ContentslistsavailableatScienceDirect
International
Journal
of
Biological
Macromolecules
jo u r n al hom e p ag e :w w w . e l s e v i e r . c o m / l o c a t e / i j b i o m a c
First
isolation
and
antinociceptive
activity
of
a
lipid
transfer
protein
from
noni
(
Morinda
citrifolia
)
seeds
Dyély
C.O.
Campos
a,
Andrea
S.
Costa
a,
Amanda
D.R.
Lima
a,
Fredy
D.A.
Silva
a,
Marina
D.P.
Lobo
a,
Ana
Cristina
O.
Monteiro-Moreira
b,
Renato
A.
Moreira
b,
Luzia
K.A.M.
Leal
c,
Diogo
Miron
c,
Ilka
M.
Vasconcelos
a,
Hermógenes
D.
Oliveira
a,∗aDepartmentofBiochemistryandMolecularBiology,FederalUniversityofCeará,CampusdoPiciProf.PriscoBezerra,60440-900Fortaleza,CE,Brazil bNUBEX—NúcleodeBiologiaExperimental,CentrodeCiênciasdaSaúde,UniversidadedeFortaleza—UNIFOR,60811-905Fortaleza,CE,Brazil cDepartmentofClinicalandToxicologicalAnalysis,FacultyofPharmacy,FederalUniversityofCeará,Fortaleza,CE,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received4November2015
Receivedinrevisedform7January2016 Accepted8January2016
Availableonline16January2016
Keywords: MorindacitrifoliaL. Rubiaceae Noni
Proteinisolation Antinociceptiveactivity Lipidtransferprotein
a
b
s
t
r
a
c
t
Inthisstudyanovelheat-stablelipid transferprotein, designatedMcLTP1,waspurifiedfromnoni (Morindacitrifolia L.) seeds,usingfour purificationstepswhichresulted inahigh-purified protein yield (72mg McLTP1 from100g of noni seeds).McLTP1 exhibited molecularmassesof 9.450and 9.466kDa,determinedbyelectrosprayionisationmassspectrometry.TheN-terminalsequenceofMcLTP1 (AVPCGQVSSALSPCMSYLTGGGDDPEARCCAGV),asanalysedbyNCBI-BLASTdatabase,revealedahigh degreeofidentitywithotherreportedplantlipidtransferproteins.Inaddition,thisproteinprovedto beresistanttopepsin,trypsinandchymotrypsindigestion.McLTP1givenintraperitoneally(1,2,4and 8mg/kg)andorally(8mg/kg)causedaninhibitionofthewrithingresponseinducedbyaceticacidin mice.Thisproteindisplayedthermostability,retaining100%ofitsantinociceptiveactivityafter30min incubationat80◦C.PretreatmentofmicewithMcLTP1(8mg/kg,i.p.andp.o.)alsodecreasedneurogenic andinflammatoryphasesofnociceptionintheformalintest.Naloxone(2mg/kg,i.p.)antagonisedthe antinociceptiveeffectofMcLTP1suggestingthattheopioidmechanismsmediatetheanalgesicproperties ofthisprotein.
©2016ElsevierB.V.Allrightsreserved.
1. Introduction
MorindacitrifoliaL.(Rubiaceae),aplantspeciesoriginatingfrom
Tropical Asia, has beenused for millennia as a sourceof food
and fabricdyes, as well asto treat disorders suchas diabetes,
rheumatoidarthritis, highblood pressure,inflammationandfor
painmanagement[1–3].Inadditiontoitsusein folkmedicine,
thereisalsoalotofevidenceofbiologicalactivityinvariousinvitro
andinvivoassaysystems[4–8].
Asaresultofvariousphytochemicalinvestigations,morethan
200secondarymetaboliteshavebeenidentifiedfromnonifruits,
roots, seeds and leaves, including anthraquinones, flavonoids,
polysaccharides, glycosides, iridoids, lignans and triterpenoids,
withthemostrepresentativebeingscopoletin,rutin,ursolicacid,
-sitosterol,asperulosideanddamnacanthal.Someofthese
com-∗Correspondingauthor.Fax:+558533669139. E-mailaddress:hermogenes@ufc.br(H.D.Oliveira).
poundshavebeensuggestedtobethesourcesofnoni’sbiological
andinvitroactivity[9–12].
Althoughnonibark,stems,leavesandfruitshave beenused
traditionallyformanydiseases,thereisalimitedamountof
infor-mationontherapeuticpropertiesof noniseeds.Air-dried seeds
constitute2.5%ofthefruit’stotalweight,howeverduringthe
pro-ductionofnonifruitpureetheyarediscarded[13].Inaddition,West
etal.[14]demonstratedthatnoniseedextractdidnotdisplayany
signoftoxicityinasubacute(28day)oraltoxicitytestin
Sprague-Dawleyrats,anddidnotexhibitgenotoxicpotentialinaprimary
DNAdamagetestinEscherichiacoliPQ37.Thus,noniseedscanbe
usedtoisolatecompoundswiththerapeuticpropertiesanda
high-addedvalue,leadingtogreatereconomicbenefitsforproducers
andanincreaseinthediversityofcompoundsderivedfromthis
species.
In contrast to their therapeutic actions, there are reports
of toxicity resulting from the consumption of noni
prod-ucts [15–18]. Secondary metabolites, such as anthraquinones,
have been suggested as the causative agents of toxicity.
1-Hydroxyanthraquinone,acomponentofnoniroots,wasshownto
http://dx.doi.org/10.1016/j.ijbiomac.2016.01.029
becarcinogenicinmaleratsandproducedadenomas,
adenocarci-nomasandbenignstomachtumoursinasmallnumberofanimals
[19].
Inthisstudywereport,forthefirsttime,theisolationand
char-acterisationofathermostablelipidtransferprotein(McLTP1)from
noniseedsthatexhibitspotentantinociceptiveactivitywhenorally
administeredtomice.McLTP1isthefirstbioactiveproteinisolated
fromthenonispeciesinrelationtothetherapeuticpropertiesof
theplant.
Plantnon-specificLTPs(nsLTPs)formaproteinfamilyofsmall
cationicpeptides ubiquitously distributed throughout theplant
kingdom[20,21].Theybelongtotheantimicrobialpeptidesgroup,
whichhasbeenincreasinglyconsideredasa sourceofpotential
therapeuticagents,exhibitingpotentialapplicationsasanalgesics,
immunomodulatorsorinthetreatmentofneurologicaldisorders
[22–25].Todate,thisisthefirstreportofalipidtransferprotein
demonstratingantinociceptiveactivityinmice.
2. Materialsandmethods
2.1. Chemicals
SephadexG-50, Superose12HR 10/30and molecularweight
markers(LMW-SDSmarkerkit)werepurchasedfromGE
Health-careLifeSciences.AgilentEclipseXBD-C18columnwaspurchased
from Agilent Technologies. Reagents and solvents for
pro-tein sequencing, acetic acid, trichloroacetic acid, indomethacin,
naloxonehydrochloride,morphinesolution(1mg/mL),diazepam
solution (1mg/mL) and formaldehyde were purchased from
Sigma–AldrichCo.,St.Louis,MO. Otherchemicalsusedwereof
analyticalgradeandobtainedfromlocalsuppliers.
2.2. Noniseeds
Noni(M.citrifoliaL.var. citrifolia)seedswerecollectedfrom
plantscultivatedattheAntonioAlbertoFarm,Ceará(3◦16′40′′S,
39◦16′08′′W) and supplied by Embrapa Agroindústria Tropical
(Embrapa—CNPAT),Ceará,Brazil.Asamplespecimen(no.44.566)
hasbeendepositedinthePriscoBezerraHerbariumoftheFederal
UniversityofCearáforfuturereference.Theseedswereground
usingakitchenblenderandtheresultingflourthoroughlydefatted
withpetroleumether(1:10,w/v)andstoredat−20◦Cuntilused.
2.3. PurificationofMcLTP1
Proteinswereextractedfromthedefattednoniseedflour(15g)
using0.050MTris–HCl/0.25MNaCl,pH8.5(1:10w/v)at4◦C.The
suspensionwasstirredfor3handthenfilteredthrough
cheese-cloth.Theresiduewasre-extractedundertheaboveconditionsfor
2handthenfiltered.Thefiltrateswerecombined,centrifugedat
10,000×g,4◦C for30minandtheclearsupernatantdesignated
ascrudeextract(CE).TheCEwassubjectedtoacidtreatmentby
theadditionoftrichloroaceticacid(TCA)toa2.5%(w/v)final
con-centration.After30minonice,the2.5%TCAsolublefractionwas
clarifiedbycentrifugationat10,000×gfor30minat4◦Candthe
supernatantdialysed(cut offMW3000) against distilled water
at4◦Candlyophilised.Thelyophilisedproteinfractionwas
dis-solved(4mg)in0.050MTris–HCl/0.15MNaCl,pH8.5andapplied
onaSephadexG-50(1.5×40cm)columnpreviouslyequilibrated
withthe same buffer. Fractions (2mL) from the protein peaks
weremonitoredat280nmandcollectedataconstantflowrateof
30mL/h.Twoproteinpeakswererecoveredusingtheequilibrium
buffer. The fractions showing antinociceptive activity (McLTP1)
wereselected,concentratedandsubjectedtoanalytical
reversed-phasehigh-performanceliquidchromatography(RP-HPLC).
2.3.1. RP-HPLC
Pooledfractionsof proteinobtainedfromgelfiltrationwere
subjectedtoreversed-phasehigh-performanceliquid
chromatog-raphy using a C18 column (Agilent Eclipse XBD-C18 column
(250×4.6mm,5m)).Proteinsweredissolvedinultrapurewater
toobtainasolutionof0.5mg/mLandwerethenfilteredthrough
a 0.22mmembrane beforebeinginjectedinto theHPLC
(vol-umeinjected:20Loffilteredproteinsample).Thecolumnwas
equilibratedwith0.02%aqueousaceticacidandtheproteinswere
elutedataflowrateof1mL/minunderisocraticmode.Elutionwas
monitoredat216nmusingadiodearraydetector(AllianceHPLC
System,Waters,Corp.,Milford,MA).Thepeakelutingat7.9min
wasvacuum-dried,dissolvedinultrapurewater andappliedon
SDS-PAGE.
2.4. Proteinquantification
Proteinconcentrations were determinedby thedye-binding
methodofBradford[26],withbovineserumalbumin(BSA)asthe
standard.
2.5. Molecularmassdetermination
ThemolecularmassofMcLTP1wasdeterminedbydenaturating
SDS-PAGEon12.5%gelsundernon-reducingconditions,
follow-ingstandardprocedures[27].Proteinbandswerevisualisedwith
CoomassieBrilliantBlueR-250(Sigma–AldrichCo.,St.Louis,MO)
foratleast2handdestained(40%methanol,50%water,10%glacial
aceticacid)for30min.
ThenativemolecularmassofMcLTP1(1mg)wasdetermined
bygelfiltrationonaSuperose12HRcolumncoupledtoanFPLC
System(GEHealthcare)and equilibratedwith0.050MTris–HCl
buffer/0.25MNaCl, pH8.5.Chromatography wascarriedout at
aconstantflow rateof0.3mL/minand1mLfractionswere
col-lected. The column was previously calibrated with proteins of
known molecular masses (BSA, 66kDa; egg albumin, 45kDa;
chymotrypsinogen,25kDa;ribonuclease,13.7kDa,andaprotinin,
6.5kDa).
Mass spectrometry of McLTP1 (0.2mg/mL dissolved in
water/acetonitrile 1:1,v/v) was carried outon a Synapt HDMS
Acquity UPLCinstrument (Waters,Manchester, UK)by
electro-sprayionisationinpositiveionmode(ESI+)andaNanoLockSpray
source.The intact massspectra wereeffectively acquiredfrom
m/z1000to4000,whichallowedtoobtainmultiplychargedmass
ions.Themassspectrometerwasoperatedinthe“V”modewith
aresolvingpowerofatleast10,000m/z.Thedatacollectionwas
performedusingMassLynx4.1software(WatersCo.,Milford,MA,
USA)andchargedistributionspectrawerethendeconvolutedby
theMaximumEntropyTechnique(Max-Ent).
2.6. N-terminalaminoacidsequenceanalysis
The N-terminal amino acid sequence was analysed on a
Shimadzu PPSQ-23A Automated Protein Sequencer, performing
EdmandegradationofMcLTP1blottedonpolyvinylidenefluoride
membraneafterSDS-PAGE.Thesequenceobtainedwassubmitted
toautomaticalignmentusingtheNCBI-BLASTsearchsystem[28].
Theproteinsequencedatareportedinthispaperwillappearinthe
UniProtKnowledgebaseundertheaccessionnumberC0HJH5.
2.7. Invitrodigestionassay
Invitrodigestibility of McLTP1 wasdeterminedaccordingto
themethodofSathe[29],withmodifications.McLTP1(1mg/mL)
wassuspended in 200L of0.1MHCl, pH1.8, forpepsin (E.C.
0.1MTris–HCl,pH8.1fortrypsin(E.C.3.4.21.4;10,100BAEEU/mg
protein,frombovinepancreas)andkeptfor10minat37◦C.Next,
40Lofenzyme(1mg/mL)wasaddedtostarttheproteindigestion
andthereactionmixturewasincubatedinashakingwaterbathat
37◦Cfor4h.Aliquotsof25Lweretakenat0and4hand25L
of0.5MTris–HClbuffer,pH6.8,containing0.1%SDS,added.The
digestswereimmediatelyheatedinboilingwater(98◦C)for5min
andanalysedbySDS-PAGE[27].
Sequentialdigestionexperimentsusingpepsin,trypsinand
chy-motrypsin(E.C.3.4.21.1;40U/mgprotein,frombovinepancreas)
werecarriedoutusingMcLTP1 (1mg/mL)dissolvedin0.1MHCl,
pH1.8andincubatedinawaterbathat37◦Cfor5min.Analiquot
ofpepsin(enzyme:protein1:10,w/w)wasaddedandthemixture
incubatedat37◦Cfor4h.Thedigestionwasimmediatelystopped
byheatingthemixtureinaboilingwater(98◦C)bathfor5min.
Next,thepepsin-digested mixturewasmixed withtrypsinand
chymotrypsin(enzyme:protein1:10,w/w)in0.1MTris–HCl,pH
8.1,forfurtherdigestionfor5h.Thereactionwasstoppedandthe
sampleanalysedbySDS-PAGEasdescribedabove.BSA(2mg/mL)
wasusedasacontrolforthedigestionreaction.Allassayswere
performedintriplicate.
2.8. Animals
Experimentalprocedureswereconductedusinggroupsof2–3
montholdSwissmalemice(25–30g)obtainedfromBiotério
Cen-tralofFederalUniversityofCeará.Theanimalswerehousedunder
standard environmentalconditions (24±1◦C, humidity45–65%
and12hlight/darkcycle)andreceivedfoodandwateradlibitum.
Groupsof6–8micewereusedineachexperimentandhabituated
tothelaboratoryconditionsforatleast2hbeforetesting.Care,
han-dlingandexperimentalprocedureswereperformedinaccordance
withtheethicalstandardsestablishedbytheNationalGuidelines
fortheUseofExperimentalAnimalsofBrazilandbytheDirective
2010/63/EUoftheEuropeanParliamentandoftheCouncilofthe
EuropeanUnion.Theexperimentalprotocolswereapprovedbythe
CommitteefortheEthicalUseofAnimalsoftheFederalUniversity
ofCeará(CEUA-UFCno.37/13).
2.9. AntinociceptiveactivityofMcLTP1
2.9.1. Aceticacid-inducedwrithingmethod
Thistestwasperformedusingamodifiedversionofthemethod
described byKosteret al.[30].Animalsweretreated
intraperi-toneally(i.p.)withnoniseedcrudeextract(10,30or90mg/kg),2.5%
TCAsolublefraction(8mg/kg),McLTP1(1,2,4or8mg/kg),vehicle
(NaCl0.15M)orindomethacin(positivecontrol;10mg/kg)30min
beforetheinjectionofa1.0%aceticacidsolution(0.1mL/10gbody
weight,i.p.).Thenumber ofabdominal writhes(pelvicrotation
followed byfull extensionof both hind legs)wascumulatively
countedovera periodof20min,soon aftertheaceticacidwas
administered.ToevaluatewhetherMcLTP1displaysoral
antinoci-ceptive activity, it was given orally (8mg/kg body weight) to
animals30or60minpriortoanaceticacidinjection.The
antinoci-ceptiveeffectwasexpressedasapercentageoftheinhibitionof
abdominalwrithing.
2.9.1.1. Thermalstability determination. Toestablishthethermal
stabilityoftheprotein,McLTP1(1mg/mL)dissolvedinNaCl0.15M
wasincubatedinawaterbathat80◦Cfor30min.Aftercooling
thetreatedMcLTP1onicefor10min,miceweretreatedwiththis
protein(8mg/kg(i.p.)),30minbeforeaceticacidsolutioninjection,
andtheantinociceptiveactivitymeasuredasdescribedinSection
2.9.1.
2.9.2. Formalin-inducedpainassay
Themethodusedwassimilartothatpreviouslydescribedwith
somemodifications[31].Thirtyminutesaftertheadministrationof
McLTP1(8mg/kgp.o.ori.p.),NaCl0.15M(vehicle;negativecontrol;
p.o.ori.p.)ormorphine5mg/kgi.p.(positiveopioidcontrol),20L
of2.5%formalin(formaldehyde36.5%solution)inasalinesolution
wasinjectedintothesubplantaroftherighthindpawsofthemice.
Animalswereplacedindividuallyinatransparentcageandthetime
(inseconds)spentlickingandbitingtheinjectedpawwastakenas
anindicatorofthepainresponse.Thepainresponsewasmeasured
for5min(firstphase)and20–25min(secondphase)afterthe
for-malininjection,representingbothneurogenicandinflammatory
painresponsesrespectively.
2.9.2.1. Involvementofopioidreceptors. Toidentifywhether
opi-oidreceptorsareinvolvedintheantinociceptiveactionofMcLTP1
(8mg/kg;p.o.ori.p.),groupsofmicewerepretreatedwitha
non-selectiveopioidreceptor antagonist,naloxone(2mg/kg),30min
beforeadministrationofMcLTP1ormorphine(5mg/kg)andtested
usingtheformalintest.
2.10. EffectofMcLTP1onlocomotoractivity
TheeffectoftheMcLTP1(8mg/kg)onspontaneouslocomotor
activityandexploratorybehaviourwasassessedusingthe
open-field test, using the methodset out by Martin et al. [32]. The
apparatus(45cmwidth×45cmlength×15cmheight)consisted
ofawoodenfieldinwhichthefloorwasdividedinto36equalareas.
MiceweretreatedwithMcLTP1 orally60minbeforethe
exper-imentor withthevehicle(NaCl 0.15M) or diazepam(2mg/kg)
intraperitoneally30minbeforethe experiment. Thenumber of
areascrossedwithallpawsandthenumberofrearingresponses
werethenrecordedduringa5minperiod.
2.11. Statisticalanalysis
Intheresultsthemean±SEMarepresented.Statisticalanalysis
wasperformedusingone-wayanalysisofvariance(ANOVA)
fol-lowedbyTukey’sposthoctests,usingGraphpadPrism6.0software
(SanDiego,CA,USA).Differencesbetweengroupswereconsidered
significantatalevelofp<0.05.
3. Resultsanddiscussion
3.1. PurificationandcharacterisationofMcLTP1
M. citrifolia L. has become popular in recent years, as it is
believedtobeabletohelppreventlifestyle-relateddiseases[33].
Nevertheless,scientific evidence forthesebenefitsis limited to
studiesdemonstratingbiologicalactivitiesofsecondary
metabo-litesfromdifferentnoniparts,whilestudiesreportingtheisolation
andcharacterisationofbioactiveproteinsfromthisspecieshave
beenlacking.
Whenadministeredintraperitoneallytomiceat dosesof10,
30 and 90mg/kg, noni seedcrude extract significantly reduced
(p<0.05)thenoxiousstimulusinducedbyaceticacid,ina
dose-dependent manner, by 23.2–41.8% compared with the control
group(Table1).Theantinociceptiveactivityofthenoniseedextract
atalldosestestedwasnotalteredafterdialysis(cutoffMW3000),
suggestingthattheeffectobservedwasrelatedtoaproteic
compo-nent.Therefore,weembarkeduponaguidedfractionationofthe
noniseedextract,seekingthisantinociceptiveagentusinganacetic
acid-inducedwrithingtestinmice.
The antinociceptive activity was also detected in the 2.5%
TCA soluble fraction after administration at a doseof 8mg/kg
Table1
SummaryofpurificationprocedureofMcLTP1fromnoniseedsguidedbyanaceticacid-inducedwrithingtestinmice.
Purificationstep Protein(mg) Yielda(%) Doseb(mg/kg) %inhibitionc Specificbiologicalactivityd Purificationindexe Crude
extract
189.04
±3.23
100 10 23.25±0.03 – –
30 37.20±0.02* – –
90 41.86±0.19* 0.46 1
2.5%TCAsolublefraction 12.80±0.95 6.77 8 81.39±0.08** 10.17 22.10
SephadexG-50column(McLTP1) 11.58±0.87 6.12 8 96.13±0.08** 12.01 26.10
aYield=(totalproteinfromthefraction/totalproteinfromthecrudeextract)
×100%.
b Micewerepretreatedintraperitoneally30minbeforeanaceticacidinjection.
c Inhibitionofaceticacid-inducedabdominalconstrictioninmice.Theresultsshowthemean
±SD(n=8).
d Specificbiologicalactivityiscalculatedbydividing“%ofmaximuminhibitoryactivity/dose(mg/kg)”ofeachstepofpurification.
ePurificationindexiscalculatedastheratiobetweenthespecificbiologicalactivityobtainedateachpurificationstepandthatofthecrudeextracttakenas1.0. * p<0.05.
** p<0.01comparedtocontrolgrouppretreatedwithNaCl0.15Mi.p.(ANOVAfollowedbyTukey’stest).
Fig.1. PurificationofMcLTP1.(A)Separationprofileof2.5%TCAsolublefractionfromnoniseedextractonaSephadexG-50column.(B)RP-HPLCchromatography.Pooled
fractionsobtainedfromgelfiltrationwereappliedtoaC18reverse-phasecolumnandruninaWatersCorp.apparatus.Elutionofproteinswasmonitoredbymeasurement oftheabsorbanceat280(A)and216nm(B).InsertCoomassie-stainedSDS-PAGE(12.5%)containing(left–right)molecularweightmarkers(lane1;14.4–97kDa),proteins extractedfromnoniseeds(lane2)and(lane3)McLTP1.
toaSephadexG-50column.AsdepictedinFig.1A,twoprotein
peakswererecoveredusingtheequilibriumbuffer.Afterbeing
col-lected,concentratedanddialysedthetopmostpartofpeak2,which
exhibitedantinociceptiveactivityinmice,thisproteinfractionwas
submittedtoanalyticalRP-HPLCusingaC18column,resultingin
onewelldefinedpeakelutedat7.9minunderisocraticconditions
(Fig.1B).Thepurifiedproteinwasshownasasinglebandon
SDS-PAGE,withanapparentmolecularmassof15.13kDaandayieldof
Fig.2.ESI-MSanalysisofnativeMcLTP1.
Nativemolecularmassoftheisolatedproteinwasalsoassessed
bygelfiltrationchromatography.Thepurifiedproteinwas
chro-matographedona Superose 12HR, showinga single peak with
a molecular mass of 10.09kDa (data not presented), which is
in agreementwiththemolecularmasses obtainedbyESI mass
spectrometryundernativeconditions,whichshowedtwopeaks
correspondingtoMWof9450.30and9466.20(Fig.2).
Thefirst33aminoacidsoftheN-terminalsequenceofthe
puri-fiedproteinshowedsignificantsimilaritiestoknownlipidtransfer
proteins(LTPs)isolatedfromvariousplants,includingfourofthe
eightcysteineresiduesconservedinotherLTPs(Table2).LTPsare
small,water-soluble,basicproteins,classifiedintotwo
subfami-liesaccordingtotheirmolecularmasses:LTP1s(9kDa)andLTP2s
(7kDa)[34].ThemolecularmassdeterminedbytheESI-MSofthe
proteinisolatedinthisstudyallowedittobeclassifiedasamember
oftheLTP1subfamily(designatedasMcLTP1).
SincethediscoveryofplantLTPsinthe1970s,anincreasing
numberoflipidtransferproteinshavebeenisolated,particularly
fromseeds[35–37].However,untilnowtherehasbeenno
infor-mationregarding theirpresence inM.citrifolia L.seeds. Zottich
etal.[38]reportedtheisolationofaLTP1fromCoffeacanephora
(Cc-LTP1—Rubiaceae)seeds,with␣-amylaseinhibitorand
antimi-crobialproperties.McLTP1,whichwasalsoisolatedfromaplant
speciesbelongingtoRubiaceaefamily,showed57%identitywith
Cc-LTP1.
The presence of two peaks withm/z values of 9450.30 and
9466.20 suggests the existence of isoforms of McLTP1. Other
authors have reported that LTPs are found in severalisoforms
indistinct planttissues andorgans coded bya multigene
fam-ily, as demonstrated by Arabidopsis thaliana and Oryza sativa
[20,34,39,40].
AlthoughthemolecularmassofMcLTP1,determinedbyESI-MS,
isconsistentwiththoseobservedforotherLTP1s,themolecular
masscalculatedusingSDS-PAGEundernon-reducingconditions
wasestimatedtobe15.13kDa.AccordingtoGorjanovicetal.[41]
LTPscanformdimersinplanttissuesandalsoundernon-reducing
Fig.3.SDS-PAGE(12.5%)profileoftheinvitrodigestibilityofMcLTP1bypepsin
(laneP),trypsin(laneT)andaftersequentialdigestionwiththecitedenzymesand chymotrypsin(laneS).BSA(lanes1and2,left–right)wasusedasacontrol.Lane 3representsMcLTP1(20g).ProteinbandswerestainedwithCoomassieBrilliant
BlueR-250.
SDS-PAGE,asdemonstratedfortheLTP1isolatedfromHordeum
vulgarewhichshowedanapparentmolecularmassof16kDawhen
calculatedusingSDS-PAGE.
Duetotheircompactstructures,whicharestabilisedbyfour
disulfidebridges,LTPstendtobeextremelystableandresistantto
thermaldenaturationanddigestionbyproteases[42–45].Inthis
study,McLTP1wassubjectedtoasimulatedgastricandintestinal
Table2
AlignmentoftheN-terminalaminoacidsequenceofMcLTP1withothernsLTPsfromplants.ProteinsequenceswerealignedwiththeBLASTalgorithm.Conservedcystein
residuesthatformdissulfidebridgesinnsLTPsarehighlightedingray.
NsLTPsource Plantfamily Residueno. Sequence Residueno. %identity SequenceID
Morindacitrifolia Rubiaceae 1 AVPCGQVSSALSPCMSYLTGGGDDPEARCCAGV 33 100 C0HJH5
Triticumaestivum Poaceae 26 AVSCGQVSSALSPCISYARGNGANPSAACCSGV 58 70 ABF14722.1
Aegilopstauschii Poaceae 26 AISCGQVSSALSPCISYARGNGANPTAACCSGV 58 67 EMT04675.1
Vitisvinifera Vitaceae 25 AVTCGQVETSLAPCMPYLTGGG-NPAAPCCNGV 56 67 XP002282792.1
Vignaradiata Fabaceae 25 AITCGQVASSLAPCISYLQKGG-VPSASCCSGV 56 61 CAQ86909.1
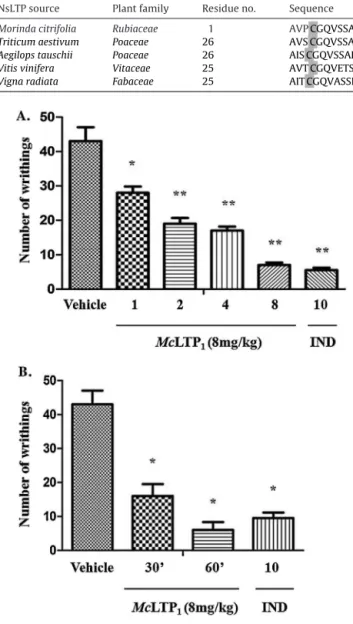
Fig.4. (A)EffectsofMcLTP1onaceticacid-inducedwrithingresponseinmice.
Vehi-cle(NaCl0.15M),McLTP1(1,2,4and8mg/kg),indomethacin(IND,10mg/kg)were
administeredi.p.30minbeforeanaceticacidinjection.(B)Antinociceptiveactivity ofMcLTP1(8mg/kg)afteroraladministrationinmice.Animalswerepretreated30
or60minbeforeanaceticacidinjection.Vehicle(NaCl0.15M)andindomethacin (IND,10mg/kg)wereorallyadministered30minbeforeanaceticacidinjection. Theresultsshowthemean±SD(n=8).Differencesbetweenthegroupswere deter-minedbyanANOVAfollowedbyTukey’stest,*p<0.05,**p<0.01whencompared tothecontrolgroup.
ofMcLTP1individuallydigestedwithpepsinandtrypsinandafter
sequentialdigestionwiththeaboveenzymesandchymotrypsin.
ThebandofMcLTP1 wasstableduringincubationwithpepsinor
trypsinfor4handwasalsodetectedevenafter9hofsequential
digestion.
3.2. AntinociceptiveactivityofMcLTP1
Whenadministeredtomiceintraperitoneally30minpriorto
acetic acid injection, McLTP1 (1, 2, 4, or 8mg/kg) significantly
reducedthenumberofabdominalconstrictionsobserved(p<0.05).
ThewrithingresponseinhibitionofMcLTP1wasdose-dependent
andatadoseof8mg/kgwascomparabletothatofindomethacin
10mg/kgi.p.(96.1%and98.3%inhibition ofabdominal
constric-tionreflexrespectively, Fig.4A).The antinociceptive activityof
Fig.5. EvaluationofthermalstabilityofthepurifiedMcLTP1usinganaceticacid
inducedwrithingresponseinmice.TheantinociceptiveeffectofMcLTP1(8mg/kg; i.p.)wasmeasuredafter0and30minpre-incubationat80◦C.Vehicle(NaCl0.15M)
andindomethacin(IND,10mg/kg)wereadministeredi.p.30minbeforeanacid aceticacidinjection.Theresultsshowthemean±SD(n=8).Differencesbetween thegroupsweredeterminedbyanANOVAfollowedbyTukey’stest,*p<0.05when comparedtothecontrolgroup.
McLTP1 was also observed after oral administration (p.o.)at a
doseof8mg/kg30(62.8%inhibition)or60min(86%inhibition)
priortotheaceticacidadministration.Theeffectobservedwhen
theprotein wasadministered60minprior toacetic acid
injec-tionwasnotsignificantlydifferentfromtheresponseobservedto
indomethacin10mg/kgp.o.(88%inhibition)(Fig.4B).Likeother
LTPs,suchasLJAMP2isolatedfromLeonurusjaponicusHoutt[46].
McLTP1displayedthermostability,retaining100%ofits
antinoci-ceptiveactivityafter1hincubationat80◦C(Fig.5).
Basedontheirinvitroactivities,numerousstudiesoverthelast
decadeshavelinkedplantLTPswitha myriadofbiological
pro-cesses,includingformationofthecuticlelayer[47],plantresponses
toabioticandbioticstresses[48–50]andasmodulatorsofplant
growthanddevelopment[51,52].However,thisisthefirstreport
ofalipidtransferproteinactingasamodulatorofthenociceptive
response.
Theacetic acid-inducedwrithingresponse isa sensitivetest
used for screening analgesic activity, regardless of the central
orperipheralcauses.Aceticacidisanirritantwhich causesthe
synthesis and release of pro-inflammatory mediators such as
prostaglandins(PGEandPGF2␣)andsympatheticnervoussystem
mediators that provoke pain nerve endings [53–55].
Intraperi-tonealororaltreatmentwithMcLTP1causedsignificantreduction
in the abdominal constriction produced by acetic acid,
indi-cating peripheral antinociception. Taissin-Moindrot et al. [56]
demonstratedthatplantLTPsareabletoaccommodatenotonly
phosholipids or fatty acids, but also a variety of hydrophobic
molecules,suchasPGB2,withoutmajorstructuralmodifications.
Thus, itcouldbeassumed that this proteininterfered withthe
blockade ortherelease of peripherallyacting endogenous
sub-stances,suchasprostaglandins,responsibleforpainfulsensations.
InordertofurtherclarifytheantinociceptiveeffectofMcLTP1,
Fig.6.Timespentonpawlickingafteroralorintraperitonealpretreatmentwith McLTP1(8mg/kg)onbothfirst(A)(0–5min)andsecond(B)(15–30min)phasesof theformalininducednociception.Dataareshownasthemean±SD(n=8). Differ-encesbetweenthegroupsweredeterminedbyanANOVAfollowedbyTukey’stest, *p<0.05vs.controlanimals.
(8mg/kg)administeredorallyorintraperitoneallycauseda
signifi-cant(p<0.05)inhibitoryeffectonbothphasesofformalininduced
pain,comparedtothecontrolcondition,withameanof51.1%(i.p.)
and32.7%(v.o.)intheearlyphaseand77.9%(i.p.)and65.1%(v.o.)
inthelatephase.Thepositivecontroldrug,morphine(5mg/kg),
significantlyattenuatedthepainresponsesofthetwophases.
Table3
EffectofMcLTP1onthetotalnumberofcrossedlinesandnumberofrearingsduring
5minofexposuretotheopen-fieldtest.Eachvaluerepresentsthemean±SEM
obtainedfrom8mice.StatisticaldifferencesweredeterminedbyANOVAfollowed byTukey’stest.
Groupa Dose(mg/kg) Crossedlines/5min Numberofrearings
Vehicle – 48.3±1.05 12.8±1.02
McLTP1 8mg/kg 39.2±2.32 12.3±1.56
Diazepam 2mg/kg 10.4±0.08** 2.01±0.04**
aMiceweretreatedwithMcLTP
1orally60minbeforetheexperiment.Vehicle
(NaCl0.15M)anddiazepam(2mg/kg)wereadministeredintraperitoneally30min beforetheexperiment.
**p<0.01(comparedwithsalinegroup).
Thefirstphaseoftheformalintest(0–5min)ischaracterised
by neurogenic pain caused by a direct stimulation of
nocicep-tors. Substance P and bradykinin are thoughtto participate in
thisphase[31].Thesecondphase(15–30min)ischaracterisedby
inflammatorypain,aprocessinwhichseveralinflammatory
medi-atorsarebelievedtobeinvolved,includinghistamine,serotonin,
prostaglandinsandbradykinin[31,57].Ingeneral,centrallyacting
drugsinhibitbothphasesequally,whileperipherallyactingdrugs
inhibitmainlythesecondphase[58].AsshowninFig.6,McLTP1
suppressedpainduringbothphases,indicatingthatthisprotein
hascentralandperipheralanalgesicproperties.
Pretreatment of animals with naloxone (2mg/kg), a
non-selective opioid receptor antagonist, partially reversed the
antinociceptiveactivityofMcLTP1,administeredorallyor
intraperi-toneally, suggestingthe involvementof theopioid routein the
observed activity. Opiates are generally believed to produce
antinociception exclusively via central mechanisms, but some
studiesindicatethat theymayalsoproduceantihyperalgesiain
peripheralsites.Thelater effectsaremediatedbyopioid
recep-torsonperipheralterminalsofsensoryneurons[59]and inthe
gastrointestinaltract[60].Thus,itispossiblethatMcLTP1bindsto
thesegastrointestinalsiteswhenadministeredorally,producing
theantinociceptiveeffect.Similarresultswerefoundforalectin
purifiedfromCanavaliabrasiliensisseeds[61].
Amajor concernin theevaluationof theanalgesicactionof
compoundsis whetherpharmacologicaltreatmentcausesother
behaviouralalterations,suchasimpairmentofmotorcoordination
or sedation, which might be misinterpretedas analgesic
activ-ity.Abehaviouralassessmentwascarriedouttodetermineifthe
antinociceptiveeffectsofMcLTP1werecausedbyanydisturbances
onthecentralnervoussystem.Locomotoractivitywasnotaffected
byMcLTP1,suggestingthattheantinociceptiveresponseobserved
washighlyselective(Table3).
4. Conclusion
In this study we purified, characterised and evaluated the
antinociceptive effect of McLTP1, a lipid transfer protein. It is
describedfor thefirst timeasapain modulator,amplifyingthe
rangeofbiologicalactivitiesdisplayedbythisgroupofmolecules
and alsoprovidingevidence ofitspotentialas asourceof new
painreliefdrugs.McLTP1demonstratedperipheralantinociceptive
activitythatwascomplementedbyitscentraleffect,dependenton
opioidergicinvolvement.Thisisalsothefirstreportoftheisolation
ofaproteinfromthenonispeciesassociatedwiththetherapeutic
propertiesoftheplant.
Conflictofinterest
Theauthorshavedeclaredthatthereisnoconflictofinterest,
includingfinancial,personaloranyotherrelationshipswithother
Acknowledgements
ThisworkwassupportedbyConselhoNacionalde
Desenvolvi-mentoCientíficoeTecnológico(CNPq)andFundac¸ãoCearensede
ApoioaoDesenvolvimentoCientíficoeTecnológico(FUNCAP).
References
[1]Y.Chan-Blanco,F.Vaillant,A.M.Perez,M.Reynes,J.M.Brillouet,P.Brat,The nonifruit(MorindacitrifoliaL.):areviewofagriculturalresearch:nutritional andtherapeuticproperties,J.FoodCompos.Anal.19(2006)645–654.
[2]A.R.Dixon,H.McMillen,N.L.Etkin,Fermentthis:thetransformationofNonia traditionalpolynesianmedicine(Morindacitrifolia,Rubiaceae),Econ.Bot.53 (1999)51–68.
[3]D.P.Sreekeesoon,M.F.Mahomoodally,Ethnopharmacologicalanalysisof medicinalplantsandanimalsusedinthetreatmentandmanagementofpain inMauritius,J.Ethnopharmacol.157(2014)181–200.
[4]R.AbouAssi,Y.Darwis,I.M.Abdulbaqi,A.A.Khan,L.Vuanghao,M.H.Laghari,
Morindacitrifolia(Noni):acomprehensivereviewonitsindustrialuses, pharmacologicalactivities,andclinicaltrials,Arab.J.Chem.(2015)(available online24.06.15).
[5]E.Dussossoy,P.Brat,E.Bony,F.Boudard,P.Poucheret,C.Mertz,J.Giaimis,A. Michel,Characterization:anti-oxidativeandanti-inflammatoryeffectsof CostaRicannonijuice(MorindacitrifoliaL.),J.Ethnopharmacol.133(2011) 108–115.
[6]S.Mahattanadul,W.Ridtitid,S.Nima,N.Phdoongsombut,P.Ratanasuwon,S. Kasiwong,EffectsofMorindacitrifoliaaqueousfruitextractanditsbiomarker scopoletinonrefluxesophagitisandgastriculcerinrats,J.Ethnopharmacol. 134(2011)243–250.
[7]S.Nima,S.Kasiwong,W.Ridtitid,N.Thaenmanee,S.Mahattanadul, GastrokineticactivityofMorindacitrifoliaaqueousfruitextractandits possiblemechanismofactioninhumanandratmodels,J.Ethnopharmacol. 142(2012)354–361.
[8]B.S.Siddiqui,F.A.Sattar,S.Begum,A.Dar,M.Nadeem,A.H.Gilani,S.R. Mandukhail,A.Ahmad,S.Tauseef,Anoteonanti-leishmanial,spasmolytic andspasmogenicantioxidantandantimicrobialactivitiesoffruits,leavesand
stemofMorindacitrifoliaLinn—animportantmedicinalandfoodsupplement
plant,Med.Aromat.Plants.3(2014),2167–0412.
[9]A.D.Pawlus,B.N.Su,W.J.Keller,A.D.Kinghorn,Ananthraquinonewithpotent quinonereductase-inducingactivityandotherconstituentsofthefruitsof
Morindacitrifolia(noni),J.Nat.Prod.68(2005)1720–1722.
[10]O.Potterat,M.Hamburger,Morindacitrifolia(Noni)fruit—phytochemistry pharmacologysafety,PlantaMed.73(2007)191–199.
[11]M.Wang,B.J.West,C.J.Jensen,D.Nowicki,C.Su,A.Palu,G.Anderson,Morinda citrifolia(Noni):aliteraturereviewandrecentadvancesinnoniresearch,Acta Pharmacol.Sin.23(2002)1127–1141.
[12]X.L.Yang,M.Y.Jiang,K.L.Hsieh,J.K.Liu,Chemicalconstituentsfromtheseeds ofMorindacitrifolia,Chin.J.Nat.Med.7(2009)119–122.
[13]S.Nelson,Noniseedhandlingandseedlingproduction.Fruitsandnuts-10. CooperativeExtensionService,CTAHR,UniversityofHawai’iatM¨anoa. [Internetdocument]<http://www.ctahr.hawaii.edu/noni/downloads/FN10. pdf>,2005(accessed13.03.15).
[14]B.J.West,C.J.Jensen,A.K.Palu,S.Deng,Toxicityandantioxidanttestsof
Morindacitrifolia(noni)seedextract,Adv.J.FoodSci.Technol.3(2011) 303–307.
[15]M.E.Carr,J.Klotz,M.Bergeron,Coumadinresistanceandthevitamin supplementNoni,Am.J.Hematol.77(2004)103.
[16]N.F.Q.Marques,A.P.B.M.Marques,A.L.Iwano,M.Golin,R.R.De-Carvalho,F.J.R. Paumgartten,P.R.Dalsenter,DelayedossificationinWistarratsinducedby
MorindacitrifoliaL.exposureduringpregnancy,J.Ethnopharmacol.128 (2010)85–91.
[17]G.Millonig,S.Stadlmann,W.Vogel,Herbalhepatotoxicity:acutehepatitis causedbyanonipreparation(Morindacitrifolia),Eur.J.Gastroenterol. Hepatol.17(2005)445–447.
[18]V.Stadlbauer,P.Fickert,C.Lackner,J.Schmerlaib,P.Krisper,M.Trauner,R.E. Stauber,Hepatotoxicityofnonijuice:reportoftwocases,WorldJ. Gastroenterol.11(2005)4758–4760.
[19]H.Mori,N.Yoshimi,H.Iwata,Y.Mori,A.Hara,T.Tanaka,K.Kawai, Carcinogenicityofnaturallyoccurring1-hydroxyanthraquinoneinrats: inductionoflargebowel,liverandstomachneoplasms,Carcinogenesis11 (1990)799–802.
[20]A.deOliveiraCarvalho,V.M.Gomes,Roleofplantlipidtransferproteinsin plantcellphysiology—aconcisereview,Peptides28(2007)1144–1153.
[21]M.Morales,M.Á.López-Matas,R.Moya,J.Carnés,Cross-reactivityamong non-specificlipid-transferproteinsfromfoodandpollenallergenicsources, FoodChem.165(2014)397–402.
[22]A.Alba,C.Lopez-Abarrategui,A.J.Otero-Gonzalez,Hostdefensepeptides:an alternativeasantiinfectiveandimmunomodulatorytherapeutics, Biopolymers98(2012)251–267.
[23]E.S.Candido,M.H.S.Cardoso,D.A.Sousa,J.C.Viana,N.G.Oliveira-Júnior,V. Miranda,O.L.Franco,Theuseofversatileplantantimicrobialpeptidesin agribusinessandhumanhealth,Peptides55(2014)65–78.
[24]C.I.Schroeder,K.J.Nielsen,D.A.Adams,M.Loughnan,L.Thomas,P.F.Alewood, R.J.Lewis,D.J.Craik,EffectsofLys2toAla2substitutionsonthestructureand potencyofomega-conotoxinsMVIIAandCVID,Pept.Sci.98(2012)345–356.
[25]E.F.Schwartz,C.B.F.Mourão,K.G.Moreira,T.S.Camargos,M.R.Mortari, Arthropodvenoms:avastarsenalofinsecticidalneuropeptides,Biopolymers 98(2012)385–405.
[26]M.M.Bradford,Arapidandsensitivemethodforthequantitationof microgramquantitiesofproteinutilizingtheprincipleofprotein-dyebinding, Anal.Biochem.72(1976)248–254.
[27]U.K.Laemmli,Cleavageofstructuralproteinsduringtheassemblyofthe bacteriophageT4,Nature227(1970)680–685.
[28]S.F.Altschul,W.Gish,W.Miller,E.W.Myers,D.J.Lipman,Basiclocal alignmentsearchtool,J.Mol.Biol.215(1990)403–410.
[29]S.K.Sathe,Solubilization,electrophoreticcharacterizationandinvitro digestibilityofalmond(Prunusamygdalus)proteins,J.FoodBiochem.16 (1992)249–264.
[30]R.Koster,M.Anderson,J.DeBeer,Aceticacidforanalgesicscreening,Fed. Proc.18(1959)412–417.
[31]S.Hunskaar,K.Hole,Theformalintestinmice:dissociationbetween inflammatoryandnon-inflammatorypain,Pain30(1987)103–114.
[32]J.R.Martin,K.Baettig,J.Bircher,Mazepatrolling:open-fieldbehaviorand runwayactivityfollowingexperimentalportacavalanastomosisinrats, Physiol.Behav.25(1980)713–719.
[33]L.Lv,H.Chen,C.T.Ho,S.Sang,ChemicalcomponentsoftherootsofNoni (Morindacitrifolia)andtheircytotoxiceffects,Fitoterapia82(2011) 704–708.
[34]J.C.Kader,Lipid-transferproteinsinplants,Annu.Rev.PlantPhysiol.Plant Mol.Biol.47(1996)627–654.
[35]S.Tsuboi,T.Osafune,R.Tsugeki,M.Nishimura,M.Yamada,Nonspecificlipid transferproteinincastorbeancotyledoncells:subcellularlocalizationanda possibleroleinlipidmetabolism,J.Biochem.111(1992)500–508.
[36]A.Carvalho,C.E.Teodoro,M.Cunha,A.L.Okorokova-Fac¸anha,L.A.Okorokov, K.V.S.Fernandes,V.M.Gomes,Intracellularlocalizationofalipidtransfer proteininVignaunguiculataseeds,Physiol.Plant.122(2004)328–336.
[37]M.S.Diz,A.O.Carvalho,S.F.F.Ribeiro,M.Cunha,L.Beltramini,R.Rodrigues, V.V.Nascimento,O.L.T.Machado,V.M.Gomes,Characterisation: immunolocalisationandantifungalactivityofalipidtransferproteinfrom chilipepper(Capsicumannuum)seedswithnovel␣-amylaseinhibitory properties,Physiol.Plant.142(2011)233–246.
[38]U.Zottich,M.DaCunha,A.O.Carvalho,G.B.Dias,N.C.M.Silva,I.S.Santos,V.V. Nacimento,E.C.Miguel,O.L.T.Machado,V.M.Gomes,Purification: biochemicalcharacterizationandantifungalactivityofanewlipidtransfer protein(LTP)fromCoffeacanephoraseedswith␣-amylaseinhibitor properties,Biochim.Biophys.Acta1810(2011)375–383.
[39]V.Arondel,C.Vergnolle,C.Cantrel,J.C.Kader,Lipidtransferproteinsare encodedbyasmallmultigenefamilyinArabidopsisthaliana,PlantSci.157 (2000)1–12.
[40]F.Vignols,G.Lung,S.Pammi,D.Tremousaygue,F.Grellet,J.C.Kader,P. Puigdomenech,M.Delseny,Characterizationofaricegenecodingforalipid transferprotein,Gene142(1994)265–270.
[41]S.Gorjanovic,E.Spillner,M.V.Beljanski,R.Gorjanovic,M.Pavlovic,G. Gojgic-Cvijanovic,Maltingbarleygrainnon-specificlipid-transferprotein (ns-LTP):importanceforgrainprotection,J.Inst.Brew.111(2005)99–104.
[42]R.Asero,G.Mistrello,D.Roncarolo,S.DeVries,M.Gautier,C.Ciurana,E. Verbeek,T.Mohammadi,V.Knul-Brettlova,J.H.Akkerdaas,I.Bulder,R.C. Aalberse,R.vanRee,Lipidtransferprotein:apan-allergeninplant-derived foodsthatishighlyresistanttopepsindigestion,Int.Arch.AllergyImmunol. 122(2001)20–32.
[43]K.Lindorff-Larsen,J.R.Winther,Surprisinglyhighstabilityofbarleylipid transferproteinLTP1,towardsdenaturant,heatandproteases,FEBSLett.488 (2001)145–148.
[44]E.Pastorello,C.Pompei,V.Pravettoni,L.Farioli,A.Calamari,J.Scibilia,A.M. Robino,A.Conti,S.Iametti,D.Fortunato,S.Bonomi,C.Ortolani,Lipid-transfer proteinisthemajormaizeallergenmaintainingIgE-bindingactivityafter cookingat100◦C,asdemonstratedinanaphylacticpatientsandpatientswith
positivedouble-blind.Placebo-controlledfoodchallengeresults,J.Allergy Clin.Immunol.112(2003)775–783.
[45]S.Scheurer,I.Lauer,K.Foetisch,M.S.Moncin,M.Retzek,C.Hartz,E.Enrique,J. Lidholm,A.Cistero-Bahima,S.Vieths,StrongallergenicityofPruav3,thelipid transferproteinfromcherry,isrelatedtohighstabilityagainstthermal processinganddigestion,J.AllergyClin.Immunol.114(2004)900–907.
[46]X.Yang,J.Li,X.Li,R.She,Y.Pei,Isolationandcharacterizationofanovel thermostablenon-specificlipidtransferprotein-likeantimicrobialprotein frommotherwort(LeonurusjaponicusHoutt)seeds,Peptides27(2006) 3122–3128.
[47]P.Sterk,H.Booij,G.A.Scheleekens,A.VanKammen,S.C.DeVries,Cell-specific expressionofthecarrotEP2lipidtransferproteingene,PlantCell3(1991) 907–921.
[48]S.Torres-Schumann,J.A.Godoy,J.A.Pintor-Toro,Aprobablelipidtransfer proteingeneisinducedbyNaClinstemsoftomatoplants,PlantMol.Biol.18 (1992)749–757.
[49]F.García-Olmedo,A.Molina,A.Segura,M.Moreno,Thedefensiveroleof nonspecificlipid-transferproteinsinplants,TrendsMicrobiol.3(1995) 72–74.
differentdevelopmentalpatternsofexpression,PlantPhysiol.116(1998) 1461–1468.
[51]A.White,M.A.Dunn,K.Brown,M.A.Hughes,Comparativeanalysisof genomicsequenceandexpressionofalipidtransferproteingenefamilyin winterbarley,J.Exp.Bot.45(1994)1885–1892.
[52]R.Ghosh,V.A.Bankaitis,Phosphatidylinositoltransferproteins:negotiating theregulatoryinterfacebetweenlipidmetabolismandlipidsignalingin diversecellularprocesses,Biofactors37(2011)290–308.
[53]I.D.G.Duarte,M.Nakamura,S.H.Ferreira,Participationofthesympathetic systeminaceticacid-inducedwrithinginmice,Braz.J.Med.Biol.Res.21 (1987)341–343.
[54]V.Martinez,S.V.Coutinho,S.Thakur,J.S.Mogil,Y.Taché,E.A.Mayer, Differentialeffectsofchemicalandmechanicalcolonicirritationon behavioralpainresponsetointraperitonealaceticinmice,Pain81(1999) 179–186.
[55]Y.Ikeda,A.Ueno,H.Naraba,S.Oh-Ishi,InvolvementofvanilloidreceptorVR1 andprostanoidsintheaceticacid-inducedwrithingresponseofmice,LifeSci. 69(2001)2911–2919.
[56]S.Taissin-Moindrot,A.Caille,J.P.Douliez,D.Marion,F.Vovelle,Thewide bindingpropertiesofawheatnonspecificlipidtransferprotein,Eur.J. Biochem.267(2000)1117–1124.
[57]M.Shibata,T.Ohkubo,H.Takahashi,R.Inoki,Modifiedformalintest: characteristicbiphasicpainresponse,Pain38(1989)347–352.
[58]A.Tjolsen,K.Hole,Animalmodelsofanalgesia,in:M.J.Besson,A.Deckeson (Eds.),ThePharmacologyofPain,Springer-Verlag,Berlin,Germany,1997,pp. 1–20.
[59]A.Barber,R.Gottschilich,Opioidagonistsandantagonists:anevaluationof theirperipheralactionsininflammation,Med.Res.Rev.12(1992)525–562.
[60]O.Pol,J.R.Palacio,M.M.Puig,Theexpressionofdelta-andkappa-opioid receptorisenhancedduringintestinalinflammationinmice,J.Pharmacol. Exp.Ther.306(2003)455–462.