Original
article
Induction
of
cancer
cell
death
by
apoptosis
and
slow
release
of
5-fluoracil
from
metal-organic
frameworks
Cu-BTC
Fla´via
Raquel
Santos
Lucena
a,*
,
Larissa
C.C.
de
Arau´jo
a,
Maria
do
D.
Rodrigues
a,
Teresinha
G.
da
Silva
a,
Vale´ria
R.A.
Pereira
b,
Gardeˆnia
C.G.
Milita˜o
c,
Danilo
A.F.
Fontes
d,
Pedro
J.
Rolim-Neto
d,
Fausthon
F.
da
Silva
e,
Silene
C.
Nascimento
aaAntibioticsDepartment,PernambucoFederalUniversity,50670-901Recife-PE,Brazil bImmunologyDepartment,PernambucoFederalUniversity,50670-901Recife-PE,Brazil
cPhysiologyandPharmacologyDepartment,PernambucoFederalUniversity,50670-901Recife-PE,Brazil dPharmaceuticalSciencesDepartment,PernambucoFederalUniversity,50590-470Recife-PE,Brazil eFundamentalChemistryDepartment,PernambucoFederalUniversity,50590-470Recife-PE,Brazil
1. Introduction
5-fluorouracil(5-FU),apyrimidineanalog,isoneofthebroad spectrum anticancer drugs [1–3] used in the treatment of malignancies like glioblastoma[4] and breast cancer [5]. Since 5-FU interferes with DNA synthesis, it principally acts as a thymidylatesynthaseinhibitor[6,7].However,shorthalf-life,wide distribution,andvarioussideeffectslimititsmedicalapplicability [8–10].Toovercometheabove-mentionedlimitations,anample numberofstudieshasbeencarriedoutonsustaineddrugdelivery systemsfor5-fluorouracil.
Rahmanetal.preparedandevaluatethecolon-specific micro-spheresof 5-fluorouracilforthetreatment ofcoloncancer. The authorsobservedthattheresultsclearlyindicatethatthereisgreat potentialindeliveryof5-FUtothecolonicregionasanalternative totheconventionaldosageform[11].
Lekha-Nair et al. studied the biological evaluation of 5-fluorouracil nanoparticles for cancer chemotherapy and its dependenceonthePLGAcarrier(nanoparticleswithdependence on the lactide/glycolide combination of PLGA). 5-FU-entrapped PLGAnanoparticlesshowedsmallersizewithahighencapsulation efficiency[12].Horcajada etal. reported theefficiencyofsome MOFsascarriersofdrugswastestedusingantiviralandantitumor drugs:bulsufan,azidothymidine-trisfofato,cidovirand doxorubi-cin. According totheauthors,incorporation of someantitumor drugs usedforcancerssuchasleukemiaand myelomaintothe MIL-100MOFwasconsideredextremelyhigh.[13].
ARTICLE INFO
Articlehistory:
Received14May2013
Accepted17June2013
Keywords:
Cu-BTCMOF
5-fluorouracil Apoptosis
Anti-inflammatoryandcytotoxicactivities
Slowrelease
ABSTRACT
Thisstudyaimedtoevaluatethemechanismassociatedwithcytotoxicactivitydisplayedbythedrug 5-fluorouracil incorporated in Cu-BTC MOF and itsslow deliveryfrom the Cu-BTCMOF. Structural characterization encompasses elementalanalysis (CHNS), differential scanning calorimetry (DSC), thermogravimetricanalysis(TG/DTG),Fourniertransforminfrared(FIT-IR)andX-raydiffraction(XRD) wasperformedtoverifytheprocessofassociationbetweenthedrug5-FUandCu-BTCMOF.Flow cytometrywasdonetoindicatethatapoptosisisthemechanismresponsibleforthecelldeath.The releaseprofileofthedrug5-FUfromCu-BTCMOFfor48hourswasobeisant.Also,theanti-inflammatory activitywasevaluatedbytheperitonitistestingandtheproductionofnitricoxideandpro-inflammatory cytokinesweremeasured.Thechemicalcharacterizationofthematerialindicatedthepresenceofdrug associatedwiththecoordinationnetworkinaproportionof0.82g5-FUper1.0gofCu-BTCMOF.The cytotoxictestswerecarriedoutagainstfourcelllines:NCI-H292,MCF-7,HT29andHL60.TheCu-BTC MOFassociateddrugwasextremelycytotoxicagainstthehumanbreastcanceradenocarcinoma (MCF-7)celllineandagainsthumanacutepromyelocyticleukemiacells(HL60),cancercellswerekilledby apoptosismechanisms.Thedrugdemonstratedaslowreleaseprofilewhere82%ofthedrugwasreleased in48hours.TheresultsindicatedthatthedrugincorporatedinCu-BTCMOFdecreasedsignificantlythe numberofleukocytesintheperitonealcavityofrodentsaswellasreducedlevelsofcytokinesandnitric oxideproduction.
ß2013ElsevierMassonSAS.Allrightsreserved.
* Correspondingauthor.AntibioticsDepartment,PernambucoFederalUniversity,
50670-901Recife-PE,Brazil.
E-mailaddress:flavia_19lucena@yahoo.com.br(F.R.S.Lucena).
Available
online
at
www.sciencedirect.com
0753-3322/$–seefrontmatterß2013ElsevierMassonSAS.Allrightsreserved.
Extensive researchwithin thelast two decadeshasrevealed that most chronic illnesses, including cancer, diabetes, and cardiovascular and pulmonary diseases, are mediated through chronicinflammation.Thus,suppressingchronicinflammationhas thepotential todelay, prevent, and even treat variouschronic diseases,includingcancer, several drugs including: dexametha-soneand doxorubicinhave beenpackagedasnanoparticlesand proven to be useful in ‘‘nano-chemoprevention’’ and ‘‘nano-chemotherapy’’[13,14].
Thusinthisperspective,thisstudyaimedtoevaluatetheaction mechanism associated with the antitumor activity of the 5-fluorouracil incorporated into Cu-BTC and a possible anti-inflammatoryactivity,sincestudiesintheliteratureshowaclose relationshipbetween inflammationand the appearanceof new cancers.
2. Materialsandmethods
2.1. Systemdrug-MOFused
Theassociationwasperformedusing5-FU99%(SigmaAldrich) andtheCu-BTCMOF– BasoliteTMC300(688814)producedby BASF.Firstthedrug(300mg)wasdissolvedin150mLofMilli-Q ultrapurewaterand100mgofCu-BTCwereadded,inaproportion of3:1(w/w–drug/MOF).Theresultingsuspensionwaskeptunder stirring at room temperature for seven days. Aliquots were removed after seven days, then centrifuged (4500rpm) for 20minutesandthesupernatantwasanalysedbyUV-Vis spectro-scopy to determine the amount of drug present in the metal organicframework.Theanalysiswasperformedintriplicate.
2.2. Chemicalcharacterization
Elementalanalysis(CHNS)measurementswereperformedina CEInstrumentselementalanalyser,modelEA1110.FIT-IRspectra were collected from KBr discs utilizing a Bruker spectrophot-ometer(IFS-66) withFouriertransform (spectralrange4000 to 400cm–1). The resultswere analyzed using the OPUS Spectro-scopicSoftwarefromBruker.TG/DTGcurveswereobtainedinthe temperaturerangebetween20–9008CusingaShimadzuTGA50 thermobalanceunderadynamicnitrogenatmosphere(50mLmin– 1) at a heating rate of 10
8C min–1 and an alumina crucible containing approximately 3mg of the sample. The instrument calibrationwasperformedbeforethetestsusingacalciumoxalate monohydrate standard, according to the American Society for TestingandMaterials.TheDSCdatawererecordedinaDSC50cell (Shimadzu)inthe25–6008Ctemperaturerangeunderadynamic nitrogen atmosphere (100mL min–1) in an alumina crucible containing2.0mgofthesample;aheatingrateof108Cmin–1was used. The powder patter XDR were obtained in a Bruker D8 AdvanceX-Raydiffractmeter(K
a
(Cu)1,54A˚),intherange58to 508,step0.028andacquisitiontime1second.2.3. Deliverystudy(invitro)
For in vitrorelease studywasusedtransparenthard gelatin capsules containing 110.97mg of incorporation, which was equivalent to 50mg of 5-FU. Dissolution (dissolutor, Varian, model VK-7000/7010/750D) was performed in PBS (pH: 6.8), volumeof 500mLat3780.5andscrewspeedof100RPM.The collectionswereperformedondays0.5,1.5,5.5,15,20,24,39,44and 48hours.Ateverygiventime,asamplewascollectedfrom2.5mLto beanalyzed onHPLC,followed byreplacementofthedissolution medium. The samples were filtered on Millipore1 membrane 0.22mminporesize,13mm(Millex).Allanalyzeswereperformed intriplicate.
Thesamplesobtainedfromdissolutionstudieswereindividually subjectedtoanalysisbyHPLC–DADusingisocraticmobilephase 85%ofacetonitrile:15%ofwater(v/v)witha2mL/minflow,the oventemperaturewas258C,withastationaryphaseC18column (2504.6mm/5
m
m),and20m
Linjectionvolumeofthesamples. TheLCsystemusedwasahighperformanceliquidchromatograph (HPLC)Shimadzu1equippedwithaquaternarypumpmodelLC– 20ADVP, powered by helium degasser model DGU – 20A, PDA detector model SPD – 20AVP, oven model CTO-20A SVP, auto samplermodelSIL–20ADVPandcontrollermodelSCL–20AVP.The datawereprocessedbysoftwareShimadzu1LCsolution2.0.2.4. Cytotoxicactivityandapoptosistestsverification
Thecytotoxictestswerecarriedoutagainstfourcelllines: NCI-H292cells(lungmucoepidermoidcarcinoma),MCF-7cells(breast adenocarcinoma),HT29cells (colon adenocarcinoma)and HL60 cells (promyelocytic leukemia). The cells were maintained in DMEM – Dulbecco’s Modified Eagle Medium. Samples were considered cytotoxic when the rates of cell growth inhibition exceeded40%,accordingtotheprotocolestablishedbyGeran[15]. Theapoptosistestwasdoneused5
m
g/mLand10m
g/mL.HL60 (3105cells/mL)wereplatedonto24-welltissuecultureplates and treated with (Cu-BTC MOF; 5-fluorouracil (5-FU) and 5-FU+Cu-BTC).Afterthe72hincubationperiod,cellswerepelleted andresuspendedin1BindingBuffer.Thepelletwasincubatedfor 10minuteswith5m
Loftheannexin-Vand10m
Lofthepropidium iodide(AnnexinV-FITCApoptosisDetectionKit-SIGMA).CellfluorescencewasdeterminedbyflowcytometryinaFACS Caliburcytometer(Becton,DickinsonandCompany,NewJersey, USA) usingthe Cell Quest software.Ten thousandevents were evaluatedperexperimentandcellulardebriswasomittedfromthe analysis.
2.5. Animalsusedinthepharmacologicaltests
Forthetests,Swissalbinofemalemice(Musmusculus)were used, weighing between 30–35g, withan average age of two months.Theanimalsweremonitoredaccordingtothenormsof the National Institute of Health Guide for Care and Use of LaboratoryAnimals.Theexperimentswereconductedaccordingto theNationalCancerInstituteprotocol[16]andapprovedbythe UFPE-Animal ExperimentationEthic Committee:23076.024149/ 2012-48.
2.6. Peritonitisinducedbycarrageenan
Inthisassay,wereusedsixgroupsofsixanimals,treatedgroups receivedorally25,50and75mg/kgof(5-FU+Cu-BTCMOF).The othersgroupreceivedorallysalinevehicle(0.1mL/10g)usedas negative control and dexamethasone (0.5mg/kg) as positive control. One hour after the treatment, the inflammation was induced by intraperitoneal (i.p) application of 0.1mL/10g of carrageenan(1%insalinesolution).
After4hourstheanimalswereeuthanizedinachamberofCO2 so was injected into the peritoneal cavity 3mL of phosphate bufferedsalinePBScontainingEDTAtocollecttheperitonealfluids. The total leukocyte number was determined in hematology analyzer Micros 601. The exudates were centrifuged and the supernatant stored at –208C for analysis of nitric oxide and citokineslevels[17].
2.7. Analysisofnitricoxideproduction
samplesweremixedwithanequalvolumeofGriessreagentina 96-wellmicrotiterplateandincubatedatroomtemperaturefor 10minutes.Theabsorbancewasreadat540nmusinganELISA reader and the nitrite concentrations were determined by comparisonwithastandardcurveofsodiumnitrite[17].
2.8. Measurementofcytokines
TheconcentrationsofTNF-
a
andIL-1b
weremeasuredusing sandwichELISA kits, specificfor mice, accordingto the manu-facturer’sinstructions (eBioscience,San Diego,California, USA). Thedetectionlimitusedwas8-1000pg/mL.Theexperimentswere doneintriplicate[18].2.9. Statisticalanalysis
Theresultsare presentedas meanstandarddeviation(SD). Datawereanalyzedusingthesoftwaregraphpadprismv5.0 One-way ANOVA followed by the Newman-Keuls test were used to evaluatethedifferencesamongthetreatments.Pvalues<0.05were
consideredstatisticallysignificant.
3. Resultsanddiscussion
Drugloading,expresseding5-FUpergramCu-BTC,afterseven daysofstirringwas0.8221.TheCu-BTCMOFcontainsC,OandH atomsbutlacksNatoms,therefore,thepresenceofthedrugonthis structure was indicated by the appearance of N atoms in the elementalanalysis.Thetheoreticalandexperimentalresultsare representedin Table1and conformtotheproportions demon-stratedintheTGAanalysis.
Fig.1showstheFTIRspectraoftheprecursorsandtheproduct obtained.IntheinfraredspectrumofCu-BTC,thereisaverystrong bandcenteredat3450cm 1assignedtotheOHstretchingfrom watermolecules. Thebroadnessof thisbandis consistentwith extensive hydrogen bonding from both coordinated and non-coordinatedwatermolecules.Bandsat1644cm 1and1373cm 1 areassignedtoasymmetricandsymmetric(OCO-)stretchingfrom carboxylategroups.Additionalbandsat1587and1450cm 1are duetoC=Cstretchingfromthearomaticrings.TheFTIRspectrum for5-FUischaracterizedbyabroadbandat3133cm 1,assignedto NHstretching,inadditiontobandsat1724cm 1and1660cm 1 arisingfromthetwodistinctcarbonylgroupsin5-FUstructure. ThespectrumforCu-BTC/5-FUsampleshowsbandsfrombothhost andguestspecies,someofthemshiftedduetotheoccurrenceof intermolecular interactions. The spectrum exhibits the NH stretching band centered at 3150cm 1 and at higher wave numbersanenvelopeofOHstretchingbands(centeredat3550, 3498,3460and3388cm 1).Thosebandsareprobablyduetothe coordinatedwatermolecules,whichwerepartiallyresolvedafter removal of the non-coordinated ones during the association process,asshallbediscussedonthebasisofthermalanalysis.At lowerwavenumbers,wecanobservethecarbonylbandsfrom 5-FUat1708and1660cm 1inadditiontothecarboxylatebands
from Cu-BTC at 1631, 1617 and 1382cm 1. Bands from both speciesinthisregionareslightlyshiftedasmentioned[19].
Thermalanalysisdatacomplementedcharacterizationallowing ustomakeapictureofthesampleCu-BTC/5-FU.Fig.2showsDSC curvesforthedrug5-fluorouracil,showingthatthefreedrugstarts todecomposeatnearly2608CwithapeakinDTGat3038C.DSCfor 5-FU shows that actually the solid drug melts at 2798C and decomposessubsequentlyaftermelting.
AnalyzingthethermalanalysiscurvesTG/DTG(Fig.3)andDSC (Fig.2)forCu-BTC,weseethatthesamplestartstoloseadsorbed andnon-coordinatedwatermoleculesbelow1008C.Afterlosing nearly30%ofthewholemass,theTGcurveshowsaplateaufrom 1508Cwhichisdisturbedbyverydiscretemasslossesat1608C
Table1
CNHSelementalanalysis.
Sample %N %C %H %S
Theoretical
Cu-BTC 0.0 25.8 4.3 0.0
Cu-BTC/5-FU 2.4 30.2 3.0 0.0
Experimental
Cu-BTC.12H2O 0.0 26.3 3.7 0.0
Cu-BTC.6H2O+0.825-FU 2.5 30.7 2.8 0.0
CU-BTC:C18H6Cu3O12/5-FU-C9H3FN2O2.
Fig.1.FTIRspectraofCu-BTCMOF.
and2258C, followedbya majormasslossstarting from3208C (peaked at 3528C and 3818C in the DTG curve), assigned to decomposition of the framework structure. This event can be assignedtobothdecompositionoftheorganicligandsandrelease/ decompositionofthecoordinatedwatermolecules.IntheCu-BTC DSCcurvetheseeventsareobservedasabroadendothermicevent centeredat 778Calong withtwo exothermic onesat 344 and 3548C.FinallyfortheCu-BTC/5-FUsample,thethermalanalysis curvesshowinterestingfeatures.
TGcurveshowsthattheinitialthermaleventobservedforthe originalMOFisabsentwhichsuggeststhatthenon-coordinated water molecules originally present in the pores are probably replacedfor5-FUmoleculesduringtheassociationprocess[20]. ThisisalsoconsistentwithobservationsmadefromFTIRanalysis. Averysmallmasslossisobservedat1638C,whichcanberelated totraceresidualnon-coordinatedwatermolecules,followedbya significantlossat286and2948C.Thelasteventisprobablyrelated todecompositionof5-FUandconsideringthatthepeakisbroad and shows two unresolved minima, it suggests two structural situationsfor thismolecule (seeDSCdiscussion). Anothermass lossisobservedat3708CduetothedecompositionoftheMOF. Boththedecompositionofthehostandoftheguestareshiftedin comparisonwiththefreesamples.
FromDSCcurveforCu-BTC/5-FU(Fig.2),wecanobservethe presenceofseveral endothermic events (108, 177,233,2798C) alongwithanexothermiceventat3528C.Thesharpendothermic
event at 2798C (drug melting) suggests that despite a careful isolationprocedure,some5-FUcrystalsmayhaveremainedsome free molecules (possibly physical adsorbed) in Cu-BTC/5-FU sample.AlthoughpreviousworkswithotherMOFshavinglarger poressuggestedthatseveralmoleculescouldoccupytheporesand thesurfacearea.Thusweproposeherethatoursamplecontainsa slight fraction of 5-FU crystals, probably as a result of the equilibriumbetweenthefreeandassociatedspecieswhichmakes difficultacompletepurification.Despitethis,itisclearthatdrug associationwiththemetalorganicframework(Cu-BTC)occurstoa greatextentascanbeinferredfromsignificantchangesintheDSC curve, in special in the interval of water release and MOF decomposition.
Theadsorptionprocessresultsfromtheinteractionbetween theguestmoleculeandtheadsorbentmaterial,inourcase,the Cu-BTC and 5-FU. Those interactions, in general, are very week (physisorption), but, in some cases, the host-gest interactions affinityisstrongandduethechemisorptionandstructuralchanges may occur. In this perspective, the X-ray powder diffraction becomesapowerfultooltoinvestigatethosecrystalline transfor-mations.
TheFig.4(inblack)showstheexperimentalpowderpatternof theCu-BTCusedinthe5-FUincorporation.Thisdiffractogram,as expected,showsallpeakscorrespondingtothe[Cu2(BTC)3(H2O)3] structure (cubic,space groupFm-3m)synthesized byWilliams etal.[12].Themostintensepeaksarelocatedin11.68,9.58and6.78 relatedtothe[222],[220]and[200]diffractionplans,respectively. After the drug incorporation, the samples was collected the powder pattern, and the result (Fig. 4, in red) shows different diffractionpeaksregardingtotheCu-BTC.Althoughthesample stillhasstrongcrystallinecharacter,thisresultsuggeststhatthere wasastructuralchangeintheCu-BTCduringtheincorporation process,probablyduetothesupramolecularinteractionsbetween thedrugandtheMOF.
The drug incorporation is confirmed by the intense signal locatedin28.88,relatedtothe[220]diffractionplaneofthe5-FU, inagreementwiththeliterature[21,22].Thisobservationisin good agreement with IR-spectroscopy, TGA and elementary analysisresults.After thedissolutiontest,the incorporated 5-FU wasreleasedand,again,wascollected thepowderpattern, shownintheFig.4(inblue).Asexpected,wasnotobservedthe diffractionpeakofthedrug,whichisingoodagreementwiththe dissolutiontestresults,andonceagain,wasdetectedstructural changesintheCu-BTC,experiencingaslightamorphizationinthe structure.
Fig.3.ThermalanalysiscurvesTG(A)andDTG(B)for5-FU;Cu-BTCand
Cu-BTC/5-FU.
Fig.4.PowderpatternXDR(black:C300)–Cu-BTCMOF;(red:incorporated)–
Thereleaseprofilewasconductedinordertoascertainwhether the drug is output from the system in the physiological environment.Theexperimentdemonstratedduringtheprolonged drugrelease 48hours. Initially,39.4% of thedrug wasreleased fromtheMOFinthefirst30minute;thiscanbeattributedtothe druginitsfreeformasindicatedinDSCcurve.Then,therelease proceededmoreslowlybeingreleasedabout60%in15hoursof study,thedissolutionreached82.0%at48hours.
Thereleaseof5-FU‘‘invitro’’foundinoursystemresemblesthe oneextendedrelease‘‘invivo’’,whichareofferedtwodosesofthe drug (Fig. 5). The first, called immediate-release initial dose, requiredtoproducethedesiredpharmacologicaleffectwithout causingdamagetothebody.Thesecond,calledmaintenancedose, isreleasedgradually,inordertoprolongtheextentof pharma-cologicalresponse[23].
Table2showstheIC50valuesforinhibitionofcellularactivity ofatleast70%.Weobservedthat5-fluorouracilexhibitedcytotoxic activityagainstMCF-7andHL-60cells.TheIC50valueforthe MCF-7cellswasverylow(1.735
m
g/mL)whenthedrugwasassociated withtheMOF,thiscanbeattributedtoprolongeddrugreleaseas indicatedin dissolutionstudy,allowingthemaintenanceof the biological effect, since the active principle of the drug being releasedslowlyandcontinues.The same was true for the HL-60 cells. The IC50 values decreased significantlywhen thedrug wasassociated withthe network,suggestingthattheassociationbetweenthe 5-fluorour-acilandtheCu-BTCMOFareresponsiblebythemodulationofthe cytotoxicactivityofthedrug(Table2).
Theapoptosistestdemonstratedthat 5-fluorouracil incorpo-ratedintoCu-BTCMOFinducedcelldeathbyapoptosismechanism tothedoseof10
m
g/mL(Fig.6).Theseresultsareaccordingwith theperitonitistestthatshowedadecreaseinlevelsofTNF-a
and NO in the tumor site, which may have contributed to the maintenance of the required levels of these substances in the activationofcaspasethatactivateapoptosis.Experiencesincethenhas indicated that when expressed locally by the cells of the immune system, TNF-
a
has a therapeutic role, however when dysregulatedandsecretedinthecirculation,TNF-a
canmediatea widevarietyof diseases,includingcancer [24].TNF-a
hasitself beenshowntobeoneofthemajormediatorsofinflammationis also producedby tumors andcan actas an endogenoustumor promoter.TheroleofTNF-a
hasbeenlinkedtoallstepsinvolvedin tumorigenesis, including cellular transformation, promotion, survival,proliferation,invasion,angiogenesis,andmetastasis[25]. Consideringthisinformation,theresultsfoundinour experi-mentsdemonstratedthat5-fluorouracilassociatedwithMOF Cu-BTCmayhavefavoredtheprocessofinductionof celldeath by apoptosis.Currently inflammatory processes are rather taken into consideration when one wants to get success in antitumor therapies,chronicinflammationthatbecauseofsomeorgan,like theliver,increasesthenumberofcelldivisionsandthustheriskof mutations.Tumorcellsproducevariouscytokinesandchemokines thatattractleukocytes[26].Thedirectevidencefortheassociation of chronic inflammation with malignant diseases is in colon carcinogenesisin individualswithinflammatorybowel diseases [27]. The emerging concept in clinical trials reveals that inflammationisapotentialtherapeutictargetincancertreatment and anti-inflammatory drugs are thereby potentially useful as adjuvanttherapy[24].
The peritonitis tests was conducted in order to verify the process of secondary mechanism of action by which the drug embeddedincoordinationnetworkhasitscytotoxicactivitywhich may be enhanced by modulation of immune cells such as polymorphonuclearleukocytes.Throughthistest,wesuggestthat thecytotoxicandantitumoractivityofthedrugisrelatedtothe routeofleukocyteactivationorsuppressionoftheinflammatory process,whichiscloselyrelatedtotheprocessesrelatedtotumor angiogenesis,parallelproductionofinflammatoryinterleukinsand TNF-
a
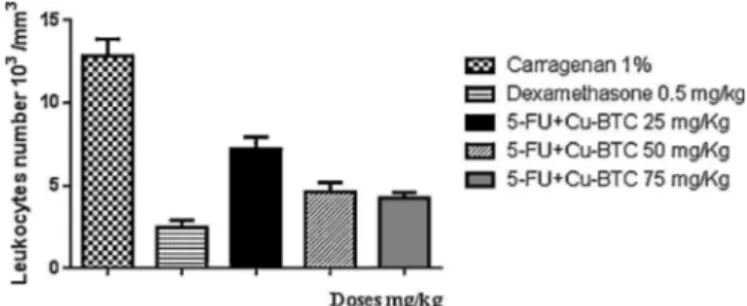
,IL-6amongothers[25].Theresultsshowedthatapossiblewayofactionofthedrug incorporatedinthenetworktocoordinatetheperformanceofthis system is as an anti-inflammatory agent, since it caused a reductionofinflammationin43.7,64and66.8%respectivelyfor dosesof25,50and75mg/kgwhencomparedtonegativecontrol groupwithinflammationinducedbycarrageenan1%insaline.The inhibitionofleukocytedataisdescribedinFig.7.
Tumor-associatedmacrophagesareasignificantcomponentof inflammatoryinfiltratesinneoplastictissuesandarederivedfrom monocytes that arerecruited largely by monocyte chemotactic protein (MCP) chemokines. The pro-inflammatory cytokines, includingtumornecrosisfactor-alpha(TNF-
a
)andinterleukin-6 (IL-6), induce direct effects on stromal and neoplastic cells in additiontotheirrolesinregulatingleukocyterecruitment[14].Fig.5.Dissolutionprofileof5-fluorouracilfromCu-BTCMOF.
Table2
IC50(mg/mL)comparedtothehumantumorlines.
Cellline CU-BTC 5-FU Cu-BTC/5-FU
MCF-7 3.49a 20 1.735b
HT-29 > 25 > 25 > 25
HL-60 > 25 8.601 4.615
NCI-H292 > 25 > 25 > 25
aVerytoxic.
b Toxic.
Thedirectevidencefortheassociationofchronic inflamma-tion with malignant diseases is in colon carcinogenesis in individualswithinflammatoryboweldiseases [25].The emer-ging concept in clinical trials reveals that inflammation is a potential therapeutic target in cancer treatment and anti-inflammatorydrugsare therebypotentiallyusefulas adjuvant therapy[28].
The exudates removed from the abdominal cavity of the animalssubjectedtothetestofcarrageenaninducedperitonitis wereanalyzed and levelsof nitricoxide and pro-inflammatory cytokineshavebeenshowninTables3and4.Itwasobservedthat therewasastatisticallysignificantdecreaseinNOproductionand cytokine treated groups when considered in doses of 25 and 75mg/kgofbodyweight.
These results being in agreement with the results on the numberofwhitebloodcellsfoundintheperitonealcavityanimals, indicatingapossibleanti-inflammatoryactionofthissystem.
4. Conclusions
As notedin theresultsobtained,5-fluorouracil incorporated intotheCu-BTCMOFappearstobeagreattoolinthetreatmentof cancer,sinceweobtainedarateoptimalencapsulation,together withacontrolledreleaseofdrugfromthenanocarrier.
Nanoparticletherapeuticsisanemergingtreatmentmodalityin cancer and other inflammatory disorders. The National Cancer Institutehasrecognizednanotechnologyasanemergingfieldwith the potential to revolutionize modern medicine for detection, treatment,andpreventionofcancer[29].Moreselectivetherapies,
suchasangiogenesis inhibitors,vascular disrupting agents,and estrogenicHER-2-targetedtherapieshavebeendevelopedtotreat cancer,theseapproacheshaveincreasedpatientsurvivalbecause oftreatmentefficacy[30].
Mostsolidtumorspossessuniquefeatures,includingenhanced angiogenesis, defective vasculature and lymphatic, increased vascular permeability, which stimulate their growth.Rationally designednanoparticlescantakeadvantageofthesetumorfeatures todeliverchemotherapeuticsselectivelyandspecifically[14].
Theseresultstogetherthedeath of cancer cells throughthe mechanismofapoptosisandanti-inflammatoryactivityassociated withdecreasedlevelsofthepro-inflammatorycytokines,directly related to the maintenance of chronic inflammation processes, indicatethissystemasaviablealternativewhenconsideringan idealsystemforcancertreatment,sinceitshowedthecontrolled release properties, low toxicity to normal cells and also an indication of anti-inflammatory activity which couldact as an adjuvanttreatment[30].
Disclosureofinterest
Theauthorshavenotdeclaredanyconflictsofinterest.
References
[1]CaiC,ZhouK,WuY,WuL.Enhancedlivertargetingof5-fluorouracilusing galactosylatedhumanserumalbuminasacarriermolecule.JDrugTarget 2006;14:55–61.
[2]CaoS,RustumYM.Synergisticantitumoractivityofirinotecanin combination-with5-fluorouracilinratsbearingadvancedcolorectalcancer:roleofdrug sequenceanddose.CancerRes2000;60:3717–21.
[3]KubotaT.Recentprogressincombinationtherapyoflow-doseCDDP/5-FUin Japan.Theoreticalbasisforlow-doseCDDP/5-FUtherapy.JpnJCancer Che-mother1999;26:1536–41.
[4]ShapiroWR,GreenSB,BurgerPC,etal.Arandomizedcomparisonof intra-arterialversusintravenouswithorwithoutintravenous5-fluorouracil,for newlydiagnosedpatientswithmalignantglioma.JNeurosurg1992;76:772– 81.
[5]Hutchins LF, GreenSJ,RavdinPM, etal.Randomized, controlledtrial of cyclophosphamide,methotrexate,andfluorouracilversuscyclophosphamide, doxorubicin,andfluorouracilwithandwithouttamoxifenforhigh-risk, node-negativebreastcancer:treatmentresultsofIntergroupProtocolINT-0102.J ClinOncol2005;23:8313–21.
[6]LongleyDB,HarkinDP,JohnstonPG.5-fluorouracil:mechanismsofactionand clinicalstrategies.NatRevCancer2003;3:330–8.
[7]ParkerWB,ChengYC.Metabolismandmechanismofactionof5-fluorouracil. PharmacolTher1990;48:381–95.
[8]FataF,RonIG,KemenyN,O’ReillyE,KlimstraD,KelsenDP. 5-fluorouracil-inducedsmallboweltoxicityinpatientswithcolorectalcarcinoma.Cancer 2000;86:1129–34.
[9]PaoloAD,DanesiR,FalconeA,etal.Relationshipbetween5-fluorouracil disposition,toxicityanddihydropyrimidinedehydrogenaseactivityincancer patients.AnnOncol2001;12:1301–6.
[10]vanKuilenburgABP,HaasjesJ,RichelDJ,etal.Clinicalimplicationsof dihy-dropyrimidinedehydrogenase(DPD)deficiencyinpatientswithsevere 5-fluorouracil-associatedtoxicity:identificationofnewmutationsintheDPD gene.ClinCancerRes2000;6:4705–12.
[11]RahmanZ,KohliK,KharRK,AliM,CharooNA,ShamsherAAA.Characterization of5-fluorouracilmicrospheresforcolonicdelivery.AAPSPharmSciTech 2006;7(2):E1–9.
[12]NairLK,JagadeeshanS,NairSA,VinodKumarGS.Biologicalevaluationof 5-fluorouracilnanoparticlesforcancerchemotherapyanditsdependenceonthe carrier,PLGA.IntJNanomed2011;6:1685–97.
[13]HorcajadaP,ChalatiT,SerreC,GilletB,SebrieC,BaatiT,etal.Porous metal-organicframeworknanoscalecarriersasapotencialplatformfordrugdelivery andimaging.NatMater2010;9:172–8.
[14]NairHB,SungB,YadavVR,KannappanR, ChaturvediMM,AggarwalBB. Deliveryofanti-inflammatorynutraceuticalsbynanoparticlesforthe preven-tionandtreatmentofcancer.BiochemPharmacol2010;80(12):1833–43.
[15]GeranRI,GreenbergNH,MacdolnaldMM,SchumacherAM,AbbottBJ. Pro-tocolsforscreeningchemicalagentsandnaturalproductsagainstanimal tumorsandotherbiologicalsystems.CancerChemother1972[Rep.3. proto-cols16and13].
[16]www1.inca.gov.br/inca/arquivos/bioterio.pdf.
[17]UchoˆaFT,SilvaTG,LimaMCA,GaldinoSL,Pitta IR,CostaTD.Preclinical pharmacokineticandpharmacodynamicevaluationofthiazolidinonePG15: ananti-inflammatorycandidate.JPharmPharmac2009;61:339–45.
[18]AggarwalBB.Sinallingpathways oftheTNFsuperfamily:adouble-edged sword.NatRevImmunol2003;3(9):745–56.
Fig.7.Peritonitistestleukocytesnumberdosesof5-FU+Cu-BTC.
Table3
Effectof5-FU+CuBTContheNOproductioninthecarrageenantest.
Treatment NO(mM)
Controlgroup 21.303.62
Dexamethasone 1.770.44a
25mg/kg(5-FU+Cu-BTC) 0,780.07a
75mg/kg(5-FU+Cu-BTC) 0,630.02a
Dataareexpressedbymeanstandarddeviation(meanstandarddeviation).
aStatisticallysignificantdifferencecomparedtocontrol(P < 0.05).
Table4
Effectof5-FU+CuBTContheTNF-aeIL-1bproductionsinthecarrageenantest
inducedperitonitis.
Compound TNF-a(pg/mL) IL-1b(pg/mL)
Control 502.113.31 751.0015.04
Dexamethasone 126.3913.15a 289.35
18.62a
75mg/kg(5-FU+Cu-BTC) 117.3937.86a 238.70
5.62a
25mg/kg(5-FU+Cu-BTC) 300.926.11a 617.18
12.62a
Dataareexpressedbymeanstandarddeviation(meanstandarddeviation).
aStatisticallysignificantdifferencecomparedtocontrol(P
[19]CamposJ,Domı´nguezJF,GalloMA,EspinosaA.Fromaclassicapproachin cancerchemotherapy towards differentiation therapy: acyclic and cyclic seven-membered5-fluorouracilO,N-acetals.CurrPharmDes2000;6:1797.
[20]ChuiSSY, LoSMF,Charmant JPH, Orpen AG,Williams ID. Achemically functionalizable nanoporous material [Cu3(TMA)2(H2O)3]n. Science 1999;26:1148–50.
[21]DhawaleSC,BankarAS,PatroMN.Formulationandevaluationporous micro-spheresof5-fluorouracilforcolontargeting.IntJPharmTechRes2010;2:1112–8.
[22]AggarwalBB,ShishodiaS,SandurSK,PandeyMK,SehtiG.Inflammationand cancer:howhotisthelink?BiochemPharmac2006;2:1605–21.
[23]AnselHC,AllenJrLV,PopovichNG.Formasfarmaceˆuticase sistemasde liberac¸a˜odefa´rmacos, 8thed.,PortoAlegre:Artmed;2007.
[24]Longhi-balbinotDT,LanznasterD,BaggioCH,SilvaMD,CabreraCH,Facundo VA,etal.Anti-inflammatoryeffectoftriterpene3b,6b,16b -trihydroxylup-20(29)-eneobtainedfromCombretumleprosumMart&Eichinmice.JEthnoph 2012;142(1):59–64.
[25]AminAR,KucukO,KhuriFR,ShinDM.Perspectivesforcancerpreventionwith naturalcompounds.JClinOncol2009;27:2712–25.
[26]ItzkowitzSH,YioX,Inflammation,cancerIV.Colorectalcancerin inflamma-toryboweldisease:theroleofinflammation.AmJPhysiolGastrointestLiver Physiol2004;287:G7–17.
[27]DaneseS,MantovaniA.Inflammatoryboweldiseaseandintestinalcancer:a paradigmoftheYin-Yanginterplaybetweeninflammationandcancer. Onco-gene2010;29:3313–23.
[28]KawasakiES,PlayerA.Nanotechnology,nanomedicine,andthedevelopment ofnew,effectivetherapiesforcancer.Nanomedicine2005;1:101–9.
[29]AnandP,SundaramC,JhuraniS,KunnumakkaraAB,AggarwalBB.Curcumin andcancer: an‘‘old-age’’disease withan‘‘age-old’’solution.CancerLett 2008;267:133–64.