w ww.e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
Qualitative
and
quantitative
analysis
of
the
phenolic
content
of
Connarus
var.
angustifolius
,
Cecropia
obtusa
,
Cecropia
palmata
and
Mansoa
alliacea
based
on
HPLC-DAD
and
UHPLC-ESI-MS/MS
Fernanda
B.
Pires
a,
Carolina
B.
Dolwitsch
a,
Valéria
Dal
Prá
a,
Henrique
Faccin
b,
Débora
Luana
Monego
b,
Leandro
M.
de
Carvalho
a,b,
Carine
Viana
a,
Osmar
Lameira
c,
Fernanda
O.
Lima
d,
Lucas
Bressan
b,
Marcelo
B.
da
Rosa
a,b,∗aProgramadePós-graduac¸ãoemCiênciasFarmacêuticas,UniversidadeFederaldeSantaMaria,SantaMaria,RS,Brazil bProgramadePós-graduac¸ãoemQuímica,UniversidadeFederaldeSantaMaria,SantaMaria,RS,Brazil
cLaboratóriodeAgrobiotecnologia,EmbrapaAmazôniaOriental,Belém,PA,Brazil dCursodeQuímica,UniversidadeFederaldaFronteiraSul,Realeza,PR,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received2November2016 Accepted28March2017 Availableonline5May2017
Keywords:
Barbatimão-do-pará Redandwhite-embaúba Cipó-d’alho
Phenoliccontent HPLC-DAD UHPLC-ESI-MS/MS
a
b
s
t
r
a
c
t
ThephenoliccontentofthemedicinalspeciesConnarusperrottettivar.angustifoliusRadlk.,Connaraceae,
CecropiaobtuseTrécul, CecropiapalmataWilld.,Urticaceae;and Mansoaalliacea(Lam.)A.H.Gentry,
Bignoniaceae,collectedinthreedifferentyearswasevaluated.Plantinfusionsandhydroalcoholic,butanol
andethylacetateextractswereanalyzedbyhigh-performanceliquidchromatographywithdiodearray
detection.Inordertoendorsetheseresults,analysisbyelectrospraymassspectrometrywasalso
per-formed.Wereidentified:gallicacid,catechin,caffeicacid,ferulicacid,rutin,quercitrinandresveratrol.
C.perrottettishowedgreaterdiversityofpolyphenols.M.alliaceahadthehigherconcentrationofcaffeic
acideventhoughitwasfoundinallspecies.Catechinwasthemajorantioxidant,butwasnotdetectedin
M.alliacea.However,wediscussthepopularuseofthesespecies,aswellastheirphenolicconstitution
andtheinterannualdistributionofphenoliccompounds.
©2017SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Thisisanopen
accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Theuseofmedicinalplantsforpreventionandrecoveryof infec-tionsaswellasforhealthpromotionisanancientpractice(Veiga etal.,2005;Schmitzetal.,2005;Alvimetal.,2006).Thisactivity grewoutofpopularknowledgeandforalongtimewastheonly alternativetreatmentforhealthproblems(Alvimetal.,2006).
Althoughmodernmedicinehasbeengreatlyadvancinginthe pastdecades,phytotherapyisstillwidelyemployed(Alvimetal., 2006).TheWorldHealthOrganizationestimatesthatabout80%of theworldpopulationusesthistraditionalmedicineinitsprimary healthcareneeds(MS,2006).AccordingtotheInstituteforApplied EconomicResearch(IPEA,2010),about30%ofcommercially avail-abledrugsderivefromnaturalsources,whileLahlou(2013)asserts thatapproximately40%ofalldrugsareeithernaturalproductsor theirsemi-syntheticderivatives.
∗ Correspondingauthor.
E-mail:marcelo.b.rosa@ufsm.br(M.B.Rosa).
Brazilholds anexpressiveplantbiodiversity, along withthe largestrainforestontheplanet:theAmazon(Paracampo,2011). Althoughtheemploymentofplantsformedicinalpurposesisvery widespreadinBrazil,knowledgeabouttheirchemical composi-tionisratherlimited,asmostofthemareappliedwithlittleor noscientificproofofaction(Campelo,2006).Inordertochange thissituation,relevantstudiesconcerningthechemical composi-tionofthesespeciesandthearisingpharmacologicalpropertiesare ofconsiderableimportance.
Differentpartsoftheplantmaybeusedinherbalmedicine,such asroots,bark,leaves,fruitsandseeds(RezendeandCocco,2002). Tea,usuallyobtainedbyinfusion,isaverypopularwayofgetting theactivecompoundsofdifferentplantproducts(Schmitzetal., 2005).
Polyphenolshavebeenincreasinglyinvestigatedandconsumed inrecentyearsduetotheirnutritionalpotentialandtherapeutic value(Ajilaetal.,2011).However,numerousspecies of medic-inal interest still need to be studied regarding this and other classesofcompounds.AmongstthemareConnarusperrottettivar.
angustifoliusRadlk.,CecropiaobtusaTrécul,CecropiapalmataWilld., Urticaceae,andMansoaalliacea(Lam.)A.H.Gentry,Bignoniaceae,all
http://dx.doi.org/10.1016/j.bjp.2017.03.004
nativespeciesoftheAmazonrainforest,wheretheyareextensively usedbythelocalswithlimitedscientificinformation.
The species C. perrottetti var. angustifolius, popularly known as “barbatimão-do-pará”, is a member of the Connaraceae family. This plant present antidiarrheal, anti-bleeding, anti-inflammatory, antibacterial, antifungal, antiviral and healing activities(Paracampo,2011),beingcommonlyusedmacerated,or asteaandsyrup,forthetreatmentofgenitourinaryinfectionsin women,uterinebleeding,vaginaldischarge,headache,gastric dis-easesandcough(Coelho-Ferreira,2009).
ThespeciesC.obtusaandC.palmata,popularlyknowninBrazilas “red-embaúba”and“white-embaúba”respectively,areboth mem-bers of the Urticaceae family (Beringhs et al., 2015). Plants of thisgenusareusuallyemployedastea,andexhibitseveral recog-nizedactivities,suchasantidiabetic(FreitasandFernandes,2006), expectorant,mucolytic,antiseptic,laxative,antimicrobial(Lameira etal.,2004),diuretic,antitussiveandanti-inflamatory(Freitasand Fernandes,2006;Lameiraetal.,2004;Costaetal.,2011).
In Brazil,especiallyin thestateof Pará,M. alliaceais popu-larly knownas “cipó-d’alho” due to the characteristic smell of garlicreleasedbyitsleaveswhenmacerated(Zoghbietal.,2009). ThisplantbelongstotheBignoniaceaefamilyandisusedbyits anti-rheumatic (Ribeiro etal.,2009), antimalarial (Pérez, 2002), antifungalandantiviral(Zoghbietal.,2009),activitiesandtothe treatmentofrespiratorydiseases(Ribeiroetal.,2009).Maceration, infusion,teapreparationanddecoctionarethemainprocesses pop-ularlyusedinthisplanttoobtainitsactivecompounds(Zoghbi etal.,2009).
Severalfactorscancoordinateorchangetherateofproduction ofphenoliccompoundsandothersecondarymetabolitesinplants. Theperiodofcollectionisoneofthem,sincetheconcentrationand evennatureofsuchcompoundsmayvaryconsiderablyoverthe year(Gobbo-NetoandLopes,2007).
Phenoliccompoundsrangefromsimpletohighlypolymerized structures,whichcanwithalbecomplexedtovariousotherplant components.Therefore,differentmethodsofextractioncombined withsolventsofdifferentpolaritiesarerequiredtoobtainthem (NaczkandShahidi,2004).
This paper aims to identify and to quantify phenolic con-tent,namely,gallicacid,catechin,caffeicacid,rutin,ferulicacid, quercitrin, myricetin, fisetin,resveratrol, quercetin, kaempferol, chrysinandflavone,presentinthespeciesC.perrottettivar. angus-tifolius, C. obtusa, C.palmata and M. alliacea.The extractswere obtainedbyultrasoundextractionusingethanol/water,n-butanol andethylacetateassolvents.Waterinfusionswerealsoanalyzed. The separation, identification and quantification of the afore-mentionedcompoundswerecarriedoutusinghigh-performance liquid chromatographywith diodearraydetection (HPLC-DAD). An ultra-high-performance liquid chromatography-electrospray ionization-tandemmassspectrometry(UHPLC-ESI-MS/MS) anal-ysis wasperformed in order to endorse theresults previously obtainedbyHPLC-DAD.
Materialsandmethods
Chemicals
Analytical standards of gallic acid, (+)-catechin, caffeic acid, rutin,quercitrin,myricetin,fisetin,quercetin,kaempferol,chrysin andflavonewerepurchasedfromSigma–Aldrich(St.Louis,MO, USA).FerulicacidwaspurchasedfromFluka(Buchs,Switzerland) andresveratrolfromTedia(RiodeJaneiro,RJ,Brazil).Allstandards usedwereofanalyticalgrade(≥95%purity).
Themobilephasewaspreparedbydilutingan85%(v/v) phos-phoricacid(PA) (F.Maya,Brazil)withultrapurewater(Milli-Q,
MilliporeSynergyUV,Bedford,MA,USA)toaconcentrationof0.1% (w/w)inavolumetricflask.Thesolutionwasthenfilteredunder vacuumsystem(Prismatec,131, 2V)througha0.2mcellulose
acetatemembranefilter(Sartorius,Goettingen,Germany). Acetoni-trilewasalsointhecompositionoftheeluentandwasobtained fromPanreacITWcompanis(Germany).
Stocksolutions of1000mg/lwereprepared bydilutingeach phenoliccompoundstudiedinHPLCgrademethanol,suppliedby Tedia(RiodeJaneiro,RJ,Brazil).TheywerestoredinFalcontubes at−30◦C.Thesesolutionswerethendilutedinmethanoltoreach
intermediateconcentrations.
Plantsampleswereweighedonadigitalanalyticalbalance (Shi-madzu/AUY220)witha0.0001gprecision.
Plantmaterial
Barks of Connarus perrottetti var. angustifolius Radlk., Con-naraceae(IAN184393)andleavesofCecropiapalmataWilld.(IAN 185556),C. obtusaTrécul,Urticaceae (IAN 185555)and M. alli-acea(Lam.)A.H.Gentry,Bignoniaceae(IAN184394)werecollected in April and May of 2012,2013 and 2014, properly identified, driedandmilled.Allplantsstudiedwereprovidedbythe herbar-iumIANlocatedattheBrazilianAgriculturalResearchCorporation (Embrapa)EasternAmazon,Belém,PA,locatedat1◦27′21′′Sand
48◦30′14′′W,atanaltitudeof10mandanaverageannual
temper-atureof30◦C.
AccordingtoAnaniasetal.(2010)theclimateoftheAmazon regionischaracterizedbyadryperiod(July–October)andarainy season(December–May),whileJuneandNovemberareconsidered transitionperiods.In addition,itis consideredahot andhumid climatewithverysmalltemperaturegradients.Therefore,samples studiedwerecollectedduringtherainyseason.
Extractionprocedure
Plant extracts were preparedby ultrasound assisted extrac-tion(Bandelin,SonorexSuperRK510H).Glasstubescontaining approximately0.2gofthesamplesreceived10mloftheextraction solvent(70%hydroethanolic,butanolorethylacetate)and were placedinanultrasonicbathfor4hatroomtemperature.Then,the supernatantwaswithdrewandtheremainingextractwasfiltered ona0.22mmembrane(SorblineTecnologie).Butanolandethyl
acetatewereevaporatedat40◦Cinaglassbeaker.Theseextracts
werelaterresuspendedinHPLCgrademethanolandfilteredina 0.22mmembrane(SorblineTecnologie).Theinitial
concentra-tionsweremaintained.Theextractedsamplesremainedstoredat
−30◦Cuntiltheanalysis.Beforeinjectionintothechromatograph,
allsamplesweredilutedto0.01g/mlwithHPLCgrademethanol.
Infusions
Avolumeof50mlwaterat90◦Cwereaddedto1.5gofdried
plant.Thirtyminuteslater,theinfusionswerefiltered,bothbya paperfilterandbya0.22mmembrane(SorblineTecnologie)and
thenstoredat−40◦Cuntilused.Thesamplesinjectedwerefirst
dilutedat0.01g/mlwithHPLCgrademethanol.
HPLC-DADapparatus
ThechromatographicanalysiswasperformedinaKnauer chro-matograph (Berlin, Germany) equipped with a manual Knauer injector(20lloop).ThesystemconsistedofaSmartlinePump
Table1
DatafromthechromatographicseparationviaHPLC-DAD.
Phenoliccompound/equationofanalyticalcurve tR(min)a (nm)b LOD(mg/kg)c LOQ(mg/kg)d
1.Gallicacid/y=9387.5x+5270.2 4.40 220 0.7 1.0
2.(+)-Catechin/y=13992x+23821 8.65 220 1.7 2.8
3.Caffeicacid/y=14013x+7961.9 10.10 320 0.3 1.0
4.Rutin/y=16178x+1196.8 12.45 254 0.6 2.4
5.Ferulicacid/y=15927x+3286.3 14.75 320 0.5 1.0
6.Quercitrin/y=18225x+5576.9 18.00 254 0.4 0.8
7.Resveratrol/y=24317x+1592.9 28.45 320 0.4 0.8
at
R:retentiontime. b :wavelength. c LOD:detectionlimit. d LOQ:quantificationlimit.
Identificationandquantificationofphenoliccompoundswere performedusingthemethodologydevelopedwhich,besidesthe lowlimitsofdetectionandquantificationpresentedbythis chro-matographicmethod(Table1),theanalyticalvalidationconfirmed itsselective,linear,preciseandaccuratecharacter(Table2).
Compoundswereseparatedwithagradientreverse-phase sys-tem.A250mm×4.6mmchromatographic columnpackedwith C18 (5m particle diameter, Phenomenex) and a guard
col-umnwithsimilarcompositionwereemployed.Themobilephase consistedoforthophosphoricacidsolution(0.1%,w/w)assolventA andacetonitrileassolventB.Theelutionconditionswere:90–80% Aand10–20%B(0–5min);80–75%Aand20–25%B(5–35min); 75–0%Aand25–100%B(35–55min).Theflowrateofthemobile phasewas0.8ml/min(0–35min)and0.8–1.0ml/min(35–55min). Roomtemperature(21±2◦C)wasmonitoredduringall
chromato-graphic runs. The wavelengths scanned were 220nm, 254nm, 320nmand360nm.
Identificationofcompoundswasbasedonthecorrespondence ofretentiontimesofchromatographicsignalsofplantextractsand referencematerials,theultraviolet(UV)spectraandbytheaddition ofthreedifferentconcentrationsofthestandardsolutionstothe samples.
Following,compoundswerequantifiedbythestandard addi-tionmethod,whichcorrelatestheconcentrationofthestandard solution(addedinmol/l)tothepeakarea.AccordingtoRibani
etal.(2004)theconcentrationof thesamplesanalyzedmaybe determinedthroughstraightlineextrapolation,correspondingto thepointwhereitcutstheaxisoftheabscissae(x).
Table1showstheequationfortheanalyticalcurve,retention timesoftheantioxidantcompoundsfoundinplantspecies,aswell asthedetectionwavelength(nm),limitofdetectionandlimitof quantificationofthechromatographicseparationviaHPLC-DAD.
Thelimitsofdetection(LOD)andofquantification(LOQ)were determinedbythesignal-to-noiseratio,inacordancetoNational Instituteof Metrology (2016). Thearea and standard deviation oftheretention times werecomputedthrough theinjectionof 7replicatesoftheblank(matrixfreeofthecompoundof inter-est), in this case methanol (HPLC grade). LOD=X+t(n−1)·s and
LOQ=X+10·s,where:X=mean ofblanks, s=standarddeviation of blanks,t=number ofinjections (7-1)=6 degrees of freedom. Consideringthedegreesoffreedom,theunilateralt,ata99% con-fidencelevel,is3.143.
Table2showsthelinearrange,thecorrelationcoefficient,the intradayprecision,expressedbytherelative standarddeviation (RSD%),theinter-dayprecision,expressedbythecoefficientof vari-ation(CV%),andtheaccuracy(%),determinedbyrecoveryassays.
UHPLC-ESI-MS/MSmethod
The method developed and applied by Faccin et al. (2016)
to detect the molecular ions through mass spectrometry was employedforconfirmationpurposes.
Resultsanddiscussion
The methodologyemployed to determinethephenolic con-stituentsbyHPLC-DADenablestheseparationandidentification ofthirteenantioxidantsasshowninFig.1(A).Amongthese,seven weredetectedinthestudiedplants,namely,gallicacid(1), cate-chin(2),caffeicacid(3),rutin(4),ferulicacid(5),quercitrin(6)and resveratrol(9).
DeterminationbyHPLC-DADofphenoliccompoundsinplant extractsfromdifferentyearsofcollection
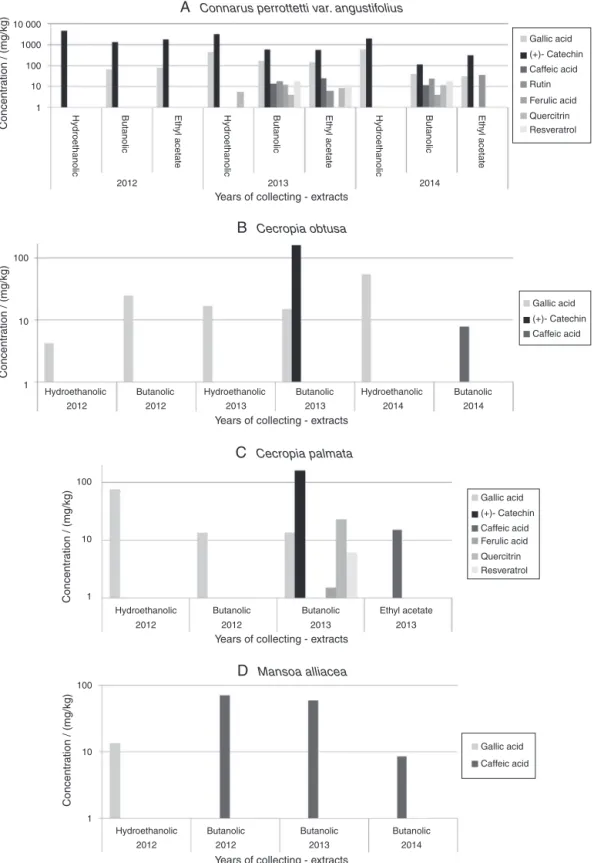
Fig.2 shows the concentrationof antioxidantsfound inthe medicinalspeciesstudiedrelatingtheextractstotherespective col-lectionperiod.Themeancoefficientofvariationoverthetriplicate extractionswas28–30%forallextractsobtained.
Connarusperrottettivar.angustifolius
Amongtheanalyzedspecies,C.perrottettivar.angustifolius pre-sented the highest diversity of phenolic compounds. Although catechin was the predominant antioxidant, corresponding to
Table2
ValidationparametersoftheHPLC–DADmethod.
Phenoliccompound Linearrange(mol/l) (r)a Intraday(RSD%)b Interday(CV%)c Accuracy(%)
1.Gallicacid 2.94–588 0.9990 0.6 1.9 104.7
2.(+)-Catechin 8.61–344.4 0.9997 1.2 3.5 83.8
3.Caffeicacid 5.5–550 0.9997 0.8 2.3 103.4
4.Rutin 0.82–164 0.9996 0.9 2.8 88.2
5.Ferulicacid 5.15–515 0.9997 0.5 1.6 97.6
6.Quercitrin 2.23–223 0.9984 1.2 3.7 94.6
7.Resveratrol 4.38–438 0.9996 0.6 1.9 100
150 135 120 105 90 75 60 45 30 15 0
–15 0 5 10 15 20 25 30 35 40 45 50 55
1 2
3
4 5
6
8 7
9
10
11 12
13
Time / min
Intensity / mA
U
Intensity / mA
U
Intensity / mA
U
Intensity / mA
U
Intensity / mA
U
Intensity / mA
U
Intensity / mA
U
Intensity / mA
U
A
B
C
D
E
F
H
G
Standard SampleStandard Sample
Standard Sample
Standard Sample
Standard Sample
Standard Sample
Standard Sample 80
60
40
20
0
200 250 300 350 400
200 250 300 350 400
200 250 300 350 400
200 250 300 350 400
40 30 20 10 0 30 20 10 10 8 6 4 2 10 8 6 4 2 6 4 2 Wavelength / nm Wavelength / nm Wavelength / nm Wavelength / nm 200 250 300 350 400
Wavelength / nm 200 250 300 350 400
Wavelength / nm 200 250 300 350 400
Wavelength / nm 12.5
10.0
7.5
5.0
2.5
Fig.1. (A)ChromatographicseparationofthethirteenantioxidantsstandardsbyDAD(20.5–218.25mol/l).1.gallicacid,2.catechin,3.caffeicacid,4.rutin,5.ferulicacid,
6.quercitrin,7.myricetin,8.fisetin,9.resveratrol,10.quercetin,11.kaempferol,12.chrysinand13.flavone;(B)Standardspectrumofgallicacid/sample:Cecropiaobtusa
hydroalcoholicextract2013;(C)Standardspectrumofcatechin/sample:Cecropiapalmatabutanolicextract2013;(D)Standardspectrumofcaffeicacid/sample:M.alliacea
butanolicextract2013;(E)Standardspectrumofrutin/sample:C.perrottettivar.angustifoliusethylacetateextract2014;(F)Standardspectrumofferulicacid/Sample:
B Cecropia obtusa
A Connarus perrottetti var. angustifolius
C Cecropia palmata
D Mansoa alliacea
10 0001000
100
10
1
Concentr
ation / (mg/kg)
Concentr
ation / (mg/kg)
Hydroethanolic Butanolic
Butanolic
Butanolic Butanolic
Eth
yl acetate
Eth
yl acetate
Eth
yl acetate
Hydroethanolic Hydroethanolic
2012 2013
Years of collecting - extracts
Years of collecting - extracts
Years of collecting - extracts
Years of collecting - extracts
2014
100
10
1
Concentr
ation / (mg/kg)
100
10
1
Concentr
ation / (mg/kg)
100
10
1 Hydroethanolic
2012
Hydroethanolic 2013
Hydroethanolic 2014 2012
Butanolic Hydroethanolic
2012 2012
Butanolic 2013
Butanolic Butanolic
Hydroethanolic
2012 2012 2013
Butanolic 2014 Ethyl acetate
2013 Butanolic
2013
Butanolic 2014
Gallic acid (+)- Catechin Caffeic acid Rutin Ferulic acid Quercitrin Resveratrol
Gallic acid (+)- Catechin Caffeic acid
Gallic acid (+)- Catechin Caffeic acid Ferulic acid Quercitrin Resveratrol
Gallic acid
Caffeic acid
Fig.2.PhenoliccompoundsfoundinthestudiedspeciesviaHPLC-DAD:(A)C.perrottettivar.angustifolius,(B)Cecropiaobtusa,(C)Cecropiapalmataand(D)M.alliacea; coefficientofvariation:28–30%overextractstriplicate.
approximately80%,allsevencompoundsdeterminedinthestudied plantswereencounteredinthisspecies.
Fig. 2 shows a decrease on the concentration of catechin betweencollectionsof2012and2014.Thisdecreasewasobserved forallextractsanalyzed,buttherewasnoclearcorrelationtothe concentration.
The highly polar phenolic compounds are not completely extracted with pure organic solvent. The addition of water to
ethanol increases thepolarity, facilitating theextraction ofthe polarconstituents (Stalikas,2007), i.e. gallic acid and catechin. Accordingly, the greatest amounts of these compounds were detected in the hydroethanolic extracts. Silveira et al. (2015)
Theuseofsolventswithlowerpolarities,suchasbutanoland ethylacetate,enabledtheidentificationoflesspolarconstituents. ThisobservationissupportedbyNaczkandShahidi(2006),who reportsthatthesolubilityofphenolic compoundsis affectedby thechemicalnatureoftheplantconstituentsandbythepolarity ofthesolventextractor.Therefore,variousorganicsolventsand aqueousmixtureswithalcoholsarereportedintheextractionof thesemetabolites(Ignatetal.,2013).
Caffeicandferulicacidwerepresentinhigherconcentrations inthesamplecollectedin2013.Rutinandquercitrin,ontheother hand,reachedhigherconcentrationsinplantscollectedin2014, whatissupportedbyFig.1(E)and(G),thatshowsthespectraused toidentifyrutinandquercitrininthespecies.
Therewasnosignificantvariationonresveratrolconcentration whencomparingbutanolicextractsofC.perrottettivar.angustifolius
collectedindifferentyears(2013and2014).Nonetheless,extracts usingethylacetateasasolventexhibitedadecreaseof approxi-mately34%onthisconcentrationfrom2013to2014.Thisisastrong evidenceoftheloweraffinitybetweenthissolventandresveratrol, whosepresenceintheextractissupportedbyFig.1(H).Fig.2(A) showsthatresveratrolwasnotdetectedinplantscollectedin2012.
Cecropiaobtusa
From2012to2014,asignificantincrease(92.2%)wasobserved ontheconcentrationofgallicacidinthehydroethanolicextractof thisspecies,asshowninFig.2(B).Thismaybearesultofsomestress sufferedbytheplant,since,asreportedinliterature,many environ-mentalfactorssuchasseasonality,temperature,wateravailability, ultraviolet radiation, nutrients and altitude may interfere with thebiosynthesisofsecondarymetabolites(Gobbo-NetoandLopes, 2007;Morais,2009).In ordertoendorsetheresultsconcerning theidentificationofgallic acid,thespectrummeasuredfor this specieswascomparedtoitsreferencespectrum.Thiscomparison isreportedinFig.1(B).
Catechin was themajor compound in C. obtusa, but it was detectedonlyinthebutanolicextractofplantscollectedin2013. Theseextractionconditionsalsodetectedcaffeicacidinthesample collectedin2014.
Besidespresentinglittlevarietyof antioxidantsin its consti-tution, C. obtusahasshown very irregularbehavior duringthe analyzedperiod.Thismayberelatedtothedefensestrategy of theplantagainsttheinfluenceofbioticand/orabioticfactors,as mentionedbyMorais(2009)andCoutinhoetal.(2010).
Cecropiapalmata
Regarding the studied compounds, C. palmata exhibited a greaterdiversitywhencomparedtotheotherspeciesofthesame genusanalyzed,asshowninFig.2(C).
Thehydroethanolicsolventextractedthehighestconcentration ofgallicacidonplantscollectedin2012.Thesimilaritybetween polarities(bothhigh)ofsolventandextractedcompoundjustifies thisresult.However,this conditiondidnotextractedanyother compoundofinterest.
ButanolicextractionofC.palmatacollectedin2013,ontheother hand,enabledtheseparationoflesspolarcompoundswhichhave notbeenidentifiedinthisplantsofar,i.e.,inthe hydroethano-licextract.Fig.1(F)showsthatferulicacidisalsopresentinthis species.Inaddition,thefollowingcompoundswereextracted: cate-chin,quercitrinandresveratrol.Thissolventwasalsoabletoextract caffeicacid,despiteitshigheraffinitywithethylacetate.
SimilarlytowhatwasobservedtoC.obtusa,catechinwasthe majorcompound,butwasonlydetectedonbutanolicextractsof thesamplecollectedin2013.Comparingbothspeciesinrelationto thecatechinconcentration,aminordifferenceof1.3%–higherfor
C.obtusa–isobserved.Fig.1(C)showsthespectrumemployedon theidentificationofcatechininC.palmata.
ThereareseveralstudiesregardingspeciesofCecropia genus reportedinliterature.Franck(1998)analyzedphenolicacidsand flavonoidsinextractsoftheleavesofC.hololeuca,C.pachystachaya
andC.glazioviiusingthin-layerchromatography.C.glazioviiwas alsostudiedusingliquidchromatographybyLuengas-Caicedoetal. (2007)who identified catechin,and byArend etal. (2011)and
Beringhs et al. (2015) who detected caffeic acid. These results supporttheonespresentedhereforthetwospeciesofCecropia
investigated.
Mansoaalliacea
Thisspeciesshowedthelowerdiversityofphenoliccompounds, asshowninFig.2(D).Incontrast,itpresentedthehighest concen-trationofcaffeicacid,detectedinthebutanolicextract,Fig.1(D). Consideringtheentireperiodofcollection,i.e.2012–2014,there wasadecreaseof87.9%intheconcentrationofsuchcompound. Similarlytotheotherplantsevaluatedinthisstudy,gallicacidwas detectedinM.alliacea,butonlyinsamplescollectedin2012.
Phytochemical studies involving the leaves of M. alliacea
revealedthepresenceofpolyphenolsandflavonoids(Ribeiroetal., 2009;Pateletal.,2013)andtheabsenceofcatechins(Ribeiroetal., 2009).Also, flavonoidslikeluteolinandapigeninweredetected intheflowers(Zoghbietal.,2009),andphenolsintheroot(Patel etal.,2013).Thechemicalcompositionofthisspeciesmaybe bet-terunderstooddue totheresultspresentedin thisstudy,since theyagreewiththeaforementionedworksandprovideadditional informationonthephenolicacidscomposition,suchascaffeicand gallic.
AccordingtotheNationalInstituteofMeteorology(INM,2015), theaveragerainfallduring theyears ofcollections (2012, 2013 and 2014) was416.7±80.4mmand thecoefficientof variation stayedaround20%.Thetemperaturesvariedbetweenaminimum of23.3±0.3◦Candamaximumof32.8±0.3◦C.Bechoetal.(2009)
showedthatcompoundswithhigherpolaritycanbeeliminated fromplantsbyleaching.Whereasthesamplesanalyzedwere col-lectedduringtherainyseason,thesedatasupportthebehavior observedconcerningtheperiodofcollection.
Althoughthechemicalcompositionofplantsislargely deter-mined by the genetic characteristics of the species, various environmentalfactors(Dinizetal.,2007)andtheemploymentof differentextractionmethods canchangeit (Naczkand Shahidi, 2004). For this reason, studies concerning these variables are paramountinordertoassurethedesiredconcentrationsofactive compounds.
AnalysisofinfusionsbyHPLC-DAD
InvestigatedcompoundsweredetectedonlyoninfusionsofC. perrottettivar.angustifoliusandC.obtusa.Consideringtheuseof thesespeciesintheformoftea,aconcentrationof0.81mg/100ml ofgallicacidwasquantifiedinsamplesofC.perrottettivar. angusti-foliuscollectedin2013and0.58mg/100mlinsamplescollected in 2014. Concentrations of catechin were considerably higher onthisspecies:7.9mg/100mlinsamplescollectedin2013and 3.5mg/100mlinsamplesfrom2014.SamplesofC.obtusacollected in2012and2013showednovariationontheconcentrationofgallic acid,whichwasof0.14mg/100ml.
Table3
Tandemmassspectrometry(MS/MS)parametersofinvestigatedphenoliccompounds.
Phenoliccompound Fragmentadorvoltage Quantificationtransitiona Confirmationtransitiona
1.Gallicacid 106 169.0>125.1(10) –
2.(+)-Catechin 134 289.1>245.1(10) 289.1>203.2(15)
3.Caffeicacid 106 179.0>135.1(10) –
4.Rutin 201 609.1>300.1(31) –
5.Ferulicacid 88 193.1>134.1(9) 193.1>178.1(7)
6.Quercitrin 164 447.1>301.1(17) –
aTheenergycollision(V)isgiveninbrackets.
pharmacologicaleffectsandespeciallyrecognizedfortheir antiox-idantactivity.
Confirmationoftheidentityofantioxidantcompoundsby UHPLC-ESI-MS/MS
Inordertoendorsetheresultsconcerningthephenolic consti-tutionofthemedicinalplantsstudied,dataobtainedbyHPLC-DAD andbyUHPLC-MS/MSwerecompared.
Negativechemical ionizationwas employed for allphenolic compoundsanalyzedand theretentiontimesrangedfrom0.39 to3.66min.Themassspectraldataobtainedaresummarizedin
Table3.
Morethan 60% of theresultsreportedin Fig.2 are in good agreement with the ones from analysis by liquid chromatog-raphy coupled to tandem mass spectrometry (LC-MS/MS). For samplescontainingcatechin and ferulic acid, thesecompounds weredetectedbyHPLC-DADandLC-MS/MS.
The results not supported by LC-MS/MS were: caffeic acid, rutinandquercitrinonsamplesofC.perrottettivar.angustifolius
collectedin2013,aswellasresveratrolforallperiodsassessed forthisspecies;gallic acidoninfusionandsamplesofC.obtusa
collectedin 2012and 2014; gallic acidonM.alliacea samples; gallic acid, quercitrin and resveratrol on C. palmata samples. Thisdisagreementisbelieved tobeduetothedifferenceinthe extractionmethods used,as allthese wereobtainedfrom cold maceration.
AccordingtoNaczkandShahidi(2006),theextractionof phe-noliccompoundsinplantsisinfluencedbyseveralfactors,suchas chemicalnatureofthespecies,extractionmethod,sampleparticle size,processing,amongstothers.Eachextractionmethodaffects theselectivityofthecompounds,especiallywhenitinvolvesthe studyofantioxidantcomponents,sincetheyaresensitivetothe actionoflight,oxygenandheat(AndreoandJorge,2006;Pereira andMeireles,2010).
Thereis nostandard or fully satisfactory procedure for the extractionofallphenoliccompoundsoraspecificclassofvegetable material(NaczkandShahidi,2004).Giventhevastnumberof stud-iesonthesubject,however,theextractionassistedbyultrasound provestobequiteeffectiveinisolatingpolyphenols(Luqueand Priego-Capote,2007;RoutrayandOrsat,2013).
AccordingtoDalPráetal.(2015),extractionassistedby ultra-soundinvolvestheformationofcavitationbubbles,whichassist therelease ofthevegetablecontent,increasingthemass trans-fer.In studieswithBrassicaoleracea, theextractionassisted by ultrasoundhadapositiveeffect,increasingtheefficiencyin approx-imately36.1±15.5%whencomparedtoconventionalextraction (maceration).
Somephenoliccompoundsareveryphotosensitive.Therefore, time-consumingextraction may result in degradation. Thereby techniquesinvolvingultrasoundhavebeenwidelyusedmainlyin ordertopreventthedegradationofresveratrol(Guamán-Balcázar etal.,2016).Resveratrolbelongstotheclassofstilbenes,which presenthighsensitivitytoexternalagentssuchasair,light and
oxidative enzymes.Accordingly, there are many foodadditives beingdevelopedaimingtheincreaseonsolubilityand photosta-bilityofthesecompounds(Silvaetal.,2014).
Studiesregardingthesolidphaseextractionofpolyphenolsin winedemonstrateda lowrecoveryrateforthegallicacid.Even tough,quantificationwasstillpossible,giventhelargeamountsof thiscompoundpresentinthismatrix(Villiersetal.,2004).Hence, whenoccurringinlowerconcentrations,asinsomeplantspecies, thedetectionmaynotbepossible,dependingontheextraction procedure.
AccordingtoJaquesetal.(2010),whostudiedthechangeon phenoliccompoundcompositionofblackberrypulpsduring stor-age,gallicacidsufferedthemajordegradation.Thisresultisrelated totheredoxreactionsresultingfromitshighlyhydroxylated struc-ture.
Furthermore, according toWaterhouse (2002), gallic acid is widelyusedasastandardintheFolin-Ciocalteumethodforthe determinationoftotalpolyphenols,becauseitisrelatively inex-pensiveand pureanditsdry formis verystable.However,the standardsolutionlosesabout5%ofitseffectivenessafteroneweek atroomtemperatureandthedecompositionisevenfasterwhen thesolutionisexposedtotheair.
Takingintoaccountallparametersinvolvingthedetermination ofthepolyphenols,whichwereheredemonstrated,alongwiththe numerousstudiesonthissubject,wecanaffirmthatboth analy-sisperformedinthisstudy,bydiodearraydetectionandbymass spectrometry,yieldedsatisfactoryresults.
Conclusions
One or more phenolic compounds, e.g. phenolic acids and flavonoids,arepresentinthechemicalconstitutionoftheextracts ofmedicinalplantsanalyzed.Amongtheinvestigatedcompounds, gallicandcaffeicacidweretheonlyantioxidantsfoundinallspecies whenanalyzingtheextractswithdiodearraydetection.InC. per-rottettivar.angustifolius,catechin,rutin,ferulicacid,quercitrinand resveratrolwerealsopresent.Amongthese,onlyrutin wasnot detectedinC.palmata.CatechinwasnotpresentonlyinM.alliacea, beingthemajorcompoundinallotherspecies.Theinfusionwith
C.perrottettivar.angustifoliusmayprovideimportantamountsof gallicacidandcatechin,supportingitsemploymentwithmedicinal purposes.
Whencomparing the results obtainedvia LC-MS/MS tothe onesobtainedbyHPLC-DAD,over 60%agreementwasreached. Ofallcompounds,onlyresveratrolcouldnotbedetectedbymass spectrometry.However,thisresult,aswellasthefactthatother componentswerealsonotdetectedinsomeperiodsofcollectionor eveninsomeplantspecies,maybeaconsequenceoftheextraction methodused.
Authors’contributions
FBPcarriedouttheresearch.Theseresultsformapartofher Masterdegreework.CBD,DM,andVDPsupportedtheultrasound extraction.HFperformedtheLC-MSanalysis.LMC,CVandMBR cre-atedtheProjectandwereresponsibleforarrangingthescholarship andfinancialsupport.MBRwastheadvisorofFBP.OLcollectedthe plantsandperformedthevoucher.FOLperformedthe chromato-graphicvalidation.Alltheauthorshavereadthefinalmanuscript andapproveditssubmission.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgement
ToCNPqforfinancialsupport.
References
Ajila,C.M.,Brar,S.K.,Verma,M.,Tyagi,R.D.,Godbout,S.,Valéro,J.R.,2011.Analysis ofpolyphenols:recenttrends.Crit.Rev.Biotechnol.31,227–249.
Alvim,N.A.T.,Ferreira,M.A.,Cabral,I.A.,Almeida,J.de,2006.Ousodeplantas medic-inaiscomorecursoterapêutico:dasinfluênciasdaformac¸ãoprofissionalàs implicac¸õeséticaselegaisdesuaaplicabilidadecomoextensãodapráticade cuidarrealizadapelaenfermeira.Rev.Latino-Am.Enfermagem.14,1–9. Ananias,D.dosS.,Souza,E.B.,Souza,P.F.S.,Souza,M.deL.,Vitorino,M.I.,Teixeira,
G.M.,Ferreira,D.daS.B.,2010.Climatologiadaestruturaverticaldaatmosfera emNovembroparaBelém-PA.Rev.Bras.Meteorol.25,218–226.
Andreo,D.,Jorge,N.,2006.Antioxidantesnaturais:técnicasdeextrac¸ão.B.doCEPPA 24,319–336.
Arend,D.P.,Santos,T.C.,Sonaglio,D.,Santos,A.L.G.,Reginatto,F.H.,Campo,A.M., 2011.Experimentaldesignasatooltoevaluatechlorogenicandcaffeicacids extractedfromCecropiaglazioviiSneth.J.Pharm.Biomed.Anal.54,58–66. Becho,J.R.M.,Machado,H.,Guerra,deM.,2009.Rutina–estrutura,metabolismoe
potencialfarmacológico.Rev.Int.Est.Exp.1,21–25.
Beringhs,A.O.,Dalmina,M.,Crecznski-Pasa,T.B.,Sonaglio,D.,2015.Responsesurface methodologyIV–optimaldesignappliedtotheperformanceimprovementofan RP-HPLC-UVmethodforthequantificationofphenolicacidsinCecropiaglaziovii products.Rev.Bras.Farmacogn.25,513–521.
Campelo,P.M.S.,2006.Medicinalplantsanditsextracts:thenecessityofcontinuous studies.Rev.Est.Biol.Amb.Divers.28,1–2.
Coelho-Ferreira,M.,2009.Medicinalknowledgeandplantutilizationinna Amazo-niancoastalcommunityofMaruda,ParáState(Brazil).J.Ethnopharmacol.126, 159–175.
Costa,G.M., Ortmann,C.F.,Schenkel, E.P.,Reginatto, F.H.,2011. AnHPLC-DAD methodtoquantificationofmainphenoliccompoundsfromleavesofCecropia species.J.Braz.Chem.Soc.22,1096–1102.
Coutinho,I.D.,Kataoka,V.M.F.,Honda,N.K.,Coelho,R.G.,Vieira,M.C.,Cardoso,C.A.L., 2010.Influênciadavariac¸ãosazonalnosteoresdeflavonóideseatividade antiox-idantedasfolhasdeCampomanesiaadamantium(Cambess.)O.Berg,Myrtaceae. Rev.Bras.Farmacogn.20,322–327.
DalPrá,V.,Dolwitsch,C.B.,Lima,F.O.,Carvalho,deL.M.,Viana,C.,Nacimento,doP.C., Rosa,daM.B.,2015.Ultrasound-assistedextractionandbiologicalactivitiesof extractsofBrassicaoleraceavar.capitata.FoodTechnol.Biotechnol.53,102–109. Diniz,A.C.B.,Astarita,L.V.,Santaré,E.R.,2007.Alterac¸ãodosmetabólitossecundários emplantasdeHypericumperforatumL.(Hypericaceae)submetidasàsecageme aocongelamento.ActaBot.Bras.21,443–450.
Faccin,H.,Viana,C.,Nascimento,P.C.do,Bohrer,D.,Carvalho,L.M.de,2016.Study ofionsuppressionforphenoliccompoundsinmedicinalplantextractsusing liquidchromatography–electrospraytandemmassspectrometry.J.Chromatogr. A1427,111–124.
Franck,U., 1998. Phytochemischeund pharmakologischeUntersuchungen der kardiovaskulärenWirkprinzipienvonCecropiahololeucaMiq.,Cercropia pachys-tachya Tréc., CecropiaglazioviiSneth.,Musanga cecropioidesR. Brown und CrataegusL.monogyna,oxyacantha.HerdeckeGCA-Verl,Alemanha. Freitas,J.C.,Fernandes,M.E.B.,2006.Usodeplantasmedicinaispelacomunidade
deEnfarrusca,Braganc¸a.Pará.Bol.Mus.Para.EmílioGoeldi,sér.Ciênc.Nat.1, 11–26.
Gobbo-Neto,G.L.,Lopes,N.P.,2007.Plantasmedicinais:fatoresdeinfluênciano conteúdodemetabólitossecundários.Quim.Nova30,374–381.
Guamán-Balcázar,M.C.,Setyaningsih,W.,Palma,M.,Barroso,C.G.,2016. Ultrasound-assistedextractionofresveratrolfromfunctionalfoods:cookiesandjams.Appl. Acoust.103,207–213.
Ignat,I.,Volf,I.,Popa,V.I.,2013.Analyticalmethodsofphenoliccompounds.In: Ramawat,K.G.,Mérillon,J.-M.(Eds.),NaturalProducts–Phytochemistry,Botany andMetabolismofAlkaloids,PhenolicsandTerpenes.Springer, Berlin,pp. 2061–2092.
IPEA,2010.SustentabilidadeambientalnoBrasil:biodiversidade,economiae bem-estarhumano.Institutode PesquisaEconômicaAplicada,http://www.ipea. gov.br/portal/images/stories/PDFs/livros/livros/livro07
sustentabilidadeambienta.pdf/(accessed02.04.16).
INM, 2015. Instituto Nacional de Meteorologia, http://www.inmet.gov.br/sim/ gera graficos.php/(accessed15.11.15).
INM, 2016.Normalizac¸ãoeQualidade Industrial: Orientac¸õessobre Validac¸ão de Métodos Analíticos –DOQ-CGCRE-008. Instituto Nacional de Metrolo-gia, http://www.inmetro.gov.br/Sidoq/Arquivos/CGCRE/DOQ/DOQ-CGCRE-8
02.pdf/(acessed13.03.16).
Jaques,A.C.,Pertuzatti,P.B.,Barcia,M.T.,Zambiazi,R.C.,Chim,J.F.,2010.Estabilidade decompostosbioativosempolpacongeladadeamora-preta(Rubusfruticosus) cv.TUPY.Quim.Nova33,1720–1725.
Lahlou,M.,2013.Thesuccessofnaturalproductsindrugdiscovery.Pharmacol. Pharm.4,17–31.
Lameira,O.A.,Paiva,J.S.,OliveiraE.C.P.de,Pinto,J.E.B.P.,2004.Fenologiaeanálise fitoquímicadeplantasmedicinaisdeocorrêncianaAmazônia.Hortic.Bras.22, 1–6.
Luengas-Caicedo,P.E.,deOliveira,A.B.,Brago,F.C.,Brandão,G.C.,2007.Seasonal andintraspecificvariationofflavonoidsandproanthocyanidinsinCecropia glazioviSneth.leaves fromnativeand cultivated specimens.J. Biosci. 62, 701–709.
LuqueM.D.,deC.,Priego-Capote,F.,2007.Ultrasound-assistedpreparationofliquid samples.Talanta72,321–334.
Morais,L.,2009.Influênciadosfatoresabióticosnacomposic¸ãoquímicadosóleos essenciais.Hortic.Bras.27,4050–4062.
MinistériodaSaúde(MS),2006.PolíticaNacionaldePlantasMedicinaise Medica-mentos Fitoterápicos, http://bvsms.saude.gov.br/bvs/publicacoes/politica
nacionalfitoterapicos.pdf/(acessed02.10.15).
Naczk,M.,Shahidi,F.,2004.Extractionandanalysisofphenolicsinfood.J. Chro-matogr.A1054,95–111.
Naczk,M.,Shahidi,F.,2006.Phenolicsincereals,fruitsandvegetables:occurrence, extractionandanalysis.J.Pharm.Biomed.Anal.41,1523–1542.
Paracampo, N.E.N.P., 2011. Connarus perrottetii var. angustifolius Radlk. (Con-naraceae): tradicionalmenteutilizada como barbatimãonoPará. Embrapa AmazôniaOriental,Belém,PA,Brasil,pp.2011.
Patel, I., Sipai,S., Rathod, D., Shrimali, G., Patel, A., Rami, E., 2013. Phyto-chemical studies on Mansoa alliacea (Lam.). Int. J. Adv. Pharm. Res. 4, 1823–1828.
Pereira, C., Meireles, M.A.A., 2010. Supercritical fluid extraction of bioactive compounds:fundamentals,applicationsandeconomicperspectives.Food Bio-processTechnol.3,340–372.
Pérez,D.,2002.EtnobotánicamedicinalybiocidasparaMalariaenlaregiónUcayali. Fol.Amazon13,87–101.
Rezende,H.A.,Cocco,M.I.M.,2002.Utilizac¸ãodefitoterapianocotidianodeuma populac¸ãorural.Rev.Esc.Enferm.USP36,282–288.
Ribani,M.,Bottoli,C.B.G.,Collins,C.H.,Jardim,C.S.F.,2004.Validac¸ãoemmétodos cromatográficoseeletroforéticos.Quim.Nova27,771–780.
Ribeiro,C.M.,Souza,K.G.daS.,Ribeiro,A.B.R.R.,Mendonc¸a,L.C.V.,Barbosa,W.L.R., 2009.Avaliac¸ãodaatividadeantimicrobianadeplantasutilizadasnamedicina populardaAmazônia.Infarma21,45–49.
Routray,W.,Orsat,V.,2013. Preparativeextractionandseparationofphenolic compounds.In:Ramawat,K.G.,Mérillon,J.-M.(Eds.),NaturalProducts– Phy-tochemistry,BotanyandMetabolismofAlkaloids,PhenolicsandTerpenes. Springer,Berlin,pp.2013–2046.
Schmitz,W.,Saito,A.Y.,Saridakis,D.H.O.,2005.Ocháverdeesuasac¸õescomo quimioprotetor.SeminaCienc.Biol.Saude26,119–130.
Silva,F.,Gallardo,A.F.E.,Nerín,C.,Domingues,F.C.,2014.Strategiestoimprovethe solubilityandstabilityofstilbeneantioxidants:Acomparativestudybetween cyclodextrinsandbileacids.FoodChem.145,115–125.
Silveira, da G.D., Motta, M.J., Müller,L.S., Lameira, O., Athayde, M.L., Pianna, M., Rosa, da M.B., Viana, C., Carvalho, de L.M., 2015. Determination of phenolic antioxidants in Amazonian medicinal plants by HPLC with pulsed amperometric detection. J. Liq. Chromatogr. Relat. Technol. 38, 1259–1266.
Stalikas,C.D.,2007.Extraction,separation,anddetectionmethodsforphenolicacids andflavonoids.J.Sep.Sci.30,3268–3295.
Veiga,V.F.J.,Pinto,A.C.,Maciel,M.A.M.,2005.Plantasmedicinais:curasegura?Quim. Nova28,519–528.
Villiers,F.L.,Lynen,A.,Crouch,S.P.,2004.Developmentofasolid-phaseextraction procedureforthesimultaneousdeterminationofpolyphenols,organicacidsand sugarsinwine.Chromatographia59,403–409.
Waterhouse,A.L.,2002.Determinationoftotalphenolics.Curr.Protoc.FoodAnal. Chem.6,1–8.