ContentslistsavailableatScienceDirect
Process
Biochemistry
j ourna l h o m e pa g e : w w w . e l s e v i e r . c o m / l o c a t e / p r o c b i o
Efficacy
of
a
membrane
composed
of
polyvinyl
alcohol
as
a
vehicle
for
releasing
of
wound
healing
proteins
belonging
to
latex
of
Calotropis
procera
Ingrid
Samantha
Tavares
de
Figueiredo
a,
Márcio
Viana
Ramos
b,
Nágila
Maria
Pontes
Silva
Ricardo
c,
Maria
Leônia
da
Costa
Gonzaga
c,
Rachel
Sindeaux
Paiva
Pinheiro
d,
Nylane
Maria
Nunes
de
Alencar
d,∗aCentroUniversitárioEstáciodoCearáViaCorpusRuaEliseuUchoaBecco,n◦600–BairroÁguaFria,CEP:60810-270,Fortaleza,Ceará,Brazil
bDepartmentodeBioquímicaeBiologiaMolecular/UFC,CampusdoPici,CaixaPostal6033,60451-970Ceará,Brazil cDepartmentodeQuímicaOrgânicaeInorgânica/UFC,CampusdoPici,CaixaPostal6021,60455-760Ceará,Brazil dDepartmentodeFisiologiaeFarmacologia/UFC,CoronelNunesdeMelo,1127RodolfoTeófilo,60430-270Ceará,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received27September2013
Receivedinrevisedform2December2013 Accepted27December2013
Availableonline18January2014
Keywords:
Drugdelivery Fibroplasia Inflammation Infrared Thermogravimetry
a
b
s
t
r
a
c
t
ThesolubleproteinsextractedfromthelatexofCalotropisprocera(LP)havebeenshowntopowerfully amelioratedifferentinflammatorydisorderssuchasarthritis,cancerandsepsis.Inthisstudy,we devel-opedapolyvinylalcohol-basedmembraneasanLPdeliverysystemanddeterminedtheefficacyofLPin tissueremodelingandhealingupontopicaltreatmentinmice.Aqueouspolyvinylalcohol(PVA,1%,w/v) solutionwasmixedwithLP(0.2%or1%)whilestirringtoobtainabiomembrane,whichwasthenapplied towounds.PVAandLP-containingPVAmembraneswerestructurallycharacterizedandstandardizedby infraredabsorptionspectroscopyandthermogravimetry.Woundhealingparametersincludedtimeof healingprogressandtissueremodeling(fibroplasiaandcollagen).LP-containingPVAmembrane(0.2% and1%),butnotPVAmembrane,significantlyacceleratedwoundhealingthroughfasterneo-tissue for-mation.Thisprocesswasaccompaniedbyintensifiedfibroplasiaandcollagendepositionasrevealedby microscopicanalyses.ItisthereforeconcludedthattopicalapplicationofLPonwoundsstimulatesthe healingprocessandthattheuseofPVAmembranestocarryLPisareliableapproachfortopicaldelivery ofpharmacologicallyactiveproteins.
©2014ElsevierLtd.Allrightsreserved.
1. Introduction
CalotropisproceraisashrubintheApocynaceaefamilythatis nativetotropicalregionswhereitisexploitedbyindigenous peo-ple.Itisalsousedasamedicinalplantinseveralcountries,most notablyinIndia,Pakistan,EgyptandMalaysia,andtoalesserextent inNortheasternBrazil.C.proceraisamilkweedplant,andthepartof theplantmostusedforfolkmedicinalpurposesisitslatex.Thelatex istraditionallyusedtotreatskininfections,inflammatory touch-iness,andotherrelatedailments[1,2].Scientificstudieswiththe wholelatexorpartiallypurifiedlatexfractionshavestrengthened thepharmacologicalpotentialofC.proceraand,toacertainextent, havevalidateditsethnopharmacologicalusages[3,4].
Chemicalanalysisofthelatexrevealedthepresenceofcalactin, calotropin,uscharin,sitosterol, andcalotoxinas phytochemicals
∗Correspondingauthor.Tel.:+558533668339.
E-mailaddresses:nylane@ufc.br,ingridfarma@gmail.com(N.M.N.deAlencar).
[5,6].Proteins,suchasantioxidativeenzymesand pathogenesis-related proteins (i.e. proteins with fungicidal or insecticidal activities),havebeenreported[7,8].Recently,proteaseshavebeen discoveredinlaticiferfluids[9],andproteasesofC.proceralatex havebeeninvestigatedinsomedetail[10].
C.proceralatexexhibitsanti-andpro-inflammatoryactivities, andtheanti-inflammatoryfractionhasbeenshowntoinhibitthe effectofthepro-inflammatoryfraction[11].Theanti-inflammatory fraction,whichiscomposedofthemajorwater-solubleproteins of the latex, has successfully reduced inflammation in several standard models of inflammation, suchas peritonitis and paw edema[12].Later,thepharmacologicalpotentialofthis fraction wasreliablyconfirmedbyitsabilitytomodulateinvivo inflamma-toryconditionsinducedbynociception,cancer,arthritisandsepsis
[13–16].
The use of pharmacologically active proteins as drugs is of interestduetotheirputativelylowefficacyassociatedwith molec-ularinstability,susceptibilitytoproteolysisandlimiteddiffusion becauseof their molecularsize. In this study,both thewound
healingpotentialoftheproteinfractionofC.proceraandthe effi-cacyofapolyvinylalcohol-basedmembraneasadeliveryvehicle forthelatexfractionweredetermined.
2. Materialsandmethods
2.1. Chemicals
PolyvinylalcoholwaspurchasedfromSIGMA(SãoPaulo,Brazil), ketaminechloridrateandxilazinechloridratewaspurchasedfrom VETBRANDS(Paulínia,SãoPaulo,Brazil),iodopovidonewas pur-chasedfromADV(SãoPaulo,Brazil),andhematoxylin–eosinwas purchasedfromMerckMillipore,SãoPaulo,Brazil.Allother chem-icalswereofanalyticalgradeunlessotherwisespecified.
2.2. Latexproteins
Latexproteinsusedinthisstudywereobtainedusingmethods describedbyAlencaretal.[11].Thesolubleproteinsrecoveredwere lyophilizedandstoredat25◦C untiluse.Biochemicaland
phar-macologicalcharacterizationofthissamplehavebeenpreviously reportedindifferentmanuscripts[11–14].
2.3. Polyvinylalcohol-basedmembrane
BiomembranecomposedofLP/PVAwastentativelypreparedby fixingPVAat1%mixedwithdifferentpercentofLPstartingat0.1% upto2%.Twofinalcompositionswerechosenforusinginbiological assays.Therefore,basedoninfraredmicroscopydataand thermo-graphyteststhebetterfilm-formingwasobtainedatthemaximum concentrationof1%LPassociatedto1%PVA.0.2%LPin1%PVAwas usedforcomparativepurpose.Thesebiomembraneswereprepared asfollows.
Initially,aPVA(1%,w/v)solutionandLP(0.2%or1%)with10mL ofdistilledwaterwereprepared.Aftercompletehomogenization, PVAwasmixedwithLPtoobtainthefollowingfinalsolutions:1% PVA+1%LPor1%PVA+0.2%LP.Thesesolutionswerestirredat 50◦Cfor2h.Theresultingmixtureswerefilteredusingafunnel
withasinteredglassplateundervacuumandthenpoured(20mL) intopetriplates(13.0cm diameter/height1.0cm).Themixtures weredried in anair circulation ovenat 40◦C untilthesolvent
wascompletelyevaporatedandadequatelyprotectedtoprevent
contamination.ThePVAmembranescontainingLP werenamed
BioMemPVA/LP0.2%or1%.TheBioMemsweresterilizedby ultra-violetlight(UV)irradiationpriortofurtherexperiments.
2.4. Chemicalmeasurements
2.4.1. Infraredspectroscopyanalysis
Fourier transform infrared spectroscopy (FTIR-ATR) of the BioMemsampleswasrecordedwithaspectrophotometer(Perkin Elmer2000)atambienttemperature(25◦C).Theresolutionwas
4cm−1intherangeof4000–400cm−1.Samplesweredriedand
powderedinthepresenceofliquidnitrogenbeforeanalyses.Allof theexperimentswereperformedasdescribedbyGomesetal.[17].
2.4.2. Thermography
FilmsofBioMemsampleswerecharacterizedintermsoftheir thermalstabilityusingmethodsreportedinCasariegoetal.[18]. ThermogravimetriccurveswereobtainedusingaShimadzu(model TGA-50H)thermogravimetricanalyzerwithatemperaturerange of25–800◦C,nitrogenflowrateof20mL/minandaheatingrateof
10◦C/min.
2.5. Biologicalassays
2.5.1. Animals
AdultmaleSwissmice,12weeksoldandweighing25±3.0g, wereobtained fromtheCentral Animal Houseof the Universi-dadeFederaldoCeara,Brazil.Theanimalswerekeptinindividual plasticcagesundercontrolledenvironmentalconditions(12/12h light/darkcycles,temperatureof25◦Candhumidity55±10%)with
waterandfoodadlibitum(commercialsterilediet–Purina, Paulí-nia,SP, Brazil).Themicewerehandledaccordingtothecurrent guideforthecareanduseoflaboratoryanimals,andassayswere approvedbytheEthicalCommitteeforAnimalUseofthe Univer-sidadeFederaldoCeara(24/2009).
2.5.2. Wound-inducedmodel
Micewereanesthetizedbysubcutaneousinjectionswith10% ketamine chloridrate (115mg/kg) and 2% xilazine chloridrate (10mg/kg)beforetheirsurgicalprocedure[19].Aftershavingthe dorsalsurfaceskin,theregionwaspreparedforasepticsurgery using1%iodopovidonefollowedby70%ethanol.Onewound,either incisionalorexcisional,wasintroduced.
Incisional wounds (0.5cm) were created by a single liner cut. Excisional wounds (1cm2) were performed with a tissue punch.AftersubcutaneousimplantationofthedifferentBioMems (1.2cm2), incisional wounds were sutured with mononylon 5-0 (PolySuture, Brazil) so that the healing occurred by primary intention.Excisionalwoundswerenotsuturedsothatthehealing occurredbysecondaryintention.
Animalswererandomlydividedintogroups(n=15pergroup),
as follows: (i) animals with 1% polyvinyl alcohol membrane
implantedintoincisionalor(ii)excisionalwounds,(iii)animals with BioMem PVA/LP 0.2% used in incisional or (iv)excisional wounds,(v)animalswithBioMemPVA/LP1%usedonlyinincisional wounds,and(vi)animalssubjectedonlytosurgicalproceduresthat didnotreceivedanyBioMem(SHAM).
2.5.3. Macroscopicanalyses
Macroscopicdifferencesinneoformedtissueinducedby
sub-cutaneously implanted BioMem were monitored 2, 7, and 14
daysaftersurgery[20].Underanesthesiaaspreviouslydescribed, the entire dorsal skin was removed, and neoformed tissues of incisional wounds were evaluated using the following scoring system: (0): absent; (1): mild; (2): moderate and (3):intense. Excisionalwoundswereevaluatedevery48haftersurgery consid-eringthefollowingaspects:edema,hyperemia,woundareaand re-epithelialization.Inflammatoryclinicalsignals,suchasedema andhyperemia,werescoredas(0):absent;(1):mild;(2):moderate and(3):intense.Woundareasofexcisionalwoundsweremeasured usingapachometerandwerecalculatedasfollows:A=×R×r, whereA,Randrarearea,largerayandsmallray,respectively[21]. Re-epithelializationwasnotedwheneverascabwasnotcovering thewounds[22].
Aftermacroscopicstudy,4mmpunchbiopsiesfromneoformed tissue and excisional wounds with adjacent normal skin were removed (n=5/group)for histological study.Then, theanimals weresacrificedwithananestheticoverdose.
2.5.4. Microscopicanalyses
Neoformed tissuesofincisional andexcisional woundswere fixedinformaldehyde(10%,v/v)andresuspendedin0.01MPBS,pH 7.2over24hinpreparationforhistopathologyprocedures.Sections (5m)werestainedwithhematoxylin–eosintoevaluatefibroblast numberandre-epithelialization.Mallory’trichromewasusedto examinecollagenfiberdeposition.
DFC280camera.Imagesof10randomfieldsofconnectivetissue werecapturedat400×,and20 regionsofinterest (ROI)ofsize 800×1536pixels (=11m×21m) were extracted toestimate thenumberoffibroblasts.Collagenfiberdepositionwas quanti-fiedinimagesobtainedfrom6randomfieldsat200×in24regions of374×260pixels(=10m×7m).ImageJ® 1.46software(U.S. NationalInstitutesofHealth,USA)wasusedtocountfibroblasts andevaluatecollagenesisbyusingthe“cellcounter”and “thresh-oldcolor”plug-ins,respectively[20].Thethicknessofthenewly formedepidermisofexcisionalwoundswasmeasuredusing18 imagesof655m×492minwoundsectionsfromday14after surgery[23].
2.6. Statisticalanalyses
All results are expressed as mean values of groups
(n=5)±standard error of the mean (S.E.M.). Statistical signif-icancewasassessedbyANOVAfollowedbyBonferroni’stestfor multiplecomparisonsofmediaorKruskal–Wallisformedian.The levelofsignificancewasdeterminedasP<0.05usingGraphpad prism,version3.02.
3. Resultsanddiscussion
Poly(vinyl alcohol) (PVA) is a synthetic water-soluble poly-mer,non-toxic,biocompatible,biodegradablewithexcellentfilm formingproperties,which hasbeenstudied widelyfor medical andbiomedicalapplications[24,25].Furthermore,reportsclearly demonstratethedevelopmentof biocompatibleand biodegrad-ableprotein-coatedPVAmatrices,includingblendedwithproteins ofvarioussources,suchasPVA/wheat-gluten,PVA/collagenand PVA/gelatin[26–28].Unprecedented,thispaperdemonstratedthe abilityofPVAinfilm-formingwithpharmacologicallyactive lati-ciferproteins,whichefficientlyimprovewoundhealing.
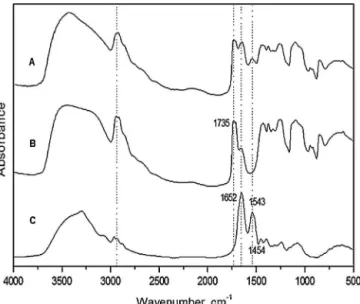
InfraredspectraobtainedofLP,PVA1%andPVA/LP1areshown inFig.1.Spectra(B)and(C)showfrequenciesthatarecharacteristic ofPVAandLP,respectively,whichdemonstratesthequalityofthese substances.
Thesignalobservedatafrequencyof1735cm−1isfroma car-bonylgroup(C O).In thePVA spectrum,thevibrations witha frequencyof854cm−1areduetoC Hbonds,whilethevibrations
as1544cm−1intheLPspectracomefromN HandC Nbonds
andarecharacteristicoftheproteins.Otherfrequenciesrecorded
Fig.1.FTIRspectra:(A)BioMemPVA/LP1%,(B)PVA1%,and(C)LP.
inthespectra(B)and(C)arecommontobothPVAandLPwithonly minorvariationsofvalues,whichiscommonforthetypeofapplied analysis.
Asimilarspectroscopicprofilewasobservedinthespectrum oftheBioMemPVA/LP1%(spectrumA),inwhichbothsubstances’ spectroscopicsignalsareobserved.Notably,thefrequencies asso-ciatedwithC O,C H,C NandN Hareidentifiable.Inaddition, somefrequenciesappearinthespectrumoftheBioMemPVA/LP1% butinneitherspectrum(B)or(C),mostlikelyindicatingthe occur-renceofVanderWaalsinteractionsandhydrogenbonding(butnot covalentbonding)betweenthetwosubstances.Therefore,thereis indicationthatPVA/LPhasreachedastabledispersion,preventing thecoagulationofproteinandkeepingitcrystalline.
OnlysmalldifferencesinthebandsoftheBioMemPVA/LP1% spectrumareobservedwhencomparedtotheisolatedsubstances’ spectra.Thisobservationconfirmsthatnosignificantchangesoccur uponformationofBioMem(Table1).
ThermogramsofBioMemsamplesunderanitrogenatmosphere arepresentedinFig.2.Thefirstmasslossobservedat50◦Cis
asso-ciatedwithadsorbedwater(asobservedintheinfraredspectra), whilesampledecompositionoccursat200,300and330◦CinPVA
1%,LP1%andBioMemPVA/LP1%,respectively.Therefore,mixing ofLPinPVAcausesBioMemPVA/PL1%tobecomemorethermally stable.Moreover,theadditionofLPinPVAledtoincreasedfilm flexibility,lesstransparencyandasallowercolor,asviewed macro-scopically.
Previousreportsdemonstratedthatdailytopicalapplicationof watersolublefractionsoflatexfromC.procerareducestheareaof excisionalwounds[29].However,thiseffectwasobtainedusingan aqueousextractofnaturallatexinsteadofapurifiedmoleculeor specificfraction,andnoreportshaveattributedthehealingeffectof thelatexfromC.proceratobioactiveproteinsexistinginthesoluble fraction.
In the present work, a BioMem was made by combining
polyvinylalcohol and soluble proteinsobtainedof the latex of
C.procera(BioMemPVA/LP)toinvestigate theefficacyofthese BioMemsasdrugdeliverysystems.Theeffectsoflatexproteinson thetissueneoformationtogetherwithwoundhealingwere evalu-ated.Initially,concentrationsof0.2%and1%ofLPwerebasedonthe effectiveconcentrationoflatexfractionsreportedpreviously[29]
andtheconcentrationsneededtoformstabledispersionsbetween thecompounds(LP)withinthePVABioMem.
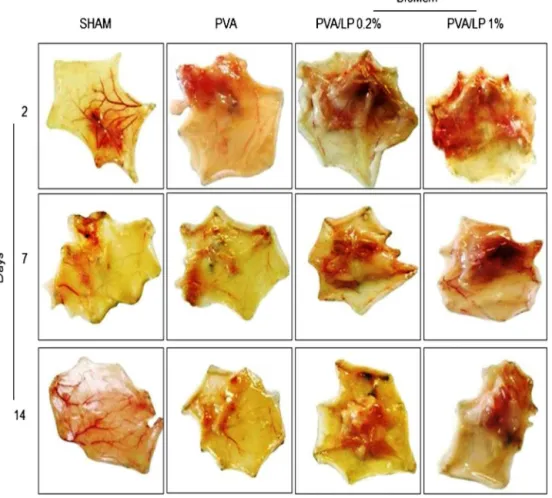
SubcutaneouslyimplantingLP-containingBioMeminincisional woundsinducedneoformedtissue(Fig.3andTable2).No signif-icantdifferencewasfoundbetweentheSHAMand PVAcontrol
groups. BioMem PVA/LP (0.2% and 1%), however, induced the
appearanceofagreateramountofneoformedtissuebythesecond dayaftersurgerywhencomparedtothePVAandSHAMcontrol groups(P<0.05).Afterlongerperiodsoftimefollowing surgery (especiallyafter14days),areductioninthequantityoftissuewas observed,butthedifferencebetweentheexperimentaland con-trolgroupswasmaintainedthroughout.Theseresultsaresimilarto
Table1
Assignmentsofthemeaningbandsoftheinfraredspectra.
Wavenumber(cm−1) Assignments
3428 O H(A,B)
2926 C HofCH2andCH3(A,B)
1725 C O(A)
1643 asCOO−(A,B)
1540 CN,␦NH(B)
1267 CN,␦NH(B)andCO(A,B)
1095 (C C)(A,B)
852 ␦C H(A)
Fig.2.Thermogram(A)andderivative(B):PVA1%,LPandBioMemPVA/LP1%.
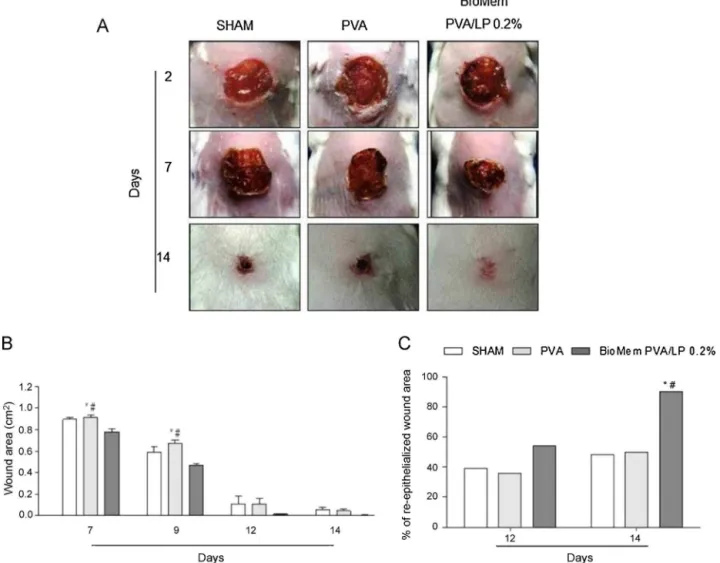
Fig.4.Healingprogressonexcisionalwounds:(A)macroscopicaspects,(B)woundareaand(C)progressofre-epithelizationonwoundingarea.Imageswereselectedfrom animalsbelongingtothecorrespondingexperimentalgroups.Dataaremeanofthreeindependentexperimentsandareexpressedasmean±standarderrormean(S.E.M.)
ofaveragewoundarea.*P <0.05indicatesstatisticaldifferencecomparedwiththeSHAMgroupand#P <0.05withPVAgroup.ANOVA–Bonferronitest;Kruskal–Wallistest followedbyDunn’stest.
thoseobservedusingnaturallatexbiomembranefromtherubber
treeHeveabrasiliensis,inwhichlatexprotein-dependentinduction
oftissueneoformationwasalsodemonstrated[20].
Astheamountofneoformedtissuewassimilarinboth0.2%and 1%BioMemPVA/LP,thelowestdoseofLPwaschosentoevaluate theeffectonexcisionalwoundhealing.Onalternatedays follow-ingthesurgery,woundsweremacroscopically evaluatedbythe presenceofflogisticsigns,woundareasizeandpresenceofcrust. Duringtheinflammatoryperiod (Day2),edema andhyperemia wereobservedinallexcisionalwounds.Animalsimplantedwith BioMemPVA/LP0.2%showedmoderateedemainrelationtothe
Table2
Semi-quantitativeevaluationofneoformedtissue.
Groups
Days SHAM PVA PVA/LP0.2% PVA/LP1%
2 0(0–1) 1(0–2) 3(2–3)* 3(2–3)*
7 0(0–1) 0(0–2) 3(1–3)*# 3(2–3)*#
14 0(0–1) 1(0–1) 2(2–3)* # 2(1–3)*
Datarepresentthemedianandrangeofscoresfromtwoseparateexperiments:(0) absent,(1)mild,(2)moderateand(3)intensetotissueneoformed.
* P<0.05indicatesstatisticaldifferencecomparedwiththeSHAMgroup(n=15 animals/group,Kruskal–WallistestfollowedbyDunn’stest).
#P<0.05indicatesstatisticaldifferencecomparedwiththePVAgroup(n=15 animals/group,Kruskal–WallistestfollowedbyDunn’stest).
SHAMandPVA controlgroups (P<0.05),withnodifferencesin hyperemia(Fig.4AandTable3).
A significant difference in wound size reduction in animals
implantedwithBioMemPVA/LP0.2%whencomparedtocontrol
groups(P<0.05)(Fig.4B)wasobservedontheseventhdayafter surgeryandpeakedontheninthday.Moreover,ahigher percent-ageofre-epithelializedwoundareawasmacroscopicallyobserved 2weeksaftersurgeryintheexperimentalgroupascomparedto controlgroups(P<0.05)(Fig.4C).
Thepost-inflammatoryphaseofhealingisfollowedby fibropla-siaandisclinicallycharacterizedbygranulationtissueformation andfibroblastaccumulation[30].Fibroblastsarethemost com-monresident cells responsiblefor themajorityof collagenand elastinsynthesis,andtheyevenorganizetheextracellularmatrix
Table3
Semi-quantitative evaluation of macroscopic flogistic signs induced by biomembranes.
Flogisticsigns SHAM PVA BioMemPVA/LP0.2%
2nd day
Edema 1(0–1) 1(0–2) 2(2–3)*#
Hyperemia 1(0–1) 1(0–2) 2(1–2)
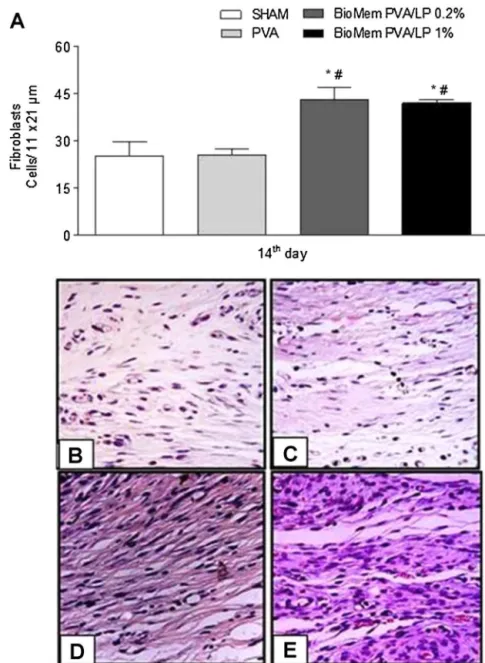
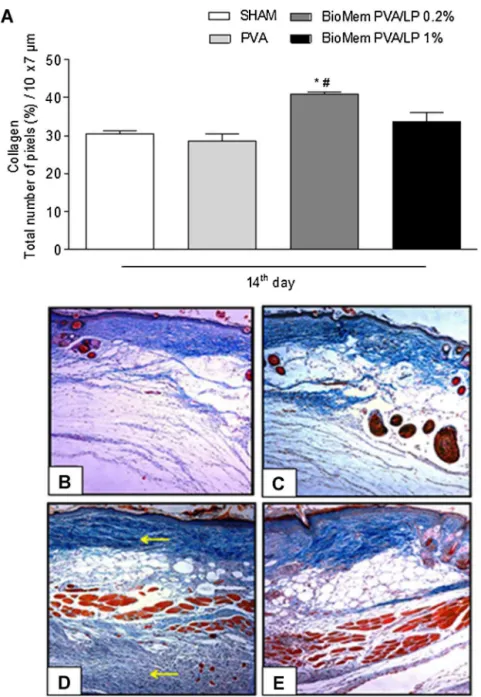
Fig.5.Fibroplasiaonincisionalwoundhealing.On14thdayaftersurgery,animalsweresacrificedandsamplesofsubcutaneoustissuewereremovedtoperformhistological analyses.Hematoxylin–eosinstainedsectionswereemployedtoestimatefibroplasia.(A)Evaluationoffibroplasiaprogressamongthedifferentexperimentalgroups.Data aremeanofthreeindependentexperimentsandareexpressedasmean±standarderrormean(S.E.M.)ofthenumberoffibroblasts.*P<0.05indicatesstatisticaldifference comparedwiththeSHAMgroupand#P<0.05withPVAgroup(ANOVA–Bonferronitest).HistologicalviewsoffibroplasiaonSHAM(B),PVA(C),BioMemPVA/LP0.2%(D)and 1%(E)experimentalgroups(400×magnification).
components [31]. Therefore, some fibroblasts differentiate into myofibroblasts,whichperformwoundcontraction[22,32].
Histological evaluation of incisional wounds showed that BioMemPVA/LPprovidedstimulusforfibroplasia,asevidencedby ahighernumberoffibroblastswhencomparedtocontrolgroups onDay14(Fig.5A–E).Accordingly,thesametrendwasobserved inexcisionalwounds(Fig.S1A–D).Moreover,slidesstainedwith Mallory’strichromewereusedtoestimatethepercentageof colla-gendeposition.AsshowninFig.6A–EandFig.S2A–D,therateof collagendeposition,estimatedaspercentageperarea,wasgreater inneoformedtissueandexcisionalwoundswhenstimulatedwith BioMemPVA/LP(P<0.05).
Previous reports have shown an effective communication
betweenmast cells and fibroblasts through gapjunction inter-cellularcommunication.Physicalorchemical factorsmayresult frommastcellsdegranulation,whichisassociatedwithfibroplasia andcollagendepositionduringcicatricialprocess[33–35].Thus, thestimulusgivenbyBioMemPVA/LPontheinflammatoryphase
mostlikelyinvolvesmacrophageactivationandmastcell degranu-lation,whichmaythereforeberelatedtotheobservedintensified fibroplasiaandcollagendeposition.Measurementofcellularand molecularmarkersinvolvedinthecourseofhealing,suchas neu-trophilinfiltration,mastcelldegranulation,macrophageactivation, and inflammatory mediator levels (e.g. cytokines and nitrites), wouldaidinunderstandingthemechanismunderlyingLPactivity inthecourseofwoundhealing.
This studyconcluded that theprotein fraction of C. procera
Fig.6. Collagendepositiononincisionalwoundhealing.On14thdayaftersurgery,animalsweresacrificedandsamplesofsubcutaneoustissuewereremovedtoperform histologicalanalyses.Mallory’strichromestainedsectionswereemployedtoestimatecollagendeposition.(A)Evaluationofcollagendepositionamongthedifferent exper-imentalgroups.Dataaremeanofthreeindependentexperimentsandareexpressedasmean±standarderrormean(S.E.M.)ofthecollagencontentestimatedbythetotal numberofpixelsinafixedarea(10m×7m),expressedinpercent.*P<0.05indicatesstatisticaldifferencecomparedwiththeSHAMgroupand#P<0.05withPVAgroup (ANOVA–Bonferronitest).HistologicalviewsofcollagendepositiononSHAM(B),PVA(C),BioMemPVA/LP0.2%(D)and1%(E)experimentalgroups(200×magnification).
cycleoffemalescouldinterferewiththemechanismoftissuerepair
[36].Thecompletepharmacologicalbenefitsofthissystemonthe courseofhealingarecurrentlyunderevaluation.Thesuccessof thisfirstbiotechnologicalapproachwillpermitfurtherevaluation oftheefficacyofpolyvinylalcohol-basedmembranesforLP deliv-eryastreatmentforcutaneouslesionsofleprosypatientsinthe future.
Acknowledgements
The study has been supported by grants from the
follow-ing Brazilian Agencies: Concelho Nacional de Desenvolvimento CientíficoeTecnológico(CNPq)andFundac¸ãoCearensedeApoio
ao Desenvolvimento Científico e Tecnológico (FUNCAP). The
Incentive of International Foundation for Science (IFS) support provided toMVR at thebeginning of his careeris also greatly
appreciated. M.V.R. and N.M.N.A. are senior researcher fellows ofCNPq.
AppendixA. Supplementarydata
Supplementarydataassociatedwiththisarticlecanbefound,in theonlineversion,atdoi:10.1016/j.procbio.2013.12.015.
References
[1]SharmaR,ThakurGS,SanodiyaBS,SavitaA,PandeyM,SharmaA,etal. Ther-apeuticpotentialofCalotropisprocera:agiantmilkweed.IntJPharmBiolSci 2012;4(2):42–57.
[2]KumarVL,AryaS.MedicinalusesandpharmacologicalpropertiesofCalotropis procera.RecProgMedPlant2006;11:373–88.
[4]KumarVL,RoyS.ProtectiveeffectoflatexofCalotropisprocerainFreund’s completeadjuvantinducedmonoarthritis.PhytotherRes2009;23(1):1–5. [5]UpadhyayRK.Plantlatex:anaturalsourceofpharmaceuticalsandpesticides.
IntJGreenPharm2011;5(3):169–80.
[6]ShakerKH,MorsyN,ZineckerH,ImhoffJ,SchneiderB.Secondarymetabolites fromCalotropisprocera(Aiton).PhytochemLett2010;3:212–6.
[7]KonnoK.Plantlatexandotherexudatesasplantdefensesystems:rolesof variousdefensechemicalsandproteinscontainedtherein.PhytochemLett 2011;72(13):1510–30.
[8]SouzaDP,FreitasCD,PereiraDA,NogueiraFC,SilvaFD,SalasCE,etal. Lati-ciferproteinsplayadefensiveroleagainsthemibiotrophicandnecrotrophic phytopathogens.Planta2011;234(1):183–93.
[9]TorresMJ,TrejoSA,NatalucciCL,LópezLM.Biochemicalcharacterizationof VQ-VII,acysteinepeptidasewithbroadspecificity,isolatedfromVasconcellea quercifolialatex.Planta2013;237(6):1651–9.
[10]RamosMV,AraújoES,JucáTL,Monteiro-MoreiraAC,VasconcelosIM,Moreira RA,etal.Newinsightsintothecomplexmixtureoflatexcysteinepeptidases inCalotropisprocera.IntJBiolMacromol2013;58:211–9.
[11]AlencarNM,OliveiraJS,MesquitaRO,LimaMW,ValeMR,EtchellsJP,etal. Pro- andanti-inflammatoryactivitiesofthe latexfrom Calotropisprocera (Ait.)R.Br.aretriggeredbycompoundsfractionatedbydialysis.InflammRes 2006;55(12):559–64.
[12]AlencarNM,FigueiredoIS,ValeMR,BitencurtFS,OliveiraJS,RibeiroRA,etal. Anti-inflammatoryeffectofthelatexfromCalotropisprocerainthreedifferent experimentalmodels:peritonitis,pawedemaandhemorrhagiccystitis.Planta Med2004;70(12):1144–9.
[13]SoaresPM,LimaSR,MatosSG,AndradeMM,PatrocínioMC,deFreitasCD,etal. AntinociceptiveactivityofCalotropisproceralatexinmice.JEthnopharmacol 2005;99(1):125–9.
[14]Oliveira JS, Costa-Lotufo LV, Bezerra DP, Alencar NM, Marinho-Filho JD, Figueiredo IS,et al. In vivo growth inhibition of sarcoma 180 by latex proteins from Calotropis procera. NaunynSchmiedebergs Arch Pharmacol 2010;382(2):139–49.
[15]KumarVL,ChaudharyP,RamosMV,MohanM,MatosMP.Protectiveeffect ofproteinsderivedfromthelatexofCalotropisproceraagainstinflammatory hyperalgesiainmonoarthriticrats.PhytotherRes2011;25(9):1336–41. [16]Oliveira RS,FigueiredoIS,FreitasLB, PinheiroRS,Brito GA,AlencarNM,
etal.Inflammationinducedbyphytomodulatoryproteinsfromthelatexof Calotropisprocera(Asclepiadaceae)protectsagainstSalmonellainfectionina murinemodeloftyphoidfever.JInflammRes2012;61(7):689–98.
[17]GomesAMM,SilvaPL,MouraCL, SilvaCEM,RicardoNMPS.Studyofthe mechanical and biodegradable properties of cassava starch/chitosan/PVA blends.MacromolSymp2011;299–300(1):220–6.
[18]Casariego A, Souza BWS, Cerqueira MA, Teixeira JÁ, Cruz L, Díaz R, et al. Chitosan/clayfilms’ properties asaffectedby biopolymerand clay micro/nanoparticlesconcentrations.Hydrocolloids2009;23:1895–902. [19]HallLW,ClarkeKW.Veterinaryanaesthesia.9thed.London:Elsevier;1991.p.
52–4.
[20]AndradeTAM,LyerA,DasPK,FossNT,GarciaSB,Coutinho-netoJ,etal.The inflammatorystimulusofanaturallatexbiomembraneimprovehealingin mice.BrazJMedBiolRes2011;44(10):1036–47.
[21]PrataMB,HaddadCM,GoldenbergS.Usotópicodoac¸úcaremferidascutâneas: estudoexperimentalemratos.ActaCirBras1998;3(2):43–8.
[22]Romana-SouzaB,OtrantoM,VieiraAM,FilgueirasCC,FierroIM,Alto-Costa AM.Rotationalstress-inducedincreaseinepinephrinelevelsdelayscutaneous woundhealinginmice.BrainBehavImmun2010;24(3):427–37.
[23]BaranowskyA,MokkapatiS,BechtelA,KrugelMJ,MiosgeN.Impairedwound healinginmicelackingthebasementmembraneproteinnidogen1.MatrixBiol 2010;29:15–21.
[24]Sudhamani SR, Prasad MS, Sankar KU. DSC and FTIR studies on gel-lan and polyvinyl alcohol (PVA) blend films. Food Hydrocolloids 2003;17:245–50.
[25]MansurHS,SadahiraCM,SouzaAN,MansurAAP.FTIRspectroscopy char-acterization of poly (vinyl alcohol) hydrogel with different hydrolysis degreeand chemicallycrosslinkedwith glutaraldehyde.Mater SciEngC 2008;28:539–48.
[26]DicharryRM,YeP,SahaG,WaxmanE,AsandeiAD,ParnasRS.Wheat gluten-thiolatedpoly(vinylalcohol)blendswithimprovedmechanicalproperties. Biomacromolecules2006;7:2837–44.
[27]SartiB,ScandolaM.Viscoelasticandthermalpropertiesofcollagen/poly(vinyl alcohol)blends.Biomaterials1995;16:785–92.
[28]CarvalhoRA,MariaTCM,MoraesICF,BergoPVA,KamimuraES,Habitante AMQB,etal.Studyofsomephysicalpropertiesofbiodegradablefilmsbased onblendsofgelatinandpoly(vinylalcohol)usingaresponse-surface method-ology.MaterSciEngC2009;29:485–91.
[29]RasikAM,RaghubirR,GuptaA,ShuklaA,DubeyMP,SrivastavaS,etal.Healing potentialofCalotropisproceraondermalwoundsinGuineapigs.J Ethnophar-macol1999;68:261–6.
[30]DiegelmannRF.Cellularandbiochemicalaspectsofnormalandabnormal woundhealing:anoverview.JUrol1997;157:298–302.
[31]LiJ,ChenJ,KirsnerR.Pathophysiologyofacutewoundhealing.ClinDermatol 2007;25(1):9–18.
[32]GabbianiG.Themyofibroblast:akeycellforwoundhealingand fibrocontrac-tivediseases.ProgClinBiolRes1981;54:183–94.
[33]MeyerM,MullerAK,YangJ,WenerS.Theroleofchronicinflammationin cuta-neousfibrosis:fibroblastgrowthfactorreceptordeficiencyinkeratinocytesas anexample.JInvestigDermatolSympProc2011;15:48–52.
[34]MarquisBJ,HaynesCL.Theeffectsofco-cultureoffibroblastsonmastcell exocytoticreleasecharacteristicsasevaluatedbycarbon-fibermicroelectrode amperometry.BiophysChem2008;137:63–9.
[35]AuSR,AuK,SaggersGC,KarneN,EhrlichHP.Ratmastcellscommunicate withfibroblastsviagapjunctionintercellularcommunications.JCellBiochem 2007;100:1170–7.