HMGCR
gene polymorphism is associated with stroke risk
in the EPIC-Norfolk study
Renata N. Freitas
a,b, Kay-Tee Khaw
b,c, Kelvin Wu
d, Richard Bowman
d,
Hannah Jeffery
d, Robert Luben
c, Nick J. Wareham
c,eand Sheila Rodwell
b,c,da
DENCS, School of Nutrition and NUPEB, Federal University of Ouro Preto, Ouro Preto, Minas Gerais,
Brazil,
bMRC Centre for Nutritional Epidemiology in Cancer Prevention and Survival (CNC),
cEPIC Norfolk,
Department of Public Health and Primary Care, University of Cambridge,
dMRC Dunn Human Nutrition Unit,
Cambridge and
eMRC Epidemiology Unit, Cambridge, UK
Received19 June 2009Accepted9 July 2009
Background Earlier, a G/T single nucleotide polymorphism (SNP) in theHMGCRgene was shown to significantly reduce the overall serum lipids response to pravastatin. This study aimed to investigate the relationship of the rs17238540 SNP with coronary heart disease, stroke and cardiovascular disease risk.
Design Cross-sectional study from the European Prospective Investigation into Cancer and Nutrition-Norfolk cohort.
Methods Genotype was determined by pyrosequencing 23 011 participants, for whom clinical and biochemical data were available. Baseline risk factors according to genotype were evaluated, and the risk for fatal and nonfatal stroke, ischaemic heart disease and all types of cardiovascular diseases were assessed by logistic regression after approximately 11 years of follow-up.
Results The G allele carriers presented 1.4 mmHg higher systolic blood pressure and 0.8 mmHg higher diastolic blood pressure than those who were TT carriers. They also presented higher risk of prevalent total (odds ratio: 1.44, 95% confidence interval: 1.05–1.97,P= 0.025) and nonfatal (odds ratio: 1.56, 95% confidence interval: 1.12–2.17,P= 0.009) stroke
events compared with the TT individuals in the multivariate models.
Conclusion An association between the rs17238540 SNP and stroke risk was observed, independent of the effect of the SNP on the blood pressure. The possible mechanisms involved, besides the effect on blood pressure, might be related to pleiotropic functions of theHMGCR, and remain to be explored. Eur J Cardiovasc Prev Rehabil 17:89–93c 2010 The
European Society of Cardiology
European Journal of Cardiovascular Prevention and Rehabilitation2010,17:89–93
Keywords:HMGCR polymorphism, hypertension, stroke risk
Introduction
Cardiovascular diseases (CVDs), mainly coronary heart disease and stroke, are the main causes of death worldwide. In 2004, stroke caused 9.7% of all deaths around the world and was listed, just after coronary heart disease, as the second cause of death worldwide [1]. Epidemiologic studies have clearly shown that modifiable factors, such as smoking, hypercholesterolaemia and hypertension, are the most common risk factors for CVD in developed and in developing countries [2–4].
Numerous trials have shown that the 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR) inhibi-tors (statins) decrease coronary events in primary and secondary prevention of coronary heart disease, and also stroke risk [5,6]. Besides the well-known effects on serum lipids, statins have pleiotropic effects [6–9], which have been proposed as potentially contributing to the observed reduction in major cardiac events [5–7].
Several polymorphisms were identified in the HMGCR gene locus [8–11], and they have been studied for associations with lipids levels. However, most of these studies did not address the effects of these polymorph-isms in the risk for CVD [12–15], apart from one recent
Correspondence to Professor Renata N. Freitas, DENCS/ENUT/UFOP, Campus Universita´rio, Morro do Cruzeiro, Ouro Preto, MG 35400-000, Brazil
publication [16]. Two tightly linked single nucleotide polymorphisms (SNPs) were found to be significantly associated with a difference in the change in the serum lipids response to pravastatin treatment, but not with differences in the basal serum lipids concentration [8]. A significant reduction in the overall efficacy of pravastatin of 22.3% was observed for SNP rs17238540 [8].
The aim of this study was to investigate the influence of the SNP rs17238540 of the HMGCR gene in coronary heart disease, stroke and CVD risk in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk cohort study.
Materials and methods
Study protocolEPIC-Norfolk is a prospective population study of White men and women recruited at the age of 45–75 years from general practice age–sex register in Norfolk, UK from 1993 to 1997. Approximately, 25 000 people participating in the baseline survey, who had filled a detailed health and lifestyle questionnaire, attended a health checkup where-in blood and urwhere-ine samples, and data on height, weight, waist circumference and blood pressure were collected as described earlier [17]. Medical history, blood lipids, smoking status and habitual physical activity were assessed as described elsewhere [18–20]. Alcohol intake was assessed by a Food Frequency Questionnaire filled by the participants before the first health checkup [21].
Participants were followed up for health end points. Data on fatal and nonfatal events were identified from death certificates or hospital discharge codes for stroke (ICD-9, 430–438), ischaemic heart disease (ICD-9, 410–414) and CVDs (ICD-9, 401–448). This study reports follow-up data until 29 February 2008. The EPIC-Norfolk Study was approved by the Norfolk Health District Ethics Committee.
Genotype determination
Genomic DNA was obtained from blood samples collected by health examinations occasion [19]. HMGCR G/T SNP (rs17238540) was assessed using pyrosequen-cing, detailed procedure of which has been published earlier [22]. The genetic analysis were repeated in separated experiments for a total of 1322 samples of 23 011 successfully genotyped participants to check the reproducibility of the method, and these analysis were 99.9% concordant.
Statistical analysis
Data on theHMGCRSNP was available for 23 011 though only 20 805 had complete data on all variables for the full multiple adjusted analyses. Allele frequencies were obtained by gene counting and differences between the observed and the expected genotype frequencies were tested byw2test.
Characteristics of people with different genotypes were compared. Differences in means were tested using analysis of variance and differences in the frequency of the categorical variables and of cardiovascular events (stroke, ischaemic heart disease and all types of CVDs) between the genotype groups were examined using w2.
Logistic regression models were used to ascertain the odds ratio (OR) for the occurrence of total, nonfatal and fatal cardiovascular events according to the genotype group. Continuous [age, systolic blood pressure (SBP), diastolic blood pressure (DBP), waist circumference, BMI, alcohol intake, triglycerides and LDL-cholesterol] and categorical (sex, smoking status, physical activity and use of antihypertensive and lipid-lowering drugs) variables were included as covariates in the models. Covariates were measured at baseline. The results are presented as ORs and 95% confidence intervals. AllP values are two-tailed and aPvalue of less than 0.05 was considered significant. The data were analysed using SPSS for Windows, version 16.0 (SPSS Inc., Chicago, Illinois, USA).
Results
Genotype frequencies of 23 011 participants for whom the genetic data were available were, TT 95.65%, TG 4.29% and GG 0.06%. The genotype frequencies were in the Hardy–Weinberg equilibrium (w2= 0.068) and did
not differ between men and women. The baseline characteristics and distribution of selected cardiovascular risk factors according to genotype are presented in Table 1. Individuals carrying the minor allele (G) were pooled in the same group, because of the low number of homozygous for this allele. Individuals carrying the G allele were older and had higher SBP and DBP than TT individuals. No other difference was observed in the variables analysed between the genotype groups, apart from a significantly higher proportion of history of stroke in the G allele carriers group. Individuals using lipid-lowering drugs were very few at the baseline (n= 340), and we do not have information on the nature of drug (whether statins or not) or how many would have started using statins during follow-up.
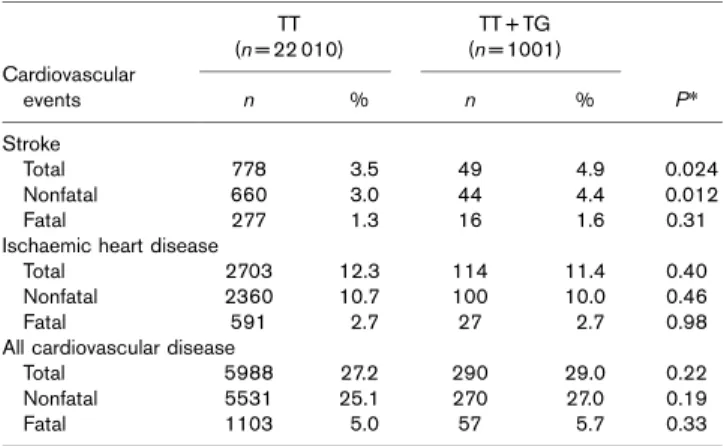
Nonfatal and total (nonfatal and fatal) stroke occurred more frequently in the G allele carriers, whereas the frequency of fatal stroke did not differ between the geno-types (Table 2). Otherwise, the frequencies of ischaemic heart and all types of CVDs (fatal, nonfatal and total) were not different between the genotype groups.
of antihypertensive and lipid-lowering drugs (Model 2) did not materially alter the results. Alcohol intake and BMI were also tested as covariates, but the results did not change and they were not included in the fully adjusted model. The odds for nonfatal stroke was also significantly more than 50% higher for the G allele group in both
models tested. The G allele carriers showed only a nonsignificant 16% higher odds for fatal stroke in the fully adjusted model. Otherwise, the OR for ischaemic heart disease and all types of CVDs did not show relationship with the polymorphism.
Discussion
Here, we observed the association between the SNP rs17238 540 in theHMGCRgene and the OR for stroke in a large prospective population study. To the best of our knowledge, this polymorphism has not been studied in relation to any end point for CVD before, although it has been reported to reduce the efficacy of pravastatin in lowering serum lipids [8]. In our study, we did not find any association of the SNP rs17238540 with the baseline serum lipids, in concordance with other studies [8,14,23].
In the past years, there has been an increasing interest in the genetic epidemiology of stroke [24–27]. Although very much attention is being paid to the search for ‘stroke genes’, [28,29], the pathogenesis of sporadic strokes is multifactorial as are most complex disorders, and mediated by several dynamic processes, which may be
Table 1 Baseline characteristics and some cardiovascular risk factors distribution according to SNP rs17238540 onHMGCR
in EPIC-Norfolk
Variables
TT TG + GG
P*
n Mean ± SD n Mean ± SD
Age (years) 22 010 58.7 ± 9.3 1001 59.3 ± 9.2 0.03 BMI (kg/m2) 21 395 26.3 ± 3.9 977 26.4 ± 4.0 0.67
Waist circumfer-ence (cm)
21 412 88.0 ± 12.4 977 88.3 ± 12.3 0.36
SBP (mmHg) 21 389 135.3 ± 18.4 974 136.7 ± 18.4 0.02 DBP (mmHg) 21 389 82.4 ± 11.2 974 83.2 ± 11.4 0.03 Total cholesterol
(mmol/l)
20 816 6.19 ± 1.17 949 6.19 ± 1.18 0.88
LDL-cholesterol (mmol/l)
20 150 3.97 ± 1.04 913 3.99 ± 1.06 0.56
HDL-cholesterol 20 149 1.43 ± 0.43 913 1.42 ± 0.41 0.77 Triglycerides
(mmol/l)
20 814 1.81 ± 1.10 949 1.78 ± 1.04 0.44
n (%) n (%)
All 22 010 95.6 1001 4.4 0.79**
Men 9512 95.7 428 4.3
Women 12 498 95.6 573 4.4
Current smokers 2514 11.5 126 12.7 0.17
Physically active (moderately active and active)
9003 40.9 392 39.2 0.27
Lipid-lowering drug users
328 1.5 12 1.2 0.46
Antihypertensive drug users
3995 18.2 192 19.2 0.40
History of myocardial infarction
634 2.9 36 3.6 0.19
History of stroke 286 1.3 23 2.3 0.007
DBP, diastolic blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SBP, systolic blood pressure; SD, standard deviation; SNP, single nucleotide polymorphism. *P value for one-way analysis of variance tests (continuous variables) or Pearson’sw2tests (categorical variables) between
genotypes groups: TT and TG + GG. **P value for Pearson’s w2 test for
differences in the genotype distribution between men and women.
Table 3 Association between SNP rs17238540 onHMGCR
and risk of cardiovascular disease events risk in EPIC-Norfolka
Cardiovascular events
TT,n= 19 930, OR (95% CI)
TG + GG,n= 905, OR (95% CI) P
Stroke Total
Model 1 1.00 (Ref) 1.48 (1.08–2.02) 0.015
Model 2 1.44 (1.05–1.97) 0.024
Nonfatal
Model 1 1.00 (Ref) 1.60 (1.15–2.22) 0.005
Model 2 1.56 (1.12–2.17) 0.009
Fatal
Model 1 1.00 (Ref) 1.21 (0.70–2.09) 0.51
Model 2 1.16 (0.67–2.01) 0.61
Ischaemic heart disease Total
Model 1 1.00 (Ref) 0.82 (0.66–1.03) 0.09
Model 2 0.82 (0.65–1.04) 0.10
Nonfatal
Model 1 1.00 (Ref) 0.86 (0.68–1.09) 0.21
Model 2 0.87 (0.68–1.10) 0.25
Fatal
Model 1 1.00 (Ref) 0.88 (0.57–1.36) 0.56
Model 2 0.87 (0.56–1.35) 0.54
All cardiovascular diseases Total
Model 1 1.00 (Ref) 1.06 (0.91–1.25) 0.45
Model 2 1.03 (0.88–1.22) 0.69
Nonfatal
Model 1 1.00 (Ref) 1.08 (0.92–1.27) 0.35
Model 2 1.06 (0.90–1.25) 0.51
Fatal
Model 1 1.00 (Ref) 1.09 (0.80–1.47) 0.60
Model 2 1.06 (0.78–1.44) 0.72
Model 1: Adjusted by age and sex. Model 2: Adjusted by age, sex, systolic blood pressure, diastolic blood pressure, waist circumference, low-density lipoprotein-cholesterol, triglycerides, smoking status, physical activity and use of antihyper-tensive and lipid-lowering drugs. CI, confidence interval; OR, odds ratio; SNP, single nucleotide polymorphism.aAnalysis restricted to subset with data for all
variables available.
Table 2 Nonfatal, fatal and total cardiovascular events according to SNP rs17238540 onHMGCRin EPIC-Norfolk
Cardiovascular events
TT (n= 22 010)
TT + TG (n= 1001)
P*
n % n %
Stroke
Total 778 3.5 49 4.9 0.024
Nonfatal 660 3.0 44 4.4 0.012
Fatal 277 1.3 16 1.6 0.31
Ischaemic heart disease
Total 2703 12.3 114 11.4 0.40
Nonfatal 2360 10.7 100 10.0 0.46
Fatal 591 2.7 27 2.7 0.98
All cardiovascular disease
Total 5988 27.2 290 29.0 0.22
Nonfatal 5531 25.1 270 27.0 0.19
Fatal 1103 5.0 57 5.7 0.33
SNP, single nucleotide polymorphism.*Pvalue for Pearson’sw2tests between
genetically determined. Recently, several studies have addressed genes involved in such processes [19,30–32], which would partially influence the phenotype.
Hypertension is one of the most powerful risk factors for stroke [33,34]. The relationship of stroke mortality to usual blood pressure is observed at all ages, with no evidence of a threshold in the range of usual SBP above 115 mmHg or of usual DBP above 75 mmHg [35]. We observed higher SBP and DBP in the G allele carriers. Individuals carrying the G allele had a 1.4 mmHg higher SBP and 0.8 mmHg higher DBP than those who were TT carriers. However, when we included established risk factors, including SBP and DBP (Model 2), in the regression model, only a minor change in the OR was observed (approximately 3%) for both total and nonfatal stroke. Thus, the magnitude of the differences in stroke risk between the genotypes did not seem to be totally explained by SBP or DBP differences. Consistent with lack of effect of the polymorphism on blood lipids, we did not find any effect of the genotype in the OR for ischaemic heart disease.
Our results indicate that the effect of theHMGCRSNP rs17238540 on the odds for stroke is independent and apparently only partially explained by its effect on the blood pressure.
The mechanisms underlying our observation are specu-lative as the biological effect of this SNP is uncertain. As the polymorphism was found to reduce lipid changes in response to pravastatin [8], it might be related to an alteration of the enzyme’s expression, activity or drug binding. TheHMGCRSNP rs17238540 might counteract some of the observed pleiotropic effects of statins, such as improved endothelial function, decreased platelet aggreg-ability and reduced vascular inflammation [4,9,36], which has been associated with significant reduction of the stroke risk and has been proposed to prevent either primary or secondary cerebrovascular events [5]. It is also possible that this polymorphism is linked to other genetic changes within functional parts of theHMGCRgene and the observed effect in our study might reflect this.
The strengths of our study are the large population and the fair number of strokes; in addition, the lost to follow-up is negligible because mortality certification in the UK is virtually complete. Our stroke end points were ascertained through routine record linkage of all partici-pants with death certification and hospital admissions all over the UK National Health Service hospitals. A validation study carried out showed high accuracy of stroke ascertainment through the death certification and hospital record linkage methods used in the EPIC-Norfolk study [37]. All the variables were measured at baseline with end points assessed during 11 years
follow-up. The strength of this approach is that the exposures are measured before the outcome, and therefore do not suffer from recall biases that are a problem in case–control studies. We have shown earlier that this polymorphism is associated with the blood pressure response to urinary sodium/potassium ratio [22]. This and the observed lower effect of the polymorphism on the pravastatin response were the basis for the a priori hypothesis of this study. As it is not a whole genome association study examining thousands of genes, independent confirmatory studies are not essential. However, as with all other research, even for well-accepted associations, further studies are always helpful but other studies will need to obtain substantial additional data to do this research.
This study has some limitations. We did not distinguish between different stroke subtypes, and our results may therefore mask differences between these subtypes and the association found. Sporadic stroke is a complex disorder that can be divided into two major categories, haemorrhagic and ischaemic stroke, each having several subcategories, which can be identified as distinct phenotypes with their own aetiologies. Whether the association found in this study is related to a specific subtype deserves further investigation. However, the end points included (fatal and nonfatal stroke) are clinically relevant in terms of population impact. Another issue is that the end points were identified through mortality data and hospital records; therefore, it is possible that some mild stroke cases that were not hospitalized have been included in the ‘comparison/control’ group. Nevertheless, stroke misclassifications are likely only to attenuate any associations observed. The single measure of variables at baseline does not take into account changes during the period of follow up. However, the random measurement error resulting from this is likely only to attenuate any association observed, not producing spurious associations. Finally, we cannot rule out the possibility that the association found is because of chance, although these analyses examining the relationship of the candidate genotype to CVD were based on a priori hypothesis.
In summary, in this large prospective study, an indepen-dent association between stroke and the HMGCR rs17238540 SNP was found. Although the polymorphism was also associated with higher blood pressure, the magnitude of its effect on the OR for stroke cannot be explained by its effect on blood pressure. The mechan-isms involved are possibly related to pleiotropic roles for HMGCRgene beyond lipids metabolism, which remain to be explored.
Acknowledgements
Conflicts of interest: none declared.
References
1 World Health Organization.The global burden of disease:2004 update. Geneva, Switzerland: WHO Press; 2008.
2 Donnan GA, Fisher M, Macleod M, Davis SM. Stroke.Lancet2008; 371:1612–1623.
3 Leys D, Deplanque D, Mounier-Vehier C, kowiak-Cordoliani MA, Lucas C, Bordet R. Stroke prevention: management of modifiable vascular risk factors. J Neurol2002;249:507–517.
4 Myint PK, Sinha S, Luben RN, Bingham SA, Wareham NJ, Khaw KT. Risk factors for first-ever stroke in the EPIC-Norfolk prospective population-based study.Eur J Cardiovasc Prev Rehabil2008;15:663–669.
5 Amarenco P, Lavallee P, Touboul PJ. Stroke prevention, blood cholesterol, and statins.Lancet Neurol2004;3:271–278.
6 Paciaroni M, Hennerici M, Agnelli G, Bogousslavsky J. Statins and stroke prevention.Cerebrovasc Dis2007;24:170–182.
7 Ma¨rz W, Ko¨enig W. HMG-CoA reductase inhibition: anti-inflammatory effects beyond lipid lowering?Eur J Cardiovasc Prev Rehabil2003;10:169–179. 8 Chasman DI, Posada D, Subrahmanyan L, Cook NR, Stanton VP Jr,
Ridker PM. Pharmacogenetic study of statin therapy and cholesterol reduction.JAMA2004;291:2821–2827.
9 Leitersdorf E, Luskey KL. HgiAI polymorphism near the HMGCR promoter. Nucleic Acids Res1990;18:5584.
10 Leitersdorf E, Hwang M, Luskey KL. ScrFI polymorphism in the 2nd intron of the HMGCR gene.Nucleic Acids Res1990;18:5584.
11 Zuliani G, Hobbs HH. A high frequency of length polymorphism in repeated sequences adjacent to alu sequences.Am J Hum Genet2008;46:963–969. 12 Hubacek JA, Pistulkova´ H, Valenta Z, Poledne R. (TTA)n repeat
polymorphism in the HMG-CoA reductase gene and cholesterolaemia.Vasa 1999;28:169–171.
13 Plat J, Mensink RP. Relationship of genetic variation in genes encoding apolipoprotein A-IV, scavenger receptor BI, HMG-CoA reductase, CETP and apolipoprotein E with cholesterol metabolism and the response to plant stanol ester consumption.Eur J Clin Invest2002;32:242–250. 14 Singer JB, Holdaas H, Jardine AG, Fellstrom B, Os I, Bermann G,et al.
Genetic analysis of fluvastatin response and dyslipidemia in renal transplant recipients.J Lipid Res2007;48:2072–2078.
15 Tong Y, Zhang S, Li H, Su Z, Kong X, Liu H,et al. 8302A/C and (TTA)n polymorphisms in the HMG-CoA reductase gene may be associated with some plasma lipid metabolic phenotypes in patients with coronary heart disease.Lipids2004;39:239–241.
16 Hindorff LA, Lemaitre RN, Smith NL, Bis JC, Marciante KD, Rice KM,et al. Common genetic variation in six lipid-related and statin-related genes, statin use and risk of incident nonfatal myocardial infarction and stroke. Pharmacogenet Genomics2008;18:677–682.
17 Khaw KT, Bingham S, Welch A, Luben R, O’Brien E, Wareham N,et al. Blood pressure and urinary sodium in men and women: the Norfolk Cohort of the European Prospective Investigation into Cancer (EPIC-Norfolk).Am J Clin Nutr2004;80:1397–1403.
18 Wareham NJ, Jakes RW, Rennie KL, Schuit J, Mitchell J, Hennings S,et al. Validity and repeatability of a simple index derived from the short physical activity questionnaire used in the European Prospective Investigation into Cancer and Nutrition (EPIC) study.Public Health Nutr2003;6:407–413. 19 Day N, Oakes S, Luben R, Khaw KT, Bingham S, Welch A,et al.
EPIC-Norfolk: study design and characteristics of the cohort. European Prospective Investigation of Cancer.Br J Cancer1999;80:95–103.
20 Wu K, Bowman R, Welch AA, Luben RN, Wareham N, Khaw KT,et al. Apolipoprotein E polymorphisms, dietary fat and fibre, and serum lipids: the EPIC Norfolk study.Eur Heart J2007;28:2930–2936.
21 Bingham S, Luben R, Welch A, Low YL, Khaw KT, Wareham N,et al. Associations between dietary methods and biomarkers, and between fruits and vegetables and risk of ischaemic heart disease, in the EPIC Norfolk Cohort Study.Int J Epidemiol2008;37:978–987.
22 Freitas RN, Khaw KT, Wu K, Bowman R, Jeffery H, Luben R,et al. AHMGCRpolymorphism is associated with relations between blood pressure and urinary sodium and potassium ratio in the EPIC-Norfolk study. J Am Soc Hypertens2009;3:238–244.
23 Polisecki E, Muallem H, Maeda N, Peter I, Robertson M, McMahon AD,et al. Genetic variation at the LDL receptor and HMG-CoA reductase gene loci, lipid levels, statin response, and cardiovascular disease incidence in PROSPER.Atherosclerosis2008;200:109–114.
24 Markus HS, Alberts MJ. Update on genetics of stroke and cerebrovascular disease 2005.Stroke2006;37:288–290.
25 Schulz UGR, Flossmann E, Rothwell PM. Heritability of ischemic stroke in relation to age, vascular risk factors, and subtypes of incident stroke in population-based studies.Stroke2004;35:819–824.
26 Flossmann E, Schulz UGR, Rothwell PM. Systematic review of methods and results of studies of the genetic epidemiology of ischemic stroke.Stroke 2004;35:212–227.
27 Jerrard-Dunne P, Cloud G, Hassan A, Markus HS. Evaluating the genetic component of ischemic stroke subtypes: a family history study.Stroke2003; 34:1364–1369.
28 Alberts MJ. Genetics of cerebrovascular disease.Stroke2004; 35:342–344.
29 Gretarsdottir S, Thorleifsson G, Reynisdottir ST, Manolescu A, Jonsdottir S, Jonsdottir T,et al. The gene encoding phosphodiesterase 4D confers risk of ischemic stroke.Nat Genet2003;35:131–138.
30 Enquobahrie DA, Smith NL, Bis JC, Carty CL, Rice KM, Lumley T,et al. Cholesterol ester transfer protein, interleukin-8, peroxisome proliferator activator receptor alpha, and Toll-like receptor 4 genetic variations and risk of incident nonfatal myocardial infarction and ischemic stroke.Am J Cardiol 2008;101:1683–1688.
31 Marciante KD, Totah RA, Heckbert SR, Smith NL, Lemaitre RN, Lumley T, et al. Common variation in cytochrome P450 epoxygenase genes and the risk of incident nonfatal myocardial infarction and ischemic stroke. Pharmacogenet Genomics2008;18:535–543.
32 Lemaitre RN, Heckbert SR, Sotoodehnia N, Bis JC, Smith NL, Marciante KD, et al. Beta1-and beta2-adrenergic receptor gene variation, beta-blocker use and risk of myocardial infarction and stroke.Am J Hypertens2008; 21:290–296.
33 Ezzati M, Lopez AD, Rodgers A, Vander HS, Murray CJ. Selected major risk factors and global and regional burden of disease.Lancet2002; 360:1347–1360.
34 Beckett NS. Prevention of stroke.Eur J Cardiovasc Prev Rehabil2001; 8:257–264.
35 Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies.Lancet2002; 360:1903–1913.
36 Bellosta S, Ferri N, Bernini F, Paoletti R, Corsini A. Non-lipid-related effects of statins.Ann Med2000;32:164–176.