Advantages of the Alpha-lipoic Acid Association with Chlorpromazine
in a Model of Schizophrenia Induced by Ketamine in Rats: Behavioral
and Oxidative Stress evidences
Luis Rafael Leite Sampaio,a,bFrancisco Maurı´cio Sales Cysne Filho,cJamily Cunha de Almeida,cDanilo dos Santos Diniz,a Cla´udio Felipe Vasconcelos Patrocı´nio,dCaren Na´dia Soares de Sousa,aManoel Cla´udio Azevedo Patrocı´nio,d
Danielle Maceˆdoaand Silvaˆnia Maria Mendes Vasconcelosa*
aNeuropsychopharmacology Laboratory, Department of Physiology and Pharmacology, Drug Research and Development Center, Faculty
of Medicine, Federal University of Ceara´ Fortaleza, CE, Brazil
bCenter of Biological and Health Sciences, Regional University of Cariri Cariri, CE, Brazil
cUniversity of Fortaleza Fortaleza, CE, Brazil
d
School of Medicine, University Center Christus-Unichristus Fortaleza, CE, Brazil
Abstract—Schizophrenia is a chronic mental disorder reported to compromise about 1% of the world’s popula-tion. Although its pathophysiological process is not completely elucidated, evidence showing the presence of an oxidative imbalance has been increasingly highlighted in the literature. Thus, the use of antioxidant sub-stances may be of importance for schizophrenia treatment. The objective of this study was to evaluate the behav-ioral and oxidative alterations by the combination of chlorpromazine (CP) and alpha-lipoic acid (ALA), a potent antioxidant, in the ketamine (KET) model of schizophrenia in rats. Male Wistar rats (200–300 g) were treated for 10 days with saline, CP or ALA alone or in combination with CP previous to KET and the behavioral (open field, Y-maze and PPI tests) and oxidative tests were performed on the last day of treatment. The results showed that KET induced hyperlocomotion, impaired working memory and decreased PPI. CP alone or in combination with ALA prevented KET-induced behavioral effects. In addition, the administration of KET decreased GSH and increased nitrite, lipid peroxidation and myeloperoxidase activity. CP alone or combined with ALA prevented the oxidative alterations induced by KET. In conclusion, the treatment with KET in rats induced behavioral impair-ments accompanied by hippocampal oxidative alterations, possibly related to NMDA receptors hypofunction. Besides that, CP alone or combined with ALA prevented these effects, showing a beneficial activity as antipsy-chotic agents.Ó2018 IBRO. Published by Elsevier Ltd. All rights reserved.
Key words: schizophrenia, Hippocampus, oxidative stress, ketamine, chlorpromazine, alpha-lipoic acid.
INTRODUCTION
Schizophrenia is a severe and disabling chronic mental disorder reported to compromise about 1% of the world’s population (Messias et al., 2007). Although the pathophysiology of schizophrenia is not completely elucidated, accumulating evidence points toward the par-ticipation of oxidative imbalance in the neurobiology of
this mental disorder (Gonzalez-liencres et al., 2014). In this regard, previous studies have shown oxidative alterations through the quantification of biomarkers such as, myeloperoxidase (MPO), malondialdehyde (MDA) and antioxidants, such as reduced glutathione (GSH), the major endogenous antioxidant, in the post-mortem brain samples and plasma of patients with schizophrenia (Al-asmari and Khan, 2014; Leza et al., 2015). With the excess of free radicals and/or the reduction of antioxidant compounds, the aggression to the DNA, lipids and cellular proteins is imminent, compromising neuronal functions and leading to cell death, contributing, thus, to the progression of the disease (Copoglu et al., 2015).
Animal models are important tools for the study of mental disorders. One of the most widely used animal model of schizophrenia is based on the repeated administration of ketamine (KET), a
N-methyl-D-https://doi.org/10.1016/j.neuroscience.2018.01.008
0306-4522/Ó2018 IBRO. Published by Elsevier Ltd. All rights reserved.
*Corresponding author. Address: Department of Physiology and Pharmacology, Federal University of Ceara´, Cel. Nunes de Melo street, 1127, 60431-270 Fortaleza, CE, Brazil.
E-mail addresses: silvania@pq.cnpq.br, silvania_vasconcelos@ yahoo.com.br(S. M. M. Vasconcelos).
Abbreviations: ALA, alpha-lipoic acid; CP, chlorpromazine; GSH,
glutathione; HClO, Hypochlorous acid; HTAB,
hexadecyltrimethylammonium bromide; KET, ketamine; MDA, malondialdehyde; MPO, myeloperoxidase; MPO, myeloperoxidase; NMDAR, N-methyl-D-aspartate receptor; NO, nitric oxide; NOS, nitric oxide synthase; PCP, phencyclidine; RM, repeated measures.
N
EUROSCIENCE
RESEARCH ARTICLE
L. R. L. Sampaio et al. / Neuroscience 373 (2018) 72–81
aspartate receptor (NMDAR) antagonist (Monte et al., 2013). In fact, hypofunction of NMDARs is a core patho-physiological alteration observed in schizophrenia (Coyle, 2012). The repeated administration of KET to rodents mimics positive, negative and cognitive symptoms of schizophrenia, accompanied by brain oxidative and inflammatory changes (Arau´jo et al., 2017). In this regard, a recent study showed that KET repeated administration was related to the following brain alterations: i) increased MPO activity and lipid peroxidation, ii) decreased GSH levels in the prefrontal, hippocampus and striatum, and iii) increased hippocampal levels of interleukin (IL)-4 and IL-6 (Arau´jo et al., 2017).
Considering the oxidative imbalance observed in schizophrenia patients, the use of antipsychotics, in a greater extension of the typical and smaller of the atypical ones, has been linked to oxidative alterations (Miljevic et al., 2010; Smaga et al., 2015). Furthermore, antipsychotic drugs are able to change the oxidative sta-tus in drug-naı¨ve animals. A study demonstrated that chronic administration of haloperidol decreased suide dismutase and catalase activity and raised lipid perox-idation, while another study demonstrated that lipid peroxidation was reduced and protein peroxidation was increased after haloperidol and clozapine treatment (Pillai et al., 2007; Smaga et al., 2015). Long-term treat-ment with the atypical antipsychotics chlorpromazine, ziprasidone and risperidone can raise the lipid peroxida-tion (Pillai et al., 2007; Smaga et al., 2015). Despite these studies, the result of the use of antipsychotics on oxidative parameters remains inconclusive (Smaga et al., 2015).
The use of antioxidant substances in the management of individuals with schizophrenia has been proposed, as means of reducing oxidative damage, whether induced by the use of antipsychotics or not. Numerous clinical and preclinical studies have demonstrated positive effects by the use of antioxidants as neuroprotective agents in schizophrenia (Mas et al., 2012; Reddy and Reddy, 2015; Magalha˜es et al., 2016).
Alpha-lipoic acid (ALA) is an endogenous disulfide synthesized in mitochondria, playing an important role in the energy metabolism as a cofactor of enzymatic complexes. The presence of ALA in dietary sources derived from animals and vegetables is very low (Do¨rsam and Fahrer, 2016). The oral administra-tion of ALA has a potent antioxidant activity, which occurs by the extinction of reactive oxygen species, regeneration of endogenous antioxidants and chelation of metal ions and repair of oxidative damaged proteins (Vidovic´ et al., 2014; Vasconcelos et al., 2015).
The ability of ALA to cross the blood–brain barrier and exert its antioxidant action, acting in a similar way to glutathione, makes this antioxidant a promising therapeutic alternative for schizophrenia (Arroll et al., 2014).
Thus, the objective of this study was to evaluate the behavioral and oxidative alterations induced by the combination of CP and ALA in rats submitted to the ketamine-induced model of schizophrenia.
EXPERIMENTAL PROCEDURES
Animals
Male Wistar rats, weighing 200–300 g, kept in a temperature controlled room (23 ± 1°C) with a cycle of 12-h light/12-h dark and food and waterad libitum were used. The experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals from the National Research Council. All protocols were approved by the Ethics Committee of the Federal University of Ceara´ (No. 92/2009).
Drugs
Ketamine hydrochloride (KET – KetalarÒ, Parke-Davis Lab, Brazil) at a dose of 10 mg/kg and chlorpromazine (CP – LongactilÒ, Crista´lia Lab, Brazil) at the doses of 1 and 5 mg/kg were diluted in 0.9% saline solution and administered intraperitoneally in 10 ml/kg body weight. Alpha-lipoic acid (ALA) was administered by gavage (Sigma–Aldrich, St. Louis, USA) at a dose of 100 mg/kg and dissolved in carboxymethylcellulose 5% (CMC). The doses of KET and ALA were based on previous studies (Arruda et al., 2008; Maceˆdo et al., 2012).
Experimental protocol
A total of 130 animals were used. For the treatment protocols the animals were randomly divided into nine groups with the number of animals in each group (N) shown in parenthesis: control group- received saline solution 0.9% (N= 14); KET group- received KET 10 mg/kg (N= 15); ALA group- received ALA 100 mg/kg (N= 16); CP1 group- received CP 1 mg/kg (N= 13); CP5 group- received CP 5 mg/kg (N= 19); CP1 + KET received CP 1 mg/kg 30-min before KET (N= 11); C P5 + KET received CP 5 mg/kg 30-min before KET (N= 15); ALA + CP1+KET received ALA 30-min before CP 1 mg/kg followed by KET, with a 30-min interval between CP and KET (N= 10); ALA + CP5+K ET received ALA 30-min before CP 5 followed by KET, with a 30-min interval between CP and KET (N= 17). Each group received treatment for 10 days.
After treatment, each group was divided into two subgroups. Subgroup 1 was submitted to PPI test, while subgroup 2 was submitted to the evaluation of Y maze and open field tests in this order. After this subdivision, the number of animals allocated per group varied from 5–10, as specified in the legend of each figure. The behavioral tests were performed on the last day of treatment immediately after the drug applications. The rats were then euthanized by decapitation and the hippocampus dissected, readily frozen and stored at -70 °C until the oxidative stress tests were performed (Fig. 1).
BEHAVIORAL TESTS
Open field test
into four equal parts, situated in a room with red light. After careful placement in the center of the set, the ani-mals were submitted to the habituation period, allowed to freely explore the scene for 1-min. The evaluated parameter was the number of squares crossed for a per-iod of 5-min.
Y-maze test
The Y-maze test was performed in order to evaluate the working memory performance. This maze consists of three identical arms (40625 cm), each converging at an equal angle. Each animal was placed at the end of one arm and left to explore the environment for 8-min. The sequence of the arms in which the animals entered was then registered and the information analyzed in order to determine the number of entries in each arm without repetition. A sequence was considered correct if the animal visited a new arm and did not return to the previously visited arm. Thus, the percentage of correct alternations was determined by the ratio between the correct alternations (n) and the number of visits performed during the observation period (n 2), multiplied by 100 (Yamada et al., 1996; Dall’Igna et al., 2007).
Prepulse inhibition of the startle reflex (PPI)
For this test, the animals were subjected to a series of 10 pulses after acclimatization to the habituation period with background noise (pulse alone [120 dB], 50 ms duration). The assay consisted of 74 pseudo-randomly trials grouped into seven different categories (with 20-s interval): 20 executions of pulse-alone (P, 120 dB, 50 ms duration), 8 executions of each prepulse alone (PP, 70, 75 or 80 dB, 3000-Hz frequency, 20-ms duration) and 10 executions of each prepulse + pulse (PP + P, with 50-ms interval). Mean amplitude of startle reflex to P and PP + P was determined for each animal. The percentage of amplitude reduction was determined for
each subject according to the following formula: %PPI = 100 [10 0(PP/P)] (Levin et al., 2011). Determination of oxidative stress parameters
Reduced glutathione (GSH). This test is based on the reaction of Ellman’s reagent (DTNB) with thiol groups. Brain areas were diluted in 0.02 M EDTA buffer (10% w/v) and
mixed with a solution of
trichloroacetic acid 50%. The samples were centrifuged (3000 rp m/15 min) and the supernatant collected and mixed with 0.4 M tris– HCl buffer, pH 8.9, and 0.01 M DTNB. GSH concentration, ng of GSH/g wet tissue, was determined by spectrophotometry at 412 nm (Sedlak and Lindsay, 1968).
Nitrite determination. To evaluate the effects of KET as well as of the drug treatments on nitric oxide (NO), the hippocampal levels of nitrite were determined by Griess reaction (Green and Goldman, 1981; Radenovic and Selakovic, 2005). The homogenates were centrifuged (800g/10 min), 100ll of the supernatant collected, added to 100lL of the Griess reagent (sulfanilamide 1 %/N-(1-naphthyl)ethylenediamine hydrochloride 0.1% / phosphoric acid 5% / distilled water, 1:1:1:1) and incu-bated at room temperature for 10 min. The preparation of the standard curve was elaborated under the same conditions, using several different concentrations of NaNO2 (0.75–100 mM). Blank was prepared by mixing 100ll of the Griess reagent with 100ll of the buffer used for the homogenate. The absorbance of the samples was determined in microplate reader at 560 nm and the nitrite concentration expressed in nM nitrite/g wet tissue.
Measurement of lipid peroxidation. The lipid peroxidation was analyzed by determining the concentration of MDA, a thiobarbituric acid reactive substance in hippocampal homogenates (Huong et al., 1998). Samples were added to a free radical forming cat-alyst system (0.01 mM FeSO4 and 0.1 mM ascorbic acid). The reaction was ended by the addition of trichlor-oacetic acid 10%. Afterward, samples were centrifuged (4°C, 14,000 rpm, 15-min), the supernatant removed, added thiobarbituric acid 0.8% and then, put in water bath for 30-min. After cooling, the absorbance was measured at 535 nm. Lipid peroxidation was expressed in lg of MDA/mg wet tissue.
Evaluation of myeloperoxidase (MPO) activity. The
homogenates were made using a solution of
hexadecyltrimethylammonium bromide (HTAB) 0.5% in 50 mM phosphate buffer pH 6.0 (1 mL/50 mg tissue) and centrifuged at 4000 rpm for 15-min at 4°C. Phosphate buffer (50 mM, pH 6) containing 0.167 mg/ml
h-dianisidine dihydrochloride and hydrogen peroxide 0.0005% was added to 30ll of the supernatant. The samples were analyzed by spectrophotometry (470 nm), at time 0 and 3-min (Bradley et al., 1982).
Statistical analysis
All analyses were performed using GraphPad Prism
software for Windows (San Diego, CA, USA).
Homoscedasticity was verified through Bartlett’s test. D’Agostino-Pearson omnibus normality test was performed to verify normal distribution. For parametric data, i.e., locomotor activity in the open field test and oxidative parameters, one-way ANOVA with Tukey post hoc test were performed. Y-maze data was evaluated by the nonparametric Kruskal–Wallis test followed by Dunn’s multiple comparisons test. In the case of PPI test, repeated measures (RM) two-way ANOVA with Bonferroni post hoc test was used. For two-way ANOVA, the within-subject factor was ‘‘PP intensities” (PP 70, 75 and 80) while the between-subject factor was ‘‘treatment groups”. The results were considered significant atp0.05 and are present in the figures as mean ± SEM.
RESULTS
Behavioral tests
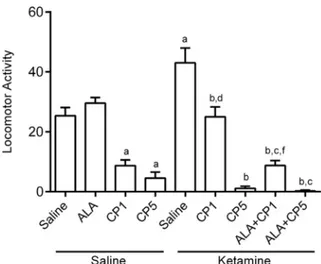
Open field test. One-way ANOVA revealed that the treatment groups presented significant alterations in locomotor activity [F(8,56) = 35.33, p< 0.0001]. Tukey post hoc test showed that the locomotor activity was decreased in rats administered CP1 alone (p< 0.01) and CP5 (p< 0.001) when compared to control group (Fig. 2). KET repeated administration increased the
spontaneous locomotor activity in relation to control group (p< 0.01). The effect of KET was prevented by the pretreatment with CP at both doses [CP1 + KET (p < 0.05) or CP5 + KET (p< 0.001)] or by the combination of ALA and CP [ALA + CP1+KET (p< 0.001) or ALA + CP5 + KET (p< 0.01)]. However,
ALA + CP+KET presented decreased locomotor
activity when compared to ALA group.
Y-maze. Kruskal–Wallis test showed that the treatment with the drugs caused significant differences in the percentage of correct alternations in the Y-maze test (H(9) = 54.31, p< 0.0001). Dunn’s post hoc test revealed that the percentage of correct alternations in animals treated with CP5 (p< 0.001), KET (p< 0.05), CP5 + KET (p< 0.001) or ALA + CP5 + KET (p< 0.001) was decreased when compared to control group (Fig. 3). Only the association of CP at the lower dose (A LA + CP1 + KET) prevented the decrease in the percentage of correct alternations induced by KET (p< 0.001).
Prepulse inhibition of the startle reflex (PPI). The evaluation of PPI by two-way ANOVA revealed significant main effects of ‘‘PP intensities” [F(2,165) = 23.20,p< 0.0001] and ‘‘treatment groups” [F(8,165) = 21.49,p< 0.0001] without significant interaction between factors. The results showed that the administration of KET decreased PPI on PP70 (p< 0.001), PP75 (p< 0.001) and PP80 (p< 0.001) when compared to control group. This reduction in PPI induced by KET was prevented by the pretreatment with CP1 or CP5 [PP70 (CP1 + KET:p < 0.001; CP5 + KET: p< 0.001); PP75 (CP1 + KET: p< 0.01; CP5 + KET: p< 0.001); PP80 (CP1 + KET: p< 0.001; CP5 + KET: p< 0.001)] or by the combination ALA + CP at both doses [PP70 (ALA + C
Fig. 2. Effects on locomotor activity by CP (1 or 5 mg/kg) and ALA (100 mg/kg), alone or combined, in an animal model of schizophrenia induced by KET (10 mg/kg) in rats. Each bar represents mean ± SEM ofn= 5–10 animals/group. a, b, c, d and fP< 0.05 compared with saline (control group), KET, ALA, CP1, CP1 + KET, respectively, according to one-way ANOVA followed by Tukey as post hoc test. Abbreviations: ALA – lipoic acid; CP – chlorpromazine; KET – ketamine.
P1+KET: p< 0.001; ALA + CP5 + KET: p< 0.001); PP75 (ALA + CP1 + KET:p< 0.001; ALA + CP5 + K ET: p< 0.001); PP80 (ALA + CP1 + KET: p< 0.001; ALA + CP5 + KET: p< 0.001)]. On PP75 the combination ALA + CP1 + KET potentiated the effects of CP1 + KET (p< 0.05) (Fig. 4).
Determination of oxidative stress parameters
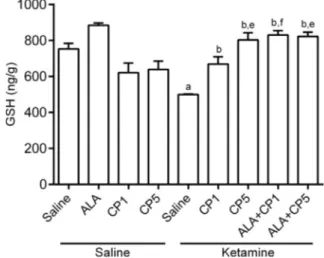
Reduced glutathione (GSH). One-way ANOVA conducted to compare the effect of the drugs on the levels of GSH revealed significant alterations in this endogenous antioxidant among the treatment groups [F( 8,62) = 13.56, p< 0.0001]. In this regard, decreased levels of GSH were observed in rats submitted to repeated administration of KET when compared to the control group (p< 0.001). This decrease was prevented by the pretreatment with CP [CP1 + KET (p< 0.05) or CP5 + KET (p< 0.001)] or by the combination ALA + CP [ALA + CP1 + KET (p< 0.001) or ALA + CP5 + KET (p< 0.001)]. In the presence of ALA, it was observed a potentiation of CP effects at the lower dose on hippocampal GSH levels when compared to CP1 + KET group (p< 0.05) (Fig. 5).
Nitrite determination. One-way ANOVA revealed significant alterations in nitrite levels among the treatment groups [F(8,58) = 40.70, p< 0.0001]. Nitrite levels were increased after the repeated administration of KET in comparison with the control group (p< 0.001). Pretreatment with CP5 + KET (p< 0.01) or with the combinations ALA + CP1 + KET (p< 0.001) or ALA + CP5 + KET (p< 0.001) prevented this effect (Fig. 6).
In the presence of ALA, there was an augmentation of CP effects at both doses, ALA + CP1 + KET (p< 0.01) or ALA + CP5 + KET (p< 0.01), when compared to
CP1 + KET and CP5 + KET groups, respectively (Fig. 6).
Measurement of lipid peroxidation. Significant alterations in lipid peroxidation were observed among the treatment groups (one-way ANOVA: [F(8,60) = 22.7 7, p< 0.0001]. Ketamine administration increased hippocampal MDA levels when compared to control group (p< 0.001). Pretreatment with CP alone, only at the higher dose CP5 + KET (p< 0.001), or combined with ALA [ALA + CP1 + KET (p< 0.001) or ALA + C
Fig. 4.Effects on the percentage of prepulse inhibition in PP70, PP75 ou PP80 by CP (1 or 5 mg/kg) and ALA (100 mg/kg), alone or combined, in an animal model of schizophrenia induced by KET (10 mg/kg) in rats. Each bar represents mean ± SEM of n= 5–10 animals/group. a, b and fP< 0.05 compared with saline (control group), KET, CP1 + KET, respectively, according to repeated mea-sures two-way ANOVA followed by Bonferroni as post hoc test. Abbreviations: ALA – lipoic acid; CP – chlorpromazine; KET – ketamine.
Fig. 5. Effects on hippocampal reduced glutathione (GSH) concen-tration by CP (1 or 5 mg/kg) and ALA (100 mg/kg), alone or combined, in an animal model of schizophrenia induced by KET (10 mg/kg) in rats. Each bar represents mean ± SEM ofn= 6–10 animals/group. a, b, e and fP< 0.05 compared with saline (control group), KET, CP5, CP1 + KET, respectively, according to one-way ANOVA followed by Tukey as post hoc test. Abbreviations: ALA – lipoic acid; CP – chlorpromazine; KET – ketamine; GSH – reduced glutathione.
Fig. 6.Effects on hippocampal nitrite concentration by CP (1 or 5 mg/ kg) and ALA (100 mg/kg), alone or combined, in an animal model of schizophrenia induced by KET (10 mg/kg) in rats. Each bar repre-sents mean ± SEM ofn= 6–10 animals/group. a, b, c, d, e, f and g
P5 + KET (p< 0.001)], prevented this increase observed with KET alone (Fig. 7).
On the other hand, the combination ALA + CP, only at the lower dose [ALA + CP1 + KET (p< 0.001)], accentuated the decrease in hippocampal MDA concentration observed in CP1 + KET group (Fig. 7).
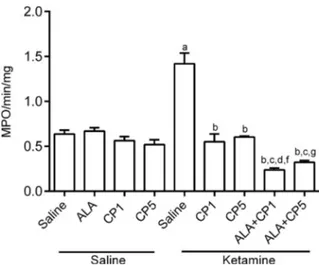
Evaluation of MPO activity. We also observed significant alterations among treatment groups in relation to MPO activity (one-way ANOVA: [F(8,46) = 3 9.41, p< 0.0001]). An increase in hippocampal MPO activity was observed with KET in relation to the control group (p< 0.001). This increase was prevented in the groups pretreated with CP [CP1 + KET (p< 0.001) or CP5 + KET (p< 0.001)] or with the combination of ALA + CP [ALA + CP1 + KET (p< 0.001) or ALA + CP5 + KET (p< 0.001)] when compared to KET group (Fig. 8).
In the combination groups ALA potentiated the effects of CP1 and CP5 [ALA + CP1 + KET (p< 0.01) or ALA + CP5 + KET (p< 0.05)] when compared to CP1 + KET and CP5 + KET groups, respectively (Fig. 8).
DISCUSSION
The results obtained in the present study replicate previous behavioral findings regarding KET as an animal model of schizophrenia (Monte et al., 2013; Vasconcelos et al., 2015; Arau´jo et al., 2017). The behav-ioral alterations were accompanied by hippocampal pro-oxidative changes. Furthermore, our results provide novel evidence to the effectiveness of the combination of antiox-idants and antipsychotics, by showing a superior effect of the combination of ALA + CP, at the low dose (ALA + CP1), against KET-induced alterations. This conclusion is based on the preventive effect of ALA + CP1 against all behavioral alterations induced by KET, an effect that
was not observed with the other treatments evaluated in the present study which were not able to prevent working memory deficits.
In our study, KET-induced hyperlocomotion was prevented by CP while an augmentation effect was observed by the combination of ALA + CP1, bringing the locomotor activity of the animals to control levels. Previous studies showed that hyperlocomotion is induced by a wide range of KET doses, i.e., from 10 (Arruda et al., 2008) to 100 mg/kg (Chatterjee et al., 2011). This hyperlocomotion relates to an inhibitory effect of KET in cortical NMDARs leading to a hyperdopaminer-gic activity in striatal structures, mainly nucleus accum-bens (Brisch et al., 2014). In vitro studies also showed that the increase in striatal dopaminergic content occurs due to an indirect dopaminergic agonist action of KET, since this drug was able to inhibit synaptosomal uptake of dopamine in the nucleus accumbens (Irifune et al., 1991). In line with KET effects in locomotion, increased motor activity is observed in healthy controls and schizophrenia patients submitted to the administration of this drug (Sigurdsson and Duvarci, 2016). Additionally, KET-induced hyperlocomotion was blocked by both typi-cal, such as haloperidol (Arruda et al., 2008), and atypical antipsychotics, such as, risperidone (Arruda et al., 2008) and clozapine (Chatterjee et al., 2011; Vasconcelos et al., 2015). In a recent study, the administration of ALA and clozapine, alone or in combination, reversed KET-induced hyperlocomotion (Vasconcelos et al., 2015). Deficits in working memory represent an important cognitive endophenotypic measure of schizophrenia (Kellendonk et al., 2009). In this context, in the Y-maze test we observed that the impairment of spatial working memory induced by KET was prevented only by the com-bination of ALA + CP1. It is well described in the litera-ture that schizophrenia patients suffer from generalized cognitive disorder that correlates with functional outcome
Fig. 7.Effects on hippocampal MDA concentration by CP (1 or 5 mg/ kg) and ALA (100 mg/kg), alone or combined, in an animal model of schizophrenia induced by KET (10 mg/kg) in rats. Each bar repre-sents mean ± SEM ofn= 5–10 animals/group. a, b, d and fP< 0.05 compared with saline (control group), KET, CP1, CP1 + KET, respectively, according to one-way ANOVA followed by Tukey as post hoc test. Abbreviations: ALA – lipoic acid; CP – chlorpromazine; KET – ketamine; TBARS – thiobarbituric acid reactive substances; MDA – malondialdehyde.
(e.g., memory deficit, daily activities, and resolution of social problems) (Sharma and Antonova, 2003), and that these deficits are treated by atypical, but not typical antipsychotics. In our results CP5 alone or CP5 + ALA caused working memory deficits. This negative effect of CP5, CP5 + KET and ALA + CP5 + KET groups in the working memory may be explained by the motor deficit observed in these animals when evaluated in the open field test, as referred above. Thus, the combination of ALA with CP only at low dose could be a therapeutic advantage in order to prevent working memory deficits.
PPI deficits are considered neurophysiological endophenotypes of schizophrenia (Braff, 2005). Consid-ering PPI test, our results demonstrated that the repeated administration of KET caused a deficit in this function. In humans, PPI deficits indicate impairment in the mecha-nisms associated with the correct operation of sensorimo-tor gating, an anticipasensorimo-tory process that prevents the sensory overload and the cognitive fragmentation (Powell et al., 2009). Deficits in PPI were also observed in other studies using KET as an animal model of schizophrenia (Monte et al., 2013; Vasconcelos et al., 2015).
In this study, CP alone or combined with ALA prevented PPI deficits at all tested doses. Similarly to the working memory results, we observed that on PP75 the combination of ALA and CP1 presented a superior effect in preventing PPI deficits. In a previous study of our research group we also observed the ability of ALA combined with clozapine in the prevention of KET-induced PPI deficits mainly on PP75 (Vasconcelos et al., 2015).
One previous study aiming to compare the effects of acute doses of typical, CP (100 mg/kg) and atypical antipsychotics, amisulpride (300 mg/kg) and risperidone (3 mg/kg), on the PPI of healthy volunteers, found that none of the tested drugs affected PPI (Barrett et al., 2004). Researchers who investigated the differences between the effects of typical (haloperidol and CP) and atypical antipsychotic drugs (seroquel and clozapine) on PPI measures in animals, showed that the capacity of antipsychotics in restoring PPI deficits in rats treated with a NMDA antagonist is not specific for clinically atypical antipsychotics (Swerdlow et al., 2008).
The repeated administration of KET, as an animal model of schizophrenia, induces oxidative imbalance in putative brain areas related to the neurobiology of this mental disorder (Maceˆdo et al., 2012; Smaga et al., 2015). In this regard, accumulating evidence points toward an intimate regulation of NMDARs activity by oxidative stress response. In other words, deficiencies in brain GSH levels cause NMDAR hypofunction. Con-versely, active neurons present an increased capacity of synthesizing GSH, which is induced by the high Ca2+ influx through synaptic NMDARs (Baxter et al., 2015). This means that NMDARs are involved in brain oxidative homeostasis.
In this study, we decided to measure hippocampal oxidative alterations since studies have shown that KET is harmful to hippocampal neurogenesis (Zheng et al., 2015; Huang et al., 2016), modifying the function of this
brain area. In fact, cognitive impairments involving episo-dic and working memory deficits, as well as attention, pro-cessing speed and problem solving are present in schizophrenia (Hasan et al., 2014). These deficits are related to decreased hippocampal volume (Geisler et al., 2015). Thus, the hippocampus is an important brain area related to the pathophysiology of schizophrenia being strongly affected by oxidative agents, which seems to affect its functioning (Zheng et al., 2015).
Taken together, our results revealed that KET repeated administration decreased hippocampal GSH content while increased hippocampal nitrite levels, lipid peroxidation and MPO activity. GSH is an important marker for impairments of antioxidant defenses in neuropsychiatric diseases (Tunc¸el et al., 2015). Preclini-cal studies have demonstrated a link between schizophre-nia and GSH deficiency in several brain regions (Silva et al., 2010; Monte et al., 2013; Vasconcelos et al., 2015). In our study, pretreatment with CP alone, at both doses, and combined with ALA prevented KET-induced GSH decrease. It is important to highlight the lack of studies evaluating changes in GSH levels induced by CP administration, what is the opposite for atypical antipsychotics (Monte et al., 2013; Vasconcelos et al., 2015). A previous study demonstrated that a single dose of CP increased GSH levels in the cerebral cortex of ani-mals (Dejanovic´ et al., 2016). However, the dose of CP used in the study ofDejanovic´ et al. (2016), 38.7 mg/kg, was almost eight times higher than the highest dose (5 mg/kg) used in this study. In agreement with our results, ALA-induced increases in brain GSH levels were previ-ously demonstrated (Goc et al., 2015; Silva et al., 2016). This effect of ALA seems to be related to a reduc-tion of cystine to cysteine, the limiting substrate for GSH production (Shay et al., 2009), elevating GSH concentra-tion (Moini et al., 2002). In addition, it was demonstrated that ALA can induce the synthesis of GSH by the activa-tion of the signaling pathway of the transcripactiva-tion factor erythroid nuclear factor 2 (Nrf2)-Antioxidant Response Element (Suh et al., 2004).
In line with the oxidative alterations in schizophrenia, an increase in the final products of NO oxidation in combination with other free radicals, may probably contribute to the neurobiology of this mental disorder (Ozyurt et al., 2014). Accordingly, a systematic and quanti-tative review of oxidative stress markers pointed toward an increase in the levels of TBARS and NO in schizophrenia patients, compared to control patients (Zhang et al., 2010). Similar to our results, high nitrite levels were found in the prefrontal cortex (Silva et al., 2010) and hippocampus (Chatterjee et al., 2012) of animals after KET administra-tion. Post-mortem studies have demonstrated elevated levels of NO and nitric oxide synthase (NOS) in brain tis-sues of individuals with schizophrenia and have suggested that NOS remains active in schizophrenia (Yao et al., 2004; Xu et al., 2005). The concentration of nitrite in the plasma of patients in the first psychotic epi-sode is significantly low and, high in outpatients (Flatow et al., 2013).
KET-induced effects in hippocampal nitrite levels. An in vitro study showed that CP (10 mg/kg) inhibits the activity of NOS in rat brain mitochondria, resulting in a reduction in mitochondrial hydrogen peroxide production (Lores-Arnaiz et al., 2004). The action of ALA seems to be attributed to its powerful antioxidant property against the formation of reactive oxygen and nitrogen species, promoting the regeneration of antioxidant agents such as vitamin E, C and A, coenzyme Q10 and GSH and act-ing as a metal chelator (Santos et al., 2010).
Oxidative lipid damage has often been used as a biomarker of oxidative damage in several diseases as well as in schizophrenia (Zhang et al., 2010). Therefore, the increase in the concentration of MDA that occurred after repeated doses of KET, as observed in this study, mimics the occurrence of lipid peroxidation observed in schizophrenia. This finding may be related to the damage on hippocampal cell membranes by KET. Similar results were found bySilva et al. (2010), showing that subanes-thetic doses of KET, 10 and 20 mg/kg, increased MDA levels in mice prefrontal cortex. Similarly, an elevation in MDA content was observed in the cerebellum, striatum and prefrontal cortex after an injection of 10 mg/kg (Oliveira et al., 2009) or 20 mg/kg (Monte et al., 2013) of KET. Another study showed a significant increase in MDA levels in the hippocampus and thalamus of perinatal animals treated with phencyclidine (PCP) (Radonjic´ et al., 2010).
As observed in relation to hippocampal nitrite content, in this study the pretreatment with CP5, ALA + CP1 and ALA + CP5 prevented KET-induced alterations in MDA content. Inhibition of brain lipid peroxidation was observed by the repeated administration of clozapine (Vasconcelos et al., 2015) or risperidone (Monte et al., 2013) for 14 days to mice. Contradictory finding showed that a single dose of CP increased MDA levels in the cere-bral cortex of animals (Dejanovic´ et al., 2016). However, as previously mentioned, the dose of CP used in the study ofDejanovic´ et al. (2016)was eight times higher than the highest dose (5 mg/kg) used in our study. Considering ALA effects, previous studies demonstrated a strong antioxidant activity of ALA against lipid peroxidation and oxidative protein damage (Marangon et al., 1999; Zembron-Lacny et al., 2009).
Hypochlorous acid (HClO), a powerful oxidant is generated by MPO. This enzyme uses the chloride ion (Cl ), in the presence of H2O2, to generate HClO, that functions as an important antimicrobial agent (Shaeib et al., 2015).
In this study, MPO levels were analyzed to verify possible inflammatory changes associated with the induction of schizophrenia by the administration of repeated doses of KET. In fact, we observed a significant increase in hippocampal MPO activity after the treatment with repeated doses of KET. A similar result was obtained by the repeated administration of KET 20 mg/kg for 14 days (Arau´jo et al., 2017). Accord-ingly, a clinical study byAl-asmari and Khan (2014) indi-cates that MPO is found in high concentrations in the serum of schizophrenia patients. Green et al. (2004) observed that the increase in MPO expression in the brain
is related to neurodegeneration. Therefore, this elevation in MPO concentration may indicate hippocampal neu-rodegeneration induced by KET.
We also observed that the increase in MPO activity induced by KET was significantly prevented by the pretreatment with CP1 or CP5 alone and combined with
ALA. These results indicate hippocampal
neuroprotection of these treatments. This finding is relevant for the treatment of schizophrenia with antipsychotics, since its connection with inflammation has been supported by several studies (Silva et al., 2010; Radonjic´ et al., 2010; Maceˆdo et al., 2012; Flatow et al., 2013; Leza et al., 2015; Borella et al., 2016). On the other hand, despite the lack of data about the effect of the combination of antipsychotics and ALA on MPO activity, other studies have demonstrated the potential effect of ALA in inhibiting the inflammatory and oxidative damage induced by this enzyme in a variety of experi-mental designs (Myzak and Carr, 2002; C¸akır et al.,
2015; Fei et al., 2016; Petronilho et al., 2016). It is also worth mentioning that ALA promotes regulation in gene expression and transcription of pro-inflammatory factors (Gorazca and Asanowicz-Antkowiak, 2009).
The present study has some limitations because we used only one model of schizophrenia induced by the repeated administration of a NMDAR antagonist. Therefore, other preclinical studies must be designed to replicate our findings in other animal models of schizophrenia, for example, models based on the repeated administration of dopaminergic agonists as well as neurodevelopmental models based on maternal immune activation.
In conclusion, KET repeated administration induced schizophrenia-like behavioral changes that resemble positive (hyperlocomotion and PPI deficits) and cognitive (working memory deficits) symptoms of schizophrenia. These behavioral alterations were accompanied by hippocampal oxidative alterations. The behavioral alterations induced by KET were mainly prevented by the administration of chlorpromazine 1 mg/ kg combined with ALA, since when used at 5 mg/kg dose this antipsychotic caused impairments in the locomotor activity and working memory. Thus, our results suggest the importance of the prevention of the side effects of typical antipsychotics by ALA.
ACKNOWLEDGMENTS
This study was supported by Grants from the Brazilian Government Institutions National Council for Scientific and Technological Development (CNPq), Coordination for the Improvement of Higher Education Personnel (CAPES), and Cearense Foundation for the Support of Scientific and Technological Development (FUNCAP).
COMPETING INTERESTS
REFERENCES
Al-asmari A, Khan MW (2014) Inflammation and schizophrenia: Alterations in cytokine levels and perturbation in antioxidative defense systems. Hum Exp Toxicol 33:115–122.
Arau´jo TS, Chaves Filho AJM, Monte AS, Queiroz AIG, Cordeiro RC, Machado MJS, Lima RF, Lucena DF, Maes M, Maceˆdo D (2017) Reversal of schizophrenia-like symptoms and immune alterations in mice by immunomodulatory drugs. J Psychiatr Res 84:49–58.
Archer J (1973) Tests for emotionality in rats and mice: a review. Anim Behav 21:205–235.
Arroll MA, Wilder L, Neil J (2014) Nutritional interventions for the adjunctive treatment of schizophrenia: a brief review. Nutr J 13:91–99.
Arruda MOV, Soares PM, Hono´rio JER, Lima RCS, Chaves EMC, Lobato RFG, Martin ALAR, Sales GTM, Carvalho KM, Assreuy ANS, Brito EN, Vasconcelos SMM (2008) Activies of the antipsychotic drugs haloperidol and risperidone on behavioural effects induced by ketamine in mice. Sci Pharm 76:673–687.
Barrett SL, Bell R, Watson D, King DJ (2004) Effects of amisulpride, risperidone and chlorpromazine on auditory and visual latent inhibition, prepulse inhibition, executive function and eye movements in healthy volunteers. J Psychopharmacol 18:156–172.
Baxter PS, Bell KF, Hasel P, Kaindl AM, Fricker M, Thomson D, Cregan SP, Gillingwater TH, Hardingham GE (2015) Synaptic NMDA receptor activity is coupled to the transcriptional control of the glutathione system. Nat Commun 6:6761–6773.
Borella CM, Seeman MV, Cordeiro RC, Santos JV, Souza RM, Fernandes NS, Monte AS, Vasconcelos SMM, Quinn JP, Lucena DF, Carvalho AF, Macedo D (2016) Gender and estrous cycle influences on behavioral and neurochemical alterations in adult rats neonatally administered ketamine. Dev Neurobiol 76:519–532.
Bradley PP, Christensen RD, Rothstein G (1982) Cellular and extracellular myeloperoxidase in pyogenic inflammation. Blood 60:618–622.
Braff DL (2005) The use of neurophysiological endophenotypes to understand the genetic basis of schizophrenia. Dialogues Clin Neurosci 7:125–135.
Brisch R, Saniotis A, Wolf R, Bielau H, Bernstein H-G, Steiner J, Bogerts B, Braun K, Jankowski Z, Kumaratilake J, Henneberg M, Gos T (2014) The role of dopamine in schizophrenia from a neurobiological and evolutionary perspective: old fashioned, but still in vogue. Front Psychiatry 5:47.
C¸akır T, Bastu¨rk A, Polat S, Aslaner A, Durgut H, Sehirli AO¨, Gu¨l M, O¨g˘u¨nc¸ AV, Gu¨l S, Sabuncuoglu MZ, Oruc¸ MT (2015) Does alfa lipoic acid prevent liver from methotrexate induced oxidative injury in rats? Acta Cir Bras 30:247–252.
Chatterjee M, Ganguly S, Srivastava M, Palit G (2011) Effect of ‘chronic’ versus ‘acute’ ketamine administration and its ‘withdrawal’ effect on behavioural alterations in mice: Implications for experimental psychosis. Behav Brain Res 216:247–254.
Chatterjee M, Verma R, Ganguly S, Palit G (2012) Neurochemical and molecular characterization of ketamine-induced experimental psychosis model in mice. Neuropharmacology 63:1161–1171.
Copoglu US, Virit O, Kokacya MH, Orkmez M, Bulbul F, Erbagci AB, Semiz M, Alpak G, Unal A, Ari M, Savas HA (2015) Increased oxidative stress and oxidative DNA damage in non-remission schizophrenia patients. Psychiat Res 229:200–205.
Coyle JT (2012) NMDA receptor and schizophrenia: a brief history. Schizophrenia Bull 38:920–926.
Dall’Igna OP, Fett P, Gomes MW, Souza DO, Cunha RA, Lara DR (2007) Caffeine and adenosine A(2a) receptor antagonists prevent beta-amyloid (25–35)-induced cognitive deficits in mice. Exp Neurol 203:241–245.
Dejanovic´ B, Stevanovic I, Ninkovic M, Stojanovic I, Lavrnja I, Radicevic T, Pavlovic M (2016) Agmatine protection against chlorpromazine-induced forebrain cortex injury in rats. J Vet Sci 1:53–61.
Do¨rsam B, Fahrer J (2016) The disulfide compounda-lipoic acid and its derivatives: A novel class of anticancer agents targeting mitochondria. Cancer Lett 371:12–19.
Fei M, Xie Q, Zou Y, He R, Zhang Y, Wang J, Bo L, Li J, Deng X (2016) Alpha-lipoic acid protects mice against concanavalin A-induced hepatitis by modulating cytokine secretion and reducing reactive oxygen species generation. Int Immunopharmacol 35:53–60.
Flatow J, Buckley P, Miller BJ (2013) Meta-analysis of oxidative stress in schizophrenia. Biol Psychiat 74:400–409.
Geisler D, Walton E, Naylor M, Roessner V, Lim KO, Schulz SC, Gollub RL, Calhoun VD, Sponheim SR, Ehrlich S (2015) Brain structure and function correlates of cognitive subtypes in schizophrenia. Psychiat Res 234:74–83.
Goc Z, Gren´ A, Kapusta E, Dziubek K, Szaroma W (2015) Antioxidative effects of a-lipoic acid in the brain, liver and kidneys in selected mouse organs exposed to zymosan. Acta Biol Hung 66:258–269.
Gonzalez-liencres C, Tas C, Brown EC, Erdin S, Onur E, Cubukcoglu Z, Aydemir O, Esen-Danaci A, Bru¨ne M (2014) Oxidative stress in schizophrenia: a case-control study on the effects on social cognition and neurocognition. BMC Psychiatry 14:1–9.
Gorazca A, Asanowicz-Antkowiak K (2009) Prophylaxis witha-lipoic acid against lipopolysaccharide-induced brain injury in rats. Arch Immunol Ther Ex 57:141–146.
Green L, Goldman P (1981) Nitrate synthesis in the germfree and conventional rat. Science 212:56–58.
Green PS, Mendez AJ, Jacob JS, Crowley JR, Growdon W, Hyman BT, Heinecke JW (2004) Neuronal expression of myeloperoxidase is increased in Alzheimer’s disease. J Neurochem 90:724–733.
Hasan A, Wobrock T, Falkai P, Schneider-Axmann T, Guse B, Backens M, Ecker UK, Heimes J, Galea JM, Gruber O, Scherk H (2014) Hippocampal integrity and neurocognition in first-episode schizophrenia: A multidimensional study. World J Biol Psychia 15:188–199.
Huang H, Liu CM, Sun J, Hao T, Xu CM, Wang D, Wu YQ (2016) Ketamine affects the neurogenesis of the hippocampal dentate gyrus in 7-day-old rats. Neurotox Res 30:185–198.
Huong NT, Matsumoto K, Kasai R, Yamasaki K, Watanabe H (1998)
In vitroantioxidant activity of Vietnamese ginseng saponin and its components. Biol Pharm Bull 21:978–981.
Irifune M, Shimizu T, Nomoto M (1991) Ketamine-induced hyperlocomotion associated with alteration of presynaptic components of dopamine neurons in the nucleus accumbens of mice. Pharmacol Biochem Behav 40:399–407.
Kellendonk C, Simpson EH, Kandel ER (2009) Modeling cognitive endophenotypes of schizophrenia in mice. Trends Neurosci 32:347–358.
Levin R, Calzavara MB, Santos CM, Medrano WA, Niigaki ST, Abı´lio VC (2011) Spontaneously Hypertensive Rats (SHR) present deficits in prepulse inhibition of startle specifically reverted by clozapine. Prog Neuro-Psychoph 35:1748–1752.
Leza JC, Garcı´a-Bueno B, Bioque M, Arango C, Parellada M, Do K, O’Donnell P, Bernardo M (2015) Inflammation in schizophrenia: A question of balance. Neurosci Biobehav Rev 55:612–626.
Lores-Arnaiz S, D’Amico G, Czerniczyniec A, Bustamante J, Boveris A (2004) Brain mitochondrial nitric oxide synthase:in vitroand
in vivo inhibition by chlorpromazine. Arch Biochem Biophys 430:170–177.
Maceˆdo DS, Medeiros CD, Cordeiro RC, Sousa FC, Santos JV, Morais TA, Hyphantis TN, McIntyre RS, Quevedo J, Carvalho AF (2012) Effects of alpha-lipoic acid in an animal model of mania induced by D-amphetamine. Bipolar Disord 14:707–718.
Magalha˜es PVS, Dean O, Andreazza AC, Berk M, Kapczinski F (2016) Antioxidant treatments for schizophrenia. Cochrane Db Syst Rev 2:1–90.
Mas S, Gasso´ P, Trias G, Bernardo M, Lafuente A (2012) Sulforaphane protects SK-N-SH cells against antipsychotic-induced oxidative stress. Fundam Clin Pharm 26:712–721.
Messias E, Chen C-Y, Eaton WW (2007) Epidemiology of Schizophrenia: Review of Findings and Myths. Psychiat Clin N Am 30:323–338.
Miljevic C, Nikolic M, Nikolic-Kokic A, Jones DR, Niketic V, Lecic-Tosevski D, Spasic MB (2010) Lipid status, anti-oxidant enzyme defence and haemoglobin content in the blood of long-term clozapine-treated schizophrenic patients. Prog Neuro-Psychoph 34:303–307.
Moini H, Packer L, Saris NEL (2002) Antioxidant and prooxidant activities of alpha-lipoic acid and dihydrolipoic acid. Toxicol Appl Pharm 182:84–90.
Monte AS, Souza GC, McIntyre RS, Soczynska JK, Santos JV, Cordeiro RC, Ribeiro BM, Lucena DF, Vasconcelos SM, Sousa FC, Carvalho AF, Maceˆdo DS (2013) Prevention and reversal of ketamine-induced schizophrenia related behavior by minocycline in mice: Possible involvement of antioxidant and nitrergic pathways. J Psychopharmacol 27:1032–1043.
Myzak MC, Carr AC (2002) Myeloperoxidase-dependent caspase-3 activation and apoptosis in HL-60 cells: protection by the antioxidants ascorbate and (dihydro) lipoic acid. Redox Rep 7:47–53.
Oliveira L, Spiazzi CMS, Bortolin T, Canever L, Petronilho F, Mina FG, Dal-Pizzol F, Quevedo J, Zugno AI (2009) Different sub-anesthetic doses of ketamine increase oxidative stress in the brain of rats. Prog Neuro-Psychoph 33:1003–1008.
Ozyurt H, Ozyurt B, Sarsilmaz M, Kus I, Songur A, Akyol O (2014) Potential role of some oxidant / antioxidant status parameters in prefrontal cortex of rat brain in an experimental psychosis model and the protective effects of melatonin. Eur Rev Med Pharmaco 18:2137–2144.
Petronilho F, Florentino D, Danielski LG, Vieira LC, Martins MM, Vieira A, Bonfante S, Goldim MP, Vuolo F (2016) Alpha-lipoic acid attenuates oxidative damage in organs after sepsis. Inflammation 39:357–365.
Pillai A, Parikh V, Terry AV, Mahadik SP (2007) Long-term antipsychotic treatments and crossover studies in rats: differential effects of typical and atypical agents on the expression of antioxidant enzymes and membrane lipid peroxidation in rat brain. J Psychiatr Res 41:372–386.
Powell SB, Zhou X, Geyer MA (2009) Prepulse inhibition and genetic mouse models of schizophrenia. Behav Brain Res 204:282–294.
Radenovic L, Selakovic V (2005) Differential effects of NMDA and AMPA/kainate receptor antagonists on nitric oxide production in rat brain following intrahippocampal injection. Brain Res Bull 67:133–141.
Radonjic´ NV, Knezevic´ ID, Vilimanovich U, Kravic´-Stevovic´ T, Marina LV, Nikolic´ T, Todorovic´ V, Bumbasirevic´ V, Petronijevic´ ND (2010) Decreased glutathione levels and altered antioxidant defense in animal model of schizophrenia: long-term effects of perinatal phencyclidine administration. Neuropharmacology 58:739–745.
Reddy R, Reddy R (2015) Antioxidant therapeutics for schizophrenia. Antioxid Redox Sign 15:2047–2055.
Santos I´MS, Freitas RLM, Saldanha GB, Tome´ AR, Jorda´n J, Freitas RM (2010) Alterations on monoamines concentration in rat hippocampus produced by lipoic acid. Arq Neuro-Psiquiat 68:362–366.
Sedlak J, Lindsay RH (1968) Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem 25:192–205.
Shaeib F, Khan SN, Ali I, Najafi T, Maitra D, Abdulhamid I, Saed GM, Pennathur S, Abu-Soud HM (2015) Melatonin prevents myeloperoxidase heme destruction and the generation of free
iron mediated by self-generated hypochlorous acid. PLoS One 10:1–15.
Sharma T, Antonova L (2003) Cognitive function in schizophrenia. Psychiat Clin N Am 26:25–40.
Shay KP, Moreau RF, Smith EJ, Smith AR, Hagen TM (2009) Alpha-lipoic acid as a dietary supplement: molecular mechanisms and therapeutic potential. Biochim Biophys Acta 1790:1149–1160.
Sigurdsson T, Duvarci S (2016) Hippocampal-prefrontal interactions in cognition, behavior and psychiatric disease. Front Syst Neurosci 9:1–18.
Silva FC, Cito MCO, Silva MI, Moura BA, Aquino Neto MR, Feitosa ML, Chaves RC, Macedo DS, Vasconcelos SM, Fonteles MMF, Sousa FC (2010) Behavioral alterations and pro-oxidant effect of a single ketamine administration to mice. Brain Res Bull 83:9–15.
Silva MC, Sousa CN, Gomes PX, Oliveira GV, Arau´jo FY, Ximenes NC, Silva JC, Vasconcelos GS, Leal LK, Maceˆdo D, Vasconcelos SM (2016) Evidence for protective effect of lipoic acid and desvenlafaxine on oxidative stress in a model depression in mice. Prog Neuro-Psychoph 64:142–148.
Smaga I, Niedzielska E, Gawlik M, Moniczewski A, Krzek J, Przegalinski E, Pera J, Filip M (2015) Oxidative stress as an etiological factor and a potential treatment target of psychiatric disorders. Part 2. Depression, anxiety, schizophrenia and autism. Pharmacol Rep 67:569–580.
Suh JH, Shenvi SV, Dixon BM, Liu H, Jaiswal AK, Liu RM, Hagen TM (2004) Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc Natl Acad Sci U S A 101:3381–3386.
Swerdlow NR, Weber M, Qu Y, Light GA, Braff DL (2008) Realistic expectations of prepulse inhibition in translational models for schizophrenia research. Psychoph S 199:331–388.
Tunc¸el O¨K, Sarısoy G, Bilgici B, Pazvantoglu O, C¸etin E, U¨nverdi E,
Avcı B, Bo¨ke O (2015) Oxidative stress in bipolar and
schizophrenia patients. Psychiat Res 228:688–694.
Vasconcelos GS, Ximenes NC, Sousa CN, Oliveira TQ, Lima LL, Lucena DF, Gama CS, Maceˆdo D, Vasconcelos SM (2015) Alpha-lipoic acid alone and combined with clozapine reverses schizophrenia-like symptoms induced by ketamine in mice: Participation of antioxidant, nitrergic and neurotrophic mechanisms. Schizophr Res 165:163–170.
Vidovic´ B, Milovanovic´ S, Dordevic´ B, Kotur-Stevuljevic´ J, Stefanovic´
A, Ivanisˇevic´ J, Miljkovic´ M, Spasic´ S, Stojanovic´ D, Pantovic´ M (2014) Effect of alpha-lipoic acid supplementation on oxidative stress markers and antioxidative defense in patients with schizophrenia. Psychiat Danub 26:205–213.
Xu B, Wratten N, Charych EI, Buyske S, Firestein BL, Brzustowicz LM (2005) Increased expression in dorsolateral prefrontal cortex of CAPON in schizophrenia and bipolar disorder. PLoS Med 10:999–1007.
Yamada K, Noda Y, Hasegawa T, Komori Y, Nikai T, Sugihara H, Nabeshima T (1996) The role of nitric oxide in dizocilpine-induced impairment of spontaneous alternation behavior in mic. J Pharmacol Exp Ther 276:460–466.
Yao JK, Leonard S, Reddy RD (2004) Increased nitric oxide radicals in postmortem brain from patients with schizophrenia. Schizophrenia Bull 30:923–934.
Zembron-Lacny A, Slowinska-Lisowska M, Szygula Z, Witkowski K, Szyszka K (2009) The comparison of antioxidant and hematological properties of N-acetylcysteine and alpha-lipoic acid in physically active males. Physiol Res 58:855–861.
Zhang M, Zhao Z, He L, Wan C (2010) A meta-analysis of oxidative stress markers in schizophrenia. Sci China Life sci 53:112–124.
Zheng X, Zhou J, Xia Y (2015) The role of TNF-a in regulating ketamine-induced hippocampal neurotoxicity. Arch Med Sci 6:1296–1302.