Mestrado Integrado em Medicina Veterinária
Ciências Veterinárias
Assessment of propofol anesthesia in the rabbit
Sónia Patrícia Seabra Campos
Orientador:
Prof. Doutor Luís Marques Antunes
Co-orientadores:
Prof. Doutora Maria Paula do Amaral Alegria Guedes de Pinho
UNIVERSIDADE DE TRÁS-OS-MONTES E ALTO DOURO VILA REAL, 2010
iii
General Abstract
The growing interests in propofol as an intravenous anesthetic agent and in particular to its
use in rabbits were the motivation for this work. The rabbit is the third most common pet, and
is also used as a biomedical research model in a wide range of science branches such as
pharmacology, toxicology, anesthesiology and surgery.
The main goal of this study was to explore the effects of Total Intravenous Anesthesia (TIVA)
with propofol in rabbits. Different infusion rates were administrated to obtain different
anesthetic depths. Blood samples were collect before, during and after anesthesia, in
previously determined moments. Propofol and propofol non-conjugated metabolites
concentrations were determined in the collected blood serum by Gas Chromatography/ Ion
Trap-Mass Spectrometry (GC/IT-MS). This information allowed the study of the drug
pharmacodynamic profile in the rabbit, and to understand the effects of propofol on the
cardio-respiratory system, clinical signs of anesthetic depth and electroencephalogram (EEG).
Furthermore, the correlation between these variables and non-conjugated propofol metabolites
plasma concentrations also help to understand the pharmacodynamic characterization of the
drug. This study will also aid the anesthetists to better understand the rabbit anesthesia with
propofol and provides important information for toxicology studies when this drug is used.
The rabbits shares many clinical signs of Propofol Infusion Syndrome (PRIS) observed in
humans. The analyses of the free propofol metabolites (diisopropyl-1,4-quinone and
2,6-diisopropyl-1,4-quinol) done in these studies, allowed to understand how they interact in the
rabbit. These are important components to be analyzed more in depth in future studies. These
iv
Resumo
O interesse crescente no uso do propofol como anestésico intravenoso e em particular a sua
aplicação no coelho foram o impulso para a realização do presente trabalho. O coelho é o
terceiro animal de estimação mais adoptado e é também usado como modelo biomédico de
investigação nas diversas áreas da ciência, sendo de salientar, a farmacologia, toxicologia,
anestesiologia e a cirurgia.
O principal objectivo deste estudo consistiu em explorar os efeitos da Anestesia Total
Intravenosa (TIVA) com propofol em coelhos. Assim, foram aplicadas diferentes taxas de
infusão para se obter diferentes profundidades anestésicas, tendo sido recolhidas amostras de
sangue em períodos de tempo previamente estabelecidos. Foram ainda realizadas análises
laboratoriais de Cromatografia Gasosa com detecção por Espectroscopia de Massa
(GC/IT-MS) para quantificar a concentração plasmática do propofol e dos seus metabolitos
não-conjugados no soro recolhido. Os dados obtidos permitiram o estudo do perfil
farmacodinâmico do propofol no coelho, de forma a clarificar os efeitos deste anestésico no
sistema cardio-respiratório, nos sinais clínicos de profundidade anestésica e no
electroencefalograma (EEG). As correlações entre os sinais clínicos, EEG e as concentrações
plasmáticas do propofol e dos seus metabolitos não-conjugados possibilitaram ainda a
caracterização farmacodinâmica do propofol, o que auxilia os anestesistas no que respeita à
anestesia de coelhos com propofol. As análises dos metabolitos livres no sangue
(2,6-diisopropil-1,4-quinona and 2,6-diisopropil-1,4-quinol) realizadas neste estudo, permitiram
uma melhor compreensão de como estes metabolitos interagem no coelho. O presente estudo
v
Acknowledgements
I would like to express my gratitude to several people who helped me while performing this
work:
To members of the IDeA Project – Integrated Design for Automation of Anesthesia
(FCT project: PTDC/EEA-ACR/69288/2006), for selecting me and for the opportunity to
carry out this study.
To Professor Luís Antunes and to Dr. Aura Silva, for their incredible and continued
guidance, inspiration and patience. Thank you both.
To Professor Paula Guedes, for letting us freely use the laboratory from Faculdade de
Farmácia da Universidade do Porto and for all support and availability.
To Professor Luísa Ferreira and to Professor Paula Branco of the Chemistry
department from Faculdade de Ciências e Tecnologia da Universidade Nova de Lisboa, in a
collaboration REQUIMTE/CBQF Lisboa, for producing the synthetic propofol metabolites.
To my parents, Patrícia e Luís, and to my brother, Luís Carlos, with all my love.
To Tita, to my grandmother, to Ana Ribeiro and especially to my aunt Claudia, for
vi
Contents
General Abstract ... iii
Resumo ... iv
Acknowledgements ... v
Index of Figures ... viii
Index of Tables ... x
Abbreviations ... xi
Chapter 1 - General Introduction ... 1
1.1 Propofol description ... 2
1.1.1 Physicochemical characteristics ...3
1.1.2 Mechanism of action ...6
1.1.3 Propofol in veterinary anesthesia ...6
1.1.4 Undesired side-effects of propofol ...7
1.1.5 Effects of propofol on cardiovascular function ...7
1.1.6 Effects of propofol on respiratory function ...8
1.1.7 Effects of propofol on cerebral activity ...9
1.2 Total Intravenous Anesthesia (TIVA) ... 10
1.3 Target Control Infusion (TCI) Systems... 11
1.4 Monitoring depth of anesthetic and closed-loops systems ... 13
1.5 Propofol pharmacokinetic properties ... 15
1.5.1 Propofol blood concentration versus time ...16
1.5.2 Plasma Protein Binding ...19
1.5.3 Distribution...19
1.5.4 Elimination ...20
1.5.4.1 Metabolism ... 21
1.5.4.2 Clearance ... 22
1.5.4.3 Excretion ... 23
1.7 The rabbit as a model for propofol studies ... 24
1.8 Pharmacodynamic characteristics of propofol ... 25
1.9 Toxicity and metabolites of propofol ... 26
Aims and objectives of the thesis ... 31
Chapter 2 – General methods and materials ... 32
2.1 Animals ... 32
2.2 Anesthesia and monitoring ... 32
2.3 Plasma propofol sampling and quantification ... 37
2.3.1 Plasma propofol sampling ...37
2.3.2 Propofol and propofol metabolites quantification in blood serum ...37
2.3.2.1 Drugs, chemicals and preparation of standard solutions ... 37
vii
2.3.2.3 Linearity study ... 41
2.3.2.4 Quantification of Limit of Detection (LOD) and of Limit of Quantitation (LOQ). ... 41
2.3.2.5 Precision study ... 41
2.3.2.6 Interferences ... 42
2.5 Data analysis ... 42
Chapter 3 - Results ... 43
3.1 Clinical responses to propofol total intravenous anesthesia: hemodynamic and electroencephalographic data ... 43
3.2 Analysis of metabolites plasma concentration ... 59
3.2.1 Validation of the technique ...59
3.2.1.1 Study of linearity ... 59
3.2.1.2 Quantification of Limit of Detection (LOD) and of Limit of Quantitation (LOQ) ... 60
3.2.1.3 Precision study ... 60
3.2.1.4 Interferences ... 60
3.2.2 Propofol and its metabolites quantification ...61
3.2.3 Correlation between propofol non-conjugated metabolites plasma concentration with propofol plasma concentration and with collected clinical signs ...63
Chapter 4 - Discussion of the results ... 67
Chapter 5 - Conclusion ... 75 References ... 77 Appendix 1 ... 85 Publications ... 87 Abstracts ... 87 Articles ... 87 Appendix 2 ... 88 Appendix 3 ... 89 Appendix 4 ... 90 Appendix 5 ... 91 Appendix 6 ... 92 Appendix 7 ... 93
viii
Index of Figures
Figure 1 - General schematic representation of a three-compartment model for an anesthetic
drug. ... 18
Figure 2 – Major biotransformation pathways for propofol into its metabolites in humans. .. 24
Figure 3- Inhibitory actions of propofol at the mitochondrial electron transport chain.. ... 27
Figure 4 – Effects of propofol on fatty acid metabolism at the mitochondria. ... 28
Figure 5 - Structure of 2,6-diisopropyl-1,4-quinol and 2,6-diisopropyl-1,4-quinone, transformed by alkali conditions.. ... 30
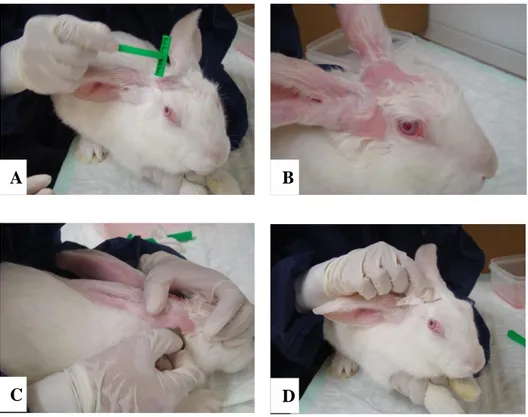
Figure 6 - Skin preparation technique for electrode placement in the rabbit. ... 33
Figure 7 – EEG baseline record in fully awake animal, using the Ioc-View monitor ... 33
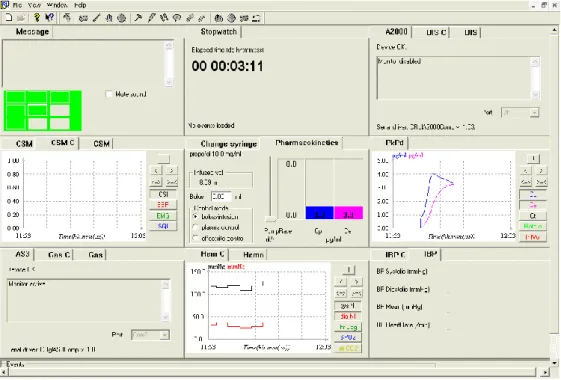
Figure 8 – Rugloop ® II Vet Software. ... 34
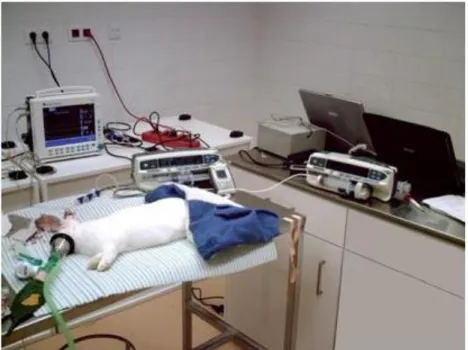
Figure 9 - The anesthetic setup. ... 35
Figure 10 - Schematic representation of blood samples (S) collection. ... 37
Figure 11 - Synthetic pathway of propofol non-conjugated metabolites production. ... 39
Figure 12 - Protocol for the extraction procedure for propofol and its metabolites. ... 40
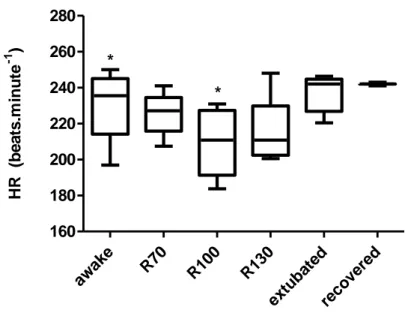
Figure 13 – The box-plots show the variation values of heart rate (HR).. ... 45
Figure 14 – The box-plots show the variation values of systolic arterial blood pressure (SABP). ... 46
Figure 15 – The box-plots show the variation values of dyastolic arterial blood pressure (DABP). ... 46
Figure 16 – The box-plots show the variation values of mean arterial blood pressure (MABP) . ... 47
Figure 17 – The box-plots show the variation values of index of consciousness (IoC) . ... 47
Figure 18 - Graphic representation of propofol plasma concentration and of propofol infusion rate scheme for rabbit 1 and 2. ... 49
Figure 19 - Graphic representation of propofol plasma concentration and of propofol infusion rate scheme for rabbit 3 and 5. ... 49
Figure 20 - Graphic representation of propofol plasma concentration and of propofol infusion rate scheme for rabbit 4. ... 50
Figure 21 - Graphic representation of propofol plasma concentration and of propofol infusion rate scheme for rabbit 6. ... 50
Figure 22 - Scatter diagram of the relationship between propofol plasma concentration (PPC) and heart rate (HR), measured in all animals during the different anesthetic episodes. ... 52
Figure 23 - Scatter diagram of the relationship between propofol plasma concentration (PPC) and mean arterial blood pressure (MABP), measured in all animals during the different anesthetic episodes ... 52
Figure 24 - Scatter diagram of the relationship between propofol plasma concentration (PPC) and respiratory rate (RR), measured in all animals during the different anesthetic episodes .. 53
Figure 25 - Scatter diagram of the relationship between propofol plasma concentration (PPC) and the clinical scale of depth of anesthesia (DoA), measured in all animals during the different anesthetic episodes ... 53
Figure 26 A and B- Scatter diagrams of the relationship between propofol plasma concentration (PPC) and the index of consciousness (IoC), measured during the different anesthetic episodes ... 54
Figure 27 - Aligned dot plot diagram of the evolution of heart rate (HR) at different ranges of propofol plasma concentration (PPC).. ... 55
Figure 28 - Aligned dot plot diagram of the evolution of mean arterial blood pressure (MABP) at different ranges of propofol plasma concentration (PPC). ... 56
ix
Figure 29 - Aligned dot plot diagram of the evolution of respiratory rate (RR) at different
ranges of propofol plasma concentration (PPC).. ... 56
Figure 30 - Aligned dot plot diagram of the evolution of arterial b oxygen saturation (SpO2)
at different ranges of propofol plasma concentration (PPC). ... 57
Figure 31 - Aligned dot plot diagram of the evolution of Index of Consciousness (IoC) at
different ranges of propofol plasma concentration (PPC). ... 57
Figure 32 - Aligned dot plot diagram of the evolution of Depth of Anesthesia (DoA) at
different ranges of propofol plasma concentration (PPC). ... 58
Figure 33 - Mass spectrum of propofol with ions m/z=163 and m/z=178;
2,6-diisopropyl-1,4-quinol with ions m/z=179 and m/z=194; 2,6-diisopropyl-1,4-quinone with ions m/z=149 and m/z=192 and thymol (internal standard) with ions m/z=135 and m/z=150. ... 61
Figure 34 - Gas chromatogram profiles of the 3th blood sample from rabbit 3, during the infusion rate of 70 mL.kg-1.h-1. ... 62
Figure 35 – Scatter diagram of the relationship between propofol plasma concentration (PPC)
and metabolites plasma concentration (MPC), measured in all animals during the different anesthetic episodes.. ... 64
Figure 36 - Scatter diagram of the relationship between metabolites plasma concentration
(MPC) and heart rate (HR), measured in all animals during the different anesthetic episodes. ... 65
Figure 37 - Scatter diagram of the relationship between metabolites plasma concentration
(MPC) and mean arterial blood pressure (MABP), measured in all animals during the different anesthetic episodes. ... 65
Figure 38 - Scatter diagram of the relationship between metabolites plasma concentration
(MPC) and clinical scale of depth of anesthesia (DoA), measured in all animals during the different anesthetic episodes. ... 66
Figure 39 – Scatter diagram of the relationship between metabolites plasma concentration
(PPC) and Index of consciousness (IoC), measured in all animals during the different anesthetic episodes. ... 66
x
Index of Tables
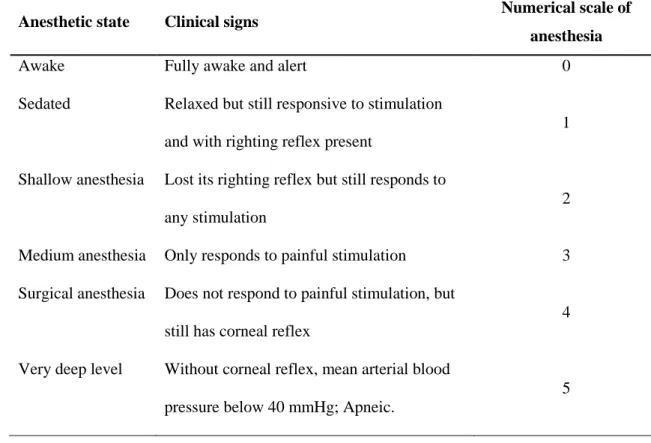
Table 1 - Pharmacokinetic parameters in different animal species. ... 17 Table 2 - Definition of anesthetic states based on clinical signs and the respective attributed
numerical scale. ... 36
Table 3 – Mean ± Standard Deviation (SD) values of clinic variables (HR, SABP, DABP,
MABP, SpO2,T, and IoC values) in the six rabbits, during the different episodes of the
experiment. ... 44
Table 4 - Propofol infusion schemes order and respective rabbit in which one was used. ... 48 Table 5 - Correlation coefficients (Pearson r and Spearman r) between the Propofol Plasma
Concentration (PPC) and the clinical parameters (HR, MABP, SpO2, RR, clinical scale of
DoA and IoC). ... 51
Table 6 - Linearity study. Calibration curves and correlation coefficients of propofol and its
metabolites. ... 59
Table 7 - Correlation coefficient (Pearson r) between the Propofol Plasma Concentration
(PPC) and Metabolites Plasma Concentration (MPC). ... 63
Table 8 - Correlation coefficients (Pearson r and Spearman r) between the Metabolites
Plasma Concentration (PPC) and the clinical parameters (HR, MABP, SpO2, clinical depth of
xi
Abbreviations
AEP ATP BIS bpm BS ClTB CNS CSI CYP DABP DoA EDTA EEG EtCO2 GABA GC/IT-MS LCT LOD LOQ HR ICU IoC IR IS MABP min mmHg MPC MTC NMDA PK/PD PONVAuditory evoked potentials Adenosine 5’-triphosfate Bispectral Index
Beats per minute Burst suppression Total body clearance Central Nervous System Cerebral state index Cytochrome
Diastolic arterial blood pressure Depth of anaesthesia
Ethylenediamine tetraacetic acid Electroencephalogram
End-tidal carbon dioxide Gama-aminobutiric acid
Gas Chromatography/ Ion Trap-Mass Spectrometry Long chain triglycerides
Limit of detection Limit of quantitation Heart rate
Intensive care unit Index of Consciousness Infusion rate
Internal Standard
Mean arterial blood pressure Minutes
Millimetres of mercury Medium chain triglycerides Metabolites Plasma Concentration N-methyl-D-aspartate
Pharmacokinetic/pharmacodynamic Post-operatory Nausea and Vomiting
xii PPC PRIS RR R70 R100 R130 SABP SE SpO2 TCI TIVA TºC UGT
Propofol Plasma Concentration Propofol Infusion Syndrome Respiratory rate
Infusion rate of 70 mg.kg-1.h-1 Infusion rate of 100 mg.kg-1.h-1 Infusion rate of 130 mg.kg-1.h-1 Systolic arterial blood pressure Spectral entropy
Arterial blood oxygen Target controlled infusion Total intravenous anesthesia Temperature (Celsius degrees)
1
Chapter 1 - General Introduction
Veterinary anesthesia demands increasingly safer techniques which enable rapid recoveries
and low incidence of adverse side effects (Smith, Terhoeve et al. 1999). Better anesthetic
techniques are dependent on the development of new-generation anesthetic drugs, such as
intravenous anesthetics with fast onset of action and rapid elimination, providing very fast
dose adjustments. The study of the drugs pharmacokinetic and pharmacodynamic profiles is
thus a very important part in anesthesia and may contribute to the advance of veterinary
anesthesia. Pharmacokinetic studies include the routes and time of absorption, distribution,
metabolism and excretion in the body, whereas pharmacodynamics analyzes the
pharmacological activity and side effects of drugs (Gibaldi and Levy 1976). The appearance
of short-acting hypnotics, including propofol, initially used for induction of anesthesia,
triggered the development of an alternative method to volatile anesthetics, and the expansion
of Total Intravenous Anesthesia (TIVA) techniques (White 2005). This procedure is routinely
used for maintenance of anesthesia in human patients and has been introduced in veterinary
medicine relatively recently. However, the precise use of this technique in animals still has
several limitations. Its proper use requires knowledge of the pharmacokinetics of the
anesthetic, for the species that is used (Beths, Glen et al. 2001; Guarracino, Lapolla et al.
2005), which is not available to most veterinary species, remaining the administration of
propofol based on a constant clinical observation of the patient, and therefore very subjective
and dependent on the experience of each professional. Through the creation of
pharmacokinetic models it becomes possible to predict the concentration of propofol in
plasma and local drug effect (the brain) so that the anesthesiologist has a more rapid, safe and
efficient anesthesia of patients (Flaherty, Reid et al. 1997; Glowaski and Wetmore 1999; Horn
and Nesbit 2004). This control is facilitated by the integration of pharmacokinetic models in
2
The option of undertaking studies in rabbits, Oryctolagus cuniculus, is justified because this is
the third most anesthetized animal species in the world, either as pets or as research model
(Brodbelt 2009), which gives these animals the opportunity to conduct basic research with
direct application to the species and translation to other species. Currently there are no clinical
studies on the pharmacokinetics of propofol in the rabbit; this drug may be an alternative to
volatile anesthetic through the implementation of Total Intravenous Anesthesia. Moreover,
studies conducted by Ypsilantis et al. (2007), report that the rabbit can serve as a model of
Propofol Infusion Syndrome (PRIS). This syndrome occurs frequently in critically ill human
patients subjected to high doses of propofol and long periods of sedation (3-4 days), with
children more affected than adults. The PRIS is characterized by bradycardia or asystole,
severe metabolic acidosis, rhadbomyolysis, renal failure and changes in lipid metabolism.
Their detection is based on clinical and biochemical signs of patients, so it has not been fully
clarified the mechanism responsible for its development (Corbett, Montoya et al. 2008). The
possibility of determining not only the plasma concentrations of propofol, but also its
metabolites, makes possible a more thorough study of this syndrome using the rabbit.
In this chapter, a general review of the state of the art regarding the characteristics of the
anesthetic propofol is performed, as well as its general administration regimens such as total
intravenous anesthesia (TIVA) and target controlled infusion (TCI). As described in previous
works, the pharmacokinetic and pharmacodynamic profile of propofol is reviewed, as well as
the Propofol Infusion Syndrome (PRIS).
1.1 Propofol description
Propofol (Lipuro®, Diprivan®, etc) is a fast-acting anesthetic of short duration, given
intravenously and suitable for induction and maintenance of general anesthesia. Nowadays, it
3
It was developed in the beginning of the 1970’s but only in 1989 it was introduced in the clinical practice. Through the last decades, this new injectable anesthetic has become more
and more popular, turning itself the choice drug from simple sedation to complex medical
procedures that require the induction and maintenance of general anesthesia, either in humans
and in animals (White 2005).
The use of this sedative-hypnotic agent in clinical routine may be achieved with simple
equipment for intravenous administration such syringes and intravenous catheters but
nowadays the use of propofol is demanding more and more the use of specific and auxiliary
equipment, such as sophisticated infusion pumps. Nevertheless, it has acquired worldwide
acceptance, mainly because of its rapid onset, short duration of action and clinical effect,
minimal side-effects, good hemodynamic stability and the quality and rate of recovery
whether it is given by a bolus or continuous infusion (Hall and Chambers 1987; Morgan and
Legge 1989; Baker and Naguib 2005). Regarding veterinary anesthesia, the applications of
propofol are mostly focused in the dog sedation, induction and maintenance of general
anesthesia. However, it has already started to be used in others species like mice, rats, pigs
and, as exposed this work, in rabbits (Short and Bufalari 1999).
1.1.1 Physicochemical characteristics
Propofol is an alkylphenol (2,6-diisopropylphenol) hypnotic drug chemically unrelated with
others intravenous anesthetic drugs (Langley and Heel 1988; Sebel and Lowdon 1989;
Ypsilantis, Mikroulis et al. 2006). It consists in a simple phenol substituted with two
isopropyl groups in each of the positions adjacent to the hydroxyl group (Baker and Naguib
2005).
The critical point in the propofol formulation was the capability to adjust a biocompatible
vehicle that ensured adequate pharmacodynamic profiles and minimal side effects. This issue
4
can only be achieved in lipophilic substances or organic solvents (Baker and Naguib 2005).
The first formulation introduced in the clinical trials was prepared with polyoxyethylated
castor oil (Cremaphor EL) (Kay and Rolly 1977) but this emulsion was unstable (Dye and
Watkins 1980), demonstrating undesirable effects as: painful injection (White 2002),
anaphylactic reactions (Cummings, Dixon et al. 1984), potential for sepsis (Baker and Naguib
2005) and hyperlipidemia-related side effects (McKeage and Perry 2003) during clinical
studies. These effects reveled the need to develop a safer alternative to this formulation
(Langley and Heel 1988; Sebel and Lowdon 1989), that consisted in 1% propofol in
Intralipid, a parental nutricional agent based on 10% soybean oil, 2.25% glycerol and 1.2%
purified egg phosphatide (Hall and Chambers 1987; Short and Bufalari 1999), where each
compound performs a specific function in forming the ultimate formulation (Baker and
Naguib 2005). Current propofol emulsion appears as a slightly viscous milky yellowish
substance that remains stable at room temperature and in the presence of light. It can be
diluted with 5% dextrose in water (Lumb and Jones 1996). Aseptic conditions are required
since the vehicle is capable of supporting bacterial growth. Some emulsions may contain
excipients as EDTA to avoid the bacterial growth (Baker and Naguib 2005). Nevertheless, the
currently available product contains no antimicrobial preservatives which means that the
emulsion must be removed from either ampoules or vials and that any contents of an opened
ampoule must be eliminated 6 hours after the opening (Short and Bufalari 1999).
Propofol in medical and veterinary use is often conjugated with fast-acting analgesics-onset anesthetics in order to promote a primarily rapid anesthesia. However, it doesn’t produce analgesia and muscle relaxation, which means that for surgical procedures, it should be used
in combination with other drugs that fulfill these drawbacks. Though, the combination of
propofol with others substances like lidocaine leads to the emulsion instability, due to an
5
In order to overcome the disadvantages of the lipid emulsion, numerous approaches to
improve propofol formulation have been recently tested. Increasing propofol concentrations in
the emulsion, creating emulsions containing less than 10% oil, creating emulsions having oils
with different fatty acid contents and modifying emulsions with protein are some
modifications that have been done to produce a compound more soluble in water (Baker and
Naguib 2005). Diprivan®, 1% propofol in 10% soybean oil emulsion has predominantly
LCT’s (Long Chain Triglycerides) when compared with Propofol-Lupiro® that contains mixed MCT (Medium Chain Triglycerides) – LCT and 1% propofol. The combination
MCT-LCT provides more rapid triglyceride elimination, does not affect the pharmacokinetics or
pharmacodynamics of propofol and is less painful when injected (Doenicke, Roizen et al.
1997; Larsen, Beerhalter et al. 2001). Nonemulsion formulations containing cyclodextrins or
poloxamers can be used to increase solubility, dissolution rate and the stability of drugs.
However, pharmacodynamic studies showed that the recovery time was longer when
compared with propofol emulsion (Trapani, Latrofa et al. 1998). At last, the water-soluble
prodrugs, such the hemisuccinate or phosphate monoesters, are the ultimate formulation of
propofol (Hendler, Sanchez et al. 2001). Prodrugs are molecular entities that are soluble in
aqueous phases and, where desired, release the anesthetic. They are hydrolyzed mostly by
action of tissue esterases and to lesser degrees by nonenzymatic mechanisms. Aquavan® (GPI
15715: phosphono-O-methyl-2,6-diisopropylphenol) is hydrolyzed by alkaline phosphatase
rather than by esterases (Banaszczyk, Carlo et al. 2002) and it releases in plasma free
propofol, phosphate and formaldehyde. This prodrug exhibits a longer half-life, increased
volume of distribution, delayed onset and has a longer duration of action (Schywalsky,
Ihmsen et al. 2003), which makes it more suitable for long-term sedation than for anesthesia
6
1.1.2 Mechanism of action
In spite of propofol being so well studied, it´s precise mechanism of action isn’t totally clear. Most of its pharmacological actions are thought to result from the direct activation of the
GABAA (Gama Amino Butyric Acid – A) receptors, involving a positive modulation of the
inhibitory synaptic function of this neurotransmitter (Trapani, Altomare et al. 2000), possibly
due to a delay in closing of the calcium (Kotani, Shimazawa et al. 2008) and/or chloride
channels (Ferreira 2007). This leads to cell membrane hyperpolarization and prevention of
further action potential propagation. It is also reported that propofol may also cause the
inhibition of the NMDA (N-methyl-D-aspartate) subgroup of glutamate receptors (Yamakura,
Sakimura et al. 1999).
1.1.3 Propofol in veterinary anesthesia
The first clinical experiments with propofol were developed in laboratory animals like
rodents, pigs, monkeys, dogs and cats, using a 1% preparation formulated in Cremophor EL,
before being tested in humans (Kay and Rolly 1977; Sebel and Lowdon 1989; Short and
Bufalari 1999).
In veterinary practice, propofol has been used for short procedures, mostly in dogs, as a sole
anesthetic or associated with other agents, either injectables or inhalants (Bayan, K. et al.
2002). It is a suitable drug for induction and/or maintenance of anesthesia in different
domestic animals (Carroll, Hooper et al. 1998; Bayan, K. et al. 2002).
The progressive improvement at controlling and monitoring physiological parameters like
blood pressure, heart and respiratory rates, during general anesthesia, allowed the
development of new anesthetic techniques. Moreover, the exact determination of the effects
of anesthetic and sedative agents on central nervous system (CNS), according to their dosage,
7
The introduction of TIVA (Total Intravenous Anesthesia) in the clinical procedures of
veterinary anesthesia, made possible the application of a Target Controlled Infusion (TCI)
system through which the anesthesiologist attempts to achieve a real-time target level of drug
in the plasma or effect site, according to the drug´s pharmacokinetics. These are the most
modern tools available for the control of anesthetic depth and they have already started to be
used in dogs (Beths, Glen et al. 2001; Guarracino, Lapolla et al. 2005).
1.1.4 Undesired side-effects of propofol
Though propofol is considered a safe and efficient anesthetic agent for sedation in humans,
there are some restrains when long-term administration is required (McKeage and Perry
2003). The most common side-effects observed are hypotention and cardiorespiratory
depression (Kotani, Shimazawa et al. 2008). Others reported consequences like vomiting,
sneezing, seizures, blood stream infections, hypertriglyceridemia and pancreatitis (Bennett,
McNeil et al. 1995; Kotani, Shimazawa et al. 2008). When given in large doses and
long-periods, more than five days in adults and two days in children, propofol may lead to the
Propofol Infusion Syndrome (PRIS) (Vasile, Rasulo et al. 2003) which can be fatal.
According to a study of Ypsilantis et al. (2007), it is believed that similar clinical signs of
PRIS in humans can also occur in the rabbit, making this animal a suitable research model for
this syndrome.
1.1.5 Effects of propofol on cardiovascular function
Propofol administration induces time and dose-dependent hypotension, negative ionotropy
and decreased system vascular resistance, resulting in the decreasing of the cardiac output
(Glowaski and Wetmore 1999; Lerche, Nolan et al. 2000; Baumgartner, Bollerhey et al.
2008). Mechanisms by which propofol induces hemodynamic changes include decreases in
8
Serrao 1989). Propofol reduces the baroreceptor reflex set point, allowing slower heart rates,
despite decreases in arterial pressure. It is important to ensure that during all the procedures
where propofol is being given, the vital signs are closely monitored (Glowaski and Wetmore
1999). These changes depend on the physiological state prior to induction, namely, the heart
rate and blood pressure as well as if it was used any kind of premedicants. Nevertheless,
changes in electrocardiographic rhythm are not very frequent with either a single bolus or a
continuous infusion (Smith, Gaynor et al. 1993). In the experiment performed by Ypsilantis et
al. (2007), where rabbits were anaesthetized for 38 consecutive hours, tachycardia was
observed during the last hours of the procedure, instead of the expected bradycardia.
1.1.6 Effects of propofol on respiratory function
According to Glowaski & Wetmore (1999), propofol causes bradypnea which may conduct to
apnea, as a main undesirable effect. The depression of the respiratory center and the absence
of the response to hypercapnea (Adetunji, Ajadi et al. 2002) that can culminate in respiratory
acidosis, are reasons why bradypnea occurs, either in human or veterinary patients. Hypoxia
and hypercapnea are dose-dependent changes commonly observed (Oklu, Bulutcu et al.
2003). Endotracheal intubation and mechanical ventilation with oxygen should be applied to
avoid these complications when propofol is used to maintenance of anesthesia.
In a previous study of Ypsilantis et al. (2007) in which rabbits were anesthetized with
propofol, macroscopic findings of the lungs at necropsy included lung enlargement and
congestion and pinky frothy edema fluid effusing from lung sections and filling the tracheal
cannula. The lungs had also a milky tincture and histological examination revealed interstitial
pneumonia and pulmonary edema. This last finding was suggested as the most probable cause
of death, in this animals, and indicated the primary involvement of the lungs in the
9
1.1.7 Effects of propofol on cerebral activity
As a sedative-hypnotic drug, propofol causes dose-dependent depression of the CNS function.
Due to lipophilic properties, it easily crosses the blood-brain barrier and depresses
electroencephalographic activity as a result of reduced neuronal activity rather than a direct
action of propofol on cerebral capillaries (Short and Bufalari 1999). Attending to Peduto et
al. (1991), propofol acts by potentiating the GABA-ergic transmission in rats, increasing the
binding of GABA to its receptor complex which is in a different site from barbiturate and
benzodiazepine receptors in these animals (Peduto, Concas et al. 1991). It reduces the cerebral
blood flow, leading to a decrease in cerebral metabolic rate and in cerebral perfusion, causing
as well, a decrease in intracranial pressure, which justifies the alternative use of propofol, face
to volatile anesthetic agents that increase the intracranial pressure (Silva 2007). Propofol is
more effective than other anesthetic agents at reducing abnormalities in ion fluxes produced
during cerebral ischemia (Weir, Goodchild et al. 1989). In similarity with barbiturates,
propofol diminishes the cerebral metabolic requirement for oxygen and the intracranial
pressure, providing neuroprotective effects (Short and Bufalari 1999), anti-inflammatory and
antioxidant properties and potential protection against brain ischemic damage (Kotani,
Shimazawa et al. 2008). In addition to these features of propofol, the lack of accumulation
and the short recovery time allow a good answer in the neurological examination after the
operative procedure (Mirski, Muffelman et al. 1995). The anticonvulsant activity of propofol
has also been studied through last years. In humans it is an efficient drug when used to control
refractory status epilepticus (Marik 2004).
During routine surgical procedures propofol is weak at controlling pain. It is important to
ensure that additional analgesic agents are used when painful procedures are required (Smith,
10
1.2 Total Intravenous Anesthesia (TIVA)
Total Intravenous Anesthesia (TIVA) became possible due to the introduction of short-acting
hypnotics with a pharmacokinetic profile that favors their use in continuous infusions (White
2005). The invention of propofol, along with new insights in its pharmacokinetic and
pharmacodynamic properties, the technological progresses in the development of
computational intravenous delivery systems and the development of the electroencephalogram
(EEG)-based monitors that give a more precise accuracy of the control of the hypnotic
component of general anesthesia, have already became a essential component of the human
anesthetic practice (Padfield 2000; White 2005; Beths 2008).
According to international treaties, the emission of volatile anesthetic agents like halothane,
isoflurane and enflurane into the atmosphere will be prohibited from the year 2030. The future
of human and veterinary anesthesia can be dependent on the development of total intravenous
anesthesia (Joubert 2009).
Basically, TIVA consists in the administration of bolus dose in order to reach initial target and
to induce anesthesia, and in the maintenance of an infusion that keeps the patient asleep by
replacing the clearance and the redistribution of the drug. It is a technique through which the
entire anesthesia is only performed with intravenous drugs, without using volatile agents
(Oliveira, Oleskovicz et al. 2007).
In one hand, the use of TIVA decreases the exposure of workers and environment to
anesthetic gases, provides a greater hemodynamic and anesthetic plane stability, reduces the
recovery time and improves the recovery profile, allows a quickly deepening or lightning of
anesthesia and revels to be the technique of choice for certain types of surgery and patient
groups (Eyres 2004; White 2005; Oliveira, Oleskovicz et al. 2007). In the other hand, it
requires the use of sophisticated user-friendly infusion delivery systems such as the infusion
11
(White 2005). Computer technology has enabled targeted plasma concentration controlled
infusions to replace manual infusion regimens and the drugs utilized in TIVA should have a
rapid onset and no tendency to accumulate in the body (Joubert 2009). Propofol is commonly
used as a continuous infusion thanks to its pharmacokinetic characteristics, which allows
anesthetic maintenance for prolonged periods and rapid recoveries (Flaherty, Reid et al. 1997;
Glowaski and Wetmore 1999; Horn and Nesbit 2004). It avoids the residual sedation, fatigue
(“hangover effect”) and cognitive impairment of others sedative-hypnotic drugs and the post-operative nausea and vomiting (PONV), which are very common side-effect of anesthesia
with volatile agents (White 2005). Furthermore, the capability to associate propofol with
potent, rapid and short-acting opioid analgesics (example, remifentanil) has also promoted the
use of TIVA, leading to a higher acceptance of this technique (White 2005).
TIVA is already routinely used in human anesthesia and is becoming a very common
procedure, especially in dogs’ anesthesia. In 2001, Beths and his research team created a
pharmacokinetic model for the use of propofol in dogs. This model made possible the
implementation a Target Control Infusion (TCI) system for dog anesthesia, allowing a more
precise titration of the anesthetic’s concentration in the body and increasing the confidence in
the use of this technique.
1.3 Target Control Infusion (TCI) Systems
According to Beths (2008), a TCI system consists in a computer, previously programmed with
a pharmacokinetic model of a specific drug to particular specie that is coupled to a syringe
driver. TCI was created from measures of the drugs’ plasma concentrations, obtained by different infusion rates, in order to obtain a PK/PD pool data (White 2005). In the last two
decades, many PK/PD studies using propofol in humans and animals, motivated by the
12
systems for intravenous anesthetics (Beths 2008). In both human and veterinary medicine,
propofol may be the anesthetic drug that has dominated the use of TCI. However, new efforts
have been done to adapt it to fentanyl congeners, especially remifentanil (De Castro, Godet et
al. 2003). TCI is becoming an increasingly common type of administration technique for
propofol. However, it requires accurate knowledge of pharmacokinetics, including the effects
of age and weight. Nowadays, the development of infusion rate control algorithms and of
mathematical models incorporated in syringe pumps, based in the pharmacokinetic
characteristics of the anesthetic drugs, allow real-time estimation of drug concentrations to be
achieved rapidly and accurately (Beths, Glen et al. 2001; Guarracino, Lapolla et al. 2005) and
it also allow an easier control of depth of anesthesia. Hence, the anesthetist is able to vary the “target” drug concentration in the plasma (blood) and effect-site (brain) according to the anesthetic plan indications inserted in the computational program. The computer controls the
pump to maintain the desired target concentration, based on the PK/PD data of the specie in
which is being used (Egan 2003; White 2005; Beths 2008). It is understandable that target
concentration needs to be adjusted according to the observed pharmacodynamic effects and
immediately before changes caused by surgical procedures (White 2005), because its goal is
to maintain and control a therapeutic window with an acceptable range of safety.
The evolution of TCI systems followed the research with closed-loop systems for automatic
control of anesthetic delivery. An idyllic automatic delivery system would continuously titrate
the anesthetic plasma concentration to the desired effect, by using a signal collected on the
patient which reflects the depth of anesthesia. Currently, TCI remains an open system because
the anesthesiologist needs to be present to evaluate the depth of anesthesia and adjust the
target plasma concentration (van den Nieuwenhuyzen, Engbers et al. 2000; White 2005). This
means that the computer’s software or the syringe pumps can predict with a strict margin of
13
association with the infusion rate, but is the anesthetist the one who controls the entire
anesthetic procedure based on the clinical signs of anesthetic depth (Ferreira 2007) .
1.4 Monitoring depth of anesthetic and closed-loops systems
Monitoring depth of anesthesia (DoA) is an essential request of modern anesthesia. One of its
main goals is to prevent awareness without inadvertently overdosing the patients with potent
drugs, ensuring an adequate DoA (Kaul and Bharti 2002). Generically, DoA is described as
the patient’s level or plane of anesthesia, characterized by fluctuating degrees of hypnosis and analgesia, and it is used to determine if the patient may undergo safe surgery with smooth
recovery (March and Muir 2005). Most anesthesiologists rely on a subjective evaluation of
clinical signs of the patient that are a reflex of a combination of components: analgesia,
consciousness and somatic and autonomic answers to noxious stimulation (White 2005). The
introduction of intravenous anesthetic drugs and techniques along with the improvement in
cardio-respiratory monitoring, allowed a more effective and easy control of DoA (Beths, Glen
et al. 2001). During propofol anesthesia, an excessive depth of sedation may be associated
with clinically significant cardiovascular and respiratory depression (Smith, Monk et al.
1994). DoA is a pharmacodynamic variable: assessing the effect of an anesthetic drug is the
essence of measuring the DoA. Many techniques have been described to measure the level of
consciousness and to maintain the patient in the adequate therapeutic window.
Electroencephalography provides a non-invasive continuous monitoring of awareness through
the exhibition of a EEG with low frequency and high amplitude waves when the patient is
undergoing hypnosis (Seth and Baars 2004; Tonner and Scholz 2006). As recorded from the
skin of the head, it gives a measurement of electrical activity in the brain cortex, thus
changing with variations in DoA, as has been demonstrated in many species (Rose, Hicks et
14
2006). The interest in the EEG increased with the introduction of new technological and
mathematical developments. Techniques based on power spectral analysis, frequency ratios,
relative band powers, burst suppression ratio (BS), are now used to quantify and interpret the
raw EEG (Silva 2007). The percentage of isoelectric periods is the burst suppression ratio
(BS) (Rampil 1998). The EEG burst suppression consists of transient sequences of high
voltage slow waves and sharp waves, altering with periods of depressed background activity,
and is encountered during deep anesthesia (Bollen and Saxtorph 2006). Modern DoA
EEG-based monitors process and obtain EEG analog signals over a period of time and display the
information in the form of histograms or numerical parameters, according to brain activity
(Kaul and Bharti 2002). Examples are: auditory evoked potentials (AEP) (Alaris AEPTM
electrodes; Alaris Medical Systems, Inc., San Diego, CA), spectral entropy (SE) (EntropyTM,
ZipPrep, Aspect Medical Systems), bispectral index (BIS) (Aspect Medical Systems, Natick,
MA), cerebral state index (CSI) (CSITM, Danmeter, Odense, Denmark) and the Index of
Consciousness (IoC) (Aircraft Medical Barcelona, Barcelona, Spain). An ideal DoA monitor
would integrate the physiologic and neurologic information from all aspects of the anesthetic
state. When utilizing TIVA, it is extremely valuable and helpful having a tool which would
reliably predict the patient’s response to surgical stimuli (White 2005). Some implementations
of closed-loop control have been described as research works in rats (Angel, Arnott et al.
2000; Tzabazis, Ihmsen et al. 2004) and in humans (Struys, De Smet et al. 2001; Absalom and
Kenny 2003). Mainly, they consist in integrated systems that can monitor the patient DoA,
predicting and maintaining the desired target-effect according to the required anesthetic dose,
while the anesthetist just needs to select the correct variables (Struys, Mortier et al. 2005).
However, they would be unsafe if they are unsupervised (O'Hara, Bogen et al. 1992). An ideal
sensor for such a system should reflect the dose-response relation of the drug and the
anesthetist observations, through continuous control of variables that can be measured (e.g.
15
becoming a more and more measurable variable of hypnosis for closed-loop systems (Locher,
Stadler et al. 2004). Using closed-loop systems in clinical practice will provide the patient’s
better anesthesia quality by the achievement of a higher stability of the hemodynamic
variables with faster adjustments to their variations, reducing the risks associated to the
anesthetic drugs and surgical procedures. Efforts to develop an ideal closed-loop system have
been done throughout the last decade. Attempting to create an accurate system capable of
providing hypnosis, optimize the control of variables and maintain the desired target level is a
great challenge in modern anesthesiology.
1.5 Propofol pharmacokinetic properties
During the last decades, the growing knowledge about the pharmacokinetic properties of
propofol allowed a continuous remodeling of pooled data, describing together information of
clinical trials and adequate modeling for the specie in which it is used (Schuttler and Ihmsen
2000; Sneyd 2004). These characteristics are very well documented in humans and, in
veterinary medicine some research groups have already reported pharmacokinetic data for
propofol, especially in the dog and the cat (Cockshott, Douglas et al. 1992; Hall, Lagerweij et
al. 1994; Beths, Glen et al. 2001; Sneyd 2004).
From a pharmacokinetic point of view, propofol remains the best controllable intravenous
hypnotic, since it has a huge body uptake and a fast elimination due to a large apparent
volume of distribution and a high clearance (Schuttler and Ihmsen 2000).
Drug delivery systems, based on pharmacokinetic and pharmacodynamic properties of
propofol, were developed allowing a reasonable real-time estimation of its plasma
16
1.5.1 Propofol blood concentration versus time
In all species, following a single bolus injection, propofol is immediately distributed from the
vascular pool to the highly perfused tissues, the brain being the key target organ for clinical
effect, but this compartment also includes the liver (Bester 2009). The rapid and curvilinear
decline in propofol plasma levels is noticed with time, the concentration gradient results in
rapid redistribution from the CNS to organs with intermediate perfusion, mainly the
abdominal viscera and muscle tissues. Finally, redistribution to the poorly perfused but
extremely high capacity adipose tissues occurs (Cockshott, Douglas et al. 1992; Short and
Bufalari 1999).
The pharmacokinetic profile of propofol is usually described through the sum of two or three
exponential functions that include: fast distribution from blood into tissues; rapid metabolic
clearance and slow return of the drug from a poorly perfused deep compartment (probably fat
tissue) into blood (Cockshott 1985).
The rapid penetration of the blood-CNS barrier and distribution into the central nervous
system (CNS) by propofol justifies its rapid uptake and onset of action (Kanto and Gepts
1989). The short duration of action results from its redistribution from the brain to other
tissues, like muscle and fat (Zoran, Riedesel et al. 1993). For these motives the propofol
plasma concentration declines within ten minutes to less than 1 μg/ml, after a single bolus
dose of 2.5 mg/kg, which is the mean value at awakening from anesthesia in humans
(Cockshott, Briggs et al. 1987; Kanto and Gepts 1989).
When the blood sampling period is adequate (i.e. 12-24h after administration), the data
collected better fit a tri-exponential function. Nevertheless, a shorter sampling period results
in the definition of only two exponential phases which means pharmacokinetic parameters for
a two-compartment model (Table 1) (Cockshott, Douglas et al. 1992). Regarding Table 1, the
17
values derived for rabbit and pig probably overestimate their true values since only two
exponential phases were defined (Cockshott, Douglas et al. 1992). The distribution of
propofol is extensive, with the initial distribution volume approximating to body volume in all
species, so demonstrating the high affinity of this lipophilic molecule for tissues (Cockshott,
Douglas et al. 1992).
Table 1 - Pharmacokinetic parameters in different animal species. T ½ α, T ½ β and T ½ ɣ are the plasma
concentration half-lives of α, β and ɣ decay curves obtained by Cockshott et al. (1992). Vdss is the apparent volume of distribution at steady-state. ClB is the total body clearance.
Re fe re n c e Specie Dose Compar- tments T ½ α (min.) T ½ β (min.) T ½ ɣ (min.) Vdss (mL.Kg-1) ClB (mL. min-1. Kg-1) Co ck sho tt et a l. 1 9 9 2 Dog 7mg/kg 3 4,2 31 303 1140 76 Rat 9.3 mg/kg 3 3,5 33 383 996 72 Pig 2.5 mg/kg 2 4,9 57 - 620 76 Rabbit 5 mg/kg 2 2,1 17 - 460 337
The blood concentration curve of propofol has been best described with a three-compartment
model, although in some animals, as shown in Table 1, only two exponential phases can be
defined. The first exponential phase mirrors the rapid onset of action, the second manifests a
high metabolic clearance, whereas the third exponential phase describes the slow elimination
of a small proportion of the drug remaining in poorly perfused tissues (Kanto and Gepts
1989).
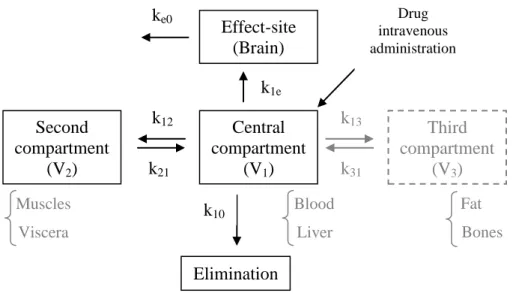
As shown in Figure 1, after an intravenous injection of propofol, it reaches the plasma very
rapidly, getting spread into the central compartment at the same time that diffuses into tissues and organs, achieving the “effect-site” (the brain). Here, it will produce the desired effect, hypnosis and general anesthesia.
18
Figure 1- General schematic representation of a three-compartment model for an anesthetic drug. V1 represents
blood or plasma; V2 indicates highly perfused tissues; V3 represents poorly perfused tissues; k12, k21, k13 and k31
are inter-compartmental rate constants; k1e is the rate constant of the drug’s passage from the central
compartment to the effect-site; k10 is the elimination rate constant from the central compartment and ke0 is the
elimination rate constant from the effect-site. Adapted from Glass et al. 2000.
The time necessary to reach the peak concentration in the plasma and in the brain is named
hysteresis. The constant k1e represents the rate constant of the drug’s passage from the central
compartment to the effect-site and the dissipation of the drug from the effect compartment is
characterized by ke0. These last constant indicates the equilibrium rate of conversion of
propofol plasma into effect-site concentration, which makes possible the estimation of the
drug concentration in the effect compartment (Brás, Bressan et al. 2008) which is very useful
in anesthesia using TCI devices, as it provides a more precise reflex of the drug effect than the
estimated plasma concentration (Struys, De Smet et al. 2000). The clearance of propofol
during the third exponential phase is constrained by the slow return of propofol from adipose
tissues into blood, whereas the elimination of propofol in the second phase was apparently
dominated by unconstrained metabolic clearance (Cockshott, Douglas et al. 1992). Effect-site (Brain) Central compartment (V1) Third compartment (V3) Second compartment (V2) Elimination k12 k21 k1e k13 k31 k10 Drug intravenous administration ke0 Muscles Viscera Blood Liver Fat Bones
19
1.5.2 Plasma Protein Binding
Propofol is a weak organic acid that remains entirely unionized at pH 7.4. In all species, it can
get extensively bound to plasma proteins (96-98%), especially to albumin (95%) (Cockshott,
Douglas et al. 1992). Plasma protein binding of propofol doesn’t seem to change with age but
very low albumin concentrations can modify propofol binding (Kirkpatrick, Cockshott et al.
1988; Aguilera, Zamacona et al. 1995).
Cockshott et al. (1992) demonstrated with in vitro data that propofol had a uniform
distribution within human blood, but its distribution within dog blood favored plasma (overall
blood/plasma ratio of 0.6), while distribution in the rabbit (ratio 13.7) and rat (ratio 3.8) blood
favored the propofol protein binding. In humans, propofol can get bound with red blood cells
but, this is taken as a part of the central compartment and is active and available for
distribution to other tissues (Mazoit and Samii 1999).
1.5.3 Distribution
As an extremely lipid soluble drug, propofol can rapidly distribute from blood into tissues and
organs (Glowaski and Wetmore 1999).
It is known that propofol distribution and pharmacokinetic depend on cardiac output (Upton,
Ludbrook et al. 1999; Bienert, Zaba et al. 2009). After its intravenous administration,
propofol makes a first pass through the heart and lungs subsystem, before getting in the
systemic arterial blood. From there on, it spreads to all the body organs, including those
responsible for drug clearance and distribution in the rest of the body (the second subsystem)
before it can re-circulate and be measured in arterial blood. Those two passages occur
simultaneously and propofol is essentially added to a stream of blood flowing at a rate
determined by the cardiac output (Upton, Ludbrook et al. 1999). Modifying the cardiac output
has demonstrated an evident effect on arterial and brain concentrations of propofol (Ludbrook
20
high hepatic extraction ratios (Kuipers, Boer et al. 1999), such as propofol. This means that
cardiac output is an important determinant of the induction dose of propofol (Upton,
Ludbrook et al. 1999) and should be contemplated as a pharmacokinetic variable.
1.5.4 Elimination
The clinical success of propofol is attributable to its unique profile of action, redistribution
and to its rapid metabolic clearance and elimination. The principal pathway of elimination is
the hepatic metabolization into sulphate and glucuronic conjugates that have no hypnotic
activity and are eliminated in urine (Bowman 1989; Murayama, Sato et al. 2005). In previous
studies, it has also been related an extrahepatic clearance of propofol in humans, thought to
occur in the lungs, brain, gut and kidneys, and/or extra-renal elimination, since the metabolic
clearance exceeds hepatic blood flow (Shafer 1993; Murayama, Sato et al. 2005). This
supposition is sustained by the presence of propofol metabolism during the anhepatic phase of
liver transplantation (Gray, Park et al. 1992; Veroli, O'Kelly et al. 1992).
The ending of effects of most anesthetic agents is related with redistribution from the brain to
the tissues and is generally much faster than the elimination rate of the drug from poorly
perfused tissues (Kanto and Gepts 1989). In a continuous infusion system, the duration of
effects continues until steady-state is reached, indicating that accumulation has occurred. At
this phase, the distribution equilibrium is reached, where the rate of introduction of the drug is
equal to the rate of removal of the drug from the body through metabolism and excretion. The
elimination half-life is representative of the duration of effect (Kanto and Gepts 1989).
Although the recovery from propofol anesthesia is also quick, the elimination half-life of the
drug is high (Zoran, Riedesel et al. 1993; Smith and White 1998). According to Shafer (1992),
this occurs because of the slow elimination of propofol from the highly lipophilic tissue
compartments (e.g. fat) and the propofol concentration reached in blood during this phase is
21
1.5.4.1 Metabolism
Metabolism of drugs normally involves biotransformation that converts lipophilic compounds
into hydrophilic ones. Thereafter, these compounds suffer conjugation and if the final
substance has a high molecular weight it is excreted by the bile but, if it has a low molecular
weight, it is eliminated in the urine (Chang and Kam 1999).
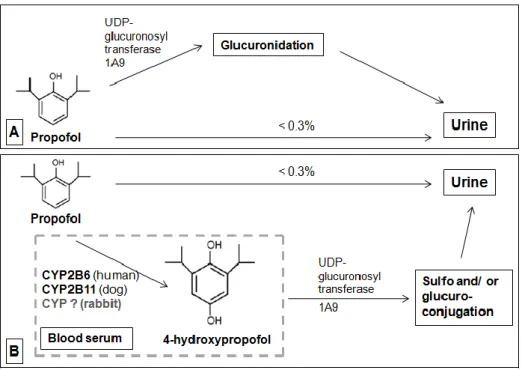
In humans, after the administration of a sub-anesthetic dose of propofol, only 0,3% is
eliminated in the urine, which means that the principal pathway of elimination is via
metabolism (Favetta, Degoute et al. 2002), mainly by glucuronidation (Benet, Kroetz et al.
1996) (Figure 2). It occurs essentially in the liver, by specific cytochrome (CYP) P450
enzymes that prevent the saturation of redistribution sites during long periods of
administration (Cockshott, Douglas et al. 1992), and results in the formation of inactive
metabolites that are excreted in the urine (Dawidowicz, Fornal et al. 2000). In humans,
CYP2B6 contributes to biotransformation of propofol and appears to be the principal
determinant of interindividual variability in the oxidation of this drug by human liver
microsomes (Court, Duan et al. 2001). In studies designed to investigate the molecular basis
for dog breed differences in propofol metabolism, CYP2B11 was assessed as the principal
hepatic propofol hydroxylase in dogs (Court, Hay-Kraus et al. 1999). So far, no studies have
been published assessing the main CYP in rabbit metabolism (Figure 2 - B). Simons et al.
(1991) and Le Guellec et al. (1995) in their studies noticed that the high metabolic activities
obtained in rabbit liver samples were consistent with those observed in vivo, indicating that
glucuronidation is also the main metabolic pathway in this specie (Le Guellec, Lacarelle et al.
1995).
Human liver microsomes exhibit greater glucuronidation activity than rabbits, followed by
rats. Concerning kidney microsomes from these animals, they were able to glucuronidate
22
(Le Guellec, Lacarelle et al. 1995). However the possible lung pathway on metabolism wasn’t
so clear, both in human and animal (Le Guellec, Lacarelle et al. 1995).
In humans, it was reported that the brain may also be a pathway of extra-hepatic metabolism,
by the expression of UGT (uridine diphosphate glucuronosyltransferase) isoforms, suggesting
the possibility of glucuronidation of propofol in the brain (Zhang, Li et al. 2001).
1.5.4.2 Clearance
Due to its high metabolic clearance rate, total body clearance of propofol is rapid in humans
(1.91 mL min-1 kg-1), dogs, rats, pigs and even faster in rabbits (340 mL min-1 kg-1)
(Cockshott, Douglas et al. 1992).
Hiraoka et al. 2005 reported that total body clearance of propofol was unaltered by changes in
protein binding due to the high hepatic extraction ratio of propofol, which indicates that the
hepatic clearance is blood flow-limited (Hiraoka, Yamamoto et al. 2005). In fact, the
metabolic clearance of propofol exceeds hepatic blood flow, indicating that this anesthetic has
extra-hepatic sites of metabolism and elimination (Ferreira 2007). One of the most important
characteristics that make propofol clinically and pharmacokinetically different from other
intravenous agents relays on its rapid metabolic clearance (Shafer 1992). The distribution
clearance of an intravenous anesthetic drug is a measure of the movement of the drug from
the central compartment (the instantaneously equilibrating tissues, including blood and highly
perfused tissues) into tissues with lower blood flow (Shafer 1992). The total distribution
clearance of propofol ranges from 3 to 4 L/min/kg, a value that is approximately 60-80% of
the cardiac output, suggesting that its distribution into body tissues depends on the cardiac
output and on the target organ blood flow (Shafer 1992). Clearance of propofol is by
metabolism with urine being the major route of metabolite elimination in rabbits and humans
23
1.5.4.3 Excretion
In a previous study of Simons et al. (1991) with rabbits, the inactive metabolites of propofol
are excreted mainly in urine, whereas in feces it is less than 2%. According to that work,
rabbit urine contains the glucuronide and sulphate conjugates of propofol, the quinol
(2,6-diisopropyl-1,4-quinol) and conjugates of hydroxylated derivatives of propofol and the quinol
(2,6-diisopropyl-1,4-quinol). The small proportion of these metabolites in feces, indicate that
the biliary route is not important in rabbit (Simons, Cockshott et al. 1991).
According to Figure 2, one of the routes of metabolism appears to be glucuronidation of the
parent compound (Figure 2 – A, Phase I). UDP-glucuronosyltransferase 1A9 is the only
enzyme that has been shown to mediate this reaction. Propofol may also undergo ring
hydroxylation by in CYP2B6 in humans, to form 4-hydroxypropofol, which is then
glucuronidated or sulphated (Figure 2 – B, Phase II).
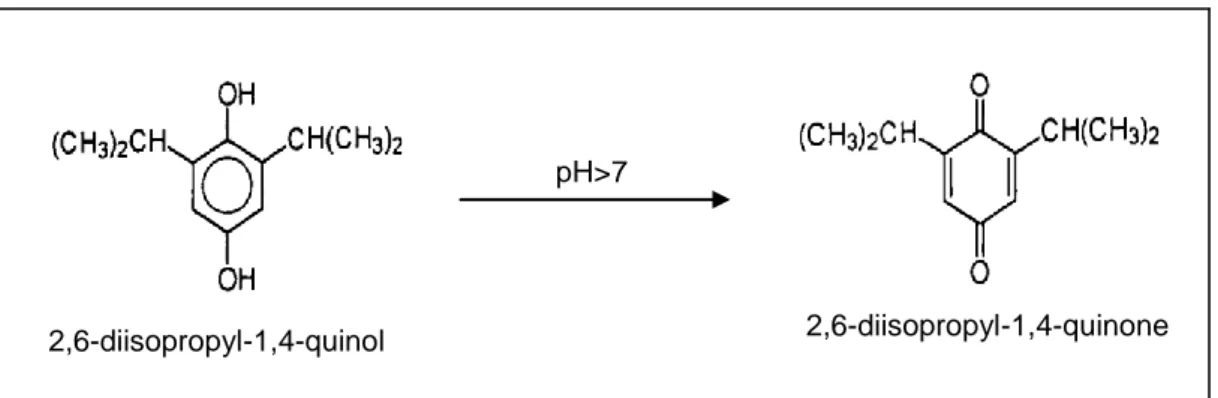
The metabolite 4-hydroxypropofol (2,6-diisopropyl-1,4-quinol) can be quantified in blood
serum and it is found in tautomeric equilibrium with 2,6-diisopropyl-1,4-quinone in alkali
conditions (Guitton, Burronfosse et al. 1997).
Propofol can be cleared by glucuronidation of the parent molecule or in sulphatation and
glucuronidation of its hydroxylated (2,6-diisopropyl-1,4-quinol) metabolite (Figure 2 - A);
hydroxylation of an isopropyl group also occurred in the rat and the rabbit (Simons, Cockshott
24
1.7 The rabbit as a model for propofol studies
Currently, there are only a few studies using the lipid formulation of propofol in rabbits. A
better understanding of propofol anesthesia in the rabbit is essential not only for its
improvement in clinical practice or research use and safety, but also for the introduction of
techniques like TIVA and TCI. Indeed, there are no PK/PD models for propofol in rabbits that
could allow the implementation of TCI.
Although rabbits have more risks of anesthetic-related death when compared to cats and dogs,
they are the third most commonly anaesthetized species (Brodbelt, 2009). Lagomorphs are
also used as biomedical research models in many scientific fields (Cruz, Carregaro et al.
2010).
As models for anesthesia and neurophysiologic trials, rabbits allow good handling and
restraining and good collection and monitoring of vital signs. They are small enough to be
easily handled while big enough to record a variety of physiologic signals, such as heart rate,
blood pressure or even the electroencephalogram (EEG) which may be obtained with good-Figure 2 – Major biotransformation pathways for propofol into its metabolites in humans. good-Figure 2-A
25
quality in this species due to the presence of a smaller muscle layer between the skull and the
head skin than in other species such as rabbits or dogs.
1.8 Pharmacodynamic characteristics of propofol
Pharmacodynamic properties of propofol depend on its therapeutic plasma concentrations.
The knowledge of pharmacokinetic models allows a more accurately prediction of the optimal
dosage (White 2005). The required plasma concentration varies with the desired
pharmacological effect (sedation, induction or maintenance of anesthesia), the simultaneous use of other drugs (opioids, muscle relaxants), the type of operation and the patient’s sensitivity to the drug (age, weight and pre-existing diseases) (White 2005). A drug
extensively metabolized as propofol implies a reduction in its dosage when there is evidence
of severe hepatic dysfunction, heart failure or renal failure because these diseases can
manifestly unbalance the pharmacokinetic variables (Servin, Cockshott et al. 1990). So, the
use of propofol demands a continuous titration of the drug infusion rate to the desired
pharmacological end point.
Although propofol continuous infusion may provide less hemodynamic depression than bolus
injection, the main reported pharmacodynamic characteristics are related with its
cardiovascular effects: transitory decrease in arterial blood pressure (due to a decrease in
peripheral vascular resistance) (Goodchild and Serrao 1989; Pagel and Warltier 1993),
sympathetic outflow, and myocardial depression (Coetzee, Fourie et al. 1989), that are likely
to occur at higher blood concentrations or due to rapid increases in infusion rates. As a
consequence, a decrease in heart rate is more commonly observed than the expected reflex
tachycardia, and results from the resetting of the baroreceptor reflex by the anesthetic agent
which was seen in humans (Sellgren, Ejnell et al. 1994). Another clinical effect is related to