremoved andthe releasedhemoglobinwasmeasuredspectrophotometricallyatan absorbanceat540nmafterincubationatroomtemperaturefor1h andcentrifugation.
Cellcultureassay
TheNHI-3T3celllineofmousefibroblastswasgrownin RPMI-1640mediumsupplementedwith10%fetalbovineserum,2mM glutamine,100g/mlstreptomycin,and100U/mlpenicillin,and incubatedat37◦Cwitha5%atmosphereofcarbondioxide(CO
2).
ThegroupstestedwereEM1,EM2,EM3,EM4,freshcopaiba oleo-resin,Tween®80(emulsionvehicle),andchlorhexidine(control).
Thecellswereplatedin96-wellplates(2.5×104cellsperwell)and
theAlamarBlueTMassaywasperformedusingpreviouslydescribed
proceduresofAhmedetal.(1994).After24h,thecompoundswere dissolvedinDMSOandaddedtoeachwelltogivefinal concen-trationsof5l/ml.Controlgroupshadfinalwellconcentrationsof 0.1%DMSO.Plateswereincubatedfor72hand3hbeforetheend oftheincubation,10lofAlamarBlueTMwasaddedtoeachwell.
Thefluorescentsignalwasmonitoredwithamultiplatereaderat excitationwavelengthof530–560nmandemissionwavelengthof 590nm.
Influenceofthetimeoftestemulsionapplicationonhumantooth colorchange
Thefabricationof150testspecimens(TS)of75humanteeth extractedfororthodonticand/orsurgicalreasonswasperformed accordingtothemethodologyproposedbyAraújoetal.(1998)and therulingsoftheEthicsCommittee.Afterextraction,theteethwere storedin2%thymolforaminimumof24hfordecontamination. Thecrownwasseparatedfromtherootatthecementumenamel junctionwiththeaidofacutter(PresiMecatome,Grenoble,France, p100)andfittedwithadouble-sideddiamonddisk.Thecrownwas sectionedinamesiodistaldirectiontoobtaintwotestspecimens of each sectioned tooth. Each specimen wasstored in individ-ualvialscontainingdeionizedwater and keptat atemperature of37◦C.
TenTSwereusedforeachtestemulsion,whichwereimmersed inthetestemulsionsforthetimeintervalsof10s,30s,and10min. Thecontrolusedinthestudywas2%chlorhexidine(FGM,Joinville, SC,Brazil).
The color measurement of TS was performed withthe Vita Easyshade(Easyshade®,Vivadent,Brea,CA,USA)
spectrophotome-terattheinitialtime(T0)withouttreatmentandexperimentaltime intervals.Colorwasdeterminedinaccordancewiththeparameters oftheCIElabsystem(L*a*b*),inwhichL*indicatesthe bright-nesswheretheaveragerangesfrom0(black)to100(white).The a*andb*representthehuewitha*asthesaturationred–green axis and b* the saturation in blue–yellow axis. Comparison of thecolorchangeofteethbeforeandafterimmersioninthetest substanceswasobservedandrepresentedbytheequation:E* ab=([L*]2+[a*]2+[b*]2)0.5.
Statisticalanalysis
The antibacterial activity was analyzed in descriptive form. Thehemolyticassaywasperformedusingtheaverage percent-agestandarddeviationandlinearsigmoidregressiontocalculate theeffectiveconcentrationof50%ofcelllysis(EC50).Thecell
cul-tureassaywasevaluatedbyone-wayanalysisofvarianceusing Dunnett’stest(p<0.05)formultiplecomparisonsadjustment.The medianandquartiles(Qi)wereusedtosummarizethechromatic changeoftheTS.Forcomparisonofthemedians,Kruskal–Wallis tests wereapplied for comparison of thedifferent groups, and Mann–Whitneytestswereusedforthecomparisonoftwomedians (p<0.05).
Resultsanddiscussion
ChromatographicanalysisofCMrevealedsesquiterpenes, espe-cially-caryophyllene (9.2%),␣-humulene (1.8%),germacrened (3.5%),caryophylleneoxide(11.5%),bisabolol(7.2%),labdane diter-peneskeleton-containingcompoundssuchascopalicacid(2.1%), 3-hydroxy-copalic(1.7%),andpinipholic(1.3%)asthemajor com-ponents.TheseresultsweresimilartotheresultsofBandeiraetal. (1999),CasconandGilbert(2000),VeigaJuniorandPinto(2002),
Limaetal.(2003),Vasconcelosetal.(2008),Souzaetal.(2011a, 2011b),andBarbosaetal.(2013),whoreportedthesesquiterpenes
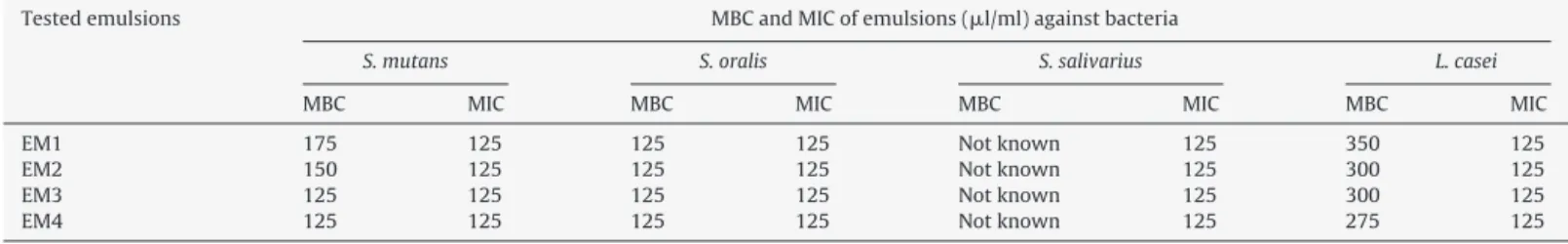
ˇ-caryophylleneandcaryophylleneoxideasthemaincomponents. TheMIC(125l/ml)ofalltestedemulsionsshowed antibac-terialactivityagainstS.mutans,S.oralis,S.salivarius,andL.casei.
Bandeiraetal.(1999),Vasconcelosetal.(2008),andPierietal. (2012)alsoreportedtheantibacterialactivityofcopaibaoilagainst microorganismsintheoralcavity.
Theantibacterialactivityobservedinemulsiontestsis possi-blyrelatedtotheCMcomponents,includingthediterpenes,afact corroboratedbyVasconcelosetal.(2008)andSouzaetal.(2011a, 2011b).TheMBCandMICanalysisresultsareshowninTable1.
Table1showsthedifferentconcentrationsofemulsionsthat showedbactericidalactivityagainstS.mutans,S.oralis,andL.casei. However, no bactericidal activity against S. salivarius could be detected.Pierietal.(2012)demonstratedthebacteriostaticactivity ofcopaibaoilsolutionsatconcentrationsupto100l/ml,whereas theresultsofthisworkshowedbactericidalactivityat concentra-tionsabove100l/ml.
Allofthe2%chlorhexidineconcentrationstestedshowed bacte-ricidalactivity,similartotheresultsofSouzaetal.(2011b),which showedthattheMBCofchlorhexidinewas3.5-foldhigherthan thatoftheextractedcopalicacidoilofC.langsdorffi,confirmingthat chlorhexidineisthegoldstandardforantibacterialactionagainst oralpathogens.
CellviabilityanalysisofEM3at9and12g/mlbyfluorescence techniqueshowed8.59%viableS.mutanscellsand6.59%viableL. caseicells,respectively.TheMICofandcellviabilityupontreatment withEM4were45l/mland11.22%forS.mutans,respectively; theMICforandcellviabilityofL.caseiwere60l/mland11.20%, respectively.CellviabilitycanbelessthantheMICfoundinthefirst
Table1
Minimumbactericidalconcentration(MBC)andMinimuminhibitoryconcentration(MIC)ofCopaiferamultijugaoilemulsionsagainstbacteria.
Testedemulsions MBCandMICofemulsions(l/ml)againstbacteria
S.mutans S.oralis S.salivarius L.casei
MBC MIC MBC MIC MBC MIC MBC MIC
EM1 175 125 125 125 Notknown 125 350 125
EM2 150 125 125 125 Notknown 125 300 125
EM3 125 125 125 125 Notknown 125 300 125
Table2
Medianscoredistributionandquartilesoffinalcolorchangeinhumanteethat10s,30sand10minimmersioninchlorhexidineandCopaiferamultijugaoilemulsions.
Groups Score pa
10s 30s 10min
Med Q1/Q3 Med Q1/Q3 Med Q1/Q3
Chlorhexidine 3.60b 1.93/4.32 3.81b 1.51/4.68 1.22c 0.85/1.80 0.009
EM1 3.21 2.24/6.11 10.00 3.98/12.72 7.41 2.66/14.69 0.059
EM2 3.88 1.96/5.66 2.70 1.97/3.57 3.08 2.20/5.52 0.703
EM3 13.73 7.28/20.12 8.35 4.05/17.87 7.49 5.12/21.86 0.532
EM4 3.48 2.08/6.19 4.39 2.12/5.62 3.03 2.04/4.06 0.802
Med,median;Q,quartile.
Italicizedvalueindicatestatisticaldifference(p<0.05).
aKruskal–Wallistest. b andc–statisticallydifferent.
testowingtodilution.Thepresenceofviablecells,suggests bacte-riostaticactivityatconcentrationsthatinducebactericidalactivity, 125l/ml.
CytotoxicityassessmentofC.multijugaoilemulsions
Hemolyticassay
Thefreshcopaibaoleoresin,thevehicleoftheemulsions,and 2%chlorhexidineshowed0.72%,0.71%,and1.87%celllysis, respec-tively.
Theresultsofhemolyticactivityindicatedthatat30l/ml,all formulationsshowedhighhemolyticactivitywith70.82%,47.58%, 71.16%, and 44.67% cell lysis with EM1, EM2, EM3, and EM4, respectively. However,owing to thehigh hemolyticactivity of thesesubstances andasasafety precaution,it isrecommended tousetheseemulsionsatlowEC50values,11.15l/mlforEM1and
16.55l/mlforEM3.
Thetestemulsionsshowedhemolyticactivity,similartothat reportedbyCosta-Lotufoetal.(2002),whoevaluatedkaurenoic acidfromC.langsdorffioleoresin.KaurenoicacidshowedanEC50of
54.6Mforhumanerythrocytes.
Thefreshcopaibaoleoresinshowednosignificanthemolytic activity,whichisindisagreementwiththeresearchofCosta-Lotufo etal.(2002),whotestedkaurenoicacid,acompoundofcopaibaoil dilutedinDMSOandwater.Thisresultmaybeattributabletothe absenceofadetergenttolysetheCMmacromolecules,preventthe contactofCMwiththesolutionoferythrocytes,andthus,induce celllysis.
Cellcultureassay
Cell culture assay showed that all test emulsions and fresh copaibaoleoresinweretoxictoNHI3T3cells,causingadecrease incellviability(Fig.1).
0 20 40 60 80 100 120
% viable cells
Control Copaiba Vehicle EM1 EM2 EM3 EM4
Fig.1.CellviabilitytestingofCopaiferamultijugaoilemulsionsinthecellline NHI3T3.
ThedecreasedcellviabilityofNHI3T3,non-neoplasticmurine fibroblasts,validatetheresultsofCosta-Lotufoetal.(2002),Lima etal.(2003),andVeigaJunioretal.(2007),whodemonstrateda decreaseinviabilityoftumorcells.Cellviabilitywithoutcopaiba was52.33%indicatingthatthehighcytotoxicityinthisstudymay berelatedtothevehicleusedintheemulsion.
ThevehicleusedintheformulationofemulsionswasTween®
80,whichisanonionicsurfactantthatistoxictobiological mem-branesandconsistsofthefattyacidester,polyoxyethylene.Toxicity ofTween®80asavehicleincellcultureispossiblyrelatedtoits
abilitytoalterthesurfacemorphologyandcellwallviaitsdetergent action(Dominguesetal.,2000).
Influenceofthetimeoftestemulsionapplicationonhumantooth colorchange
Influenceoftimeoftestemulsionapplicationonhumanteeth colorchangeisshowninTable2.
ResultsofEvaluesequaltoorlowerthan3.3areconsidered clinicallyinsignificantfromthedailyclinicalperspectiveofvisual appearance;thatistosay,thecolorchangeofhumanteethofthis magnitudewouldbeclinicallyimperceptible(Vichietal.,2004). Basedonthesefacts,thetestemulsionsforwhichthecolorchanges ofteethwouldnotbeclinicallyperceptiblewereEM1at10s,EM2, at30s,and2%chlorhexidine,EM2,andEM4at10min.
Chlorhexidinewasusedinthepresentexperimentasasolution for cavity and tooth surface cleaning and for short-term non-stainingproceduresofthetoothstructure.
Useofcopaibaoleoresinemulsions,abiotechnologicalproduct fromtheAmazonianregion,contributedsignificantlytothe reduc-tionofcolorchange(expressedinEvalues);however,nostudies reportedintheliteraturesupportthedataobtainedinthisresearch. The copaiba emulsion tests demonstrated the potential of copaibaemulsionsforuseindentistry,especiallyEM1andEM2. Furtherresearchontheclinicalapplicationisnecessary.
Ethicaldisclosures
Protectionofhumanandanimalsubjects. Theauthorsdeclare thattheproceduresfollowedwereinaccordancewiththe regula-tionsoftherelevantclinicalresearchethicscommitteeandwith thoseoftheCodeofEthicsoftheWorldMedicalAssociation (Dec-larationofHelsinki).
Confidentialityofdata. Theauthorsdeclarethatnopatientdata appearinthisarticle.
Authors’contributions
CNCDB,FCS,CT,andNCOCcontributedtothedevelopmentof theexperimentalphasefortheemulsionformulationsand antibac-terialactivity.VVJcontributedtothechemicalanalysisoftheraw material.MCVandGSBcontributedtothecytotoxicityassay.MFCLB coordinatedtheprogressofallexperimentalphasesanddata anal-ysis.CNCDB,LGM,andGNVdraftedthearticle.Alltheauthorshave readthefinalmanuscriptandapprovedthesubmission.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgments
This study was supported by CNPq. Project MCT/CNPq/CT-Amazon (Process N◦ 575752/2008-4 and Process N◦ 406457/
2013-1).
References
Ahmed,S.A.,Gogal,R.M.,Walsh,J.E.,1994.Anewrapidandsimplenon-radioactive assaytomonitoranddeterminetheproliferationoflymphocytesanalternative to[3H]thymidineincorporationassay.J.Immunol.Methods170,211–224. Andrews,J.M.,2001.Determinationofminimuminhibitoryconcentration.J.
Antimi-crob.Chemoth.48,5–16.
Araújo,M.A.J.,Rode,S.M.,Villela,L.C.,Gonc¸alves,R.D.,1998.Avaliac¸ãoqualitativa doefeitodeagentesdelimpezanacamadadelamadentinária:estudo ultra-estruturalemmicroscopiaeletrônicadevarredura.Rev.Odontol.Univ.SãoPaulo 12,99–104.
Bandeira,M.F.C.L.,Oliveira,M.R.B.,Benatti-Neto,C.,CamelliLia,R.C.,1999.Estudo comparativodacompatibilidadebiológicaemmolaresderatodoóleoessencial edaresinadaCopaiferamultijuga(óleodeCopaíba)associadosaohidróxidode cálcio.J.Bras.Clin.Estet.Odontol.16,42–49.
Barbosa,P.C.S.,Wiedemann,L.S.M.,Medeiros,R.S.,Sampaio,P.T.B.,Vieira,G., Veiga-Junior,V.F.,2013.Phytochemicalfingerprintsofcopaibaoils(Copaiferamultijuga Hayne)determinedbymultivariateanalysis.Chem.Biodiv.10,1350–1360. Brasil,2010.FarmacopeiaBrasileiraVol.2,AgênciaNacionaldeVigilânciaSanitária.
Brasília:Anvisa.
Cascon,V.,Gilbert,B.,2000.Characterizationofthechemicalcompositionof ole-oresinsofCopaiferaguianensis Desf.CopaiferaduckeiDwyer andCopaifera multijugaHayne.Phytochemistry55,773–778.
CLSI,2007.PerformanceStandardsforAntimicrobialSuceptibilityTesting; Seven-teenthInformationalSupplementM100-S17,vol.27.CLSI,pp.1–177. Costa-Lotufo,L.V.,Cunha,G.M.A.,Farias,P.A.M.,Viana,G.S.B.,Cunha,K.M.A.,Pessoa,
C.,Moraes,M.O.,Silveira,E.R.,Gramosa,N.V.,Rao,V.S.N.,2002.Thecytotoxic andembryotoxiceffectsofkaurenoicacid,aditerpeneisolatedfromCopaifera langsdorffiióleo-resin.Toxicon40,1231–1234.
Domingues,F.C.,Queiroz,J.A.,Cabral,J.M.,Fonseca,L.P.,2000.Theinfluenceof cul-tureconditionsonmycelialstructureandcellulaseproductionbyTrichoderma reeseiRutC-30.EnzymeMicrob.Technol.26,394–401.
Fejerskov,O.,Kidd,E.,2011.CárieDentária:ADoenc¸aeseuTratamentoClínico. Santos,SãoPaulo.
Filoche,S.K.,Coleman,M.J.,Angker,L.,Sissons,C.H.,2007.Afluorescenceassayto determinetheviablebiomassofmicrocosmdentalplaquebiofilms.J.Microbiol. Methods69,489–496.
Jimenez,P.C.,Fortier,S.C.,Lotufo,T.M.C.,Pessoa,C.,Moraes,M.E.A.,Moraes,M.O., Costa-Lotufo,L.V.,2003.Biologicalactivityinextractsofascidians(Tunicata, Ascidiacea)fromthenortheasternBraziliancoast.J.Exp.Mar.Biol.Ecol.287, 93–101.
Kidd,E.,2011.Theimplicationsofthenewparadigmofdentalcaries.J.Dent.39,s2, s3–s8.
Lima,S.R.,Junior,V.F.,Christo,H.B.,Pinto,A.C.,Fernandes,P.D.,2003.Invivoand invitrostudiesontheanticanceractivityofCopaiferamultijugaHayneandits fractions.Phytother.Res.17,1048–1053.
Pieri,F.A.,Mussi,M.C.M.,Fiorini,J.E.,Moreira,M.A.S.,Schneedorf,J.M.,2012. Bacte-riostaticeffectofcopaibaoil(Copaiferaofficinalis)againstStreptococcusmutans. Braz.Dent.J.23,36–38.
Reis,A.,Loguercio,A.D.,2007.Materiaisdentáriosdiretos–dosfundamentosà aplicac¸ãoclínica.Santos,SãoPaulo.
Sampaio,F.C.,Pereira,M.S.V.,Dias,C.S.,Costa,V.C.O.,Conde,N.C.O.,Buzalaf,M.A.R., 2009.InvitroantimicrobialactivityofCaesalpiniaférreaMartiusfruitsagainst oralpathogens.J.Ethnopharmacol.124,289–294.
Souza,A.B.,Martins,C.H.G.,Souza,M.G.M.,Furtado,A.J.C.,Heleno,V.C.G.,Sousa,J.P.B., Rocha,E.M.P.,Bastos,J.K.,Cunha,W.R.,Veneziani,R.C.S.,Ambrósio,S.R.,2011a. AntimicrobialactivityofterpenoidsfromCopaiferalangsdorffiiDesf.against car-iogenicbacteria.Phytother.Res.25,215–220.
Souza,A.B.,Souza,M.G.M.,Moreira,M.A.,Moreira,M.R.,Furtado,N.A.J.C.,Martins, C.H.G.,Bastos,J.K.,Santos,R.A.,Heleno,W.C.G.,Ambrósio,S.R.,Veneziani,R.C.S., 2011b.AntimicrobialavaluationofditerpenesfromCopaiferalangsdorffi oleo-resinagainstperiodontalanaerobicbacteria.Molecules16,9611–9619. Vasconcelos,K.R.,VeigaJunior,V.F.,Rocha,W.C.,Bandeira,M.F.C.L.,2008.Invitro
assessmentofantibacterialactivityofadentalcementconstitutedofaCopaifera multijugaHayneoil-resin.Rev.Bras.Farmacogn.18,733–738.
VeigaJunior,V.F.,Pinto,A.C.,2002.OGêneroCopaiferaL.Quim.Nova25,273–286. Veiga Junior,V.F.,Rosas,E.C.,Carvalho,M.V.,Henriques,M.G.M.O.,Pinto, A.C.,
2007.Chemicalcompositionandanti-inflammatoryactivityofcopaibaoilsfrom CopaiferacearensisHuberexDucke,CopaiferareticulataDuckeandCopaifera multijugaHayne–acomparativestudy.J.Ethnopharmacol.112,248–254. Vichi,A.,Ferrari,M.,Davidson,C.L.,2004.Colorandopacityvariationsinthree