October 2015
David Rodrigues Palhares
Synthesis and biological evaluation of mono and
bis-naphthalimide derivatives against SH-SY5Y,
human brain cancer cells
Master Thesis
Masters in Medicinal Chemistry
Under the supervision of
Dr António Gil Fortes
and
Nome: David Rodrigues Palhares
Endereço electrónico: davidpalhares1990@gmail.com Telefone: (+351) 912566090
Título da dissertação: Synthesis and biological evaluation of mono and bis
naphthalimide derivatives against SH-SY5Y, human brain cancer cells
Orientadores:
Professora Doutora Maria José Alves Professor Doutor António Gil Fortes
Ano de Conclusão: 2015
Designação do Mestrado: Mestrado em Química Medicinal
DE ACORDO COM A LESGISLAÇÃO EM VIGOR, NÃO É PERMITIDA A REPRODUÇÃO DE QUALQUER PARTE DESTA TESE/TRABALHO
Universidade do Minho, / /
iii
Acknowledgements
I’d like to thank my supervisors, Dr Maria José Alves and Dr António Gil Fortes whose support me, for their transmitted knowledge and for helping me during this project.
To the Dr Paul Kong Thoo Lin for his orientation along my stay at Robert Gordon University, for the support he gave me, his knowledge that he transmitted to me and always present when I lived in Aberdeen. Also for the help to my project is concluded with success.
To Dr.a Elisa Pinto and Dr.a Vânia Azevedo for their hard work showed on realization of Nuclear Magnetic Resonance spectrums.
For all my fellow graduate students for all support and help me along this 2 years and mainly in this last year.
My truly regards to all my friends, my girlfriend and my brother, who always supported, guided me in any respect during this last year always with kind words.
Lastly, I’d like to thank my family for their continuous support, especially to my parents and my godfather. They always believe in me, and help me always possible and make my travel to RGU a dream come true, thing that I will never forget.
v
Abstract
Naphthalimides (1H-benzo[de]isoquinoline-1,3-(2H)-diones) consists of a flat, generally π-deficient aromatic or heteroaromatic system and show strong hydrophobicity. These types of compounds with this moiety demonstrate inherent fluorescence and biological properties such as anticancer, antimicrobial, antitrypanosomal, analgesic, antioxidative and antiviral properties. The naphthalimide compounds are also known to be very good DNA intercalators, since the planar naphthalimido moiety binds by perpendicular insertion between the base pairs of the double helix of DNA.
Previous work had already shown that mono and bis naphthalimido derivatives exhibit strong activity against different cancer cell lines. Here in this work will be demonstrate that the alkyl chain, i.e. the linker between the naphthalimido groups or the substituent attached at the end of the linker chain, do have an impact on the biological and DNA binding properties.
Therefore the synthesis of a series of new mono-naphthalimide derivatives were prepared in moderate to good yields by reaction of aminonaphthalimides containing different alkyl chain length with carious aromatic aldehydes. The new bis-naphthalimides were prepared by an N-alkylation reaction of different linkers with the corresponding O-tosyl alkylnaphthalimides.
The biological activity of the newly synthesized compounds includes their ability to bind DNA, their toxicity against SH-SY5Y human brain cancer cells in vitro, cell morphology and cellular uptake were tested.
As expected the bis-naphthalimide derivatives gave better results when compared to the mono-naphthalimides in all tests. For the mono naphthalimides, longer the length of the alkyl chain, better are the results; also the nature of the aromatic aldehyde interfere with the results. For the bis-naphthalimides the type of linker has influence on biological activity and binding studies. It has been shown when the linker has more flexibility, the biological activity and binding studies gave better results.
vii
Resumo
As naftalimidas (1H-benzo[de]isoquinoline-1,3-(2H)-dionas) consistem num sistema planar aromático ou heteroaromático deficiente em electrões π e mostram forte hidrofobicidade. Estes tipos de compostos apresentam fluorescência e propriedades biológicas como anticancerígenos, antimicrobianos, antitrypanosomal, analgésica, antioxidante e antiviral. Os compostos de naftalimida também são conhecidos como intercalantes do ADN, pois o seu núcleo naftalimido planar liga-se através de inserção perpendicular entre os pares de bases da dupla hélice do ADN.
Em trabalhos anteriores mostraram que os derivados mono e bis naftalimidas exibiram forte atividade contra diferentes linhas celulares cancerígenas. Neste trabalho foi possível demonstrar que a cadeia alquílica, por exemplo a cadeia ligando, tem ou não impacto nas propriedades quer biológicas quer na ligação do ADN.
A síntese de novos derivados de naftalimida foram preparados com rendimentos de moderados a bons através da reação de aminonaftalimidas contendo diferentes tamanhos de cadeia alquílica com aldeídos aromáticos. As novas bis naftalimidas foram preparados por uma reação de N-alquilação com diferentes ligandos com os correspondentes O-tosil alquilnaftalimidas.
A atividade biológica dos novos compostos sintetizados incluindo a capacidade de ligação ao ADN, sua toxicidade in vitro contra células humanas do cancro do cérebro, SH-SY5Y, a morfologia celular e a absorção celular foram testadas.
Como esperado os derivados de bis-naftalimida apresentaram melhores resultados quando comparadas com as mono-naftalimidas em todos os testes efetuados. Para as mono-naftalimidas, os resultados mostraram que o aumento da cadeia alquílica, tal como a natureza do aldeído aromático influenciam os resultados na atividade biológica e nos estudos de ligação ao ADN. Para as bis-naftalimidas, o tipo de flexibilidade do ligando tem influência nos resultados de atividade biológica e nos estudos de ligação ao ADN..
ix
Table of Contents
Acknowledgements ... iii
Abstract ... v
Resumo ... vii
List of Figures ... xiii
List of Tables ... xvii
List of Schemes ... xix
Abbreviations and symbols ... xxi
Chapter 1 - Introduction
1. Introduction...
3 1.1 Cancer ... 3 1.2 Target Therapy ... 4 1.3 Neuroblastoma ... 4 1.4 Cell Death ... 7 1.5 DNA ... 9 1.6 DNA Intercalators ... 14 1.7 Naphthalimides ... 16 1.8 Bis-Naphthalimides ... 22 1.9 Aims ... 25Chapter 2 - Results and Discussion
2. Results and Discussion...
292.1 Introduction ... 29
x
2.1.2 Synthesis of compounds 2a – p ... 32
2.1.3 Synthesis of compounds 3a – p ... 40
2.1.4 Synthesis of naphthalimides 4a – c ... 44
2.1.5 Synthesis of tosyl compounds 5a – c ... 46
2.1.6 Synthesis of Mesitylamines 6a and 6b ... 48
2.1.7 Synthesis of bis-naphthalimide derivatives 7a – c ... 51
2.1.8 Synthesis of 8a – c derivatives ... 54
2.2 DNA Binding Studies ... 57
2.2.1 Fluorescence Binding ... 57
2.2.2 Discussion of the values obtained for mono-naphthalimide derivatives ... 59
2.2.3 Discussion of the values obtained for bis-naphthalimide derivatives ... 67
2.3 Biological Activity... 70
2.3.1 Cell Morphology ... 70
2.3.2 Cytotoxicity ... 72
2.4 Cellular Uptake ... 79
Chapter 3 - Experimental Procedure
3. Experimental Procedure...
833.1 General Details ... 83
3.1.1 Chemical Synthesis – Analytical Techniques ... 83
3.1.2 Binding Studies ... 84
3.1.3 Cell Culture and Biological Activity ... 84
3.2 General synthesis of mono-naphthalimide derivatives ... 85
3.3 Reaction of 1,8-naphthalic anhydride with alkyl diamines ... 86
3.3.1 General procedure ... 86
3.4 Reaction of amines 1a – c with aldehydes ... 88
3.4.1 General procedure ... 88
3.5 Reduction of imines 2a – p ... 98
3.5.1 General procedure ... 98
xi
3.7 Reaction of 1,8-naphthalic anhydride with alkyl aminoalcohols ... 109
3.7.1 General procedure ... 109
3.8 Reaction of 4a – c with p-toluenesulfonyl chloride ... 111
3.8.1 General procedure ... 111
3.9 Reaction of alkyl diamines 10 and 11 with 2-mesitylenesulfonyl chloride (linker synthesis) ... 113
3.9.1 General procedure ... 113
3.10 Reaction of compounds 5b and 5c with 6a and 6b (N-Alquilation reaction) . 114 3.10.1 General procedure ... 114 3.11 Deprotection reaction of 7a – c ... 117 3.11.1 General procedure ... 117 3.12 Binding Studies ... 120 3.13 Biological Activity ... 122 3.13.1 Cell Maintenance ... 122 3.13.2 Cell Counting ... 123 3.14 Cytotoxicity ... 124
Chapter 4 - Conclusion
4. Conclusion...
129Chapter 5 - Future Work
5. Future Work...
133Chapter 6 - Bibliography
6. Bibliography...
137xiii
List of Figures
Figure 1: Stages of cellular response to a stress stimuli and harmful6 ... 8
Figure 2: Cellular characteristics of necrosis (at left) and apoptosis (at right)6 ... 9
Figure 3: Components of nucleic acids: bases, sugars and phosphates (adapted)23 ... 10
Figure 4: Formation of DNA chain23 ... 11
Figure 5: The two common Watson-Crick base pairs of DNA23 ... 12
Figure 6: Representation of major and minor groove of DNA structure ... 13
Figure 7: Intercalation model of aromatic ring system into DNA. A) monointercalation; B) bisintercalation28 ... 14
Figure 8: Structures of atypical and typical intercalators and groove binders ... 15
Figure 9: A – Cytotoxicity compounds considered in the design of Naphthalimides; B – First series of naphthalimides synthetized for possible antitumor activity1... 19
Figure 10: Structure of mono-naphthalimide derivatives... 19
Figure 11: Structure of Scriptaid... 21
Figure 12: General structure of 2-metoxybenzyl derivatives ... 21
Figure 13: General structure of bis-naphthalimides ... 22
Figure 14: Deformation of the DNA helix by a monointercalating agent (at left) and scheme of intercalation between a bisintercalator and DNA (at right)30 ... 23
Figure 15: Structure of Elinafide and Bisnafide ... 23
Figure 16: Mono and Bis-Naphthalimides derivatives synthetized ... 25
Figure 17: Structure of compounds 1a – c ... 30
Figure 18: 1H NMR of imine 2a ... 33
Figure 19: DEPT 135 of imine 2a... 33
Figure 20: Structure of compounds 2d and 2i ... 34
Figure 21: Structure of compounds 2a, 2e and 2j ... 35
Figure 22: Structure of compounds 2b, 2f and 2k ... 36
Figure 23: Structure of compounds 2b, 2g and 2l ... 37
Figure 24: Structure of compounds 2h and 2m ... 38
Figure 25: Structure of compounds 2n-p ... 39
Figure 26: 1H NMR spectrum of amine 3a ... 41
Figure 27: DEPT 135 spectrum of amine 3a ... 41
Figure 28: Structures of compounds 3a – p ... 42
xiv
Figure 30: Structures of compounds 4a – c... 45
Figure 31: 1H NMR spectrum of tosyl compound 5b ... 47
Figure 32: Structure of compounds 5a – c ... 47
Figure 33: Structure of compound 6a ... 49
Figure 34: Structure of compound 6b ... 50
Figure 35: 1H NMR of compound 7c ... 52
Figure 36: Structure of compounds 7a, 7b and 7c ... 53
Figure 37: 1H NMR spectrum of compound 8b ... 54
Figure 38: Structure of compound 8a ... 55
Figure 39: Structure of compounds 8b and 8c ... 55
Figure 40: MS spectrum of compound 8a ... 56
Figure 41: MS spectrum of compound 8c ... 56
Figure 42: Structure of Ethidium Bromide (EtBr) ... 57
Figure 43: Mechanism of action of displacement studies ... 58
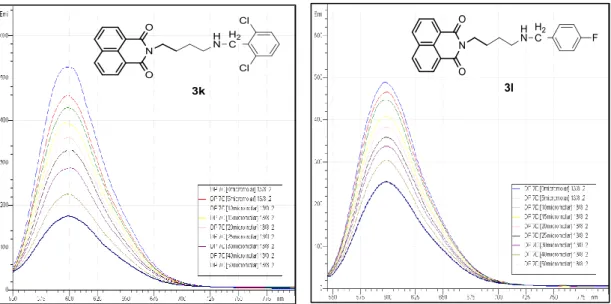
Figure 44: The effect of varying 3i and 3j concentration (0-50 µM) on fluorescence intensity of EtBr bound DNA ... 59
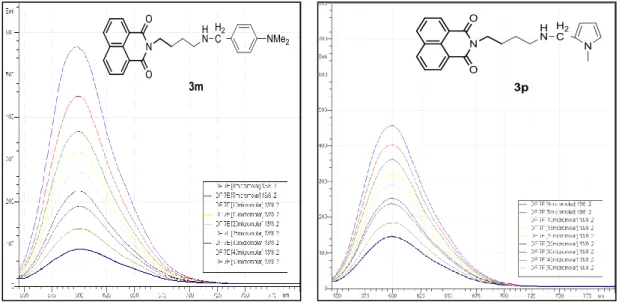
Figure 45: The effect of varying 3k and 3l concentration (0-50 µM) on fluorescence intensity of EtBr bound DNA ... 59
Figure 46: The effect of varying 3m and 3p concentration (0-50 µM) on fluorescence intensity of EtBr bound DNA ... 60
Figure 47: The effect of concentration of mono-naphthalimide derivatives with 4 carbon atoms chain on % of fluorescence intensity ... 60
Figure 48: The effect of varying 3d and 3e concentration (0-50 µM) on fluorescence intensity of EtBr bound DNA ... 62
Figure 49: The effect of varying 3f and 3g concentration (0-50 µM) on fluorescence intensity of EtBr bound DNA ... 62
Figure 50: The effect of varying 3o concentration (0-50 µM) on fluorescence intensity of EtBr bound DNA ... 63
Figure 51: The effect of concentration of mono-naphthalimide derivatives with 3 carbon atoms chain on % of fluorescence intensity ... 63
Figure 52: The effect of varying 3a and 3b concentration (0-50 µM) on fluorescence intensity of EtBr bound DNA ... 64
Figure 53: The effect of varying 3c and 3n concentration (0-50 µM) on fluorescence intensity of EtBr bound DNA ... 65
xv
Figure 54: The effect of concentration of mono-naphthalimide derivatives with 2 carbons atom
chain on % of fluorescence intensity ... 65
Figure 55: The effect of varying 8a and 8b concentration (0-25 µM) on fluorescence intensity of EtBr ... 67
Figure 56: The effect of varying 8c concentrations (0-25 µM) on fluorescence intensity of EtBr ... 67
Figure 57: The effect of concentration of bis-naphthalimide derivatives on % of fluorescence intensity ... 68
Figure 58: Morphological changes of brain cancer cells treated with mono-naphthalimide 3l at different concentrations (0 – 150 µM) (green arrows = viable cells, red arrow = dead cells ... 71
Figure 59: Morphological changes of brain cancer cells treated with bis-naphthalimide 8c at different concentrations (0 – 40 µM) (green arrows = viable cells, red arrow = dead cells ... 72
Figure 60: Reduction in MTT tetrazolium dye with bis-naphthalimide derivatives ... 73
Figure 61: Concentration variation on % of absorbance intensity in brain cancer SH-SY5Y cells, treated by mono-naphthalimide derivatives (4 carbons atoms chain) ... 74
Figure 62: Concentration variation on % of absorbance intensity in brain cancer SH-SY5Y cells, treated by mono-naphthalimide derivatives (3 carbons atoms chain) ... 75
Figure 63: Concentration variation on % of absorbance intensity in brain cancer SH-SY5Y cells, treated by mono-naphthalimide derivatives (2 carbons atoms chain) ... 76
Figure 64: Concentration variation on % of absorbance intensity in brain cancer SH-SY5Y cells, treated by bis-naphthalimide derivatives ... 77
Figure 65: Fluorescence present in brain cancer, SH-SY5Y, cells after treatments with different concentrations of mono-naphthalimide derivatives ... 79
Figure 66: Fluorescence present in brain cancer SH-SY5Y cells after treatments with bis-naphthalimide derivatives ... 80
Figure 67: Scheme of chamber in haemocytometer ... 123
Figure 68: Section to count the cells... 123
xvii
List of Tables
Table 1: The International Neuroblastoma Staging System (INSS)18 ... 6
Table 2: 1H NMR signals of protons in derivatives 1a – c in CDCl3; J in Hz; δH in ppm at 400 MHz ... 31
Table 3: 13C NMR signals of protons in derivatives 1a – c in CDCl3; δC in ppm at 100.6 MHz... 31
Table 4: 1H NMR signals of 2d and 2i derivatives in CDCl3, J in Hz, δH in ppm at 400 MHz ... 34
Table 5: 13C NMR signals of 2d and 2i derivatives in CDCl3, δC in ppm at 100.6 MHz ... 34
Table 6: 1H NMR signals of 2a, 2e and 2j derivatives in CDCl3, J in Hz, δH in ppm at 400 MHz .... 35
Table 7: 13C NMR signals of 2a, 2e and 2j derivatives in CDCl3, δC in ppm at 100.6 MHz ... 35
Table 8: 1H NMR signals of 2b, 2f and 2k derivatives in CDCl3, J in Hz, δH in ppm at 400 MHz ... 36
Table 9: 13C NMR signals of 2b, 2f and 2k derivatives in CDCl3, δC in ppm at 100.6 MHz ... 36
Table 10: 1H NMR signals of 2c, 2g and 2l derivatives in CDCl3, J in Hz, δH in ppm at 400 MHz .. 37
Table 11: 13C NMR signals of 2c, 2g and 2l derivatives in CDCl3, δC in ppm at 100.6 MHz... 37
Table 12: 1H NMR signals of 2h and 2m derivatives in CDCl3, J in Hz, δH in ppm at 400 MHz ... 38
Table 13: 13C NMR signals of 2h and 2m derivatives in CDCl3, δC in ppm at 100.6 MHz ... 38
Table 14: 1H NMR signals of 2n – p derivatives in CDCl3,J in Hz, δH in ppm at 400 MHz ... 39
Table 15: 13C NMR signals of 2n – p derivatives in CDCl3, δC in ppm at 100.6 MHz ... 39
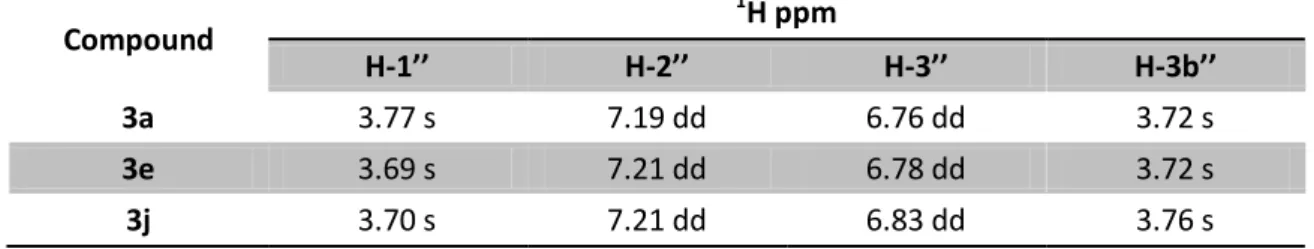
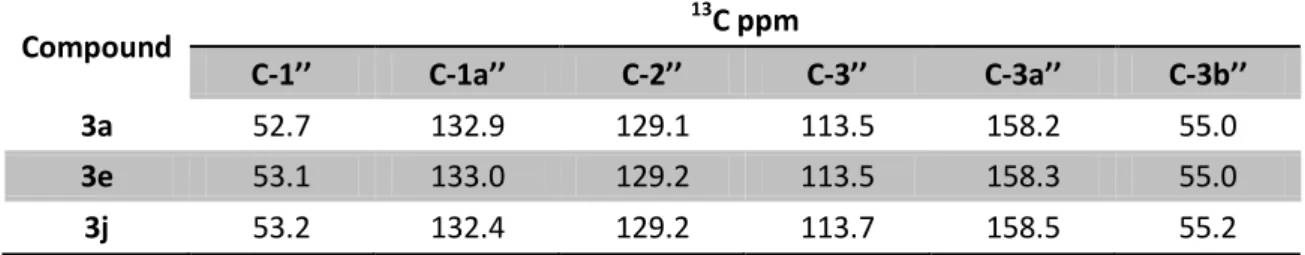
Table 16: 1H NMR signals of 3d and 3i derivatives in CDCl3, J in Hz, δH in ppm at 400 MHz ... 42
Table 17: 13C NMR signals of 3d and 3i derivatives in CDCl3, δC in ppm at 100.6 MHz ... 42
Table 18: 1H NMR signals of 3a, 3e and 3j derivatives in CDCl3, J in Hz, δH in ppm at 400 MHz .. 42
Table 19: 13C NMR signals of 3a, 3e and 3j derivatives in CDCl3, δC in ppm at 100.6 MHz ... 43
Table 20: 1H NMR signals of 3b, 3f and 3k derivatives in CDCl3, J in Hz, δH in ppm at 400 MHz .. 43
Table 21: 13C NMR signals of 3b, 3f and 3k derivatives in CDCl3, δC in ppm at 100.6 MHz ... 43
Table 22: 1H NMR signals of 3c, 3g and 3l derivatives in CDCl3,J in Hz, δH in ppm at 400 MHz ... 43
Table 23: 13C NMR signals of 3c, 3g and 3l derivatives in CDCl3, δC in ppm at 100.6 MHz... 43
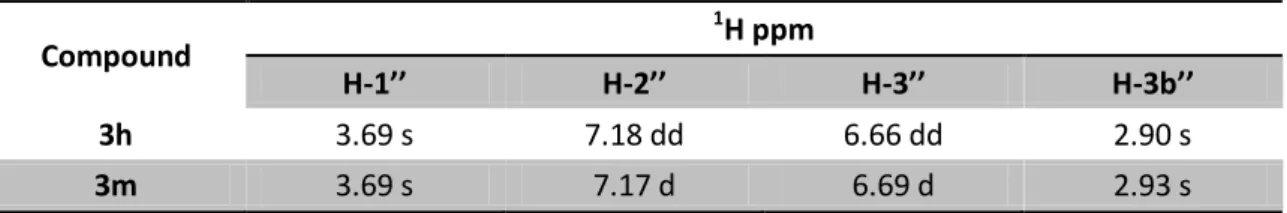
Table 24: 1H NMR signals of 3h and 3m derivatives in CDCl3, J in Hz, δH in ppm at 400 MHz ... 44
Table 25: 13C NMR signals of 3h and 3m derivatives in CDCl3, δC in ppm at 100.6 MHz ... 44
Table 26: 1H NMR signals of 3n – p derivatives in CDCl3, J in Hz, δH in ppm at 400 MHz ... 44
Table 27: 13C NMR signals of 3n – p derivatives in CDCl3, δC in ppm at 100.6 MHz ... 44
Table 28: 1H NMR signals of 4a – c derivatives in CDCl3, J in Hz, δH in ppm at 400 MHz ... 46
Table 29: 13C NMR signals of 4a – c derivatives in CDCl3, δC in ppm at 100.6 MHz ... 46
Table 30: 1H NMR signals of 5a – c derivatives in CDCl3, J in Hz, δH in ppm at 400 MHz ... 48
xviii
Table 32: 1H NMR signals of 6a derivatives in CDCl3, J in Hz, δH in ppm at 400 MHz ... 49
Table 33: 13C NMR signals of 6a derivatives in CDCl3, δC in ppm at 100.6 MHz ... 50
Table 34: 1H NMR signals of 6b derivatives in CDCl3, J in Hz, δH in ppm at 400 MHz ... 50
Table 35: 13C NMR signals of 6b derivatives in CDCl3, δC in ppm at 100.6 MHz ... 50
Table 36: 1H NMR signals of 7a derivatives in CDCl3, J in Hz, δH in ppm at 400 MHz ... 53
Table 37: 1H NMR signals of 7b and 7c derivatives in CDCl3, J in Hz, δH in ppm at 400 MHz ... 53
Table 38: 13C NMR signals of 7a derivatives in CDCl3, J in Hz, δC in ppm at 100.6 MHz ... 53
Table 39: 13C NMR signals of 7b and 7c derivatives in CDCl3, J in Hz, δC in ppm at 100.6 MHz ... 53
Table 40: 1H NMR signals of 8a derivatives in DMSO, J in Hz, δH in ppm at 400 MHz ... 55
Table 41: 13C NMR signals of 8a derivatives in DMSO, δC in ppm at 100.6 MHz... 55
Table 42: 1H NMR signals of 8b and 8c derivatives in DMSO, δC in ppm at 400 MHz ... 55
Table 43: 13C NMR signals of 8a and 8c derivatives in DMSO, δC in ppm at 100.6 MHz ... 55
Table 44: Mono-naphthalimide derivatives with 4 carbon atoms chain C50 values (µM) ... 61
Table 45: Mono-naphthalimide derivatives with 3 carbon linker chain C50 values (µM) ... 64
Table 46: Mono-naphthalimides derivatives with 2 carbons of linker chain C50 values (µM) .... 66
Table 47: Comparison of Mono naphthalimide compounds with the same substituent/chain . 66 Table 48: Bis-naphthalimide derivatives C50 values (µM) ... 68
Table 49: Mono-naphthalimide derivatives (4 carbon atoms chain) IC50 values (µM) after 24 hours incubation ... 74
Table 50: Mono-naphthalimide derivatives (3 carbon atoms chain) IC50 values (µM) after 24 hours incubation ... 75
Table 51: Mono-naphthalimide derivatives (2 carbon atoms chain) IC50 values (µM) after 24 hours incubation ... 76
Table 52: Comparison of mono-naphthalimide derivatives same substituent/chain ... 77
Table 53: Bis-naphthalimide derivatives IC50values (µM) after 24 hours incubation ... 78
Table 54: Preparation of Test Solutions for Fluorescence binding Studies ... 121
xix
List of Schemes
Scheme 1: General scheme of synthesis of mono-naphthalimide derivatives ... 17
Scheme 2: General scheme of synthesis Mitonafide and Amonafide; i) H2SO4, HNO3, 5-20ºC; ii) N,N,-dimethylethylelediamine, EtOH, reflux; iii) 10% Pd/C, H2, EtOH ... 20
Scheme 3: Synthesis of Scriptaid. a) propanoic acid, aminopropanoic ξ-acid, reflux, 4h; b) Et3N, THF, 0ºC, 15min, followed by NH2OH, MeOH, r.t., 1h ... 21
Scheme 4: Synthesis of 2-metoxibezyl derivatives; a) EtOH, reflux, 24h; b) 2-MeOC6H4CHO, toluene, reflux, 3h; c) NaBH4, EtOH, r.t., 3h ... 22
Scheme 5: Synthesis of Elinafide. a) dioxane, reflux ... 24
Scheme 6: Synthesis of Bisnafide. a) THF, B2H6; b) EtOH, reflux, metasulfonic acid ... 24
Scheme 7: Reaction between 1,8-napthalic anhydride and alkyl diamines. i) EtOH, reflux ... 29
Scheme 8: Mechanism between 1,8-naphthalic anhydride and alkyl diamine... 30
Scheme 9: Reaction between naphthalimides 1a – c and aldehydes; i) EtOH, reflux ... 32
Scheme 10: Mechanism between compounds 1a – c and an aldehyde ... 32
Scheme 11: Reduction of imines 2a – p; i) THF:MeOH (1:1), NaBH4 ... 40
Scheme 12: Imines reduction mechanism ... 40
Scheme 13: Reaction between 1,8-naphthalic anhydride and alkyl aminoalcohols; i) DMF, 85°C ... 44
Scheme 14: Reaction between 4a – c and p-toluenesulfonyl chloride; i) Py, 4°C ... 46
Scheme 15: Mechanism between alcohols 4a – c and tosyl chloride ... 47
Scheme 16: Mesitylation of alkyl diamine; i) Mts-Cl, Py, 0°C ... 48
Scheme 17: Reaction between the mesityl linkers 6a and 6b with tosylnaphthalimides; i) DMF ii) Cs2CO3 ... 51
Scheme 18: Mechanism between linkers n and 6b and the tosylnaphthalimides n and 5c ... 52
Scheme 19: Deprotection of compounds 7a – c; i) DCM, HBr/gCH3CO2H (20 %) ... 54
Scheme 20: Reaction of reduction of MTT... 73
Scheme 21: General scheme for the synthesis of mono-naphthalimide derivatives; i) EtOH 3-4h, reflux; ii) EtOH/Aldehyde 4-6 h, reflux; iii) THF/MeOH/NaBH4 overnight, r.t. ... 85
Scheme 22: General scheme for the synthesis of bis-naphthalimide derivatives; i) DMF/DBU 4h, 85 °C; ii) Py/Ts-Cl 12hrs, 4°C; iii) Py/Mts-Cl 1h, 0°C (for 6a and 6b), DMF/Cs2CO3, 12h, 60°C; iv) DCM/HBr in glacial CH3CO2H, 24h, r.t. ... 108
xxi
Abbreviations and symbols
13C NMR Carbon-13 Nuclear Magnetic Resonance spectroscopy 1
H NMR Proton Nuclear Magnetic Resonance spectroscopy
A Adenine
CNS Central Nervous System
Comp. Compound
C Cytosine
d Doublet
dd Double Doublet
δ Chemical shift in ppm
DEPT 135 Distortionless Enhancement by Polarization Transfer 135
DBU 1,8-Diazabicyclo-[5.4.0]-undec-7-ene
DCM Dichloromethane
DMF N,N-Dimethylformamide
DMSO Dimethyl Sulfoxide
DNA Deoxyribonucleic Acid
dsDNA Double stranded DNA
equiv Molar equivalents
EtOH Ethanol
Et-Br Ethidium Bromide
FBS Foetal Bovine Serum
G Guanine
h Hours
Hz Hertz
IC50 Half maximal inhibitory Concentration
INSS International Neuroblastoma Staging System
J Coupling constant
m Multiplet
M.p. Melting point
MDR Multiple Drug Resistance
xxii
Mts-Cl Mesitylenesulfonyl Chloride
MeOH Methanol
MTT 3-(4,5-dimethylthuazol-2-yl)-2,5-diphenyltetrazolium bromide
NB Neuroblastoma
NMR Nuclear Magnetic Resonance
PBS Phosphate Buffered Saline
ppm Parts per million
Py Pyridine
r.t. Room temperature
RNA Ribonucleic Acid
s Singlet
SAR Structure-Activity Relationship
SSC Saline-Sodium Citrate
ssDNA Single stranded DNA
THF Tetrahydrofuran
TLC Thin Layer Chromatography
TOPO II Topoisomerase II
Ts-Cl Toluenesulfonyl Chloride
Chapter 1
3
1. Introduction
1.1 Cancer
Nowadays, cancer is one of the highest problems on worldwide health, representing the 2nd major cause of death around the world and in 2030, nearly 12 million deaths are estimated due to cancer.1,2
This disease is characterized by uncontrolled cellular growth3, because cancer cells have this unique ability to replicate indefinitely and spread to others tissues due invasion or metastasis.4,5 Due to these factors, cancer is now an unsolved problem and
represents a real crisis of public health across the world.4
Neoplasm, or more ordinary, tumour is when the cancer cells have a certain degree of autonomy and a constant way to increase their size independent of environment and of the nutritional stage of the host.6
Tumours showed higher frequency on older people. They can be classified into two types: benign: when the cells are very similar and can behave like normal cells. Besides, their growth is localized and don’t have the ability to metastasize or invade other tissues, however these cells start to be problematic when almost all the cells interfere with the normal behaviour or start to secrete excessive quantities of active biologic substances as hormones; malignant: are characterized by their rapid cellular growth more than normal and this cells have the ability to invade and/or metastasize.7,8
The main reasons that turns the cancer a difficult disease to treat are: there are over 200 different types of cancer; in most cases their aetiology is unknown; which results in difficulties in creating/designing selective drugs against the transformed cells; pre-clinics models are generally difficult to extrapolate; and, in the end, these type of cells develop resistance against multiple anticancer drugs (MDR).3
4
1.2 Target Therapy
Over the years, the researchers have learned more about the genetic alterations in cells that cause cancer and with that in mind, were able to develop drugs that target on these changes. This type of treatment is known as targeted therapy.9
The rationally behind this therapy was formulated 15 years ago and comprises in the design and applications of drugs that act directly and specifically on targets and become critical to the survival of the tumor without compromise the normal organs and tissues.10
These types of drugs are able to attack the cancer cells with less damage to normal cells. This process is used to prevent the growth and propagation of the cancer, in other words, this mechanism breaks the process of carcinogenesis (process which the normal cells turn into cancer cells). The drugs act on certain parts of the cell and interfere with signals which are needed for the development of cancer growth. Different drugs are used to treat the cancer and may operate in different areas depending on the phase in which the cancer is located: a) heal, slow down the growth, and kill the cancer cells to avoid the spread; b) attenuate the symptoms caused by the cancer.9,11
1.3 Neuroblastoma
Neuroblastoma (NB) is one of the most common solid tumors which occur in childhood.12,13 It is the 3rd most common in children getting behind only leukemia and Central Nervous System (CNS) tumors14,15 and is also responsible for about 10% of all
pediatric cancers. This disease is diagnosed around 17/18 months and can occur throughout the sympathetic nervous system, and more insidious at adrenal medulla. 12,16
The pathologist J.H. Wright, in 1910, introduce for the first time the term neuroblastoma, describing as an ensemble of tumors at childhood with characteristics of neuronal origin. It is believed that NBs derive from immature cells originating in the neural crest, where these cells differentiate inappropriately and the primary tumors are mainly found in the adrenal medulla or across the paraspinal sympathetic ganglia. Clinically, it is a heterogeneous disease which makes a great challenge for the
5
investigation. The most common sites of metastization are the bones, bone marrow and lymph nodules; however a separate metastatic pattern, confined to the liver and skin, is seen in children. Multiple factors such as the age at which it is diagnosed, the stage of the disease and the genetic profile of the tumor, together with other molecular characteristics determine the clinical outcome of the disease.14,17
The most common primary tumors (65%) appears at abdomen, with at least half of them appear at medulla adrenal. Chest, pelvis and neck are also usual sites where NB tumor can appear.13,16
The symptoms of NB are generally diffuse and depend on the primary tumor site and also the presence and location of metastasis. A patient with a localized disease sometimes showed several abdominal pain, besides in other cases, the patient doesn’t have localized symptoms and the tumor is found by chance. In the other hand, patients with metastatic NB are typically quite ill at the time of diagnosis, showing some unspecific symptoms like fever, pallor or anorexia. For the metastatic specific symptoms, these are characterized by bone pain and sometimes even marrow failure related to bone or bone marrow metastases are also common.16
Another way to diagnosis the NB is due the histopathological characterization of tumor tissue and also evaluate the levels of catecholamines in urine or serum if they increased.17
In order to implement one system of consensual staging, in 1986, it was implemented the International Neuroblastoma Staging System (INSS). In this system, the age at which the tumor was diagnosed, the extension of the disease, and the resectability of the tumor, classified the tumors into four stages, 1 till 4, where the stage 4 is the most aggressive. Also have a special stage called 4s. This stage is for children with less than 1 year old with primary tumors localized and restricted metastasis in liver, skin and/or bone marrow. (Table 1)
6
Table 1: The International Neuroblastoma Staging System (INSS)18 Stage Description
1
Localized tumor with complete gross excision, with or without microscopic residual disease; representative ipsilateral lymph nodes negative for tumor microscopically (nodes attached to and removed with the primary tumor may be positive).
2A Localized tumor with incomplete gross excision; representative ipsilateral
nonadherent lymph nodes negative for tumor microscopically.
3B
Localized tumor with or without incomplete gross excision, with ipsilateral nonadherent lymph nodes positive for tumor. Enlarged contralateral lymph nodes must be negative microscopically.
3
Unresectable unilateral tumor infiltrating across the midline*, with or without regional lymph node involvement; or localized unilateral tumor with contralateral regional lymph node involvement; or midline tumor with bilateral extension by infiltration (unresectable) or by lymph node involvement
4 Any primary tumor with dissemination to distant lymph nodes, bone, bone
marrow, liver, skin and/or other organs (except as defined by stage 4S)
4s
Localized primary tumor (as defined for stage 1, 2A or 2B), with dissemination limited to skin, liver, and/or bone marrow† (limited to infants < 1 year of age)
These outcomes could be very different between the subtypes of NBs varying from regressive low risk till aggressive high risk. The low risk is characterized by tumor with small or no risk and have favorable clinic prognostic. (stage 1 2 and 4s). The NBs with high risk are aggressive and are metastatic tumors with a poor diagnosis with low or no response to chemotherapy and show outcomes not favorable (stage 3 and 4).18
Following a general tendency in the evolution of medicine, the treatment of neuroblastoma requires an interdisciplinary work involving surgeons, radiation therapists, chemotherapists and chemists. Such an approach is best adapted to the construction of the treatment programme. The therapy for neuroblastoma is adapted to each patient depending, for example, on the disease stage, and patient age. The
7
available options range from combined modality of surgery, chemotherapy and radiotherapy.15
Due of heterogeneity of this disease, it is a challenge to find the cure for the patients with high risk but also to avoid the overtreatment to the patients with favorable diagnosis. The surgical removal of primary tumor remains the most important treatment of NB. For localized tumor, surgery is an option, but for the metastatic tumor the use is debatable. Chemotherapy is, generally used for the treatment of subtypes of intermediate NB and high risk that require one systemic approach due a metastasis. The objective of chemotherapy is reducing the size of the tumor, to make it easy for the surgical removal and eliminate the metastatic diseases. This is the predominant treatment for the treating of NB.19 There are many alkylating drugs use as chemotherapeutic agents in the treatment of NB: cyclophosphamide, (busulphan, melphalan), vinca-alkaloids (vincristine), antracyclines (doxorubicin), and platinum analogues (cis-platinum, Carboplatinum).17 Currently, therapy options for neuroblastoma comprise combined modality of surgery, chemotherapy and radiotherapy. This approach is individually modified depending on disease stage and patients age at presentation.16,18
In spite of the advances of the actual treatment, the clinical trials on course and investigations of basic science NB still to be a complex medical challenge with a clinical curse unpredictable and dismal overall outcome for advance stage disease.
1.4 Cell Death
The cells, due the extracellular stress or because their necessities, they are continuously adjusting their function and structure to maintain their normal behavior.6
When the cells are subject to pathological stimuli and physiological stress, they adapt and continue with their function and duty viability or don’t support this type of damage and take the process called cell death (Figure 1).
The study of cell death comes over than a century. The increase knowledge of different mechanisms of the apoptotic process clearly showed the complexity and the difficulty of distinguishing the different forms of cell death, necrosis, apoptosis and/or oncosis.20,21
8
Figure 1: Stages of cellular response to a stress stimuli and harmful6
Depending on the type of damage that the cell suffers, the cells could die through two processes: apoptosis – the way to cell death that is introduced by a suicide program tightly regulated where the cells are destined to die, and there is activation of enzymes capable to degrading the nuclear DNA of cells and also the cytoplasmic proteins. This process is characterized by the shrinking, condensation and margination chromatin and riffling of the plasmatic membrane, called budding. The cell is divided into apoptotic bodies, becoming these fragments targets of the phagocytes preventing the leakage of them; necrosis – this process results from degradative action of enzymes to damage cells. The main characteristics of this process are, firstly, the cellular swelling, the condensation of chromatin and the inability to maintain the integrity of the cell membrane and their constituents are leak out inducing inflammation. However, these two processes can coexist because the apoptosis induce pathological stimuli which in turn induces the activation process of necrosis (Figure
9 Figure 2: Cellular characteristics of necrosis (at left) and apoptosis (at right)6
The process of cell death is one of the most important processes in the evolution of diseases of any organ or tissue. Besides that, it is also important that during the development of the organs, homeostasis is maintained. It is believed that the development of malign tumors results due to deregulated proliferation or the incapacity the cells suffer apoptotic cell death.22
The disruption and the anomaly regulation of cell death and cell growth is one of the reasons that cause cancer. Recent studies showed that the induction of apoptosis by therapeutics agents in cancer cells is critical to the tumor developing.21
1.5 DNA
The nomenclature known nowadays of deoxyribonucleic acid (DNA) has undergone a major evolution. The DNA is organized into a double-helical structure and consists on a very long sequence of base pairs. The nucleic acids are a long chain or a polymer of repeated subunits, called nucleotides. Each nucleotide consists of 3 parts: one sugar of 5 carbons, one phosphate group and one nitrogenous base. (Figure 3)
10
Figure 3: Components of nucleic acids: bases, sugars and phosphates (adapted)23
The formation of DNA chain occurs in 3 steps: 1st each base is connected to the sugar in C1’, forming the nucleoside; 2nd when the phosphate group is connected to the C5’ of the same sugar, the nucleotides are formed; 3rd in the end, these nucleotides polymerize by condensations reactions to form the DNA chain. (Figure 4).23
11 Figure 4: Formation of DNA chain23
DNA is a nucleic acid polymerized which contains genetic information that specifies the biological development of all cellular life forms. The molecule of DNA is responsible for the genetic propagation of all traces, so it also call as molecule of heredity. During the process of reproduction, DNA is replicated and transmitted to the offspring. The ribonucleic acid (RNA) is accountable to the transcription process (process where the DNA sequence is copied), and this molecule are used in protein synthesis to code a sequence of specific proteins (translation).
12
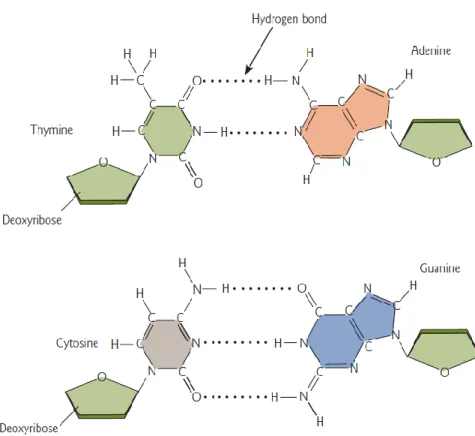
The double stranded DNA (dsDNA) is the structure in which the DNA presents the double stranded antiparallel and this structure is held together by hydrogen bound interactions between complementary base pairs: Adenine (A), Thymine (T), Guanine (G) and Cytosine (C). The C only can interact with G and A with T. Adenine and Guanine are purines besides Cytosine and Thymine are pyrimidines (Figure 5). In biological systems, the DNA structure presents as dsDNA but also can show as single stranded DNA (ssDNA) but this case is not usual.
Figure 5: The two common Watson-Crick base pairs of DNA23
The existence of several exposed reactive spots on the surface of double helix is one of the characteristics of DNA (Figure 6). For example, the amino group N2 of Guanine in minor groove of DNA is liable to action of the drugs. The specific binding of the drugs to DNA mainly involves the recognition of the G at minor groove and also by hydrogen bonds interactions of the N2 exocyclic amino group. However, this amino group is normally stericly hindered that causes a decrease of the affinity. Also in minor groove, the N3 of Guanine is a good spot for the drugs. but the most reactive spot is N7 of Guanine where a number of metallic ions and alkylating agents can attack.24
13 Figure 6: Representation of major and minor groove of DNA structure
The key of developing of new chemotherapy therapeutics is understand at molecular level how the genetic information is expressed and how to stimulate or prevent the genetic expression. Once the human genome was completely sequenced there has been a great increase on the study of the genetic sequence of organisms. To prevent the appearance certain diseases a lot of efforts have been made to control specific gene expression.
Nowadays, the study of biochemical sensor technologies that focus on the direct detection of nucleic acids (DNA and RNA) has generated a lot of interest and they play a major role in different scientific areas like forensics, pharmaceutical applications, medical diagnosis, genetic screening, rational drug design, diagnosis of drug resistance, food and agricultural analysis, etc. A new exciting field of research has develop to understand this principles to the rational design, synthesis and applications of new DNA intercalators.24
Targeting DNA is a highly important approach to the development of novel therapies. The interaction with DNA may interfere with transciption and hence with translation. Also, DNA replication is essential for the cell cycle progression. The nucleic acids interact reversibly with a great amount of chemical species, including water, and metallic ions and their complexes, organic molecules and proteins. These molecules may interact with the duplex nucleic acids in 3 primary forms: a) binding along the outside of the helix through non-specific interactions and primarily electrostatics; b)
14
specific interactions of groove binding with a bound molecule with the edges of base pairs like in minor and major groove of NA and c) intercalation of aromatic cyclic systems between base pairs.25
1.6 DNA Intercalators
The search for new anticancer drugs and the development of new molecules able to bind to DNA and show anticancer activity has received great attention nowadays.26 DNA is one of the most important biological targets to design anticancer drugs.27 Exist
3 primary ways by which some molecules and ions interact with nucleic acids duplex: a) binding along the exterior of the helix trough interactions which are usually non-specific and are primarily electrostatic; b) non-specific groove-binding interactions of the bound molecule with the edges of base-pairs in either of the (major or minor) grooves of nucleic acids; and c) intercalation of planar or approximately planar aromatic ring systems between base-pairs.28
The process of intercalation consists of inserting molecules between the base pairs. This is a very important process, especially with regards to the function of many anticancer drugs and also the way intercalators interact with the DNA structures represent new potential anticancer drugs (Figure 7).29,30
Figure 7: Intercalation model of aromatic ring system into DNA. A) monointercalation; B)
15
The intercalation process begins with the transfer of the intercalating molecule from an aqueous environment to the hydrophobic space between 2 adjacent base pairs of DNA. The disruption of the organized shell of water molecules around the ligand also called the hydrophobic effect causes the positive entropy, which leads to a process that is thermodynamically favoured.30
In structural terms, the intercalating molecules are typical fused bi/tricyclic ring structures or atypical molecules with nonfused rings systems. (Figure 8).31
Figure 8: Structures of atypical and typical intercalators and groove binders
These molecules are characterized (Figure 8) by a planar flat π-deficient aromatic or heteroaromatic ring systems, a moiety of approximately the size and shape of a DNA base pairs.30,32,33
They insert perpendicularly to the axis of the helix into the DNA, between adjacent base pairs, without forming any covalent bonds, and the formed complex is stabilized by hydrophobic interactions, van der Walls forces, hydrogen bonds and charge transfer forces.30,32 These molecules bind reversibly to double helical DNA. This process leads to changes at sugar phosphate torsional angles in order to accommodate the intercalating compound. The DNA back bone conformation suffer some changes inducing the unwinding, lengthening, and stiffening of the double helix interfering with DNA protein interaction or may affect the replication processes of the cells, leading to the cellular death and genotoxic effects or even may lead to the retardation, inhibition
16
of transcription and replication. The DNA intercalators may be mutagenic through interference with the molecular recognition and function of DNA binding proteins, such as polymerases, transcription factors, DNA repair systems, and, specially, topoisomerases.28,31,34
The intercalators are oriented parallel to the base pairs, commonly π-stacking in major groove, although some bindings seem to occur preferentially in the minor groove of DNA. In dsDNA helix, the nucleic acids are located in almost coplanar arrangement, which allows planar aromatic molecules to intercalate between two base pairs. When intercalated, it is possible note π-stack interactions (intercalated moiety), hydrogen-bonding, van der Waals interactions, hydrophobic interactions and steric hindrance effects.3,33
Comparing the DNA intercalators with minor groove binding agents, these are less sequence selective, and also show propensity for G-C regions. The main reason of this selectivity is due to complementary hydrophobic or electrostastic interactions, which are due to substituents attached to the chromophore within the major or minor grooves.30
In general the stronger the interactions of the intercalator with DNA, the higher the antitumor activity of the drug will be. In order to use any active substance as a drug, the recognition of all possible interactions with target molecules is essential.34
1.7 Naphthalimides
An active field of research of searching of new chemotherapeutic agents and also new approaches to treat cancer is stimulated by the discovery of new biological targets and the possibility of obtain new drugs without undesirable secondary effects.32
Naphthalimide (1H-benzo[de]isoquinoline-1,3-(2H)-diones) is a polycyclic amide consisting of a flat, and π-deficient aromatic or heteroaromatic system with high hydrophobicity (Scheme 1).35,36
17 Scheme 1: General scheme of synthesis of mono-naphthalimide derivatives
Naphthalimide derivatives have been investigated through their great potential in medicinal chemistry, assembly and supramolecular reorganization and in material science. This type of structure interacts with many active targets in biological system by non-covalent forces like π-π stacking or hydrogen bonding with different enzymes and receptors. The majority of these compounds which contain this moiety are fluorescents and exhibit biologic properties such as antitrypanosomal, antiviral (herpes and HIV), antimicrobial, antioxidative,36 anesthetics locals, analgesics, antagonists activity in serotonin 5-HT3 and 5-HT4 receptors as chemosensory etc. Furthermore these derivatives are also used in some other non-biologic applications like, optic brighters, non-biologic sensor, fluorescent probes, fluorescent dyes and for the synthesis of polymers, lucifer dyes, solar energy, etc.26,34,37
The naphthalimide derivatives can act as photo-reagents, which can induce damage to DNA molecules and with that they are capable to kill the cells when these are activated. This opens the possibility of this type of compounds act at photo-therapy.
In terms of synthesis these compounds are easily synthetized with high purity and in good yields.26
Many derivatives with different substituents have been synthesized in order to produce new potential anticancer drugs with cytotoxic activity and low toxicity. These results also catalyzed the use of derivatives for different pharmacological purposes. Considering the amount of patents and research articles, it is obvious that the naphthalimides have many biologic and non-biologic applications. The major application of these derivatives is related to their capacity to act as anticancer agents.1
The naphthalimides represent an important class of drugs characterized by their high cytotoxic activity against a great variety of murine and tumoral human cells. They showed their biologic activity through the formation of ternary complexes
DNA-18
Intercalant-topo II or by inhibiting other enzymes and/or transcption factors which act upon DNA. The strong interactions with the DNA, as was said before, play an important rule to their pharmacological properties.38
The naphthalimide derivatives are DNA intercalating agents, because they bind to DNA through insertion between base pairs of double helix. The main forces to bind the DNA are the interaction by charge transfer and the stacking between base pairs.25
However, the small molecules can bind to DNA in different ways like: through binding at grooves (most at minor groove) or externally (especially if the molecules show good capacity for stacking). It is also possible that the binding mode depends on the DNA sequence. The technics of absorption of UV/visible and fluorescence spectroscopy are excellent to monitoring the binding to the nucleic acids.26
Nowadays, the antitumor activity is related to the capacity to inhibit the human DNA topo II. Besides that, the bis-1,8-naphthalimides analogs kill the eukaryotic cells through the stabilization of the cleaved complex of topo II with the DNA.39
Numerous derivatives of mono and bis naphthalimides have shown to exhibit potent anticancer properties against a great variety of murine and human tumor cells. These observations with structural optimization due to the maintenance the naphthalimide moiety and add/change appropriate functional groups can help to reduce the systemic toxicity.25,40,41
The first series of naphthalimide derivatives with biological activity appeared in 1973. These compounds resulted from the combination of important structural characteristics of existing anti-tumoral drugs as acid aristocholic, tilorone, ciclohexamide and morpholino-β-thalidomide in one molecule (Figure 9).1,42
19 Figure 9: A – Cytotoxicity compounds considered in the design of Naphthalimides; B – First series of
naphthalimides synthetized for possible antitumor activity1
The naphthalimide derivatives that had the greatest impact were the amonafide (3-amino-1,8-naphthlimide), mitonafide (3-nitro-1,8-naphthalimide) and azonafide. (Figure 10).28,39,43 Amonafide and mitonafide have been tested in clinic trials for the treatment of solid tumors. Both entered into phase II clinical trials and showed high antitumor activity with IC50 values 0.47mM and 8.8mM respectively against HeLa cell lines.26
Figure 10: Structure of mono-naphthalimide derivatives
Both derivatives bind to dsDNA by intercalation. When they bind to DNA there is an increased of viscosity of sonicated rod-like DNA fragments and consequent increase in length. The mode of action of these two drugs is inducing a topo II mediated DNA cleavage at nucleotide nº 1830 on pBR322 DNA.
The other naphthalimide derivatives didn’t show this specific cleavage. The explanation is the lacking of the basic side chain because this could interacts sterically with the enzyme active site in the ternary complex.28
20
Some SAR (structure-activity relationship) studies have shown that some parameters are essential and influence the anticancer properties of naphthalimide derivatives. The main characteristics are the presence of a basic terminal group in the side chain and a 2 or 3 methylene groups separating the N-terminal of the side chain from the naphthalene ring. These characteristics showed to play a key role in anticancer activity.1
However, they have never been employed in therapeutics because nearly all clinical trials failed because of several factors: low water solubility, poor therapeutic index, dose-limiting bone marrow toxicity (mitonafide) or unexpected central neurotoxicity, hematotoxicity and limited efficiency (amonafide).30,25, 43
With these results and observations, some changes were made to optimize the activity and reduce the toxicity by keeping the key naphthalimide moiety intact while adding suitable functional groups.40 They are also used as leads to design bis-intercalators.30
Amonafide and Mitonafide can be synthetize in the same reaction. 1,8-naphthalic anhydride react with the HNO3 in presence of H2SO4 to give the nitro derivative. Next, was added the amine, N,N,-dimethylethylelediamine, to give the Mitonafide. With the mitonafide is possible to obtain the amonafide by reduction of nitro group to amino group. (Scheme 2).43
Scheme 2: General scheme of synthesis Mitonafide and Amonafide; i) H2SO4, HNO3, 5-20ºC; ii)
N,N,-dimethylethylelediamine, EtOH, reflux; iii) 10% Pd/C, H2, EtOH
Another naphthalimide derivative, Scriptaid, showed biological activity as a potent histone deactylase (HDAC) inhibitor because of its structural similarity to hidroxamic acid-containing HDAC inhibitors (Figure 11).1,44 Also the accumulation proteins as
21
Acetylated H3 and H4 histone in certain type of cells, confirm the effect of Scriptaid as HDAC inhibitor.45
This compound has used for the treatment of several diseases like cancer, infectious diseases, immune deficiency ischemic injury, etc1
Figure 11: Structure of Scriptaid
The synthesis of Scriptaid starts with the reaction between 1,8 naphthalic anhydride with aminopropanoic ξ acid in reflux in acid medium. The resulting compounds, was treated by ethyl chloroformat in triethylamine. In the end the mixture obtain is treated with NH2OH to give the Scriptaid (Scheme 3).45
Scheme 3: Synthesis of Scriptaid. a) propanoic acid, aminopropanoic ξ-acid, reflux, 4h; b) Et3N, THF, 0ºC,
15min, followed by NH2OH, MeOH, r.t., 1h
Several naphthalimide derivatives were synthetized using a 2-metoxibenzyl group as a substituent and variating the alkyl chain between 2 and 10 carbons (Figure 12). Previous work demonstrated structures with polymethylene chains where the length of the chain can change may have relevance for the anticancer activity.32
22
The synthesis of this type of compounds starts with the reaction between 1,8-naphthalic anhydride with a alkyldiamine with different chain length. After that the 2-metoxibenzaldehyde was added creating the imine and after the imine is reduce using sodium borohydride. (Scheme 4)
Scheme 4: Synthesis of 2-metoxibezyl derivatives; a) EtOH, reflux, 24h; b) 2-MeOC6H4CHO, toluene,
reflux, 3h; c) NaBH4, EtOH, r.t., 3h
1.8 Bis-Naphthalimides
One way to enhance the binding constant was to create bifunctional, or even polyfunctional intercalators based on their corresponding monomers. (Figure 13) The idea of synthesizing this type of compounds has stimulated by the idea that the pharmacological activity of intercalating drugs can be significantly increased with higher DNA binding constants and a slower dissociation rates from DNA expected for bisintercalators relative to monointercalators. Another reason may be the increase the global size occupied by the ligand could afford greater opportunities for sequence selective but this reason is not clear.28,46
Figure 13: General structure of bis-naphthalimides
Bis-naphthalimide derivatives are a type of compounds that comprise two mono naphthalimides units and they are connected by a polyamine spacer (linker).3,26 They showed higher affinity and selectivity in binding when compare to their mono counterparts. The main reason can be attributed to the increased local concentration of the active moiety (Figure 14).39,47
23 Figure 14: Deformation of the DNA helix by a monointercalating agent (at left) and scheme of
intercalation between a bisintercalator and DNA (at right)30
The two bis-naphthalimido derivatives that deserve more attention and were the first ones to reach the clinical trials against solid tumors were Elinafide and Bisnafide. (Figure 15) They exhibited very high in vivo and in vitro activity.48,49
Figure 15: Structure of Elinafide and Bisnafide
Elinafide (LU79553) showed high activity against a variety of human xenograft models such as LX-1 (lung), CX-1 (colon), and LOX (melanoma).26
The synthesis of this compound consists in the reaction between of 1,8 naphthalic anhydride with the diamine in dioxane (Scheme 5).
24
Scheme 5: Synthesis of Elinafide. a) dioxane, reflux
Some reports showed that the Bisnafide analogues kill the eukaryotic cells by stabilizing the cleavage complex of topo II with DNA but this is still controversial since in another studies, Elinafide was found not to be a poison of topo II.38
The synthesis of Bisnafide start with a reaction between ethylenediamine with a carbonyl group protected. To the intermediate formed was added acid to remove the protecting group. To the resulting product was added B2H6 to reduce the carbonyl group. In the end the diamine produced react with the nitro naphthalimide (Scheme
6).50
Scheme 6: Synthesis of Bisnafide. a) THF, B2H6; b) EtOH, reflux, metasulfonic acid
The presence of positive charges on bis-naphthalimide derivatives creates a good intercalation because the positive charges interact with the negatively charged backbone of the DNA providing enhanced DNA stability.48
Some SAR studies and analysis showed that the methylene middle chain is important for the activity. Longer the methylene middle chain of at least 8 methylene groups
25
between the two naphthalimides rings is important for example for the activity against anti-Plasmidium.49
The major problem of this type of compounds is that they are very insoluble and it would be very difficult to test them in biological assays. One way to overcome this problem is to introduce more than two nitrogen atoms in the linker chain to improve the solubility without compromise the biological activity.51
1.9 Aims
The aim of this work is to synthesize a series of mono and bis-naphthalimides derivatives with potential anticancer activity. These novel derivatives will be tested in brain cancer cells, SH-5YSY, to determine their cytotoxicity and DNA binding properties (Figure 16).
Chapter 2
29
2. Results and Discussion
2.1 Introduction
The aim/objective for the synthesis of these naphthalimides was to test them against cancer cells, study the effect of modifications in the linker chain, either in length and substituents. The project included binding studies of the molecules to DNA.
Earlier work showed that certain types of linkers, the length or the nature of the substituent was extremely important for enhanced solubility and effective biological activity against colon cancer cells.52,53
2.1.1 Synthesis of naphthalimides 1a – c
Scheme 7: Reaction between 1,8-napthalic anhydride and alkyl diamines. i) EtOH, reflux
1,8-Naphthalimides (1a – c) were obtained by a straight forward method in good yields (58 – 86 %), combining 1,8-naphthalic anhydride with alkyl diamines (Scheme 7). The nitrogen of alkyl diamines acts as nucleophile attacking the carbonyl of the naphthalic anhydride, with ring opening to generate an intermediate stabilized by resonance. Next, the same nitrogen attacks the other carbonyl group to give the desire derivatives 1a – c by water elimination. (Scheme 8)
30
Scheme 8: Mechanism between 1,8-naphthalic anhydride and alkyl diamine
To confirm and determine the structures of the products obtained, 1H and 13C NMR spectroscopy was used(Figure 17).
Figure 17: Structure of compounds 1a – c
1
H NMR showed the presence of the -CH2 attached to the naphthalimide ring in all derivatives. The protons H-1’ appeared between δH = 4.20 – 4.30 ppm as triplets (t) with a J vicinal between δH = 6.4 – 7.0 Hz for all derivatives. The H-2’ of 1a appeared as a triplet at δH = 3.08 ppm with a J vicinal = 6.8 Hz. Compounds 1b and 1c showed internal methylenic protons as multiplets (m) at δH = 1.77 – 1.95 ppm. The methylene unit near to the nitrogen of the naphthalimide ring appeared at lower field, due to deprotection effect of nitrogen atom connected to the amide. At aromatic region the protons H-4 appeared as double doublets (dd) δH = 8.61 – 8.62 ppm with a J meta = 1.2 Hz and J ortho δH = 7.4 – 8.4 Hz. The H-5 appeared as double doublets δH = 7.76 – 7.77
31
ppm with a J ortho = 7.2 – 8.4 Hz. Finally H-6 appeared as double doublets at δH = 8.21 – 8.22 ppm with a J meta= 0.8 – 1.2 Hz and J ortho = 8.0 - 8.4 Hz. (Table 2)
Table 2: 1H NMR signals of protons in derivatives 1a – c in CDCl3; J in Hz; δH in ppm at 400 MHz
Compound 1 H ppm H-4 H-5 H-6 H-1’ H-2’ H-3’ H-4’ 1a 8.62 dd 7.76 dd 8.22 dd 4.29 t 3.08 t --- --- 1b 8.61 dd 7.77 dd 8.22 dd 4.30 t 1.88-1.95 2.78 t --- 1c 8.61 dd 7.76 dd 8.21 dd 4.20 t 1.77-1.82 1.55-1.63 2.79 t 13
C NMR spectra, including DEPT 135 confirms the methylene units. In the aliphatic region all the C-1’ appeared at δc = 37.4 – 43.1 ppm and C-2’ at δc = 25.1 – 40.9 ppm. The C-3’ peaks of 1b and 1c show up between δc = 30.8 – 39.5 ppm and the C-4’ of the
1c derivative appeared at δc = 41.6 ppm. At the aromatic region signals due to C-4 appeared δc = 131.2 – 131.5 ppm, to C-5 at δc = 126.8 – 126.9 ppm, to C-6 at δc = 133.9 ppm, to C-3a at δc = 122.4 – 122.5 ppm, to C-6a at δc = 131.2 – 131.5 ppm, to C-10 at δc = 128.0 – 128.1 ppm and to C-1 at δc = 164.2 – 164.5 ppm. (Table 3)
Table 3: 13C NMR signals of protons in derivatives 1a – c in CDCl3; δC in ppm at 100.6 MHz
Comp. 13 C ppm C-1/3 C-4 C-5 C-6 C-3a C-6a C-10 C-1’ C-2’ C-3’ C-4’ 1a 164.5 131.3 126.9 133.9 122.5 131.2 128.1 43.1 40.9 --- --- 1b 164.2 131.2 126.8 133.9 122.4 131.5 128.0 37.4 31.9 39.5 --- 1c 164.3 131.5 126.8 133.9 122.4 131.2 128.0 39.8 25.1 30.8 41.6
32
2.1.2 Synthesis of compounds 2a – p
Scheme 9: Reaction between naphthalimides 1a – c and aldehydes; i) EtOH, reflux
Compounds 2a – p were obtained by a straight forward method by reaction of 1a –
c with aldehydes. The respective imines were obtained and isolated in good yields 54 –
97 % (Scheme 9). In this reaction, the amine attacks the C=O group forming the intermediate showed in Scheme 10. A water molecule was eliminated to give the iminic derivatives 2a – p.
33
The structures of the molecules synthesized were confirmed by NMR spectroscopy. The 1H NMR of compound 2a is shown in Figure 18 and it’s DEPT 135 in Figure 19.
1
H NMR
Figure 18: 1H NMR of imine 2a
DEPT 135
Figure 19: DEPT 135 of imine 2a
H-2’ H-1’ OCH3 Naphthalimide ring H-4/9 H-5/8 H-6/7 + H-1’’ H-3’’/4’’ H-2’’/5’’
CH
2N=CH
34
Figure 20: Structure of compounds 2d and 2i
For compounds 2d and 2i (Figure 20) the 1H NMR, the main peaks are: at aromatic region appeared the H-2’’/H-5’’ protons at δH = 7.67 – 7.77 ppm as a doublet (d) with J
ortho = 8.4 Hz and the H-3’’/H-4’’ at δH = 8.06 – 8.15 ppm as a doublet with a J ortho =
7.2 – 8.8 Hz. The iminic proton, H-1’’, in all derivatives appeared between δH = 8.35 – 8.39 ppm as a singlet. (Table 4)
Table 4: 1H NMR signals of 2d and 2i derivatives in CDCl3, J in Hz, δH in ppm at 400 MHz
Compound 1 H ppm H-1’ H-2’’/5’’ H-3’’/4’’ 2d 8.35 s 7.67 d 8.06 d 2i 8.39 s 7.77 d 8.15 d 13
C RMN spectrum showed the iminic carbon, C-1’’, δC = 160.2 – 161.0 ppm and C-2’’/C-5’’ and C-3’’/C-4’’ appeared at δC = 128.6 – 129.7 ppm and 124.2 – 125.4 ppm, respectively. (Table 5)
Table 5: 13C NMR signals of 2d and 2i derivatives in CDCl3, δC in ppm at 100.6 MHz
Compound 13 C ppm C-1’’ C-1a’’ C-2’’/5’’ C-3’’/4’’ C-3a’’ 2d 160.2 129.5 129.0 125.4 148.9 2i 161.0 129.2 128.6 124.2 148.7