Alpha-lipoic acid alone and combined with clozapine reverses
schizophrenia-like symptoms induced by ketamine in mice:
Participation of antioxidant, nitrergic and neurotrophic mechanisms
Germana Silva Vasconcelos
a, Naiara Coelho Ximenes
a, Caren Nádia Soares de Sousa
a,
Tatiana de Queiroz Oliveira
a, Laio Ladislau Lopes Lima
a, David Freitas de Lucena
a, Clarissa Severino Gama
b,
Danielle Macêdo
a,⁎
, Silvânia Maria Mendes Vasconcelos
aaNeuropsychopharmacology Laboratory, Department of Physiology and Pharmacology, Faculty of Medicine, Post Graduate Program in Pharmacology, Federal University of Ceará, Rua Coronel Nunes de Melo 1127, Fortaleza, CE, Brazil
bLaboratory of Molecular Psychiatry, INCT for Translational Medicine-CNPq, Hospital de Clínicas de Porto Alegre, Universidade Federal do Rio Grande do Sul, Post Graduate Program in Medicine, Department of Psychiatry, Universidade Federal do Rio Grande do Sul, Ramiro Barcelos, 2350, Porto Alegre CEP 90035-903, Brazil
a b s t r a c t
a r t i c l e
i n f o
Article history:
Received 5 November 2014 Received in revised form 9 April 2015 Accepted 12 April 2015
Available online 30 April 2015
Keywords:
Schizophrenia Alpha-lipoic acid Clozapine Oxidative stress Nitrite Ketamine
Oxidative stress has important implications in schizophrenia. Alpha-lipoic acid (ALA) is a natural antioxidant synthesized in human tissues with clinical uses. We studied the effect of ALA or clozapine (CLZ) alone or in com-bination in the reversal of schizophrenia-like alterations induced by ketamine (KET). Adult male mice received saline or KET for 14 days. From 8th to 14th days mice were additionally administered saline, ALA (100 mg/kg), CLZ 2.5 or 5 mg/kg or the combinations ALA + CLZ2.5 or ALA + CLZ5. Schizophrenia-like symptoms were evaluated by prepulse inhibition of the startle (PPI) and locomotor activity (positive-like), social preference (negative-like) and Y maze (cognitive-like). Oxidative alterations (reduced glutathione—GSH and lipid peroxi-dation—LP) and nitrite in the prefrontal cortex (PFC), hippocampus (HC) and striatum (ST) and BDNF in the PFC were also determined. KET caused deficits in PPI, working memory, social interaction and hyperlocomotion. De-creased levels of GSH, nitrite (HC) and BDNF and inDe-creased LP were also observed in KET-treated mice. ALA and CLZ alone reversed KET-induced behavioral alterations. These drugs also reversed the decreases in GSH (HC) and BDNF and increase in LP (PFC, HC and ST). The combination ALA + CLZ2.5 reversed behavioral and some neuro-chemical parameters. However, ALA + CLZ5 caused motor impairment. Therefore, ALA presented an antipsychotic-like profile reversing KET-induced positive- and negative-like symptoms. The mechanism partially involves antioxidant, neurotrophic and nitrergic pathways. The combination of ALA + CLZ2.5 improved most of the parameters evaluated in this study without causing motor impairment demonstrating, thus, that possibly when combined with ALA a lower dose of CLZ is required.
© 2015 Elsevier B.V. All rights reserved.
1. Introduction
Schizophrenia is a highly disabling and multifaceted illness with positive, negative and cognitive symptom domains (Carpenter, 2007). The pathophysiology of this mental disorder is related, but not limited to: i) alterations in neurotransmitter systems, such as dopaminergic and glutamatergic (Coyle et al., 2010; Miyake et al., 2011); ii) alterations in neurotrophins (Favalli et al., 2012); iii) oxidative imbalance (Zhang et al., 2010) and iv) alterations in nitric oxide (NO) signaling (Bernstein et al., 2005).
Ketamine (KET) repeated administration to rodents mimics positive, negative and cognitive symptoms of schizophrenia (Meltzer et al., 2013; Monte et al., 2013). These behavioral alterations are accompanied by oxidative imbalance and nitrite alterations (Monte et al., 2013).
The predictive validity of the KET-induced model of schizophrenia is achieved only by atypical antipsychotics (Becker and Grecksch, 2004). Clozapine (CLZ) was thefirst atypical antipsychotic drug developed and presents a unique mechanism of action when compared to other drugs of this class (Leo and Regno, 2000). This drug transiently occupies D2 receptors (D2Rs) besides interacting with other neurotransmitter systems. Due to its unique mechanism of action, CLZ is the most effec-tive drug for treatment-resistant schizophrenia (Leucht et al., 2013). Clozapine seems to have anti-inflammatory properties in putative brain areas related to schizophrenia, but in some studies it was not effi -cient in the reversal of oxidative alterations in animal models of schizo-phrenia (Ribeiro et al., 2013). On the other hand, CLZ is implicated in ⁎ Corresponding author at: Department of Physiology and Pharmacology, Universidade
Federal do Ceará, Rua Cel. Nunes de Melo 1127, 60431-270 Fortaleza, CE, Brazil. Tel.: +55 85 3366 8337; fax: +55 85 3366 8333.
E-mail addresses:daniellesilmacedo@gmail.com,danielle.macedo@ufc.br
(D. Macêdo).
http://dx.doi.org/10.1016/j.schres.2015.04.017
0920-9964/© 2015 Elsevier B.V. All rights reserved.
Contents lists available atScienceDirect
Schizophrenia Research
pro-inflammatory alterations in insulin responsive cells and in obesity-associated cell types, possible mechanisms related to the development of metabolic syndrome by this drug (Contreras-Shannon et al., 2013). Indeed, CLZ is associated with important side effects depending on the dose and plasma concentration (Yusufiet al., 2007).
Alpha-lipoic acid (ALA) or thioct acid is a natural acid synthesized in human tissues. This drug is used for symptomatic diabetic neuropathy (Moini et al., 2002) and cardiac autonomic neuropathy (Ziegler and Gries, 1997). Mechanistic evidences suggest that ALA (Maczurek et al., 2008; Deslauriers et al., 2014): i) inhibits the formation of hydroxyl rad-icals and also scavenges reactive oxygen species, thereby increasing the levels of reduced glutathione (GSH); ii) scavenges lipid peroxidation (LP) products; iii) down-regulates the expression of redox-sensitive pro-inflammatory proteins including TNF and inducible NO synthase; and iv) decreases D2Rs. Thefirst suggestion for ALA in schizophrenia emerged in the 50s (Giamattei, 1957). In the last decade a clinical study suggested that ALA can ameliorate the adverse metabolic effects induced by antipsychotic drugs (Kim et al., 2008). A recent study report-ed that the combination of ALA and omega-3 polyunsaturatreport-ed fatty acids was not effective in the relapse prevention after antipsychotic dis-continuation infirst-episode schizophrenia (Emsley et al., 2014). On the other hand, preclinical evidences suggest that ALA administration dur-ing preadolescence/adolescence period could prevent schizophrenia-like behavioral alterations in mice (Deslauriers et al., 2014).
Thus, based on the importance of oxidative stress to the pathophys-iology of schizophrenia (Zhang et al., 2010) and on the evidences that ALA would present antipsychotic effects, we hypothesized that: i) the administration of ALA could reverse the behavioral and neurochemical (oxidative, neurotrophic and nitrergic) alterations induced by KET and ii) the combination of CLZ and ALA could reverse the behavioral and neurochemical (oxidative, neurotrophic and nitrergic) alterations in-duced by KET with better results than each drug alone.
2. Material and methods
2.1. Ethics
The study followed legal and ethical requirements provided by the NIH Guide for the Care and Use of Laboratory Animals (NIH 1996) and the Brazilian College of Animal Experimentation (COBEA) Law no. 11794/2008. The local ethical committee of Federal University of Ceara approved the protocol.
2.2. Animals
Adult male Swiss mice (25–30 g) maintained at a controlled temper-ature (23 ± 1 °C) with a 12 h dark/light cycle (lights on at 7:00 AM) and free access to water and food were used.
2.3. Drugs and treatment
Alpha-lipoic acid was orally administered (Sigma-Aldrich, St. Louis, USA, ALA 100 mg/kg). Ketamine hydrochloride (König, Brazil, KET 20 mg/kg) or clozapine (Leponex® Novartis, Brazil, CLZ 2.5 or 5 mg/kg) was intraperitoneally administered. The doses of ALA (Macêdo et al., 2012), CLZ (Moreira and Guimarães, 2005) and KET (Monte et al., 2013) were based on previous studies.
2.4. Study design
After randomization each animal received one daily injection of KET or saline for 14 days to simulate an acute treatment of psychotic episodes (Byrne, 2007; Monte et al., 2013). Additionally from 8th to 14th days the animals were subdivided in groups to which each of the following drugs was added 30 min after saline or KET: saline, ALA, CLZ 2.5 or 5 mg/kg. The combination groups received ALA + CLZ2.5 or
ALA + CLZ5 30 min after saline or KET. Therefore, a total of twelve groups were used in the present study. Clozapine was used as standard antipsychotic.
Thirty minutes after the last drug administration prepulse inhibition of the startle reflex (PPI), locomotor activity (openfield test), working memory (Y-maze task) and social interaction were registered. After-wards mice were sacrificed by decapitation and the prefrontal cortex (PFC), hippocampus (HC) and striatum (ST) dissected, rapidly frozen and stored at−70 °C until assayed.
2.5. Behavioral tests
2.5.1. Prepulse inhibition of the startle reflex (PPI)
After acclimatization to the background noise, mice were presented with a series of 10 stimuli (pulse alone—120 dB, 50 ms duration— habituation phase). Thereafter, the PPI modulation of the acoustic star-tle was tested using 74 trials pseudo-randomly divided into seven dif-ferent categories (20 s interval): 20 presentations of pulse alone (120 dB, 50 ms duration), 8 presentations of each prepulse (PP) intensi-ty alone (70, 75 or 80 dB, 3000 Hz frequency, 20 ms duration) and 10 presentations of each prepulse intensity + pulse (with 50 ms interval). Mean amplitude of startle response to pulse-alone (P) and prepulse-pulse (PP + P) trials was calculated for each subject. The level of PPI in each mice was determined by expressing the PP + pulse startle am-plitude as a percentage decrease from pulse-alone startle amam-plitude, ac-cording to the following formula: %PPI = 100−[100 × (PP/P)] (Levin et al., 2011). Startle amplitude was used to assess motor alterations.
2.5.2. Open-field test
An arena with nine squares (Archer, 1973). The observed parame-ters were the number of squares crossed (horizontal activity) and number of rearings (vertical activity).
2.5.3. Y-maze test
Each mouse was allowed to freely move through the maze during 8 min. The series of arm entries was recorded visually. An alternation was defined as entries in all three arms on consecutive occasions. The percentage of alternation was calculated as total of alternations/(total arm entries−2) (Yamada et al., 1996; Dall'Igna et al., 2007).
2.5.4. Social interaction test (SIT)
The apparatus consisted of a box divided into three chambers with a small opening in the dividers. In each of the two side chambers an iron cage was placed with a probe mice or empty. Mice were allowed 5 min of exploration. Afterwards an unfamiliar, same-sex probe mouse from the same experimental group was placed in one of two restraining cage (Radyushkin et al., 2009). Social preference was defined as: (% time spent in the social chamber)−(% time spent in the opposite chamber).
2.6. Determination of oxidative stress parameters
2.6.1. Reduced glutathione (GSH)
The method was based on Ellman's reagent (DTNB) reaction with free thiol groups as described elsewhere (Sedlák and L' Hanus, 1982). GSH level was expressed asμg of GSH/g wet tissue.
2.6.2. Lipid peroxidation (LP)
2.6.3. Nitrite determination
Nitrite levels, an indirect measure of NO, were determined based on Griess reaction (Green and Goldman, 1981; Radenovic and Selakovic, 2005) and expressed as nM/g wet tissue.
2.6.4. Determination of BDNF levels
Brain derived neurotrophic factor (ELISA; Millipore, USA) was deter-mined in the PFC by enzyme immunoassay according the manufacturer's directions. Results are expressed as pg/g wet tissue.
2.7. Statistical analysis
In all %PPI data analyses, when a drug treatment × PP intensity inter-action was detected, post hoc ANOVA was performed at each level of PP intensity. Behavioral and neurochemical data were analyzed using reg-ular two-way ANOVA followed by Tukey's post hoc tests. The two fac-tors used for the analyses were“KET treatment”(0 and 20 mg/kg) and “antipsychotic drug treatment”(CLZ: 0, 2.5 and 5 mg/kg; ALA: 0, 100 mg/kg and their combination ALA + CLZ2.5 and ALA + CLZ5). BDNF levels were analyzed by one-way ANOVA followed by Tukey's post hoc test. Before ANOVA, D'Agostino–Pearson omnibus test was conducted to verify the normal distribution of the data. All results are expressed as means ± standard error of the mean (SEM). For all analy-ses, the significance level was set at p≤0.05. Data analyses were per-formed using GraphPad Prism software, version 6.0f for Mac (San Diego, California, USA).
3. Results
In the analysis of %PPI data we observed a significant PP intensity × drug treatment interaction with main effect of PP intensity. Two-way ANOVA of %PPI data at PP80 (Fig. 1A) revealed a significant interaction between KET treatment × antipsychotic drug treatment [F(5,74) = 5.267, P = 0.0003] with significant main effect of antipsychotic drug
treatment [F(5,74) = 13.31, Pb0.0001]. Post hoc test showed that the repeated administration of KET caused deficits in PPI when compared to saline (Pb0.001). Clozapine2.5 (Pb0.01), CLZ5 (Pb0.01), ALA (Pb0.001) or ALA + CLZ2.5 (Pb0.01) reversed KET-induced PPI defi -cits. Significant decrease in PPI was observed with KET + ALA + CLZ5 when compared to saline, KET + CLZ5 or KET + ALA (Pb0.0001). Saline + ALA + CLZ5 significantly decreased PPI levels when compared to saline (Pb0.01).
A significant main effect of antipsychotic drug treatment was ob-served in the evaluation of startle amplitude [F(5,74) = 17.41, Pb0.0001] (Fig. 1B). Post hoc test showed increased startle amplitude in animals administered saline + ALA when compared to saline (Pb0.0001) and in animals administered KET + ALA when compared to saline (Pb0.001) or KET (Pb0.001).
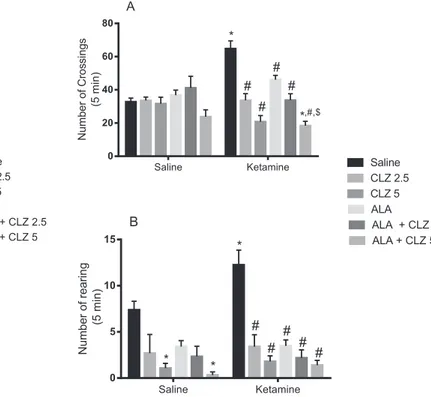
In the analysis of the number of crossings (Fig. 2A) a significant in-teraction between KET treatment and antipsychotic drug treatment [F(5, 160) = 10.53, Pb0.0001] was observed with significant main ef-fect of antipsychotic drug treatment [F(5,160) = 14.18, Pb0.0001]. Post hoc test showed that KET caused hyperlocomotion when compared to saline (Pb0.0001). Clozapine2.5 (Pb0.0001), CLZ5 (Pb0.0001), ALA (Pb0.001), ALA + CLZ2.5 (Pb0.0001) or ALA + CLZ5 (Pb0.0001) re-versed KET-induced hyperlocomotion. The largest decrease in locomo-tor activity was observed in KET + ALA + CLZ5 group. The decrease in this group was significant when compared to saline (Pb0.05) or KET + ALA-treated (Pb0.0001) animals. In the evaluation of the num-ber of rearings a significant main effect of antipsychotic drug treatment was detected [F(5,160) = 11.08, Pb0.0001] (Fig. 2B). Ketamine in-creased the number of rearings when compared to saline (Pb0.05). All post-treatments reversed this increase (Pb0.0001). Saline + CLZ5 or saline + ALA + CLZ5 decreased rearings when compared to saline (Pb0.05).
As illustrated inFig. 3, two-way ANOVA of working memory data (Fig. 3A) showed a significant KET treatment × antipsychotic drug
Fig. 1.(A) Percent of prepulse inhibition of startle (PPI) and (B) startle amplitude of ani-mals submitted to the model of schizophrenia induced by repeated administration of KET and subjected to the reversal treatment with ALA, CLZ or their combination. Bars rep-resent mean ± SEM of the percent of PPI (n = 6–8 animals/group). *Pb0.05 versus saline;
#P
b0.05 versus KET + saline;&P
b0.05 versus KET + CLZ5;$P
b0.05 versus KET + ALA according to two-way ANOVA followed by Tukey's post hoc test. ALA = alpha lipoic acid; CLZ2.5 or 5 = clozapine 2.5 or 5 mg/kg; KET = ketamine.
Fig. 2.Number of crossings (A) and rearings (B) of animals submitted to the KET-induced model of schizophrenia and subjected to the reversal treatment with ALA, CLZ or their combination. Bars represent mean ± SEM of the number of crossings or rearings (n = 16 animals/group). *Pb0.05 versus saline;#P
b0.05 versus KET + saline;$P
treatment interaction [F(5,87) = 6.486, Pb0.0001] with significant main effect of antipsychotic drug treatment [F(5,87) = 4.757, P = 0.0007]. Ketamine caused working memory deficits when compared to saline (Pb0.0001). The administration of CLZ2.5 (Pb0.05), CLZ5 (Pb0.01) or ALA + CLZ2.5 (Pb0.001) significantly reversed the deficit induced by KET. In the SIT (Fig. 3B) there was a significant interaction between KET treatment and antipsychotic drug treatment [F(5,56) = 10.12, Pb0.0001] with significant main effects of KET administration [F(1,56) = 7.025, P = 0.0104] and antipsychotic drug treatment [F(5,56) = 9.703, Pb0.0001]. Ketamine significantly decreased the %so-cial preference when compared to saline (Pb0.001). The administration of CLZ2.5 (Pb0.01), ALA (Pb0.001) or ALA + CLZ2.5 (Pb0.0001) sig-nificantly reversed the decrease induced by KET. In the groups KET + CLZ5 and KET + ALA + CLZ5 a significant decrease in the %social preference was observed when compared to saline (Pb0.001).
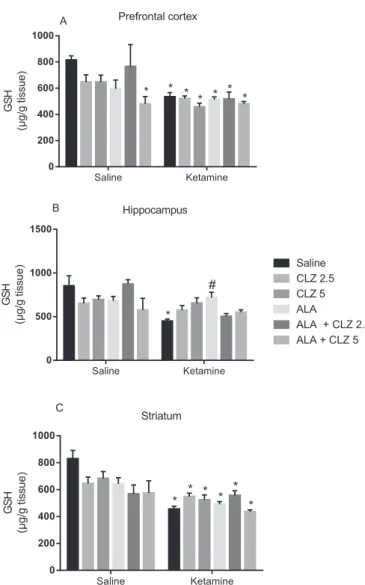
Regarding neurochemical alterations as illustrated inFig. 4, a signif-icant interaction between KET treatment and antipsychotic drug treat-ment was observed in the HC [F(5,85) = 3.815, P = 0.0036] and ST [F(5,105) = 2.525, P = 0.0336] with significant main effect of KET ad-ministration. In the HC (Fig. 4B) KET significantly decreased GSH levels when compared to saline (Pb0.001), while ALA reversed this decrease (Pb0.05). In the PFC (Fig. 4A) and ST (Fig. 4C) the decrease in GSH levels induced by KET was not reversed by the drug treatments proposed.
In the evaluation of LP (Fig. 5) a significant interaction between KET treatment × antipsychotic drug treatment was observed in the PFC [F(5,64) = 15.39, Pb0.0001], HC [F (5, 72) = 6.043, P = 0.0001] and ST [F (5, 80) = 44.29, Pb0.0001] with significant main effects of both factors. In the PFC (Fig. 5A), HC (Fig. 5B) and ST (Fig. 5C) KET signifi cant-ly increased the levels of MDA, the LP marker, when compared to saline (Pb0.0001). The two doses of CLZ, ALA or their combination signifi cant-ly reversed this increase (Pb0.0001).
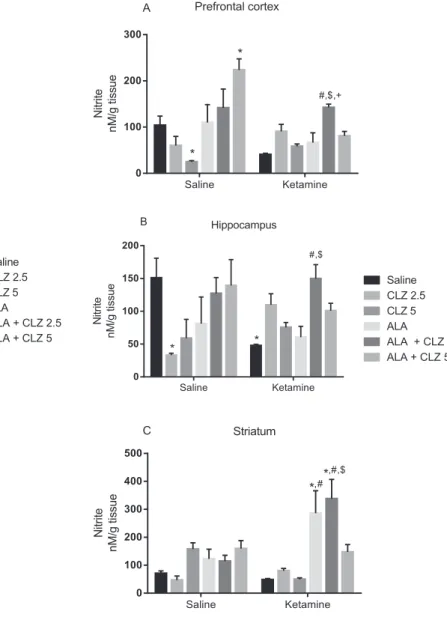
Significant interaction between KET treatment × antipsychotic drug treatment was observed in the evaluation of nitrite levels (Fig. 6) in the PFC [F (5, 75) = 8.789, Pb0.0001], HC [F (5, 73) = 4.038, P = 0.0027]
and ST [F (5, 79) = 4.598, P = 0.0010]. Post hoc test showed that KET significantly decreased nitrite levels in the HC (Fig. 6B) when compared to saline (Pb0.05). In the PFC (Fig. 6A) and HC, KET + ALA + CLZ2.5 in-creased nitrite levels when compared to KET-treated or KET + ALA mice (Pb0.01). In the ST (Fig. 6C) KET + ALA or KET + ALA + CLZ2.5 signif-icantly increased nitrite levels when compared to saline- or KET-treated mice (Pb0.05). The administration of saline + CLZ2.5 or CLZ5 signifi -cantly decreased nitrite levels in the HC and PFC, respectively, when compared to saline, while saline + ALA + CLZ5 significantly increased nitrite levels in the PFC when compared to saline (Pb0.05).
As illustrated inFig. 7, KET administration decreased prefrontal BDNF when compared to saline [F(6,36) = 11.01, Pb0.05]. Clozapine2.5 (Pb0.001), CLZ5 (Pb0.05), ALA (Pb0.001), ALA + CLZ2.5 (Pb0.001) or ALA + CLZ5 (Pb0.01) significantly reversed the BDNF alteration induced by KET. The highest levels of BDNF were observed in KET + CLZ2.5 animals being this result significant in relation to saline (Pb0.01), KET + CLZ5 (Pb0.01) and KET + ALA + CLZ2.5 (Pb0.001).
4. Discussion
Herein we demonstrated that ALA and CLZ alone or in combination (ALA + CLZ2.5) reversed some of the behavioral alterations induced Fig. 3.(A) Percent of correct alternations in the Y maze task and (B) percent of social
pref-erence of animals submitted to the KET-induced model of schizophrenia and subjected to the reversal treatment with ALA, CLZ or their combination. Bars represent mean ± SEM (n = 5–8 animals/group). *Pb0.05 versus saline;#Pb0.05 versus KET + saline according
to two-way ANOVA followed by Tukey's post hoc test. ALA = alpha lipoic acid; CLZ2.5 or 5 = clozapine 2.5 or 5 mg/kg; KET = ketamine.
Fig. 4.Levels of reduced glutathione (GSH) in the prefrontal cortex (A), hippocampus (B) and striatum (C) of animals submitted to the KET-induced model of schizophrenia and subjected to the reversal treatment with ALA, CLZ or their combination. Bars represent mean ± SEM of the % of correct alternations (n = 6–8 animals/group). *Pb0.05 versus
sa-line;#P
by KET repeated administration. More specifically, ALA and CLZ alone reversed the deficits in PPI and social interaction and the hyperlocomotion induced by KET. These behavioral parameters are re-lated to positive- and negative-like symptoms of schizophrenia (Thaker, 2007; Monte et al., 2013). Regarding the neurochemical pa-rameters, ALA and CLZ alone or in combination reversed some pro-oxidant, nitrergic and neurotrophic alterations induced by KET in the PFC, HC and ST.
Ketamine repeated administration to rodents is an important phar-macological model of schizophrenia since it presents face, construct and predictive validity (Chindo et al., 2012). Nevertheless, the face va-lidity of positive symptoms in animal models of schizophrenia is contro-versial; these symptoms are evaluated on the basis of hyperactivity and PPI deficits (van den Buuse, 2010).
In the present study ALA alone reversed positive- and negative-like symptoms induced by KET with results comparable to those obtained with CLZ alone. PPI deficits and hyperlocomotion are also induced by drugs that facilitate dopaminergic (DA) activity, for instance,D -amphetamine (Swerdlow et al., 1990). We previously demonstrated
the prevention and reversal ofD-amphetamine-induced mania in mice by ALA (Macêdo et al., 2012). Importantly, besides being a NMDA antag-onist KET is also a potent D2R agantag-onist (Kapur and Seeman, 2002).
A two-hits model of schizophrenia (combination of a prenatal im-mune challenge with poly I:C and juvenile restraint stress during ado-lescence) showed that 50 mg/kg ALA administered before restraint stress prevented the PPI deficits and D2R increase in the PFC of mice (Deslauriers et al., 2014). Besides evaluating only the preventive effect of ALA, these authors (Deslauriers et al., 2014) did not evaluate param-eters related to working memory (cognitive-like symptoms) and social interaction (negative-like symptoms). Since schizophrenia is a clinical syndrome (Carpenter, 2007), its different domains, as far as possible, should be addressed in the preclinical research.
In our results the working memory impairment induced by KET was not reversed by ALA alone, only social impairment was reversed. On the other hand, both doses of CLZ and ALA + CLZ2.5 reversed the working memory deficits induced by KET. Both cognitive and negative symp-toms of schizophrenia are related to pro-oxidant and inflammatory al-terations (Do et al., 2009). Indeed, previous studies revealed that ALA Fig. 5.Levels of lipid peroxidation of animals submitted to the KET-induced model of
schizophrenia and subjected to the reversal treatment with ALA, CLZ or their combination in the prefrontal cortex (A), hippocampus (B) and striatum (C). Bars represent mean ± SEM of the % of correct alternations (n = 7–8 animals/group). *Pb0.05 versus control; #P
b0.05 versus KET + saline according to two-way ANOVA followed by Tukey's post hoc test. ALA = alpha lipoic acid; CLZ2.5 or 5 = clozapine 2.5 or 5 mg/kg; KET = ketamine.
Fig. 6.Nitrite levels in the prefrontal cortex (A), hippocampus (B) and striatum (C) of an-imals submitted to the KET-induced model of schizophrenia and subjected to the reversal treatment with ALA, CLZ or their combination. Bars represent mean ± SEM of the % of cor-rect alternations (n = 7–8 animals/group). *Pb0.05 versus saline;#Pb0.05 versus
KET + saline;$P
b0.05 versus KET + ALA;+P
presents anti-inflammatory (Maczurek et al., 2008) and antioxidant (Biewenga et al., 1997; Macêdo et al., 2012) properties.
Another important observation related to our study was that only the combination of ALA + CLZ2.5 reversed most of the changes induced by KET. On the contrary, the combination of ALA + CLZ5 caused motor impairment as can be observed by the decrease in 43.9% in the number of crossings in KET + ALA + CLZ5-treated animals when compared to saline ones and also by the decrease in the number of rearings in saline + ALA + CLZ5 group when compared to saline. Thus, we can propose that the deficits in PPI and social interaction observed in the an-imals administered ALA + CLZ5 were possibly due to a motor impair-ment caused by an interference of both drugs with D2Rs (Deslauriers et al., 2013, 2014). Indeed, animals treated with D2R antagonists as well as D2R knockout animals present motor impairments in the open field test (Kelly et al., 1998). It was previously shown in SH-SY5Y cells a partially synergy between ALA and haloperidol on the same pathway related to D2R (Deslauriers et al., 2013). As far as we know there are no reports about a possible pharmacodynamic interaction between ALA and CLZ. Regarding the oxidative and neurotrophic parameters evaluat-ed in our study we did not detect alterations in these parameters that could justify the behavioral deficits observed in ALA + CLZ5 treated an-imals, therefore future studies evaluating the effects of this combination on D2Rs must be conducted.
We did not observe a potentiation of effects by the administration of ALA + CLZ2.5. Indeed, the animals administered KET + CLZ, KET + ALA or KET + ALA + CLZ2.5 mostly reached the same results, which were comparable to those obtained with saline-treated animals. This called our attention for a possible ceiling effect of the drugs alone, which prevented us from seeing a potentiation of effects in the combination group (ALA + CZP2.5).
Besides being a noncompetitive NMDA receptor antagonist, it was previously demonstrated that 20 mg/kg KET increase glutamate outflow in the PFC (Moghaddam et al., 1997). In line with this evidence genome-wide association studies and gene set enrichment analysis revealed the participation of glutamate metabolism pathway in schizophrenia (Jia et al., 2010). Glutamate is metabolized to gamma-aminobutyric acid or converted to GSH. Impairment in GSH synthesis is associated with schizophrenia (Gysin et al., 2007). Conversely, in rodents, a
schizophrenia-like phenotype is observed by GSH postnatal depletion (Cabungcal et al., 2006) and by genetically compromised GSH synthesis (Kulak et al., 2012). We recently reported that KET repeated administra-tion to adult mice decreases GSH levels in the PFC and ST (Monte et al., 2013).
Here we observed an increase in GSH levels only in the HC of mice treated with KET + ALA. A previous study showed that ALA induces GSH synthesis by the activation of the transcription factor, nuclear factor erythroid 2-related factor (Nrf2) (Suh et al., 2004).
The oxidative damage of lipids is a biomarker of oxidative stress in schizophrenia (Zhang et al., 2010). In fact, our group observed incre-ment in MDA levels in the PFC, HC and ST of mice after repeated admin-istration of KET (Monte et al., 2013).
In the present study we replicated our previousfindings (Monte et al., 2013) of increased MDA levels after KET administration in the PFC, HC and ST. Additionally we observed that the administration of ALA, CLZ or their combination reversed this increase in all brain areas.
We recently observed an anti-inflammatory effect of CLZ 25 mg/kg in the PFC, HC and ST of adult male rats neonatally challenged with poly I:C. However, CLZ failed to reverse the brain oxidative alterations (Ribeiro et al., 2013). Probably in our previous study the dose of CLZ may have contributed to the pro-oxidant alterations found. In the present study, we did not observe lipid peroxidation in the groups administered CLZ 2.5 or 5 after KET.
In schizophrenic patients ALA supplementation caused no signifi -cant alterations on oxidative damage, although in healthy controls ALA decreased lipid peroxidation, oxidative damage of proteins and im-proved non-enzymatic antioxidant capacity (Vidovićet al., 2014). This
cited study did not evaluate schizophrenia symptoms.
One of the many candidates linked to schizophrenia pathophysiolo-gy is NO (Bernstein et al., 2005). Neuronal NO synthase gene polymor-phism may result in increased susceptibility to this mental disorder (Shinkai et al., 2002). In schizophrenic patients both decreases and in-creases in NOS activity were observed (Bernstein et al., 2005).
Here the repeated administration of KET decreased nitrite while in-creased levels were detected in KET-treated animals post-treated with ALA alone (ST) and ALA + CLZ2.5 (PFC, HC and ST). Interestingly, in re-cent years the administration of NO donors to schizophrenic patients is bringing important results in the control of acute symptoms (Hallak et al., 2013).
Oxidative imbalance (Chong et al., 2005) and alterations in NO (Böhme et al., 1991) compromises synaptic plasticity and cell survival. BDNF plays multiple roles in regulating the differentiation, survival, and plasticity of several different populations of neurons (Cheng et al., 2003; Baydyuk and Xu, 2014), being essential for normal brain development.
Thus, on the basis of the pro-oxidant alterations and decreased ni-trite levels observed in the animals administered KET we decided to evaluate the levels of BDNF in the PFC of these mice. KET repeated administration decreased the levels of BDNF while ALA, CLZ or their combination significantly reversed this alteration.
Decreased plasma levels of BDNF were observed in schizophrenic patients (Chen et al., 2009; Zhang et al., 2012). A recent meta-analysis revealed moderately reduced peripheral BDNF levels in the serum and plasma of schizophrenic patients compared with control and increased levels after antipsychotic treatment (Fernandes et al., 2014). The repeat-ed administration of KET to adolescent rats causrepeat-ed no difference in the mRNA BDNF expression in the PFC, HC and ST (Gama et al., 2012).
The mainfinding of the present study was the determination of ALA effects alone and combined with CLZ in the reversal of three core behav-ioral alterations that resemble schizophrenia. The main limitation was that we could not assess a synergic effect with the combination of CLZ and ALA, probably because each drug alone reached a ceiling effect. Therefore, new studies must be conducted with lower doses of CLZ.
In conclusion, we determined that the endogenous compound ALA is effective in the reversal of positive- and negative-like symptoms of Fig. 7.Levels of brain-derived neurotrophic factor (BDNF) in the prefrontal cortex of
ani-mals submitted to the KET-induced model of schizophrenia and subjected to the reversal treatment with ALA, CLZ or their combination. Bars represent mean ± SEM of the levels of BDNF (n = 5–6 animals/group). *Pb0.05 versus saline;#P
b0.05 versus KET;+P b0.05 versus KET + CLZ5;&P
schizophrenia induced by KET repeated administration, behaving, thus, as an antipsychotic drug. Furthermore, we did not observe a potentia-tion of effects by the administrapotentia-tion of CLZ and ALA because it seems that both drugs alone reached a ceiling effect. Yet, the combination of ALA + CLZ5 caused motor impairments in the openfield test without causing pro-oxidant alterations or decrease BDNF levels. This calls at-tention for a possible alteration in D2R induced by this combination that needs to be addressed in future studies. Overall, our results give ev-idences of a dose reduction of CLZ when administered in combination with ALA, although this observation must be replicated in future studies. The present study brings additional evidences for the use of the antiox-idant ALA and antipsychotics in combination, opening a venue for con-firmation of these results in clinical trials.
Role of funding source
Brazilian Institutions, CNPq, CAPES and FUNCAP partially funded this study.
Contributors
Authors GSV, NCX, CNSS, TQO and LLLL performed the experiments and gave helpful input in the experimental design. Authors DM, CSG, SMMV and DFL designed the study, analyzed the data and wrote the paper. Authors GSV and DM undertook the statistical analysis. All authors have contributed to and approved thefinal manuscript.
Conflict of interests
The authors declare no conflict of interests
Acknowledgment
We thank the Brazilian Institutions, CNPq, CAPES and FUNCAP for thefinancial support of this study.
References
Archer, J., 1973.Tests for emotionality in rats and mice: a review. Anim. Behav. 21, 205–235.
Baydyuk, M., Xu, B., 2014. BDNF signaling and survival of striatal neurons. Front. Cell. Neurosci. 8, 254.http://dx.doi.org/10.3389/fncel.2014.00254.
Becker, A., Grecksch, G., 2004. Ketamine-induced changes in rat behaviour: a possible an-imal model of schizophrenia. Test of predictive validity. Prog. Neuropsychopharmacol. Biol. Psychiatry 28, 1267–1277.http://dx.doi.org/10.1016/j.pnpbp.2004.06.019. Bernstein, H.-G., Bogerts, B., Keilhoff, G., 2005. The many faces of nitric oxide in
schizo-phrenia. A review. Schizophr. Res. 78, 69–86.http://dx.doi.org/10.1016/j.schres. 2005.05.019.
Biewenga, G.P., Haenen, G.R., Bast, A., 1997.The pharmacology of the antioxidant lipoic acid. Gen. Pharmacol. 29, 315–331.
Böhme, G.A., Bon, C., Stutzmann, J.M., Doble, A., Blanchard, J.C., 1991.Possible involve-ment of nitric oxide in long-term potentiation. Eur. J. Pharmacol. 199, 379–381.
Byrne, P., 2007. Managing the acute psychotic episode. BMJ 334, 686–692.http://dx.doi. org/10.1136/bmj.39148.668160.80.
Cabungcal, J.-H., Nicolas, D., Kraftsik, R., Cuénod, M., Do, K.Q., Hornung, J.-P., 2006. Gluta-thione deficit during development induces anomalies in the rat anterior cingulate GABAergic neurons: relevance to schizophrenia. Neurobiol. Dis. 22, 624–637.http:// dx.doi.org/10.1016/j.nbd.2006.01.003.
Carpenter, W.T., 2007. Schizophrenia: disease, syndrome, or dimensions. Fam. Process 46, 199–206.http://dx.doi.org/10.1111/j.1545-5300.2007.00204.x.
Chen, D.C., Wang, J., Wang, B., Yang, S.C., Zhang, C.X., Zheng, Y.L., Li, Y.L., Wang, N., Yang, K.B., Xiu, M.H., Kosten, T.R., Zhang, X.Y., 2009. Decreased levels of serum brain-derived neurotrophic factor in drug-naïvefirst-episode schizophrenia: relationship to clinical phenotypes. Psychopharmacology (Berlin) 207, 375–380.http://dx.doi. org/10.1007/s00213-009-1665-6.
Cheng, A., Wang, S., Cai, J., Rao, M.S., Mattson, M.P., 2003. Nitric oxide acts in a positive feedback loop with BDNF to regulate neural progenitor cell proliferation and differen-tiation in the mammalian brain. Dev. Biol. 258, 319–333.http://dx.doi.org/10.1016/ S0012-1606(03)00120-9.
Chindo, B.A., Adzu, B., Yahaya, T.A., Gamaniel, K.S., 2012. Ketamine-enhanced immobility in forced swim test: a possible animal model for the negative symptoms of schizo-phrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 38, 310–316.http://dx.doi. org/10.1016/j.pnpbp.2012.04.018.
Chong, Z.Z., Li, F., Maiese, K., 2005. Oxidative stress in the brain: novel cellular targets that govern survival during neurodegenerative disease. Prog. Neurobiol. 75, 207–246.
http://dx.doi.org/10.1016/j.pneurobio.2005.02.004.
Contreras-Shannon, V., Heart, D.L., Paredes, R.M., Navaira, E., Catano, G., Maffi, S.K., Walss-Bass, C., 2013. Clozapine-induced mitochondria alterations and inflammation in brain and insulin-responsive cells. PLoS ONE 8, e59012.http://dx.doi.org/10.1371/journal. pone.0059012.
Coyle, J.T., Balu, D., Benneyworth, M., Basu, A., Roseman, A., 2010.Beyond the dopamine receptor: novel therapeutic targets for treating schizophrenia. Dialogues Clin. Neurosci. 12, 359–382.
Dall'Igna, O.P., Fett, P., Gomes, M.W., Souza, D.O., Cunha, R.A., Lara, D.R., 2007. Caffeine and adenosine A(2a) receptor antagonists prevent beta-amyloid (25–35)-induced
cogni-tive deficits in mice. Exp. Neurol. 203, 241–245.http://dx.doi.org/10.1016/j. expneurol.2006.08.008.
Deslauriers, J., Desmarais, C., Sarret, P., Grignon, S., 2013.α-Lipoic acid interaction with dopamine D2 receptor-dependent activation of the Akt/GSK-3βsignaling pathway induced by antipsychotics: potential relevance for the treatment of schizophrenia. J. Mol. Neurosci. 50, 134–145.http://dx.doi.org/10.1007/s12031-012-9884-4. Deslauriers, J., Racine, W., Sarret, P., Grignon, S., 2014. Preventive effect ofα-lipoic acid on
prepulse inhibition deficits in a juvenile two-hit model of schizophrenia. Neurosci-ence 272, 261–270.http://dx.doi.org/10.1016/j.neuroscience.2014.04.061. Do, K.Q., Cabungcal, J.H., Frank, A., Steullet, P., Cuenod, M., 2009. Redox dysregulation,
neurodevelopment, and schizophrenia. Curr. Opin. Neurobiol. 19, 220–230.http:// dx.doi.org/10.1016/j.conb.2009.05.001.
Emsley, R., Chiliza, B., Asmal, L., du Plessis, S., Phahladira, L., van Niekerk, E., van Rensburg, S.J., Harvey, B.H., 2014. A randomized, controlled trial of omega-3 fatty acids plus an antioxidant for relapse prevention after antipsychotic discontinuation infirst-episode schizophrenia. Schizophr. Res. 158, 230–235.http://dx.doi.org/10.1016/j.schres.2014. 06.004.
Favalli, G., Li, J., Belmonte-de-Abreu, P., Wong, A.H.C., Daskalakis, Z.J., 2012. The role of BDNF in the pathophysiology and treatment of schizophrenia. J. Psychiatr. Res. 46, 1–11.http://dx.doi.org/10.1016/j.jpsychires.2011.09.022.
Fernandes, B.S., Steiner, J., Berk, M., Molendijk, M.L., Gonzalez-Pinto, A., Turck, C.W., Nardin, P., Gonçalves, C.-A., 2014. Peripheral brain-derived neurotrophic factor in schizophrenia and the role of antipsychotics: meta-analysis and implications. Mol. Psychiatryhttp://dx.doi.org/10.1038/mp.2014.117.
Gama, C.S., Canever, L., Panizzutti, B., Gubert, C., Stertz, L., Massuda, R., Pedrini, M., de Lucena, D.F., Luca, R.D., Fraga, D.B., Heylmann, A.S., Deroza, P.F., Zugno, A.I., 2012. Ef-fects of omega-3 dietary supplement in prevention of positive, negative and cognitive symptoms: a study in adolescent rats with ketamine-induced model of schizophre-nia. Schizophr. Res. 141, 162–167.http://dx.doi.org/10.1016/j.schres.2012.08.002. Giamattei, L., 1957.Thioctic acid in therapy of schizophrenia. Osp. Psichiatr. 25, 221–228.
Green, L., Goldman, P., 1981. Nitrate synthesis in the germfree and conventional rat. Sci-ence 212 (80), 56–58.http://dx.doi.org/10.1126/science.6451927.
Gysin, R., Kraftsik, R., Sandell, J., Bovet, P., Chappuis, C., Conus, P., Deppen, P., Preisig, M., Ruiz, V., Steullet, P., Tosic, M., Werge, T., Cuénod, M., Do, K.Q., 2007. Impaired gluta-thione synthesis in schizophrenia: convergent genetic and functional evidence. Proc. Natl. Acad. Sci. U. S. A. 104, 16621–16626.http://dx.doi.org/10.1073/pnas. 0706778104.
Hallak, J.E.C., Maia-de-Oliveira, J.P., Abrao, J., Evora, P.R., Zuardi, A.W., Crippa, J.A.S., Belmonte-de-Abreu, P., Baker, G.B., Dursun, S.M., 2013. Rapid improvement of acute schizophrenia symptoms after intravenous sodium nitroprusside: a randomized, double-blind, placebo-controlled trial. JAMA Psychiatry 70, 668–676.http://dx.doi. org/10.1001/jamapsychiatry.2013.1292.
Jia, P., Wang, L., Meltzer, H.Y., Zhao, Z., 2010. Common variants conferring risk of schizo-phrenia: a pathway analysis of GWAS data. Schizophr. Res. 122, 38–42.http://dx.doi. org/10.1016/j.schres.2010.07.001.
Kapur, S., Seeman, P., 2002. NMDA receptor antagonists ketamine and PCP have direct ef-fects on the dopamine D(2) and serotonin 5-HT(2) receptors—implications for models of schizophrenia. Mol. Psychiatry 7, 837–844.http://dx.doi.org/10.1038/sj. mp.4001093.
Kelly, M.A., Rubinstein, M., Phillips, T.J., Lessov, C.N., Burkhart-Kasch, S., Zhang, G., Bunzow, J.R., Fang, Y., Gerhardt, G.A., Grandy, D.K., Low, M.J., 1998.Locomotor activity in D2 dopamine receptor-deficient mice is determined by gene dosage, genetic back-ground, and developmental adaptations. J. Neurosci. 18, 3470–3479.
Kim, E., Park, D.-W., Choi, S.-H., Kim, J.-J., Cho, H.-S., 2008. A preliminary investigation of alpha-lipoic acid treatment of antipsychotic drug-induced weight gain in patients with schizophrenia. J. Clin. Psychopharmacol. 28, 138–146.http://dx.doi.org/10. 1097/JCP.0b013e31816777f7.
Kulak, A., Cuenod, M., Do, K.Q., 2012. Behavioral phenotyping of glutathione-deficient mice: relevance to schizophrenia and bipolar disorder. Behav. Brain Res. 226, 563–570.http://dx.doi.org/10.1016/j.bbr.2011.10.020.
Leo, R.J., Regno, P. Del, 2000.Atypical antipsychotic use in the treatment of psychosis in primary care. Prim. Care Companion J. Clin. Psychiatry 2, 194–204.
Leucht, S., Cipriani, A., Spineli, L., Mavridis, D., Orey, D., Richter, F., Samara, M., Barbui, C., Engel, R.R., Geddes, J.R., Kissling, W., Stapf, M.P., Lässig, B., Salanti, G., Davis, J.M., 2013. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophre-nia: a multiple-treatments meta-analysis. Lancet 382, 951–962.http://dx.doi.org/10. 1016/S0140-6736(13)60733-3.
Levin, R., Calzavara, M.B., Santos, C.M., Medrano, W.A., Niigaki, S.T., Abílio, V.C., 2011. Spontaneously Hypertensive Rats (SHR) present deficits in prepulse inhibition of startle specifically reverted by clozapine. Prog. Neuropsychopharmacol. Biol. Psychia-try 35, 1748–1752.http://dx.doi.org/10.1016/j.pnpbp.2011.06.003.
Macêdo, D.S., Medeiros, C.D., Cordeiro, R.C., Sousa, F.C., Santos, J.V., Morais, T., a, Hyphantis, T.N., McIntyre, R.S., Quevedo, J., Carvalho, A.F.,, 2012. Effects of alpha-lipoic acid in an animal model of mania induced byD-amphetamine. Bipolar Disord. 14, 707–718.http://dx.doi.org/10.1111/j.1399-5618.2012.01046.x.
Maczurek, A., Hager, K., Kenklies, M., Sharman, M., Martins, R., Engel, J., Carlson, D.A., Münch, G., 2008. Lipoic acid as an anti-inflammatory and neuroprotective treatment for Alzheimer's disease. Adv. Drug Deliv. Rev. 60, 1463–1470.http://dx.doi.org/10. 1016/j.addr.2008.04.015.
Meltzer, H.Y., Rajagopal, L., Huang, M., Oyamada, Y., Kwon, S., Horiguchi, M., 2013. Trans-lating the N-methyl-D-aspartate receptor antagonist model of schizophrenia to treat-ments for cognitive impairment in schizophrenia. Int. J. Neuropsychopharmacol. 16, 2181–2194.http://dx.doi.org/10.1017/S1461145713000928.
Moghaddam, B., Adams, B., Verma, A., Daly, D., 1997.Activation of glutamatergic neuro-transmission by ketamine: a novel step in the pathway from NMDA receptor block-ade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J. Neurosci. 17, 2921–2927.
Moini, H., Packer, L., Saris, N.-E.L., 2002.Antioxidant and prooxidant activities of alpha-lipoic acid and dihydroalpha-lipoic acid. Toxicol. Appl. Pharmacol. 182, 84–90.
Monte, A.S., de Souza, G.C., McIntyre, R.S., Soczynska, J.K., dos Santos, J.V., Cordeiro, R.C., Ribeiro, B.M.M., de Lucena, D.F., Vasconcelos, S.M.M., de Sousa, F.C.F., Carvalho, A.F., Macêdo, D.S., 2013. Prevention and reversal of ketamine-induced schizophrenia re-lated behavior by minocycline in mice: possible involvement of antioxidant and nitrergic pathways. J. Psychopharmacol. 27, 1032–1043.http://dx.doi.org/10.1177/ 0269881113503506.
Moreira, F.A., Guimarães, F.S., 2005. Cannabidiol inhibits the hyperlocomotion induced by psychotomimetic drugs in mice. Eur. J. Pharmacol. 512, 199–205.http://dx.doi.org/ 10.1016/j.ejphar.2005.02.040.
Ohkawa, H., Ohishi, N., Yagi, K., 1979.Assay for lipid peroxides in animal tissues by thio-barbituric acid reaction. Anal. Biochem. 95, 351–358.
Radenovic, L., Selakovic, V., 2005. Differential effects of NMDA and AMPA/kainate receptor antagonists on nitric oxide production in rat brain following intrahippocampal injec-tion. Brain Res. Bull. 67, 133–141.http://dx.doi.org/10.1016/j.brainresbull.2005.06. 019.
Radyushkin, K., Hammerschmidt, K., Boretius, S., Varoqueaux, F., El-Kordi, A., Ronnenberg, A., Winter, D., Frahm, J., Fischer, J., Brose, N., Ehrenreich, H., 2009. Neuroligin-3-defi -cient mice: model of a monogenic heritable form of autism with an olfactory deficit. Genes Brain Behav. 8, 416–425.http://dx.doi.org/10.1111/j.1601-183X.2009.00487.x. Ribeiro, B.M.M., do Carmo, M.R.S., Freire, R.S., Rocha, N.F.M., Borella, V.C.M., de Menezes, A.T., Monte, A.S., Gomes, P.X.L., de Sousa, F.C.F., Vale, M.L., de Lucena, D.F., Gama, C.S., Macêdo, D., 2013. Evidences for a progressive microglial activation and increase in iNOS expression in rats submitted to a neurodevelopmental model of schizophre-nia: reversal by clozapine. Schizophr. Res. 151, 12–19.http://dx.doi.org/10.1016/j. schres.2013.10.040.
Sedlák, J., L' Hanus, 1982.Changes of glutathione and protein bound SH-groups concen-tration in rat adrenals under acute and repeated stress. Endocrinol. Exp. 16, 103–109.
Shinkai, T., Ohmori, O., Hori, H., Nakamura, J., 2002. Allelic association of the neuronal ni-tric oxide synthase (NOS1) gene with schizophrenia. Mol. Psychiatry 7, 560–563.
http://dx.doi.org/10.1038/sj.mp.4001041.
Suh, J.H., Shenvi, S.V., Dixon, B.M., Liu, H., Jaiswal, A.K., Liu, R.-M., Hagen, T.M., 2004. De-cline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthe-sis, which is reversible with lipoic acid. Proc. Natl. Acad. Sci. U. S. A. 101, 3381–3386.
http://dx.doi.org/10.1073/pnas.0400282101.
Swerdlow, N.R., Mansbach, R.S., Geyer, M.A., Pulvirenti, L., Koob, G.F., Braff, D.L., 1990. Am-phetamine disruption of prepulse inhibition of acoustic startle is reversed by deple-tion of mesolimbic dopamine. Psychopharmacology (Berlin) 100, 413–416.http:// dx.doi.org/10.1007/BF02244616.
Thaker, G.K., 2007. Schizophrenia endophenotypes as treatment targets. Expert Opin. Ther. Targets 11, 1189–1206.http://dx.doi.org/10.1517/14728222.11.9.1189. Van den Buuse, M., 2010. Modeling the positive symptoms of schizophrenia in genetically
modified mice: pharmacology and methodology aspects. Schizophr. Bull. 36, 246–270.http://dx.doi.org/10.1093/schbul/sbp132.
Vidović, B., Milovanović, S., Dorđević, B., Kotur-Stevuljević, J., Stefanović, A., Ivanišević, J., Miljković, M., Spasić, S., Stojanović, D., Pantović, M., 2014.Effect of alpha-lipoic acid supplementation on oxidative stress markers and antioxidative defense in patients with schizophrenia. Psychiatr. Danub. 26, 205–213.
Yamada, K., Noda, Y., Hasegawa, T., Komori, Y., Nikai, T., Sugihara, H., Nabeshima, T., 1996.
The role of nitric oxide in dizocilpine-induced impairment of spontaneous alternation behavior in mice. J. Pharmacol. Exp. Ther. 276, 460–466.
Yusufi, B., Mukherjee, S., Flanagan, R., Paton, C., Dunn, G., Page, E., Barnes, T.R.E., 2007. Prevalence and nature of side effects during clozapine maintenance treatment and the relationship with clozapine dose and plasma concentration. Int. Clin. Psychopharmacol. 22, 238–243.http://dx.doi.org/10.1097/YIC.0b013e32819f8f17. Zhang, M., Zhao, Z., He, L., Wan, C., 2010. A meta-analysis of oxidative stress markers in
schizophrenia. Sci. China Life Sci. 53, 112–124. http://dx.doi.org/10.1007/s11427-010-0013-8.
Zhang, X.Y., Liang, J., Chen, D.C., Xiu, M.H., Yang, F. De, Kosten, T.A., Kosten, T.R., 2012. Low BDNF is associated with cognitive impairment in chronic patients with schizophrenia. Psychopharmacology (Berlin) 222, 277–284. http://dx.doi.org/10.1007/s00213-012-2643-y.