H
2
S is a key antisecretory molecule against cholera toxin-induced
diarrhoea in mice: Evidence for non-involvement of the AC/cAMP/PKA
pathway and AMPK
Francisca B.M. Sousa
a,f, Luan K.M. Souza
a,f, Nayara A. Sousa
a,f, Thiago S.L. Araújo
a,f,
Simone de Araújo
b,f, Dvison M. Pací
fi
co
c, Irismara S. Silva
d, Renan O. Silva
e,
Lucas A.D. Nicolau
e, Fabiana M. Souza
a, Marcelo C. Filgueiras
a,f, Jefferson S. Oliveira
a,
Marcellus H.L.P. Souza
e, Jand Venes R. Medeiros
a,b,f,*aBiotechnology and Biodiversity Center Research, BIOTEC, Federal University of Piauí, Parnaíba, Piaui, Brazil bMedicinal Plant Research Center, NPPM, Federal University of Piaui, Teresina, Piauí, Brazil
cDepartment of Morphology, Faculty of Medicine, Federal University Ceara, Fortaleza, Ceara, Brazil dPhysiology and Pharmacology, Federal University of Minas Gerais, Belo Horizonte, Minas Gerais, Brazil eDepartment of Physiology and Pharmacology, Federal University of Ceara, Fortaleza, Ceara, Brazil fLaboratory of Experimental Physiopharmacology, Federal University of Piauí, Parnaíba, Piauí, Brazil
a r t i c l e
i n f o
Article history:
Received 24 May 2017 Received in revised form 18 September 2017 Accepted 20 September 2017 Available online xxx
Keywords:
Gaseous mediators Diarrhea diseases Cholera AMPK
a b s t r a c t
Hydrogen sulphide (H2S) is a gasotransmitter that participates in various physiological and pathophys-iological processes within the gastrointestinal tract. We studied the effects and possible mechanism of action of H2S in secretory diarrhoea caused by cholera toxin (CT). The possible mechanisms of action of H2S were investigated using an intestinalfluid secretion model in isolated intestinal loops on anaes-thetized mice treated with CT. NaHS and Lawesson's reagent andL-cysteine showed antisecretory activity
through reduction of intestinalfluid secretion and loss of Cl induced by CT. Pretreatment with an in-hibitor of cystathionine-g-lyase (CSE),DL-propargylglycine (PAG), reversed the effect ofL-cysteine and
caused severe intestinal secretion. Co-treatment with PAG and a submaximal dose of CT increased in-testinalfluid secretion, thus supporting the role of H2S in the pathophysiology of cholera. CT increased the expression of CSE and the production of H2S. Pretreatment with PAG did not reverse the effect of SQ 22536 (an AC inhibitor), bupivacaine (inhibitor of cAMP production), KT-5720 (a PKA inhibitor), and AICAR (an AMPK activator). The treatment with Forskolin does not reverse the effects of the H2S donors. Co-treatment with either NaHS or Lawesson's reagent and dorsomorphin (an AMPK inhibitor) did not reverse the effect of the H2S donors. H2S has antisecretory activity and is an essential molecule for protection against the intestinal secretion induced by CT. Thus, H2S donor drugs are promising candi-dates for cholera therapy. However, more studies are needed to elucidate the possible mechanism of action.
©2017 Elsevier Inc. All rights reserved.
1. Introduction
Cholera, a disease characterised by acute secretory diarrhoea, is caused by intestinal infection from the gram-negative bacterium
Vibrio choleraeserogroups O1 and O139[1]. This disease causes
large epidemics worldwide and is a serious threat to public health, particularly in developing countries[2]. The main virulence factor responsible for the dehydration observed during cholera is cholera toxin (CT), which is secreted byV. choleraeinto the small intestine [3].
CT causes severe diarrhoea through a direct effect on intestinal epithelial cells. More specifically, CT binds to intestinal enterocytes via interaction offive identical B-subunits with the GM1 ganglio-side receptor, which is then internalized through retrograde endocytosis[4]. Within the cell, the A subunit causes constitutive *Corresponding author. Av. S~ao Sebastiao, 2819, CEP: 64202-020, Parnaíba, PI,~
Brazil.
E-mail address:jandvenes@ufpi.edu.br(J.V.R. Medeiros).
Contents lists available atScienceDirect
Nitric Oxide
j o u r n a l h o m e p a g e :w w w . e l s e v i e r . c o m / l o c a t e / y n i o x
https://doi.org/10.1016/j.niox.2017.09.007
activation of Adenylyl cyclase (AC) by inactivation of the stimula-tory G protein Gs
a
, resulting in elevated levels of intracellular cAMP [5]. The increase in intracellular cAMP results in activation of pro-tein kinase A (PKA) and subsequent Cl channel opening [cysticfibrosis transmembrane conductance regulator (CFTR)], causing an excessive secretion of Cl accompanied by the osmotic movement of a large quantity of water into the intestinal lumen[6]. Thefluid loss is often so rapid and large that patients can die if left untreated [7].
The treatment of cholera involves replacing water and lost electrolytes, and consists of the administration of oral rehydration solution (ORS). Although this solution is effective for hydration and reduces mortality, ORS neither inhibits cholera toxin-mediated excessive secretion nor eliminates the infection from V. cholera [8]. Consequently, ORS does not decrease diarrhoea in the short term. Because of these factors, currently there are no pharmaco-logical approaches to treat cholera; treatment could be achieved by investigation of signalling molecules that can inhibit the increase of secretion induced by cholera toxin. Among the molecules that play many important physiological and pathophysiological roles in hu-man health, gaseous mediators such as hydrogen sulphide (H2S) are of particular interest.
H2S is a gaseous signalling molecule endogenously generated mainly fromL-cysteine through the activity of the enzymes cys-tathionine-
g
-lyase (CSE) and cystathionine-b
-synthetase (CBS), although there are alternative sources (e.g., cysteine aminotrans-ferase and/or 3-mercaptosulfotransaminotrans-ferase[9]. Studies have shown that H2S has important biological effects on the intestinal epithe-lium, local microcirculation, and inflammatory processes, and promotes changes in gastrointestinal smooth muscle [10e12]. In addition, recent data have shown that H2S inhibits the AC/cAMP pathway [13,14] and promotes the activation of AMP-activated protein kinase (AMPK)[15], an important kinase that phosphory-lates the CFTR channel and inhibits the secretion of chloride ions in intestinal epithelial cells[16]. These effects indicate that H2S may have beneficial effects on the pathophysiology of cholera, which involves increased activation of AC. Therefore, based on this back-ground information and owing to the absence of reports about the role of H2S in the secretory diarrhoea induced by cholera toxin, we evaluated the potential antisecretory effect of H2S in secretory diarrhoea induced by the enterotoxin ofV. choleraeand the possible mechanisms involved in this effect.2. Materials and methods
2.1. Chemicals and drugs
All drugs were purchased from Sigma Chemical Company, St. Louis, MO, USA. 5-Aminoimidazole-4-carboxamide 1-
b
-D -ribofur-anoside, (acadesine; N1-(b
-D -ribofuranosyl)-5-aminoimidazole-4-carboxamide; AICAR), 9-(tetrahydro-2-furanyl)-9H-purin-6-amine (9-THF-Ade; SQ 22536), Forskolin and bupivacaine HCl were dis-solved in 0.9% saline and administered directly on the loops at concentrations of 1 mM, 0.01 M, 20m
M and 100m
M, respectively. (9S,10S, 12R)-2,3,9,10,11,12-Hexahydro-10-hydroxy-9-methyl-1-oxo-9,12-epoxy-1H-diindolo[1,2,3-fg:30,20,10-kl]pyrrolo[3,4-i] [1,6] benzodiazocine-10-carboxylic acid hexyl ester (KT 5720) and 6-[4-(2-piperidin-1-ylethoxy)phenyl]-3-pyridin-4-ylpyrazolo [1,5-a]py-rimidine (dorsomorphin; compound C) were dissolved in PBS and administered directly to the loops at 1m
g and 30m
M, respectively. These doses were selected from published studies and the previous work of our research group[17e20].L-cysteine (H2S precursor),DL -propargylglycine (PAG; inhibitor of CSE) and NaHS (H2S donor) were dissolved in 0.9% saline and were administered by gavage [21]. Lawesson's reagent (H2S donor) was suspended in 1% carboxymethylcellulose and also administered orally. Cholera toxin was dissolved in PBS and administered directly to the loops (1m
g). All other chemicals and reagents were of analytical grade and ob-tained from standard commercial suppliers.The pH of the studied solutions are in the range of 4e5. More specifically the pH of NaHS was 4.67,L-cysteíne 5.38 and Lawesson's reagent 4.12, which was adjusted to pH 3. The pH adjust of the Lawesson's reagent solution was necessary in view of the fact that the literature shows that GYY4137, or morpholin-4-ium 4 methoxyphenyl(morpholino) phosphinodithioate, a Lawesson's reagent derivative, has a greater release at acidic pH like pH 3.0 and very slowly release under physiological conditions (pH 7.4)[22,23].
2.2. Animals
All in vivo work was subject to internal ethical review and conducted in accordance with Home Office requirements under the Animals Scientific Procedures Act (1986), with the approval of the Local Ethical Committee (No. 079/2015, Ethics Committee in Research of the Federal University of Piauí, Brazil). Swiss mice of both sexes (weight: 25e30 g) were used in this study. They were maintained in cages under laboratory conditions at a temperature of (23±1)C under a 12 h light/dark cycle with free access to a standard pellet diet and drinking tap water ad libitum. Mice were randomly assigned into groups of six to eight. They were deprived of food for 24 h before the experiments, but still allowed free access to water.
2.3. Cholera toxin-inducedfluid secretion in closed intestinal loops
The antisecretory effect of H2S was measured by assay of the intestinalfluid secretion induced by cholera toxin inoculation, as previously described by Ref.[24], with some modifications. Mice were treated by gavage withL-cysteine (10 or 50 mg kg 1), NaHS (3, 9 or 27
m
mol kg 1), Lawesson's reagent (3, 9 or 27m
mol kg 1), or saline (2.5 mL kg 1). Another group receivedDL-propargylglycine (PAG) 100 mg kg 1by gavage, an inhibitor of CSE, 30 min before administration ofL-cysteine (50 mg kg 1by gavage). After 30 min, the mice were intraperitoneally anaesthetized with a combination of xylazine hydrochloride (8 mg kg 1) and ketamine (40 mg kg 1), and a median laparotomy was performed to expose the small in-testine. A portion of the jejunum was isolated and closed with double ties to form an intestinal loop measuring approximately AbbreviationsAMPK AMP-activated protein kinase AC Adenylyl cyclase
cAMP Cyclic adenosine monophosphate CBS Cystathionine-
b
-synthetaseCFTR Cysticfibrosis transmembrane conductance regulator
2e3 cm. Intestinal loops were inoculated with 100
m
L of phosphate-buffered saline (PBS) (for the negative control group treated with saline) or 100m
L of CT dissolved in PBS at a dose of 1m
g/loop for the groups treated with saline (positive control for intestinal secretion), L-cysteine alone, PAGþL-cysteine, and H2S donors. The intestinal loops were returned to the abdominal cavity, the abdominal inci-sion was closed with sutures, and the mice were allowed to recover from the anaesthesia. Four hours after injecting anaesthesia, the mice were euthanized, and the closed loops were rapidly removed from the abdominal cavity. The fluid secretion was measured indirectly as the ratio of loop weight to length expressed in g cm 1. Tissue samples of isolated intestinal loops were collected and weighed for immediate analysis of H2S tissue levels. Other sample was fixed in 10% formalin immediately after their collection for subsequent assessment of intestinal CSE expression by immuno-histochemistry. The intestinal contents accumulated in each closed loop were collected separately to allow measurement of chloride ion (Cl ) concentration.In another set of experiments, we evaluated the direct effect of H2S donors against CT induced diarrhoea via intraluminal admin-istration using an isolated intestinal loop model. The mice were anaesthetized and the loops were inoculated with 50
m
L of the NaHS (27m
M) or Lawesson's reagent (27m
M), and 5 min later, the loops were inoculated again with 100m
L of the CT (1m
g/loop). The intestinal loops were returned to the abdominal cavity andfluid secretion was measured as described above.We also evaluated the effect of H2S on physiological secretion in the same model of intestinal loop isolated, with the treatment of the mice by gavage with NaHS 27
m
mol kg 1or Lawesson's reagent 27m
mol kg 1and the loops were inoculated with 100m
L of PBS.2.4. Effect of PAG pretreatment onfluid secretion in closed intestinal loops with a submaximal cholera toxin dose
Mice were treated by gavage with saline (2.5 mL kg 1) or PAG (100 mg kg 1). After 30 min, the animals were anaesthetized and diarrhoea induction in the isolated intestinal loop was conducted as described above. Intestinal loops were inoculated with 100
m
L of the PBS for the negative control group treated with saline alone, and 100m
L of the CT at a dose of 0.5m
g/loop for the groups treated with saline and PAG. The loops were returned to the abdominal cavity and the abdominal incision was closed with sutures. Four hours after anaesthesia, the mice were euthanized and the closed loops were rapidly removed from the abdominal cavity. Thefluid secretion was measured was measured as described in item 2.3.2.5. Determination of chloride ion concentration in intestinalfluid secretion
Fluid secretion accumulated in each closed loop of the animals pretreated by gavage with L-cysteine 50 mg kg 1, NaHS 27
m
mol kg 1, Lawesson's reagent 27m
mol kg 1, or saline 2.5 mL kg 1and inoculated in the loops with PBS (for the negative control group treated with saline) or CT for the other groups, ob-tained previously in item 2.3 described, were used to determine the intestinal concentration of chloride ions. This analyse was per-formed by standard procedures according to the manufacturer's instructions (Labtest, Lagoa Santa, MG, Brazil). Briefly, the super-natant of the samples was collected by centrifugation at 4000 rpm for 10 min. Next, the secretion was diluted in a volume ratio of 1:2 with distilled water. The samples were mixed with specific reagent kits for 2 min. After this time period, the absorbance was measured at 470 nm on a spectrophotometer and the values obtained were expressed in mEq L 1.2.6. Evaluation of intestinal absorption on closed loops
The effect of H2S on intestinal absorption was determined following the method previously described by Ref.[25]. Initially, the animals and intestinal loops were prepared as previously described. The loops were inoculated with 200
m
L of PBS (negative control for absorption), 200m
L of PBS containing 10 mM glucose (positive control for absorption), or 200m
L of PBS containingL -cysteine (50 mg kg 1), 200m
L of NaHS 27m
M, and 200m
L of Law-esson's reagent 27m
M. The intestinal loops were returned to the abdominal cavity and the abdominal laparotomy median was closed with sutures. At 30 min after inoculation of the loops the animals were euthanized, the abdominal cavity was reopened, and the closed intestinal loops were removed. The loops were weighed with and without the luminal contents, and the absorbed mass was calculated by subtraction of the two values. The percentagefluid absorption was measured indirectly from the loop weight to length ratio.2.7. Immunohistochemistry for cystathionine-
g
-lyase (CSE)Isolated bowel samples of the animals pretreated by gavage with saline 2.5 mL kg 1 and inoculated in the loops with PBS or CT, obtained previously in item 2.3 described, were used to determine the expression of CSE. For this formalin-fixed paraffin-embedded intestinal loop tissues were sliced into sections of 5
m
m. After deparaffinisation and rehydration, the sections were placed in Target Retrieval Solution (S1700, DAKO), pH 9.0, for 30 min in a 95 C water bath. Endogenous peroxidase was blocked with 3% H2O2for 20 min to reduce nonspecific binding, and then incubated with an anti-CSE primary antibody (Abnova Corporation; 1:200 dilution) for 1 h at 25C. Next, the sections were incubated for 30 min with a polymer (EnVisionþDual Link System-HRP; K4061, DAKO). The antibody binding sites were visualized by incubation with 3,30-diaminobenzidine (DAB) (K3467, DAKO) solution.Intestinal tissue cells were identified based on their specific morphology, localisation and staining behaviour. The total cell count was obtained at 40X magnification. Each microscope slide comprised two specimens of the intestinal segment. The immu-nopositive cells from the intestinal villi or crypts and other intes-tinal layers from each intesintes-tinal segment were counted using a light microscope by a blinded pathologist.
2.8. Measurement of H2S in bowel tissue
2.9. Roles of adenylate cyclase (AC), cAMP, and protein kinase A (PKA) in the antisecretory effect of H2S
To identify the roles of AC, cAMP, and PKA in the antisecretory effect of H2S, mice were treated by gavage with PAG (100 mg kg 1) or saline (2.5 mL kg 1). After 25 min, the animals were anaes-thetized and a median laparotomy was performed to expose the small intestine. A portion of the jejunum was isolated and loops were directly inoculated with 50
m
L of SQ 22536 (0.01 M/loop), bupivacaine (100m
M/loop), or KT-5720 (1m
g/loop), which are in-hibitors of AC, cAMP, and PKA, respectively. After 5 min, intestinal loops were then inoculated with 100m
L of PBS for the negative control group treated with saline alone and 100m
L of CT (1m
g/loop) for all other groups. The intestinal loops were returned to the abdominal cavity and the fluid secretion was measured as described in item 2.3. Although bupivacaine is frequently used with a local anaesthetic, it can also inhibit cAMP production as observed in a bupivacaine cardiotoxicity assay[17].To validate previous studies and to investigate the role of AC on the antisecretory effect of H2S, we used forskolin, an AC activator [27]. The mice were anaesthetized and a median laparotomy was performed to expose and isolate the small intestinal loop as described above. The loops were inoculated with 50
m
L of forskolin 20m
M or 100m
L of PBS. After 5 min, the loops were inoculated with 50m
L of NaHS 27m
M or 50m
L of Lawesson's reagent 27m
M. Finally, 5 min later, the intestinal loops were inoculated with 100m
L of PBS for the negative control group and 100m
L of CT (1m
g/loop) for all the other groups. The intestinal loops were returned to the abdominal cavity. After 4 h, the mice were euthanized, and closed loops were removed in order to evaluate the fluid secretion as previously in item 2.3.2.10. Assay of cyclic AMP levels
Isolated bowel samples of the mice treated with saline and PBS (100
m
L/loop), saline and cholera toxin (1m
g/loop), NaHS 27m
mol kg 1 and cholera toxin and Lawesson's reagent 27m
mol kg 1and cholera toxin, were used to determine the levels of cAMP. After completion of the treatment described above, the tissue samples were rapidly frozen in liquid nitrogen. Frozen tissue samples were homogenized in 10% trichloroacetic acid at 2e8C, followed by centrifugation at 2000gfor 15 min at 4C. Pellets were neutralized in 1 N NaOH for protein determination by the Bio-Rad method, with bovine serum albumin as standard. The supernatant was recovered and washed four times with 5 vol of water-saturated diethyl ether. The remaining aqueous extract was dried and frozen at 80 C to cAMP assays. For determination of cAMP levels in tissue samples, an enzyme immunoassay kit from Amersham Bio-sciences (Piscataway, NJ) was used. Briefly, antibodies to cAMP were added to each sample and allowed to incubate at 3e5C for 2 h. Next, samples were treated with cAMP-peroxidase conjugate and allowed to incubate for 60 min, followed by washing. Imme-diately after the final wash, enzyme substrate was added and samples were incubated at room temperature for 60 min. Reactions were terminated with 100m
l of 1 M H2SO4 and optical densities were determined in a plate reader at 450 nm.2.11. Role of AMP-activated protein kinase (AMPK) in the antisecretory effect of H2S
To evaluate the role of AMPK in the antisecretory effect of H2S, alternative experimental protocols were used involving AICAR, an AMPK activator, or dorsomorphin, an AMPK inhibitor. In the experiment with AICAR, mice were treated by gavage with PAG (100 mg kg 1) or saline (2.5 mL kg 1). After 25 min, the animals
were anaesthetized and a median laparotomy was performed to expose the small intestine. A portion of the jejunum was isolated and loops were directly inoculated with 50
m
L of AICAR 1 mM or 100m
L of PBS. After 5 min, the intestinal loops were then inocu-lated with 100m
L of PBS for the negative control group treated with saline alone and 100m
L of CT (1m
g/loop) for all other groups. The intestinal loops were returned to the abdominal cavity and thefluid secretion was measured apreviously described in item 2.3.In the second experimental protocol, dorsomorphin was used. For this, the mice were anaesthetized and a median laparotomy was performed to expose and isolate the small intestinal loop as described above. A portion of the jejunum was isolated and loops were inoculated with 50
m
L of dorsomorphin 30m
M or 100m
L of PBS. After 5 min, the intestinal loops were then inoculated with 50m
L of NaHS or Lawesson's reagent (both at 27m
M). Finally, after a further 5 min, the intestinal loops were inoculated again with 100m
L of PBS for the negative control group treated with saline alone and 100m
L of CT (1m
g/loop) for all the other groups. The in-testinal loops were returned to the abdominal cavity and thefluid secretion was measured apreviously described in item 2.3.2.12. Data analysis
The data and statistical analyses comply with the recommen-dations on experimental design and analysis in pharmacology. Data were expressed as mean ± SEM, and sample size in this study varied between n¼6e8. Statistical analysis was performed using GraphPad Prism (Version 5.0) software. Statistical significance of the differences between groups was determined by one-way analysis of variance (ANOVA) followed by multiple comparisons using the Student-Newman-Keuls test. To analyse the H2S levels in intestinal tissues, the number of immunostained cells perfield in the immunohistochemical assays were compared by a non-parametric test (t-test) followed by the Mann-Whitney test. P values of<0.05 were considered to be statistically significant in all the analyses.
3. Results
3.1. H2S decreases intestinalfluid accumulation in cholera toxin-treated intestinal closed loops
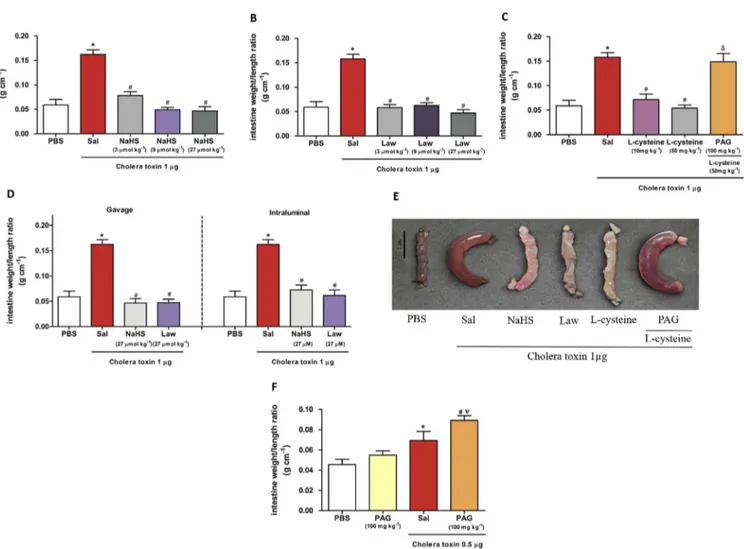
A significant decrease (p< 0.001) in the accumulation of in-testinalfluid secretion was observed in the groups treated with the NaHS 3
m
mol kg 1 (0.078 ± 0.008 g cm 1), NaHS 9m
mol kg 1 (0.049 ± 0.005 g cm 1) and NaHS 27m
mol kg 1 (0.047 ± 0.009 g cm 1), Lawesson's reagent 3m
mol kg 1 (0.058 ± 0.006 g cm 1), Lawesson's reagent 9m
mol kg 1 (0.063 ± 0.005 g cm 1) and Lawesson's reagent 27m
mol kg 1 (0.047±0.006 g cm 1) (Fig. 1A,Fig. 1B) and the precursor of H2S synthesis, L-cysteine 10 mg kg 1 (0.071 ± 0.011 g cm 1) and 50 mg kg 1(0.054±0.006 g cm 1) (Fig. 1C) as compared to the group in which the loop was injected with cholera toxin (0.162±0.009 g cm 1) in what was observed an excessivefluid accumulation (loop weight/length ratio), as expected. We also evaluated the effect of the 1% carboxymethylcellulose solution by gavage in the loops without the administration of cholera toxin, and was not observed any significant difference in intestinal secretion as compared to the negative control (PBS) (data not shown).
other models of gastrointestinal diseases. Likewise, this dose is not considered high for applications in pathological processes in the gastrointestinal tract[21].
InFig. 1C it was also observed that the administration of PAG, a CSE inhibitor, reversed the antisecretory effect of L-cysteine (0.148±0.016 g cm 1). InFig. 1E, we have shown an intestinal loop isolated from each study group. To confirm the antisecretory effect observed by gavage administration of the H2S donors, we tested NaHS and Lawesson's reagent directly inoculated into the loops. NaHS (27
m
M) and Lawesson's reagent (27m
M) administered intraluminal also significantly reduced (p<0.001,Fig. 1D) the in-testinal secretion (0.072±0.010 g cm 1) and (0.062±0.011 g cm 1) respectively.We also evaluated thefluid secretion in the presence of CBS inhibitor (carboxymethylhydroxylamine hemihydrochloride (CHH - 20 mg/kg, i.p.)). The inhibition of the CBS did not reverse the antisecretory effect ofL-cysteine (data not shown). Likewise, the treatment with NaHS and Lawesson's reagent both 27
m
mol kg 1 and PBS in the loops did not present significant difference in in-testinal secretion as compared to the negative control (PBS) (data not shown). This date indicate that H2S not show secretory effect onphysiological secretion, in this model.
3.2. PAG increases secretion of intestinalfluid in combination with a submaximal dose of cholera toxin
To determine the role of H2S in intestinal secretion induced by cholera toxin, mice were pretreated with PAG (100 mg kg 1) fol-lowed by intraluminal administration of a submaximal dose of cholera toxin (0.5
m
g/loop). This was necessary because PAG did not worsen intestinal secretion in the loop when administered at a CT dose of 1m
g/loop. This may have occurred because this dose of cholera toxin resulted in the maximum secretion that can occur in the tissue. As shown inFig. 1F, PAG in combination with CT (0.5m
g/ loop), promoted a significant increase (0.089±0.005 g cm 1) in intestinalfluid secretion as compared to the toxin group (p<0.01) and PAG alone (without the administration of the toxin) (0.055±0.004 g cm 1, p<0.001) that did not cause an increase in intestinal secretion. Cholera toxin at this dose (0.5m
g/loop) pro-moted a significant increase in intestinal secretion (0.069 ± 0.009 g cm 1) as compared to PBS group (0.046±0.005 g cm 1, p<0.01).Fig. 1.Effect of hydrogen sulphide on intestinalfluid accumulation in cholera toxin-treated intestinal closed loops. Mice were pretreated by gavage with saline (2.5 mL kg 1, n¼6),
NaHS (3, 9 and 27mmol kg 1, n¼6 (A)), Lawesson's reagent (3, 9 and 27mmol kg 1, n¼6 (B)),L-cysteine (10 and 50 mg kg 1, n¼6 (C)), and PAG (100 mg kg 1) andL-cysteine
(50 mg kg1, n
¼6 (C)) for 30 min before the induction of diarrhoea by CT (1mg/loop). The control groups were pretreated with saline, and the negative control group received PBS
(100mL) in the isolated loop and the positive control group received CT. (D) Effect of intraluminal administration versus gavage administration of H2S donors, observed in previous
trials, against CT-induced diarrhoea. (E) Intestinal loops of each study group. In F, mice were pretreated by gavage with PAG (100 mg kg1, n¼6) or saline (2.5 mL kg 1, n¼6) 30 min
before the induction of diarrhoea of CT (0.5mg/loop). All errors bars indicate SEM. *P<0.05 versus the group treated with PBS in the loops;#P<0.05 versus positive control group
(pretreated with saline and CT in the loops);dP<0.05 vs. group pretreated withL-cysteine (50 mg kg1)JP<0.05 versus the group pretreated with PAG (100 mg kg1) and treated
3.3. H2S reduces chloride ion concentration in intestinalfluid secretion
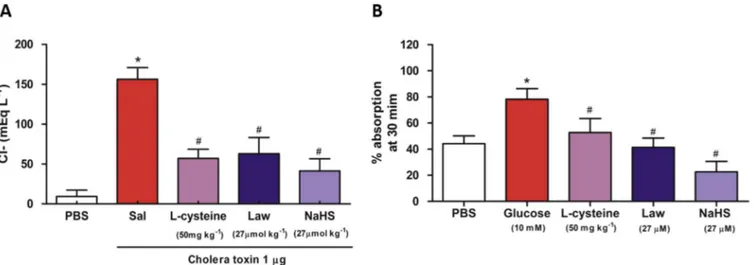
To confirm the antisecretory effect of H2S observed in previous assays, we analysed the concentration of chloride ions in the in-testinal fluid secretion accumulated in each closed loop. The intense secretion of chloride ions into the intestinal lumen caused by cholera toxin is responsible for increased intestinal secretion. In the present study, the concentration of the chloride ions was significantly high (p < 0.001) in intestinal loops injected with cholera toxin (156.5±14.51 mEq L 1) compared with loops injected only with PBS (156.5 ± 14.51 mEq L 1). Treatment with NaHS, Lawesson's reagent, and L-cysteine significantly decreased the levels of chloride in the loops (41.47±15.07 mEq L 1, 63.16±20.07 mEq L 1and 57.27±11.35 mEq L 1, p<0.001,Fig. 2A) respectively.
3.4. Effect of H2S on intestinalfluid absorption
The effect of H2S on intestinalfluid absorption was studied to evaluate whether the protective effect of H2S on intestinal fluid secretion stimulated by CT was involved in increasing intestinal absorption.Fig. 2B shows that administration of glucose solution (10 mM), promotes a significant intestinal absorption (78.25±8.06%, p<0.001) as compared to the basal level of intes-tinal absorption observed in the PBS group (44.26 ± 5.90%). A similar response occurred in loops treated with NaHS (22.64±8.03%), Lawesson's reagent (41.33±7.08%), orL-cysteine (52.75±10.73%), in which it was not observed a significant effect on intestinal absorption when compared to the PBS group.
3.5. Cholera toxin increased expression of CSE and levels of H2S in mouse small intestine
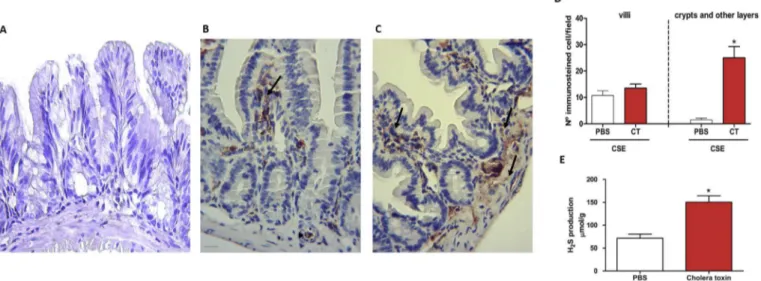
Fig. 3B show the presence of immunostaining in the loops treated with PBS, especially on the lamina propria layer of the mucosa of the intestinal villi (arrow;Fig. 3B), although some im-munostaining was also observed in the blood vessels (arrowhead; Fig. 3B). However, when the intestinal loops were inoculated with CT (Fig. 3C), staining was observed in other layers. In addition to intense staining for CSE in the mucosal layers of the intestinal villi
there was also staining in the crypts and some staining in the deeper layers of intestinal tissue (muscular and serous) (arrows; Fig. 3B).Fig. 3A shows the negative control, without primary anti-body, in the loops of mice treated with PBS. The data from the quantitative analysis of patterns of immunostaining are summa-rized inFig. 3D. An unpairedt-test revealed that immunostaining of villous mucosal cells was highly similar between the groups treated with CT (13.50±1.56) and PBS (10.75 ± 1.75) and no statistical differences were observed between them. However, analysis of the number of immunostained cells in the mucosal intestinal crypts and other layers found a significant increase of immunostained cells in loops treated with CT (25.00±4.30) and PBS (1.5±0.64, p<0.001). Based on these data, we next examined the levels of the H2S in the loops by a sulphide ion microelectrode.Fig. 3E shows a significant increase (p<0.001) occurred in the levels of H2S on the loops inoculated with CT (150.30 ± 14.04
m
mol g 1 of tissue) compared with the loops treated with PBS (71.65±8.94m
mol g 1of tissue).3.6. Roles of AC, cAMP, and PKA in the H2S antisecretory effect
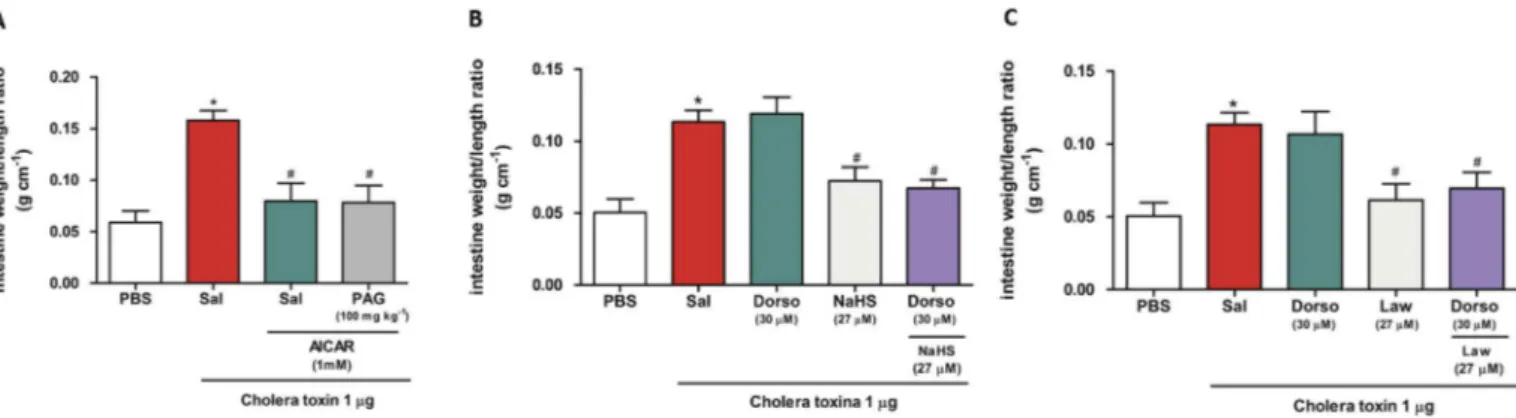
To evaluate the involvement of AC in the antisecretory effect of H2S (Fig. 4A), SQ 22536 (0.01 M), a specific inhibitor of AC, was administered directly into the isolated loop. As expected, this produced a significant reduction in intestinal fluid secretion induced by CT (0.086±0.012 g cm 1, p< 0.001). However, pre-treatment with PAG (100 mg kg 1) did not reverse the protective effect of SQ 22536 (0.085±0.011 g cm 1, p<0.001). These data suggest that the antisecretory effect of H2S is independent of AC. To confirm the non-involvement of AC, we also tested a specific AC activator, forskolin. As shown inFig. 4B and C, a significant increase in intestinal secretion was observed in the intestinal loops treated with forskolin (0.103±0.014 g cm 1, p<0.01) when compared to the PBS group. This effect was expected with forskolin as AC acti-vation causes an increase in the secretion. We also observed a significant increase in the intestinal secretion in the loops treated only with CT (0.115±0.009 g cm 1, p<0.001) or forskolin and CT (0.120±0.011 g cm 1, p<0.01), when compared to the PBS group. In another set of experiments, we tested the non-involvement of AC by treating the loops directly with forskolin, followed by NaHS or
Fig. 2.Effect of hydrogen sulphide on the levels of chloride ions and intestinal absorption. (A) Effect of hydrogen sulphide on the levels of chloride ions present in loops treated with CT. Mice were pretreated by gavage with saline (2.5 mL kg 1, n¼6), NaHS (27mmol kg 1, n¼6), Lawesson's reagent (27mmol kg 1, n¼6) orL-cysteine (50 mg kg1, n¼6) for
30 min before the induction of diarrhoea by CT (1mg/loop). (B) Effect of hydrogen sulphide on the intestinal absorption. Mice were treated directly into the isolated intestinal loop with PBS (200mL) and glucose solution (10 mM) or PBS (200mL) containingL-cysteine (50 mg kg1), NaHS (27mmol kg1), or Lawesson's reagent (27mmol kg1) 30 min before
removal of the loops. All error bars indicate the SEM. *P<0.05 versus the PBS-treated group in the loops,#P<0.05 versus the positive control group (pretreated with saline and CT
in the loops), and#P<0.05 versus the glucose-treated group. Values were determined by one-way ANOVA followed by a Newman
Lawesson's reagent and CT. We observed a significant reduction in the intestinal secretion when compared to the control group treated with CT only (0.058±0.009 g cm 1, p<0.001,Fig. 4B) and (0.072±0.009 g cm 1, p<0.01,Fig. 4C) respectively. These data corroborate with the previous results and show that activation of AC by forskolin does not reverse the antisecretory effects mediated by H2S donors, Lawesson's reagent and NaHS.
We also evaluated the effect of bupivacaine on intestinal secretion considering its side effect inhibiting cAMP production contrary to the pathophysiological mechanism of cholera causing an increase in cAMP. Due to the probable inhibition of cAMP, we observed a significant reduction in the intestinal secretion when the loops were treated with bupivacaine (100
m
M) and CT (0.080±0.020 g cm 1, p<0.001,Fig. 4D). This prompted us to useFig. 3.Immunohistochemical staining for cystathionineg-lyase (CSE) (magnification: 400) in mouse small intestine. (A) Negative control (without primary antibody) in loops of mice treated with PBS. (B) Immunostained for CSE in loops of mice treated with PBS or CT (C). Scale bar¼50mm. (D) Quantitative analysis of immunostained cells observed in the loops treated with PBS and CT in relation the villi, crypts, and others layers of intestinal tissue. Cholera toxin significantly increased the expression of CSE in the crypts and others layers of intestinal tissue. (E) H2S levels in intestinal loops of mice treated with PBS or CT. All errors bars indicate the SEM. *P<0.05 versus the group treated with PBS. Values were
determined byt-test followed by a Mann-Whitney test.
Fig. 4.Roles of AC, cAMP, and PKA in the antisecretory effect of hydrogen sulphide on diarrhoea induced by CT. Mice were pretreated by gavage with saline (2.5 mL kg1, n¼6) or
PAG (100 mg kg 1, n¼6), and after 25 min the following compounds were administered directly to the loops: (A) SQ 22536 (0.01 M), an inhibitor of AC; (D) bupivacaine (100mM),
inhibitor of cAMP production; (E) KT-5720 (1mg), a PKA inhibitor. After a further 5 min, the intestinal loops were inoculated with PBS or CT (1mg/loop). Mice also were inoculated in the loops with Forskolin (FSK - 20mM), an AC activator, followed by intraluminal administration of NaHS (B) or Lawesson's reagent (C) (both at 27mM) and CT (1mg/loop) later. (F) cAMP levels of the isolated bowel samples of the mice treated with saline and PBS (100mL/loop); saline and CT (1mg/loop); NaHS 27mmol kg1and CT (1mg/loop); Lawesson's
reagent 27mmol kg1and CT (1mg/loop). All error bars indicate the SEM. *P<0.05 versus the PBS-treated group, #P<0.05 versus the positive control group pretreated with saline
bupivacaine to evaluate whether H2S has an antisecretory effect via inhibition of cAMP. For this, the animals were pre-treated with PAG, followed by intraluminal administration of bupivacaine and CT, respectively. The pretreatment with PAG did not reverse the effects of bupivacaine (0.043±0.004 g cm 1, p<0.001,Fig. 4D). In addi-tion, a specific inhibitor of PKA, KT-5720 (1
m
g), was also used to modulate the effect of H2S. As shown inFig. 4E, KT-5720 signifi -cantly reduced the secretion of intestinal fluid induced by CT (0.066 ± 0.005 g cm 1, p < 0.001). Additionally, PAG failed to reverse the antisecretory effect of KT-5720 (0.043±0.004 g cm 1, p<0.001).3.7. Measurement of cAMP levels
To confirm the non-involvement of AC in the antisecretory effect of H2S observed in previous assays, we also analysed the concen-tration of the cAMP levels in bowel samples of the loops.Fig. 4F shows the basal levels of cAMP in the gut samples of the animals treated with saline and PBS in the loops (1.140±0.387 pmol/mg protein). As expected the animals treated with saline and Cholera toxin in the loops significantly increased the cAMP levels (7.373±1386 pmol/mg protein, p<0.001) as compared the PBS group, but the animals treated with NaHS or Lawesson's reagent both 27
m
mol kg 1and Cholera toxin in the loops did not reverse this levels (6.598 ± 0.849 pmol/mg protein, p < 0.001) and (7.166 ± 1.262 pmol/mg protein, p < 0.001) respectively. Thesefindings corroborate with the previous results, indicating the non-involvement of the AC/cAMP pathway.
3.8. Role of AMP-activated protein kinase (AMPK) in the H2S antisecretory effect
AMPK is a kinase that phosphorylates the CFTR channel, inhib-iting the secretion of chloride ions in intestinal epithelial cells[28]. We determined the role of AMPK by use of an activator and a specific inhibitor of AMPK: AICAR (Fig. 5A) and dorsomorphin (Fig. 5B and C), respectively. In this assay, the mice administered AICAR were pretreated with PAG (100 mg kg 1). The treatment with AICAR (1 mM) produced a significant reduction in the intes-tinalfluid secretion induced by CT (0.079±0.017 g cm 1, p<0.001, Fig. 5A). However, pretreatment with PAG did not abolish the protective effect of AICAR (0.078±0.017 g cm 1, p<0.01,Fig. 5A). Administration of dorsomorphin directly into the loop was used to confirm the previous result. As expected for a specific inhibitor of AMPK, treatment with dorsomorphin promoted an excessive
accumulation of intestinalfluid (0.119±0.011 g cm 1,Fig. 5B and C). Administration of NaHS (27
m
M) (Fig. 5B) and Lawesson's reagent (27m
M) (Fig. 5C) directly into the loop significantly reduced the intestinalfluid accumulation induced by CT (0.072±0.009 g cm 1, p < 0.01) and (0.062± 0.011 g cm 1, p< 0.01) respectively. In contrast, dorsomorphin administration followed by NaHS or Law-esson's reagent directly into the loop did not reverse the anti-secretory effect of hydrogen sulphide donors (0.067±0.006 g cm 1, p<0.001) and (0.070±0.011 g cm 1, p<0.01) respectively.4. Discussion
In the present study, we demonstrated for thefirst time that NaHS (which instantly release H2S in aqueous media because their dissociation give Naþand HS or S2 in water, that react with Hþto give H2S) and Lawesson's reagent (that are used as an H2S donor by spontaneous hydrolysis)[23] andL-cysteine (a substrate for H2S synthesis) demonstrated broad antidiarrheal efficacy on intestinal
fluid accumulation in the cholera toxin-treated isolated loops, significantly reducing the intestinalfluid and the loss of extracel-lular Cl (Fig. 6). This was also indicated by PAG-induced reversal of theL-cysteine antidiarrheal effect, which resulted in severe intes-tinal secretion. These findings led us to the hypothesis that hydrogen sulphide was essential for protection against the damaging effects of CT. We confirmed this by tests involving a submaximal dose of CT (0.5
m
g/loop). We observed that pretreat-ment with PAG plus a submaximal dose of CT promoted a higher increase than that observed for the control group of the CT in in-testinal secretion levels. PAG has been commonly used as a putative inhibitor of CSE, employed to inhibit H2S production at the cellular or tissue level, and also in some in vivo experiments using different models of animal disease where usually PAG inhibits the protective effect of H2S donors[29].Among the heterogeneous mechanisms of action accounting for the protective effects of H2S, the activation of potassium channels seem to play a relevant role obtained by many researchers[11]. In our study, we observed another effect for H2S, the decrease of in-testinal Cl secretion under cholera toxin effect. We believe that this effect could have occurred via direct inhibition of the CFTR channel, or indirectly via inhibition of other activators of the CFTR channel.
In the intestine, all segments have mechanisms for absorption and secretion of water and electrolytes, and the fluid transport occurring in the intestine is secondary to active salt transport across the epithelium[30]. Fluid absorption in the small intestine is
Fig. 5.Role of AMPK in the antisecretory effect of hydrogen sulphide on diarrhoea induced by CT. (A) Mice were pretreated by gavage with saline (2.5 mL kg 1, n¼6) or PAG
(100 mg kg1, n¼6), and after 25 min, AICAR (1 mM) was administered directly into the loops. After a further 5 min, the intestinal loops were inoculated with PBS or TC (1mg/loop).
(B) and (C) Mice were pretreated by gavage with saline (2.5 mL kg 1, n¼6) and, after 20 min, received PBS (50mL) or dorsomorphin (30mM) in the isolated loops. Five minutes later,
driven by Naþ
coupled transport mechanisms at the luminal membrane, andfluid secretion is determined by active Cl trans-port on the apical side of the enterocytes[31]. In fact literature shows that intestinal ion transport is controlled by neurotrans-mitters and hormones, but also gasotransneurotrans-mitters such as CO, NO, and H2S[32]. In contrast to our observations, certain researchers have shown that H2S evoked Cl secretion in guinea pigs and hu-man colon. In these tissues, the primary site of action of H2S donors are enteric neurons, where H2S acts at capsaicin-sensitive cation channels of the type transient receptor potential vanilloid receptor 1 (TRPV1) [33]. As a consequence, substance P is released and secretomotor submucosal neurons are activated, which finally induce epithelial anion secretion[34]. Another mechanism of ac-tion of NaHS was observed in rat colon where it induced colonic ion secretion by stimulation of apical and basolateral epithelial Kþ channels[35]. On the contrary, in our model, H2S exerted an anti-secretory action. The effect of H2S on intestinal functions is thus controversial. In a study using different H2S-generating compounds [36], assessed chloride secretion by rat enterocytes and demon-strated that local H2S concentration seems to critically determine whether this gasotransmitter exerts a prosecretory or an anti-secretory action in the gastrointestinal mucosa. Similar prosecre-tory and antisecreprosecre-tory epithelial mechanism of actions described for NO suggest that the kinetics of production, degradation, and even formation of reactive intermediates determines the physio-logical effects of gasotransmitters[37].
We also investigated the role of H2S in intestinal absorption. This was necessary to exclude the hypothesis that H2S may decrease the accumulation of intestinal fluid by increasing the absorption of intestinalfluid. Our results showed that H2S did not interfere with thefluid absorption process; this indicated that it might act by other mechanisms, perhaps by interfering in the pathophysiological process of the cholera. More specifically could be interfering with the AC/cAMP/PKA pathway that is highly acti-vated by cholera toxin, which results in an excessive opening of the CFTR channel, promoting an increase in intestinal secretion. Although the role of H2S as a signalling molecule is emerging, its
effects on the epithelial transport of Naþ
and consequently on the processes of intestinal absorption is largely unknown[38].
H2S is mainly synthesized fromL-cysteine by two key enzymes: CSE and CBS. In some tissues, both are needed for H2S generation; in others, one enzyme is sufficient. In our study, we used immu-nohistochemistry to demonstrate that mice intestinal closed loops treated with CT resulted in increased CSE expression. Similarly, CT also caused an increase in H2S production. The local concentration in the intestinal wall is unknown[39]. These data suggest that the gut can compensate the damaging effect of CT by an increase of H2S production to enhance the self-defence mucosal protective and anti-secretory mechanisms, probably by increasing the CSE expression in intestinal crypts with a greater secretion of intestinal
fluid. This observation is in keeping with previousfindings from other pathophysiological processes in the gastrointestinal tract [40]. In a rat model of acetic acid-induced ulcers it was demon-strated that shortly after induction of an ulcer there was a marked upregulation of expression of CSE at the site of injury and elevated capacity for H2S synthesis[41]. The same effect was observed in colitis in rats[42]and in ethanol-induced gastric mucosal damage in mice [43]. Additionally, direct enhancement of antioxidant enzyme glutathione peroxidase (GSH-Px) could be another possible mechanism, by which H2S could be protecting the intestinal mu-cosa. In fact, several studies show that H2S can produce protective effects by increasing the activity of the antioxidant system in different pathophysiological processes[44e46]. Based on this, we evaluated GSH levels in the intestinal loops treated with the L -cysteine and H2S donors in CT-induced diarrhoea and PBS control. However, we did not observe any differences in GSH levels between the analysed samples (data not shown).
In contrast to that observed with CSE, the inhibition of the CBS did not reverse the antisecretory effect of L-cysteine (data not shown). This data indicated that CBS possible does not interfere in the secretion induced by CT. The gastrointestinal tract expresses both CSE and CBS and has the ability to generate H2S, but CSE seems to be the main enzyme involved in the H2S generation in these tissues[43].
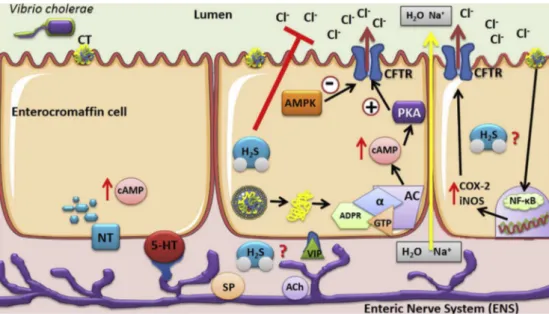
Fig. 6.Schematic diagram of the antisecretory effect of H2S. H2S reduces the CT-induced loss of Cl- and consequently the secretion of intestinalfluid (red arrows). This effect
probably does not involve the AC/cAMP/PKA pathway and AMPK. This diagram also showed other possible mechanisms (question mark) through which hydrogen sulphide could be acting, such as involvement in the enteric nerve system (ENS) induced by the CT through their action in enterochromaffin cells or in the pathway involving nuclear factor NFkB, an
The increase in intestinal fluid secretion requires cooperative functions of several transport proteins, including apical Cl chan-nels (e.g., CFTR, cyclic AMP activated Cl channel), basolateral Kþ channels (e.g., cAMP-activated Kþ
channel), and basolateral trans-porters (e.g., NKCC, the Naþ
/Kþ
/Cl cotransporter). Increases in intracellular cAMP induce the opening of corresponding apical Cl channels and basolateral Kþ
channels, which enables trans-epithelial Cl secretion[47]. Suppression of intestinal Cl secretion may occur via the inhibition of any one of these transport proteins, as well as enterocyte signalling effectors (e.g., cAMP). These sig-nalling pathways have been proven as potential antisecretory tar-gets by the efficacy of a small molecule phosphodiesterase (PDE) activator, which reduces cAMP levels, in a closed loop model of intestinalfluid secretion[48]. Based on these important targets we evaluated the roles of AC, cAMP, and PKA in the antisecretory effect of H2S. Despite lack of reports on the effects of H2S that occur through the inhibition of AC in gastrointestinal tract disorders, recent studies have indicated that H2S donors such as NaHS have the ability to inhibit AC activity, thereby reducing the formation of cAMP in different cells and tissue types, such as kidney, neuronal, and smooth muscle cells, and the aorta and cerebral artery of rats [14,49e51]. Thus, it is reasonable to speculate the involvement of the AC/cAMP pathway and measure intracellular the cAMP level in bowel samples in the various H2S effects. However, despite these literature reports, our study showed that the inhibitory effect of H2S in secretory diarrhoea induced by CT probably did not occur through action on the AC/cAMP/PKA pathway (Fig. 6).
Amongst the alternative strategies focused on the pharmaco-logical modulation of the inhibition the augmented enterotoxin induced chloride flux, AMP-activated protein kinase (AMPK) emerged as a potential candidate [16]. The kinase AMPK is considered an energy sensor that controls cellular energy homeo-stasis. AMPK phosphorylates target proteins and modulates their functional activities in response to an increase in the intracellular AMP-ATP ratio[52]. In intestinal epithelial cells, AMPK phosphor-ylates the CFTR channel, which inhibits the secretion of Cl [28]. Many effects of H2S, including inhibition of vascular inflammation in diabetes[53], anti-osteoporosis activity[50], and suppression of neuroinflammation[54]have been found to occur due to activation of AMPK[55]. also showed that H2S has cardioprotective effects when administered after myocardial ischemia and suggested that this effect was due to the activation of AMPK, because the treat-ment with dorsomorphin (an AMPK inhibitor) abolished the car-dioprotection offered by H2S donors. Our study demonstrated contrasting results, as dorsomorphin treatment did not abolish the inhibitory effect of H2S on diarrhoea induced by CT. Thus, these data suggest that H2S likely reduces intestinal secretion induced by CT by a mechanism independent of AMPK (Fig. 6).
Additional studies are therefore necessary to elucidate the possible mechanism of action of H2S on diarrhoea induced by CT. The enteric nervous system (ENS) also plays an important role in intestinal secretions induced by cholera [56]. An alternative mechanism by which CT induces the increase of intestinal secretion is through binding to mucosal enterochromaffin cells, which re-leases large amounts of 5-HT that subsequently activates secreto-motor neurons to release their transmitters onto the epithelial cells [57]. Thus, 5-HT-mediated effects on the epithelial cells induce both an increase in secretion and an inhibition of absorption, resulting in a massive loss of electrolytes and water during cholera[58]. Other studies suggest that CT can stimulate a modest intestinal infl am-matory response mediated by the release of IL-1
b
and TNF-a
in intestinalfluid[59,60]. Recently, it was also shown that the path-ogenesis of diarrhoea involves inflammatory responses mediated by NFkB, which promote an increase in expression of the enzymescyclooxygenase-2 (COX-2) and nitric oxide synthase (iNOS) that
appears to be related to intestinal barrier disruption and increased intestinal fluid secretion by the CFTR channel [61](Fig. 6). The discovery of these new pathways for CT activation led us to believe that H2S probably acts by inhibition of these, as H2S is considered an important anti-inflammatory molecule capable of suppressing cytokine expression and NFkB activation[40,62].
5. Conclusion
In conclusion, ourfindings showed that H2S displayed antidi-arrheal activity in a model of secretory diarrhoea induced by CT in isolated intestinal loops of mice, inhibiting thefluid secretion and migration of chloride ions from enterocytes into the intestinal lumen. The data also suggested that the antisecretory effect of H2S was not responsible for the increased intestinal absorption, and it probably occurs by a mechanism independent of AC/cAMP/PKA and AMPK. Furthermore, it was shown that CT induces an increase in the expression of CSE in mice, as well as the production of H2S. Thus, this study showed the importance of antidiarrheal activity of this gaseous mediator, and indicated the potential of H2S donor drugs in cholera therapy. More studies will be conducted by our research group to elucidate the possible mechanism of action of H2S on diarrhoea induced by the enterotoxin ofV. cholerae.
Author contributions
F.B.M.S., L.K.M.S., T.S.L.A., F.M.S., S.A., D.M.P., I.S.S., R.O.S., and L.A.D.N. performed the experiments, analysed the data, and pre-pared the manuscript. J.S.O., M.H.L.P.S, and J.V.R.M. designed the experiments, provided the drugs and other facilities, and critically corrected the manuscript.
Acknowledgments
The authors gratefully acknowledge the National Counsel of Technological and Scientific Development, CNPq (Process 441859/ 2014-3, Brazil) and the Research Foundation for the State of Piauí-Brazil FAPEPI, forfinancial support for this work. Dr Medeiros and Dr Souza are recipients of CNPq fellowships.
References
[1] J.B. Harris, R.C. Larocque, F. Qadri, E.T. Ryan, B.S. Calderwood, Cholera, Lancet 379 (2012) 2466e2476,http://dx.doi.org/10.1016/S0140-6736(17)30559-7.
[2] G. Moradi, M.A. Rasouli, P. Mohammadi, E. Elahi, H. Barati, A cholera outbreak in Alborz Province, Iran: a matched case-control study, Epidemiol. Health 38 (2016) e2016018,http://dx.doi.org/10.4178/epih.e2016018.
[3] I. Basu, C. Mukhopadhyay, Insights into binding of cholera toxin to GM1 containing membrane, Langmuir 30 (2014) 15244e15252,http://dx.doi.org/ 10.1021/la5036618.
[4] J.R. Thiagarajah, A.S. Verkman, New drug targets for cholera therapy, Trends Pharmacol. Sci. 26 (2005) 172e175, http://dx.doi.org/10.1016/ j.tips.2005.02.003.
[5] W.I. Lencer, Microbes and microbial toxins: paradigms for microbial-mucosal toxins. V. cholera: invasion of the intestinal epithelial barrier by a stably fol-ded protein toxin, Am. J. Physiol. Gastrointest. Liver Physiol. 280 (2001) G781eG786.
[6] C. Muanprasat, V. Chatsudthipong, Cholera: pathophysiology and emerging therapeutic targets, Future Med. Chem. 5 (2013) 781e798,http://dx.doi.org/ 10.4155/fmc.13.42.
[7] D.A. Sack, R.B. Sack, G.B. Nair, A.K. Siddique, Cholera, Lancet 363 (2004) 223e233,http://dx.doi.org/10.1016/S0140-6736(17)30559-7.
[8] H.J. Binder, I. Brown, B.S. Ramakrishna, G.P. Young, Oral rehydration therapy in the second decade of the twenty-first century, Curr. Gastroenterol. Rep. 16 (2014) 376,http://dx.doi.org/10.1007/s11894-014e;0376e2.
[9] M.D. Hartle, M.D. Pluth, A practical guide to working with H2S at the interface of chemistry and biology, Chem. Soc. Rev. 45 (2016) 6108,http://dx.doi.org/ 10.1039/C6CS00212A.
[10] S. Fiorucci, E. Distrutti, G. Cirino, J.L. Wallace, The emerging roles of hydrogen sulfide in the gastrointestinal tract and liver, Gastroenterology 131 (2006) 259e271,http://dx.doi.org/10.1053/j.gastro.2006.02.033.
P.J.C. Magalh~aes, A.A. Santos, Role of KATP channels and TRPV1 receptors in hydrogen sulfide-enhanced gastric emptying of liquid in awake mice, Eur. J. Pharmacol. 693 (2012) 57e63,http://dx.doi.org/10.1016/j.ejphar.2012.07.004.
[12] B. Gemici, W. Elsheikh, K.B. Feitosa, S.K. Costa, M.N. Muscara, J.L. Wallace, H2S-releasing drugs: anti-inflammatory, cytoprotective and chemopreventative potential, Nitric. Oxide 46 (2015) 25e31, http://dx.doi.org/10.1016/ j.niox.2014.11.010.
[13] M. Yang, Y. Huang, J. Chen, Y.L. Chen, J.J. Ma, P.H. Shi, Activation of AMPK participates hydrogen sulfide-induced cyto-protective effect against dexa-methasone in osteoblastic MC3T3-E1 cells, Biochem. Biophys. Res. Commun. 454 (2014) 42e47,http://dx.doi.org/10.1016/j.bbrc.2014.10.033.
[14] S. Li, N.N. Ping, L. Cao, Y.N. Mi, Y.X. Cao, H2S induces vasoconstriction of rat
cerebral arteries via cAMP/adenylyl cyclase pathway, Toxicol. Appl. Pharma-col. 289 (2015) 389e396,http://dx.doi.org/10.1016/j.taap.2015.10.021.
[15] J. Liu, J. Wu, A. Sun, Y. Sun, X. Yu, N. Liu, S. Dong, F. Yang, L. Zhang, X. Zhong, C. Xu, F. Lu, W. Zhang, Hydrogen sulfide decreases high glucose/palmitate-induced autophagy in endothelial cells by the Nrf2-ROS-AMPK signaling pathway, Cell. Biosci. 23 (2016) 6e33, http://dx.doi.org/10.1186/s13578-016-0099-1.
[16] A.C. Rogers, L. Huetter, N. Hoekstra, D. Collins, A. Collaco, A.W. Baird, D.C. Winter, N. Ameen, J.P. Geibel, S. Kopic, Activation of AMPK inhibits cholera toxin stimulated chloride secretion in human and murine intestine, PLoS One 8 (2013) e69050,http://dx.doi.org/10.1371/journal.pone.0069050. [17] J.F. Butterworth, R.C. Brownlow, J.P. Leith, R.C. Prielipp, L.R. Cole, Bupivacaine
inhibits cyclic-3',5'-adenosine monophosphate production. A possible contributing factor to cardiovascular toxicity, Anesthesiology 79 (1993) 88e95.
[18] H.B. Kandilci, B. Gumusel, H. Lippton, Intermedin/adrenomedullin-2 (IMD/ AM2) relaxes rat main pulmonary arterial rings via cGMP-dependent pathway: role of nitric oxide and large conductance calcium-activated po-tassium channels (BK(Ca)), Peptides 29 (2008) 1321e1328,http://dx.doi.org/ 10.1016/j.peptides.2008.04.008.
[19] J.E. Kim, S.E. Song, Y.W. Kim, J.Y. Kim, S.C. Park, Y.K. Park, S.H. Baek, I.K. Lee, S.Y. Park, Adiponectin inhibits palmitate-induced apoptosis through sup-pression of reactive oxygen species in endothelial cells: involvement of cAMP/ protein kinase A and AMP-activated protein kinase, J. Endocrinol. 207 (2010) 35e44,http://dx.doi.org/10.1677/JOE-10-0093.
[20] K.Z. Shen, V. Yakhnitsa, A.C. Munhall, S.W. Johnson, AMP kinase regulates K-ATP currents evoked by NMDA receptor stimulation in rat subthalamic nu-cleus neurons, Neuroscience 274 (2014) 138e152,http://dx.doi.org/10.1016/ j.neuroscience.2014.05.031.
[21] J.V. Medeiros, V.H. Bezerra, A.S. Gomes, A.L. Barbosa, R.C.P. Lima Jr., P.M.G. Soares, G.A.C. Brito, R.A. Ribeiro, F.Q. Cunha, M.H.L.P. Souza, Hydrogen sulfide prevents ethanol-induced gastric damage in mice: role of ATP-sensitive potassium channels and capsaicin-ATP-sensitive primary afferent neu-rons, J. Pharmacol. Exp. Ther. 330 (2009) 764e770,http://dx.doi.org/10.1124/ jpet.109.152801.
[22] L. Li, M. Whiteman, Y.Y. Guan, K.L. Neo, Y. Cheng, S.W. Lee, Y. Zhao, R. Baskar, C.H. Tan, P.K. Moore, Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide, Circulation 117 (2008) 2351e2360, http://dx.doi.org/ 10.1161/CIRCULATIONAHA.107.753467.
[23] J. Kang, D.L. Neill, M. Xian, Phosphonothioate-based hydrogen sulfide releasing reagents: chemistry and biological applications, front, Pharmacol 8 (2017),http://dx.doi.org/10.3389/fphar.2017.00457.
[24] J.R. Thiagarajah, T. Broadbent, E. Hsieh, A.S. Verkman, Prevention of toxin-induced intestinal ion andfluid secretion by a small-molecule CFTR inhibi-tor, Gastroenterology 126 (2004) 511e519, http://dx.doi.org/10.1053/ j.gastro.2003.11.005.
[25] L. Tradtrantip, E.A. Ko, A.S. Verkman, Antidiarrheal efficacy and cellular mechanisms of a Thai herbal remedy, PLoS Negl. Trop. Dis. 8 (2014) e2674,
http://dx.doi.org/10.1371/journal.pntd.0002674.
[26] Q. Gu, B. Wang, X.F. Zhang, Y.P. Ma, J.D. Liu, X.Z. Wang, Contribution of hydrogen sulfide and nitric oxide to exercise-induced attenuation of aortic remodeling and improvement of endothelial function in spontaneously hy-pertensive rats, Mol. Cell. Biochem. 375 (2013) 199e206,http://dx.doi.org/ 10.1007/s11010-012-1542-1.
[27] S.N. Quinn, S.H. Graves, C.D. McGahee, E.M. Friedman, H. Hassan, P. Witkowski, M.E. Sabbatini, Adenylyl cyclase 3/adenylyl cyclase-associated protein 1 (CAP1) complex mediates the anti-migratory effect of forskolin in pancreatic cancer cells, Mol. Carcinog. 56 (2016) 1344e1360, http:// dx.doi.org/10.1002/mc.22598.
[28] J. Walker, H.B. Jijon, T. Churchill, M. Kulka, K.L. Madsen, Activation of AMP-activated protein kinase reduces cAMP-mediated epithelial chloride secre-tion, Am. J. Physiol. Gastrointest. Liver Physiol. 285 (2003) G850eG860,http:// dx.doi.org/10.1152/ajpgi.00077.2003.
[29] C. Szabo, Hydrogen sulphide and its therapeutic potential, Nat. Rev. Drug Discov. 6 (2007) 917e935,http://dx.doi.org/10.1038/nrd2425.
[30] M. Field, Intestinal ion transport and the pathophysiology of diarrhea, J. Clin. Invest. 111 (2003) 931e943,http://dx.doi.org/10.1172/JCI200318326.
[31] J.R. Thiagarajah, A.S. Verkman, CFTR inhibitors for treating diarrheal disease, Clin. Pharmacol. Ther. 92 (2012) 287e290, http://dx.doi.org/10.1038/ clpt.2012.114.
[32] E. Pouokam, J. Steidle, M. Diener, Regulation of colonic ion transport by gasotransmitters, Biol. Pharm. Bull. 34 (2011) 789e793, http://dx.doi.org/
10.1248/bpb.34.789.
[33] R. Schicho, D. Krueger, F. Zeller, C.W.H.V. Weyhern, T. Frieling, H. Kimura, I. Ishii, R. De Giorgio, B. Campi, M. Schemann, Hydrogen sulfide is a novel prosecretory neuromodulator in the Guinea-pig and human colon, Gastro-enterology 131 (2006) 1542e1552, http://dx.doi.org/10.1053/ j.gastro.2006.08.035.
[34] D. Krueger, M. Foerster, K. Mueller, F. Zeller, J. Slotta-Huspenina, J. Donovan, D. Grundy, M. Schemann, Signaling mechanisms involved in the intestinal prosecretory actions of hydrogen sulphide, Neurogastroenterol. Motil. 22 (2010) 1224e1231,http://dx.doi.org/10.1111/j.1365-2982.2010.01571.x.
[35] B. Hennig, M. Diener, Actions of hydrogen sulphide on ion transport across rat distal colon, Br. J. Pharmacol. 158 (2009) 1263e1275, http://dx.doi.org/ 10.1111/j.1476-5381.2009.00385.x.
[36] E. Pouokam, M. Diener, Modulation of ion transport across rat distal colon by cysteine, Front. Physiol. 3 (2012) 43, http://dx.doi.org/10.3389/ fphys.2012.00043.
[37] E. Pouokam, M. Althaus, Epithelial electrolyte transport physiology and the gasotransmitter hydrogen sulfide, Oxid. Med. Cell. Longev. 2016 (2016) 4723416,http://dx.doi.org/10.1155/2016/4723416.
[38] Althaus M, Gasotransmitters: novel regulators of epithelial Na (þ) transport?
Front. Physiol. 3 (2012) 83,http://dx.doi.org/10.3389/fphys.2012.00083. [39] B. Olas, Hydrogen sulfide in signaling pathways, Clin. Chim. Acta 439 (2015)
212e218,http://dx.doi.org/10.1016/j.cca.2014.10.037.
[40] M.V. Chan, J.L. Wallace, Hydrogen sulfide-based therapeutics and gastroin-testinal diseases: translating physiology to treatments, Am. J. Physiol. Gas-trointest. Liver Physiol. 305 (2013) G467eG473, http://dx.doi.org/10.1152/ ajpgi.00169.
[41] J.L. Wallace, M. Dicay, W. McKnight, G.R. Martin, Hydrogen sulfide enhances ulcer healing in rats, Faseb J. 21 (2007) 4070e4076,http://dx.doi.org/10.1096/ fj.07-8669.
[42] K.L. Flannigan, J.G. Ferraz, R. Wang, J.L. Wallace, Enhanced synthesis and diminished degradation of hydrogen sulfide in experimental colitis: a site-specific, pro-resolution mechanism, PLoS One 8 (2013) e71962, http:// dx.doi.org/10.1371/journal.pone.0071962.
[43] J.V. Medeiros, P.M. Soares, G.A.C. Brito, M.H. Souza, Immunohistochemical approach reveals localization of cystathionine-g-lyase and cystathionine-b -synthetase in ethanol-induced gastric mucosa damage in mice, Arq. Gastro-enterol. 50 (2013) 157e160, http://dx.doi.org/10.1590/S0004-28032013000200027.
[44] Y. Kimura, Y. Goto, H. Kimura, Hydrogen sulfide increases glutathione pro-duction and suppresses oxidative stress in mitochondria, Antioxid. Redox Signal 12 (2010) 1e13,http://dx.doi.org/10.1089/ars.2008.2282.
[45] Y. Yuan, J. Zheng, T. Zhao, X. Tang, N. Hu, Hydrogen sulfide alleviates uranium-induced acute hepatotoxicity in rats: role of antioxidant and antiapoptotic signalling, Environ. Toxicol. 32 (2016) 581e593, http://dx.doi.org/10.1002/ tox.22261.
[46] X. Liu, Z. Fu, Y. Wu, X. Hu Jr., T. Zhu Jr., C. Jin Jr., Neuroprotective effect of hydrogen sulfide on acute cauda equina injury in rats, Spine J. 16 (2016) 402e407,http://dx.doi.org/10.1016/j.spinee.2015.10.046.
[47] P. Pongkorpsakol, P. Wongkrasant, S. Kumpun, V. Chatsudthipong, C. Muanprasat, Inhibition of intestinal chloride secretion by piperine as a cellular basis for the anti-secretory effect of black peppers, Pharmacol. Res. 100 (2015) 271e280,http://dx.doi.org/10.1016/j.phrs.2015.08.012.
[48] L. Tradtrantip, B. Yangthara, P. Padmawar, C. Morrison, A.S. Verkman, Thio-phenecarboxylate suppressor of cyclic nucleotides discovered in a small-molecule screen blocks toxin-induced intestinalfluid secretion, Mol. Phar-macol. 75 (2009) 134e142,http://dx.doi.org/10.1124/mol.108.050567.
[49] J.J. Lim, Y.H. Liu, E.S. Khin, J.S. Bian, Vasoconstrictive effect of hydrogen sulfide involves downregulation of cAMP in vascular smooth muscle cells, Am. J. Physiol. Cell. Physiol. 295 (2008) C1261eC1270,http://dx.doi.org/10.1152/ ajpcell.00195.2008.
[50] H.Y. Yang, Z.Y. Wu, M. Wood, M. Whiteman, J.S. Bian, Hydrogen sulfide at-tenuates opioid dependence by suppression of adenylate cyclase/cAMP pathway, Antioxid. Redox Signal 20 (2014) 31e41,http://dx.doi.org/10.1089/ ars.2012.5119.
[51] B.V. Nagpure, J.S. Bian, Hydrogen sulfide inhibits A2A adenosine receptor agonist inducedb-amyloid production in SH-SY5Y neuroblastoma cells via a cAMP dependent pathway, PLoS One 9 (2014) e88508, http://dx.doi.org/ 10.1371/journal.pone.0088508.
[52] C. Muanprasat, P. Wongkrasant, S. Satitsri, A. Moonwiriyakit, P. Pongkorpsakol, T. Mattaveewong, R. Pichyangkura, V. Chatsudthipong, Activation of AMPK by chitosan oligosaccharide in intestinal epithelial cells: mechanism of action and potential applications in intestinal disorders, Bio-chem. Pharmacol. 96 (2015) 225e236, http://dx.doi.org/10.1016/ j.bcp.2015.05.016.
[53] P. Manna, S.K. Jain, L-cysteine and hydrogen sulfide increase PIP3 and AMPK/ PPARgexpression and decrease ROS and vascular inflammation markers in high glucose treated human U937 monocytes, J. Cell. Biochem. 114 (2013) 2334e2345,http://dx.doi.org/10.1002/jcb.24578.
[54] X. Zhou, Y. Cao, G. Ao, L. Hu, H. Liu, J. Wu, X. Wang, M. Jin, S. Zheng, X. Zhen, N.J. Alkayed, J. Jia, J. Cheng, CaMKKb-dependent activation of AMP-activated protein kinase is critical to suppressive effects of hydrogen sulfide on neu-roinflammation, Antioxid. Redox Signal 21 (2014) 1741e1758, http:// dx.doi.org/10.1089/ars.2013.5587.
Hydrogen sulfide protects against myocardial ischemia and reperfusion injury by activating AMP-activated protein kinase to restore autophagicflux, Bio-chem. Biophys. Res. Commun. 458 (2015) 632e638, http://dx.doi.org/ 10.1016/j.bbrc.2015.02.017.
[56] M. Jodal, S. Holmgren, O. Lundgren, A. Sjoqvist, Involvement of the myenteric€
plexus in the cholera toxin-induced netfluid secretion in the rat small in-testine, Gastroenterology 105 (1993) 1286e1293,http://dx.doi.org/10.1016/ 0016-5085(93)90130-5.
[57] R.M. Gwynne, M. Ellis, H. Sj€ovall, J.C. Bornstein, Cholera toxin induces sus-tained hyperexcitability in submucosal secretomotor neurons in Guinea pig jejunum, Gastroenterology 136 (2009) 299e308,http://dx.doi.org/10.1053/ j.gastro.2008.09.071.
[58] O. Lundgren, Enteric nerves and diarrhoea, Pharmacol. Toxicol. 90 (1) (2002) 09e120,http://dx.doi.org/10.1034/j.1600-0773.2002.900301.x.
[59] F. Qadri, R. Raqib, F. Ahmed, T. Rahman, C. Wenneras, S.K. DasIncreased, N.H. Alam, M.M. Mathan, A.M. Svennerholm, Increased levels of inflammatory
mediators in children and adults infected withVibrio choleraeO1 and O139, Clin. Diagn. Lab. Immunol. 9 (2002) 221e229, http://dx.doi.org/10.1128/ CDLI.9.2.221-229.2002.
[60] M.F. Rocha, J.E. Aguiar, J.J. Sidrim, R.B. Costa, R.F. Feitosa, R.A. Ribeiro, A.A. Lima, Role of mast cells and pro-inflammatory mediators on the intestinal secretion induced by cholera toxin, Toxicon 42 (2003) 183e189, http:// dx.doi.org/10.1016/S0041-0101(03)00131-4.
[61] S. Satitsri, P. Pongkorpsakol, P. Srimanote, V. Chatsudthipong, C. Muanprasat, Pathophysiological mechanisms of diarrhea caused by theVibrio choleraeO1 El Tor variant: an in vivo study in mice, Virulence 24 (2016) 1e17,http:// dx.doi.org/10.1080/21505594.2016.1192743.